Acute Circadian Disruption Due to Constant Light Promotes Caspase 1 Activation in the Mouse Hippocampus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Analysis of Locomotor Activity Rhythms
2.3. Preparation of Peripheral Blood Films and White Blood Cell (WBC) Counting
2.4. RNA Isolation, Reverse Transcription (RT), and Real-Time PCR (rtPCR)
2.5. Immunoblot Analysis
2.6. Immunofluorescence
2.7. Statistical Analysis
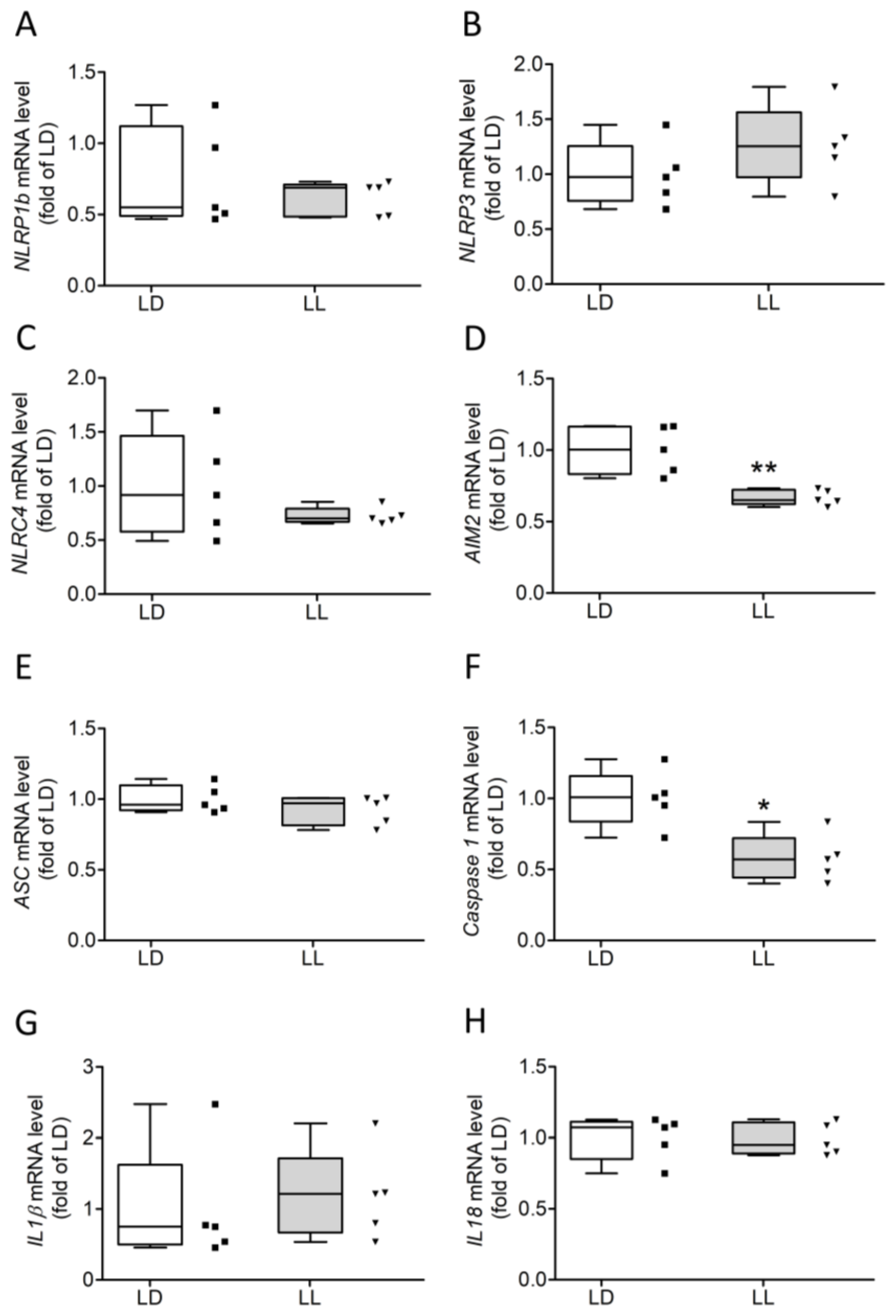
3. Results
3.1. Constant Light Significantly Affects Rhythmic Activity but Not Body Weight and White Blood Cell Composition
3.2. Exposure to LL Mildly Affects Glial Activation
3.3. Constant Light Induces Cleavage of Caspase 1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AIM2 | Absent in melanoma 2 |
| ALAN | Artificial light at night |
| ALS | Amyotrophic laterals sclerosis |
| ARG1 | Arginase 1 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| DAMP | Damage-associated molecular pattern |
| FFT | Fast Fourier transform analysis |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GFAP | Glial fibrillary acidic protein |
| HSP90 | Heat shock protein 90 |
| IBA1 | Ionized calcium-binding adapter molecule 1 |
| IL | Interleukin |
| NOS2 | Nitric oxide synthase 2 |
| LL | 12:12 light/light, constant light |
| LPS | Lipopolysaccharides |
| mtROS | Mitochondrial reactive oxygen species |
| NF-κB | Nuclear factor kappa B |
| NLR | NOD-like receptor |
| PAMP | Pathogen-associated molecular pattern |
| PD | Parkinson’s disease |
| PRR | Pattern recognition receptor |
| ROS | Reactive oxygen species |
| Rev-erba | Nuclear receptor subfamily 1 group D member 1 (NR1D1) |
| SCN | Suprachiasmatic nucleus |
| TLR | Toll-like receptor |
References
- Carter, S.J.; Durrington, H.J.; Gibbs, J.E.; Blaikley, J.; Loudon, A.S.; Ray, D.W.; Sabroe, I. A matter of time: Study of circadian clocks and their role in inflammation. J. Leukoc. Biol. 2016, 99, 549–560. [Google Scholar] [CrossRef]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian Clock Proteins and Immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- Kolbe, I.; Oster, H. Chronodisruption, Metabolic Homeostasis, and the Regulation of Inflammation in Adipose Tissues. Yale J. Biol. Med. 2019, 92, 317–325. [Google Scholar] [PubMed]
- Manfredini, R.; Fabbian, F.; Cappadona, R.; Modesti, P.A. Daylight saving time, circadian rhythms, and cardiovascular health. Intern. Emerg. Med. 2018, 13, 641–646. [Google Scholar] [CrossRef]
- Hergenhan, S.; Holtkamp, S.; Scheiermann, C. Molecular Interactions Between Components of the Circadian Clock and the Immune System. J. Mol. Biol. 2020, 432, 3700–3713. [Google Scholar] [CrossRef] [PubMed]
- Timmons, G.A.; O’Siorain, J.R.; Kennedy, O.D.; Curtis, A.M.; Early, J.O. Innate Rhythms: Clocks at the Center of Monocyte and Macrophage Function. Front. Immunol. 2020, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R. Comparison of light, food, and temperature as environmental synchronizers of the circadian rhythm of activity in mice. J. Physiol. Sci. 2015, 65, 359–366. [Google Scholar] [CrossRef]
- Aschoff, J. Freerunning and Entrained Circadian Rhythms. In Biological Rhythms; Aschoff, J., Ed.; Springer: Boston, MA, USA, 1981; pp. 81–93. [Google Scholar]
- Korf, H.-W.; von Gall, C. Circadian Physiology. In Neuroscience in the 21st Century, 3rd ed.; Pfaff, D.W., Volkow, N.D., Eds.; Springer Science + Business Media: New York, NY, USA, 2021. [Google Scholar]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Finger, A.-M.; Dibner, C.; Kramer, A. Coupled network of the circadian clocks: A driving force of rhythmic physiology. FEBS Lett. 2020, 594, 2734–2769. [Google Scholar] [CrossRef]
- Shearman, L.P.; Sriram, S.; Weaver, D.R.; Maywood, E.S.; Chaves, I.; Zheng, B.; Kume, K.; Lee, C.C.; van der, G.T.J.; Horst; et al. Interacting Molecular Loops in the Mammalian Circadian Clock. Science 2000, 288, 1013–1019. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Okuliarova, M.; Mazgutova, N.; Majzunova, M.; Rumanova, V.S.; Zeman, M. Dim Light at Night Impairs Daily Variation of Circulating Immune Cells and Renal Immune Homeostasis. Front. Immunol. 2020, 11, 614960. [Google Scholar] [CrossRef]
- Mishra, I.; Knerr, R.M.; Stewart, A.A.; Payette, W.I.; Richter, M.M.; Ashley, N.T. Light at night disrupts diel patterns of cytokine gene expression and endocrine profiles in zebra finch (Taeniopygia guttata). Sci. Rep. 2019, 9, 15833. [Google Scholar] [CrossRef]
- Tapia-Osorio, A.; Salgado-Delgado, R.; Angeles-Castellanos, M.; Escobar, C. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Behav. Brain Res. 2013, 252, 1–9. [Google Scholar] [CrossRef]
- Castanon-Cervantes, O.; Wu, M.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef]
- Mizutani, H.; Tamagawa-Mineoka, R.; Yasuike, R.; Minami, Y.; Yagita, K.; Katoh, N. Effects of constant light exposure on allergic and irritant contact dermatitis in mice reared under constant light conditions. Exp. Dermatol. 2021, 30, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.E.; Chiu, W.C. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock 2008, 29, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 2015, 47, e148. [Google Scholar] [CrossRef]
- Fifel, K.; Videnovic, A. Circadian alterations in patients with neurodegenerative diseases: Neuropathological basis of underlying network mechanisms. Neurobiol. Dis. 2020, 144, 105029. [Google Scholar] [CrossRef]
- Fifel, K.; Videnovic, A. Circadian and Sleep Dysfunctions in Neurodegenerative Disorders—An Update. Front. Neurosci. 2020, 14, 627330. [Google Scholar] [CrossRef]
- Valero, J.; Bernardino, L.; Cardoso, F.L.; Silva, A.P.; Fontes-Ribeiro, C.; Ambrósio, A.F.; Malva, J.O. Impact of Neuroinflammation on Hippocampal Neurogenesis: Relevance to Aging and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, S161–S168. [Google Scholar] [CrossRef] [PubMed]
- Schain, M.; Kreisl, W.C. Neuroinflammation in Neurodegenerative Disorders—A Review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; De Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.-W.; Lee, S.-Y.; Manzanero, S.; Tang, S.-C.; Gelderblom, M.; Chunduri, P.; Bernreuther, C.; Glatzel, M.; Cheng, Y.-L.; Thundyil, J.; et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013, 4, e790. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 Inflammasome Links Systemic Low-Grade Inflammation to Functional Decline in Aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef]
- Johann, S.; Heitzer, M.; Kanagaratnam, M.; Goswami, A.; Rizo, T.; Weis, J.; Troost, D.; Beyer, C. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 2015, 63, 2260–2273. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, M.; Du, R.-H.; Qiao, C.; Jiang, C.-Y.; Zhang, K.-Z.; Ding, J.-H.; Hu, G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Muruve, D.A.; Power, C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014, 15, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Thundyil, J.; Lim, K.-L. DAMPs and neurodegeneration. Ageing Res. Rev. 2015, 24, 17–28. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. The inflammasomes. PLoS Pathog. 2009, 5, e1000510. [Google Scholar] [CrossRef]
- Halberg, F.; Johnson, E.A.; Brown, B.W.; Bittner, J.J. Susceptibility Rhythm to E. coli Endotoxin and Bioassay. Exp. Biol. Med. 1960, 103, 142–144. [Google Scholar] [CrossRef]
- Cermakian, N.; Lange, T.; Golombek, D.; Sarkar, D.; Nakao, A.; Shibata, S.; Mazzoccoli, G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol. Int. 2013, 30, 870–888. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gerashchenko, D.; Karpova, S.A.; Konanki, V.; McCarley, R.W.; Sutterwala, F.S.; Strecker, R.E.; Basheer, R. The NLRP3 inflammasome modulates sleep and NREM sleep delta power induced by spontaneous wakefulness, sleep deprivation and lipopolysaccharide. Brain Behav. Immun. 2017, 62, 137–150. [Google Scholar] [CrossRef]
- Beynon, A.L.; Coogan, A.N. Diurnal, age, and immune regulation of interleukin-1β and interleukin-1 type 1 receptor in the mouse suprachiasmatic nucleus. Chronobiol. Int. 2010, 27, 1546–1563. [Google Scholar] [CrossRef]
- Pourcet, B.; Zecchin, M.; Ferri, L.; Beauchamp, J.; Sitaula, S.; Billon, C.; Delhaye, S.; Vanhoutte, J.; Mayeuf-Louchart, A.; Thorel, Q.; et al. Nuclear Receptor Subfamily 1 Group D Member 1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce the Severity of Fulminant Hepatitis in Mice. Gastroenterology 2018, 154, 1449–1464.e20. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Krueger, J.M. Sleep and innate immunity. Front. Biosci. 2011, 3, 632–642. [Google Scholar]
- Cavadini, G.; Petrzilka, S.; Kohler, P.; Jud, C.; Tobler, I.; Birchler, T.; Fontana, A. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc. Natl. Acad. Sci. USA 2007, 104, 12843–12848. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Cheung, Y.M.; Cao, X.; Wu, Y.; Li, C.; Tian, X.Y. REV-ERBα agonist SR9009 suppresses IL-1β production in macrophages through BMAL1-dependent inhibition of inflammasome. Biochem. Pharmacol. 2021, 192, 114701. [Google Scholar] [CrossRef]
- Kou, L.; Chi, X.; Sun, Y.; Han, C.; Wan, F.; Hu, J.; Yin, S.; Wu, J.; Li, Y.; Zhou, Q.; et al. The circadian clock protein Rev-erbα provides neuroprotection and attenuates neuroinflammation against Parkinson’s disease via the microglial NLRP3 inflammasome. J. Neuroinflamm. 2022, 19, 133. [Google Scholar] [CrossRef]
- Song, A.-Q.; Gao, B.; Fan, J.-J.; Zhu, Y.-J.; Zhou, J.; Wang, Y.-L.; Xu, L.-Z.; Wu, W.-N. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J. Neuroinflamm. 2020, 17, 178. [Google Scholar] [CrossRef]
- Liraz-Zaltsman, S.; Alexandrovich, A.G.; Trembovler, V.; Fishbein, I.; Yaka, R.; Shohami, E.; Biegon, A. Regional sensitivity to neuroinflammation: In vivo and in vitro studies. Synapse 2011, 65, 634–642. [Google Scholar] [CrossRef]
- Pfeffer, M.; Zimmermann, Z.; Gispert, S.; Auburger, G.; Korf, H.-W.; von Gall, C. Impaired Photic Entrainment of Spontaneous Locomotor Activity in Mice Overexpressing Human Mutant α-Synuclein. Int. J. Mol. Sci. 2018, 19, 1651. [Google Scholar] [CrossRef]
- Kõressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef] [PubMed]
- Hummel, C.; Leylamian, O.; Pösch, A.; Weis, J.; Aronica, E.; Beyer, C.; Johann, S. Expression and Cell Type-specific Localization of Inflammasome Sensors in the Spinal Cord of SOD1(G93A) Mice and Sporadic Amyotrophic lateral sclerosis Patients. Neuroscience 2021, 463, 288–302. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.M.; Malkani, R.G.; Zee, P.C. Circadian disruption and human health: A bidirectional relationship. Eur. J. Neurosci. 2020, 51, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Vaughn, C.A.; Galan, A.; Daye, G.; Weil, Z.; Nelson, R.J. Nocturnal Light Exposure Impairs Affective Responses in a Wavelength-Dependent Manner. J. Neurosci. 2013, 33, 13081–13087. [Google Scholar] [CrossRef] [PubMed]
- Coomans, C.P.; Houben, T.; Klinken, J.; Pronk, A.C.M.; Havekes, L.M.; Romijn, J.A.; Dijk, K.W.; Biermasz, N.R.; Meijer, J.H.; Berg, S.A.A.v.D.; et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013, 27, 1721–1732. [Google Scholar] [CrossRef]
- Lucassen, E.A.; Coomans, C.P.; van Putten, M.; de Kreij, S.R.; van Genugten, J.H.; Sutorius, R.P.; de Rooij, K.E.; van der Velde, M.; Verhoeve, S.L.; Smit, J.W.; et al. Environmental 24-hr Cycles Are Essential for Health. Curr. Biol. 2016, 26, 1843–1853. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef]
- Fonken, L.K.; Aubrecht, T.G.; Meléndez-Fernández, O.H.; Weil, Z.M.; Nelson, R.J. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythm. 2013, 28, 262–271. [Google Scholar] [CrossRef]
- Borniger, J.C.; Maurya, S.K.; Periasamy, M.; Nelson, R.J. Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol. Int. 2014, 31, 917–925. [Google Scholar] [CrossRef]
- Cissé, Y.M.; Peng, J.; Nelson, R.J. Effects of Dim Light at Night on Food Intake and Body Mass in Developing Mice. Front. Neurosci. 2017, 11, 294. [Google Scholar] [CrossRef]
- Kooijman, S.; van den Berg, R.; Ramkisoensing, A.; Boon, M.R.; Kuipers, E.N.; Loef, M.; Zonneveld, T.C.M.; Lucassen, E.A.; Sips, H.C.M.; Chatzispyrou, I.A.; et al. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc. Natl. Acad. Sci. USA 2015, 112, 6748–6753. [Google Scholar] [CrossRef]
- Kennaway, D.J. Melatonin research in mice: A review. Chronobiol. Int. 2019, 36, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; von Gall, C.; Wicht, H.; Korf, H.-W. The Role of the Melatoninergic System in Circadian and Seasonal Rhythms—Insights from Different Mouse Strains. Front. Physiol. 2022, 13, 883637. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Weil, Z.M.; Nelson, R.J. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav. Immun. 2013, 34, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Jerigova, V.; Zeman, M.; Okuliarova, M. Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 13722. [Google Scholar] [CrossRef]
- Polidarová, L.; Houdek, P.; Sumova, A. Chronic disruptions of circadian sleep regulation induce specific proinflammatory responses in the rat colon. Chronobiol. Int. 2017, 34, 1273–1287. [Google Scholar] [CrossRef]
- Inokawa, H.; Umemura, Y.; Shimba, A.; Kawakami, E.; Koike, N.; Tsuchiya, Y.; Ohashi, M.; Minami, Y.; Cui, G.; Asahi, T.; et al. Chronic circadian misalignment accelerates immune senescence and abbreviates lifespan in mice. Sci. Rep. 2020, 10, 2569. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Gritsenko, A.; Green, J.P.; Brough, D.; Lopez-Castejon, G. Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef]
- Martinon, F.; Tschopp, J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007, 14, 10–22. [Google Scholar] [CrossRef]
- Lopez-Castejon, G. Control of the inflammasome by the ubiquitin system. FEBS J. 2020, 287, 11–26. [Google Scholar] [CrossRef]
- Song, N.; Li, T. Regulation of NLRP3 Inflammasome by Phosphorylation. Front. Immunol. 2018, 9, 2305. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, A.; Yu, S.; Martin-Sanchez, F.; Diaz-Del-Olmo, I.; Nichols, E.-M.; Davis, D.; Brough, D.; Lopez-Castejon, G. Priming Is Dispensable for NLRP3 Inflammasome Activation in Human Monocytes in vitro. Front. Immunol. 2020, 11, 565924. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, S.L.; Feltham, R.; Rashidi, M.; Frank, D.; Liu, Z.; Simpson, D.S.; Ebert, G.; Vince, A.; Herold, M.J.; Kueh, A.; et al. The ubiquitylation of IL-1β limits its cleavage by caspase-1 and targets it for proteasomal degradation. Nat. Commun. 2021, 12, 2713. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.M.; Coll, R.C. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J. Leukoc. Biol. 2020, 108, 937–952. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, S.; Rizvi, S.I. Redox homeostasis in a rodent model of circadian disruption: Effect of melatonin supplementation. Gen. Comp. Endocrinol. 2019, 280, 97–103. [Google Scholar] [CrossRef]
- Verma, A.K.; Singh, S.; Rizvi, S.I. Age-dependent effect of continuous ‘artificial light at night’ on circadian rhythm in male rats: Neuroprotective role of melatonin. Biogerontology 2021, 22, 531–545. [Google Scholar] [CrossRef]
- Adamiak, M.; Ciechanowicz, A.; Skoda, M.; Cymer, M.; Tracz, M.; Xu, B.; Ratajczak, M.Z. Novel Evidence that Purinergic Signaling-Nlrp3 Inflammasome Axis Regulates Circadian Rhythm of Hematopoietic Stem/Progenitor Cells Circulation in Peripheral Blood. Stem Cell Rev. Rep. 2020, 16, 335–343. [Google Scholar] [CrossRef]
- Cearley, C.; Churchill, L.; Krueger, J.M. Time of day differences in IL1beta and TNFalpha mRNA levels in specific regions of the rat brain. Neurosci. Lett. 2003, 352, 61–63. [Google Scholar] [CrossRef]
- Zhang, J.; Pei, L.; Zang, D.; Xue, Y.; Wang, X.; Chen, Y.; Li, J.; Yu, J.; Gao, Q.; Di, W.; et al. Gender Differences of NLRP1 Inflammasome in Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 512097. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocr. 2014, 35, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Markman, J.L.; Shimada, K.; Crother, T.R.; Lane, M.; Abolhesn, A.; Shah, P.K.; Arditi, M. Sex-Specific Effects of the Nlrp3 Inflammasome on Atherogenesis in LDL Receptor-Deficient Mice. JACC Basic Transl. Sci. 2020, 5, 582–598. [Google Scholar] [CrossRef] [PubMed]





| Primer | Sequence (5′ to 3′) | |
|---|---|---|
| AIM2 | Sense: Antisense: | GCAAAACAAAGTGCGAGGAA TTCAAGGAGCAGCATCAGGA |
| ARG1 | Sense: Antisense: | CTCCAAGCCAAAGTCCTTAGAG AGGAGCTGTCATTAGGGACATC |
| ASC | Sense: Antisense: | CTTGTCAGGGGATGAACTCAAAA GCCATACGACTCCAGATAGTAGC |
| Caspase 1 | Sense: Antisense: | CCGTGGAGAGAAACAAGGAGT CCCCTGACAGGATGTCTCCA |
| GFAP * | Sense: Antisense: | CGGAGACGCATCACCTCTG AGGGAGTGGAGGAGTCATTCG |
| GAPDH | Sense: Antisense: | AGGTCGGTGTGAACGGATTTG TGTAGACCATGTAGTTGAGGTCA |
| HSP90 | Sense: Antisense: | TACTACTACTCGGCTTTCCCGT TCGAATCTTGTCCAGGGCATC |
| IBA1 | Sense: Antisense: | ATCAACAAGCAATTCCTCGATGA CAGCATTCGCCTCAAGGACATA |
| IL18 | Sense: Antisense: | TGCCAAAAGGAAGATGATGC ACACAAACCCTCCCCACCTA |
| IL1b | Sense: Antisense: | GACGGACCCCAAAAGATGAA TCCACAGCCACAATGAGTGA |
| NLRC4 | Sense: Antisense: | ATCGTCATCACCGTGTGGAG GCCAGACTCGCCTTCAATCA |
| NLRP1b | Sense: Antisense: | AGCCCTCAAAGATGCCCCTT TTGTGTTCTCAGCCCGCACT |
| NLRP3 | Sense: Antisense: | TGACCCAAACCCACCAGTGT TGTGCAGACCTCCCCAATGT |
| NOS2 | Sense: Antisense: | ACATCGACCCGTCCACAGTAT CAGAGGGGTAGGCTTGTCTC |
| Antibody | Host | Company, Order Number | WB | Target Size (kDa) |
|---|---|---|---|---|
| AIM2 ASC | Rabbit Rabbit | Bioss, Woburn, MA, USA, bs-5986R Adipogen, Fuellinsdorf, Switzerland, AG-25B-0006-C100 | 1:1000 1:1000 | 40 20 |
| Caspase 1 GAPDH GFAP IBA1 IL18 IL1β | Mouse Mouse Goat Rabbit Rabbit Rabbit | Adipogen, Fuellinsdorf, Switzerland, AG-20B-0042-C100 Santa Cruz, Dallas, TX, USA, sc-32233 Abcam, Cambridge, UK, ab53554 Fujifilm Wako, Neuss, Deutschland, 019-19741 Santa Cruz, Dallas, TX, USA, sc-7954 Novus, CO, USA, NB600-633 | 1:1000 1:10,000 1:10,000 1:1000 1:1000 1:1000 | 20, 45 35 55 17 18, 24 17, 31 |
| NLRC4 NLRP1 NLRP3 NF-κB p65 P-NF-κB p65 Vinculin | Rabbit Rabbit Rabbit Rabbit Rabbit Mouse | Merck, Darmstadt, Germany, 06-1125 Novus, CO, USA, NB100-56148 Bioss, Woburn, MA, USA, bs-10021R Cell signaling, Danvers, MA, USA, 8242S Cell signaling, Danvers, MA, USA, 3039S Santa Cruz, Dallas, TX, USA, sc-73614 | 1:1000 1:1000 1:1000 1:1000 1:1000 1:1000 | 116 136 118 65 65 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketelauri, P.; Scharov, K.; von Gall, C.; Johann, S. Acute Circadian Disruption Due to Constant Light Promotes Caspase 1 Activation in the Mouse Hippocampus. Cells 2023, 12, 1836. https://doi.org/10.3390/cells12141836
Ketelauri P, Scharov K, von Gall C, Johann S. Acute Circadian Disruption Due to Constant Light Promotes Caspase 1 Activation in the Mouse Hippocampus. Cells. 2023; 12(14):1836. https://doi.org/10.3390/cells12141836
Chicago/Turabian StyleKetelauri, Pikria, Katerina Scharov, Charlotte von Gall, and Sonja Johann. 2023. "Acute Circadian Disruption Due to Constant Light Promotes Caspase 1 Activation in the Mouse Hippocampus" Cells 12, no. 14: 1836. https://doi.org/10.3390/cells12141836
APA StyleKetelauri, P., Scharov, K., von Gall, C., & Johann, S. (2023). Acute Circadian Disruption Due to Constant Light Promotes Caspase 1 Activation in the Mouse Hippocampus. Cells, 12(14), 1836. https://doi.org/10.3390/cells12141836







