Dopamine Transporter Deficiency Syndrome (DTDS): Expanding the Clinical Phenotype and Precision Medicine Approaches
Abstract
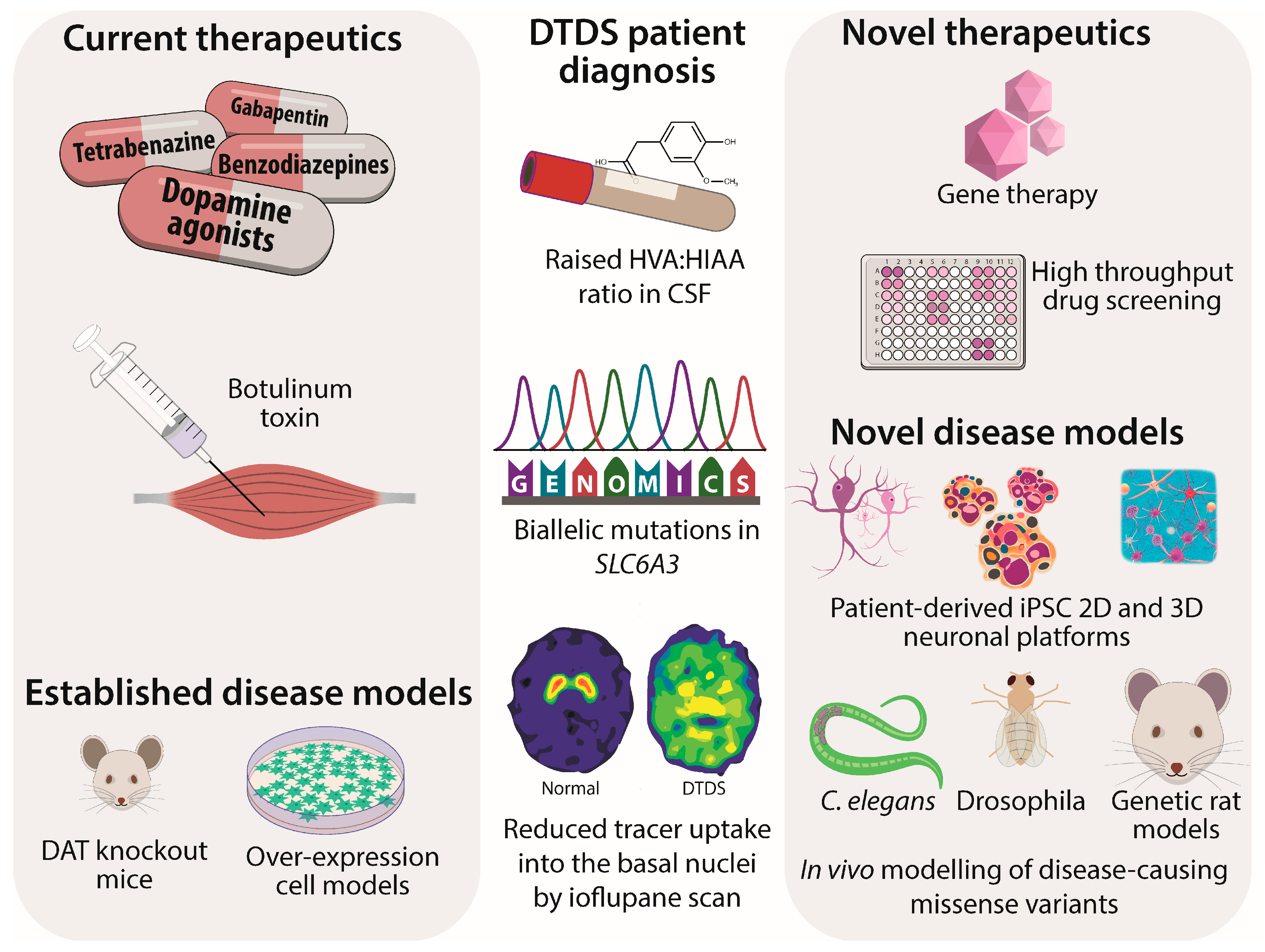
1. Introduction
2. Clinical Spectrum of DTDS
2.1. Classical Early Onset DTDS
2.2. Atypical Later-Onset DTDS
3. Dominant-Negative SLC6A3 Variants in Neurological and Neuropsychiatric Disease
4. DTDS Molecular Genetics and Disease Mechanisms
5. Pharmacochaperone Therapy Development
6. Reducing DAT Lysosomal Degradation with Chloroquine
7. Viral Gene Therapy Development
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iversen, S.D.; Iversen, L.L. Dopamine: 50 years in perspective. Trends Neurosci. 2007, 30, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.B.; Barker, R.A. Recent developments in treatments in Parkinson’s Disease. F1000Research 2020, 9, F1000, Faculty Rev-862. [Google Scholar] [CrossRef]
- Brennenstuhl, H.; Jung-Klawitter, S.; Assmann, B.; Opladen, T. Inherited Disorders of Neurotransmitters: Classification and Practical Approaches for Diagnosis and Treatment. Neuropediatrics 2019, 50, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Papandreou, A.; Heales, S.J.; Kurian, M.A. Monoamine neurotransmitter disorders—Clinical advances and future perspectives. Nat. Rev. Neurol. 2015, 11, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Cortès-Saladelafont, E.; Abela, L.; Termsarasab, P.; Mankad, K.; Sudhakar, S.; Gorman, K.M.; Heales, S.J.R.; Pope, S.; Biassoni, L.; et al. DNAJC6 Mutations Disrupt Dopamine Homeostasis in Juvenile Parkinsonism-Dystonia. Mov. Disord. 2020, 35, 1357–1368. [Google Scholar] [CrossRef]
- Niemann, N.; Jankovic, J. Juvenile Parkinsonism: Differential diagnosis, genetics and treatment. Park. Relat. Disord. 2019, 67, 74–89. [Google Scholar] [CrossRef]
- Assmann, B.E.; Robinson, R.O.; Surtees, R.A.; Bräutigam, C.; Heales, S.J.; Wevers, R.A.; Zschocke, J.; Hyland, K.; Sharma, R.; Hoffmann, G.F. Infantile Parkinsonism-dystonia and elevated dopamine metabolites in CSF. Neurology 2004, 62, 1872–1874. [Google Scholar] [CrossRef]
- Kurian, M.A.; Zhen, J.; Cheng, S.Y.; Li, Y.; Mordekar, S.R.; Jardine, P.; Morgan, N.V.; Meyer, E.; Tee, L.; Pasha, S.; et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. J. Clin. Investig. 2009, 119, 1595–1603. [Google Scholar] [CrossRef]
- Kurian, M.A.; Li, Y.; Zhen, J.; Meyer, E.; Hai, N.; Christen, H.J.; Hoffmann, G.F.; Jardine, P.; von Moers, A.; Mordekar, S.R.; et al. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: An observational cohort and experimental study. Lancet Neurol. 2011, 10, 54–62. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.; Jaber, M.; Giros, B.; Wrightman, R.M.; Caron, M.G. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 4029–4034. [Google Scholar] [CrossRef]
- Hamilton, P.J.; Campbell, N.G.; Sharma, S.; Erreger, K.; Herborg, H.F.; Saunders, C.; Belovich, A.N.; Daly, M.J.; Gibbs, R.A.; Boerwinkle, E.; et al. De Novo Mutation in the Dopamine Transporter gene associates dopamine dysfunction with Autism Spectrum Disorder. Mol. Psychiatry 2013, 18, 1315–1323. [Google Scholar] [CrossRef]
- Campbell, N.G.; Shekar, A.; Aguilar, J.I.; Peng, D.; Navratna, V.; Yang, D.; Morley, A.N.; Duran, A.M.; Galli, G.; O’Grady, B.; et al. Structural, Functional, and Behavioral insights of Dopamine dysfunction revealed by a deletion in SLC6A3. Proc. Natl. Acad. Sci. USA 2019, 116, 3853–3862. [Google Scholar] [CrossRef]
- Sakrikar, D.; Mazei-Robison, M.S.; Mergy, M.A.; Richtand, N.W.; Han, Q.; Hamilton, P.J.; Bowton, E.; Galli, A.; VeenstraVanderWeele, J.; Gill, M.; et al. Attention Deficit/Hyperactivity Disorder-Derived Coding Variation in the Dopamine Transporter disrupts microdomain targeting and trafficking Regulation. J. Neurosci. 2012, 32, 5385–5397. [Google Scholar] [CrossRef]
- Reith, M.E.A.; Kortagere, S.; Wiers, C.E.; Sun, H.; Kurian, M.A.; Galli, A.; Volkow, N.D.; Lin, Z.C. The dopamine transporters gene SLC6A3: Multidisease risks. Mol. Psychiatry 2022, 27, 1031–1046. [Google Scholar] [CrossRef]
- Ng, J.; Zhen, J.; Meyer, E.; Erreger, K.; Li, Y.; Kakar, N.; Ahmad, J.; Thiele, H.; Kubisch, C.; Rider, N.L.; et al. Dopamine transporter deficiency syndrome: Phenotypic spectrum from infancy to adulthood. Brain 2014, 137, 1107–1119. [Google Scholar] [CrossRef]
- Hansen, F.H.; SkjØrringe, T.; Yasmeen, S.; Arends, N.V.; Sahai, M.A.; Erreger, K.; Andreassen, T.F.; Holy, M.; Hamilton, P.J.; Neergheen, V.; et al. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J. Clin. Investig. 2014, 124, 3107–3120. [Google Scholar] [CrossRef]
- Yildiz, Y.; Pektas, E.; Tokatli, E.; Haliloglu, G. Hereditary dopamine transporter deficiency syndrome: Challenges in diagnosis and treatment. Neuropediatrics 2017, 48, 49–52. [Google Scholar]
- Schiff, M.; Cano, A.; Amsallem, D.; Barnerias, C.; Chaumette, B.; Plaze, M.; Slama, A.; Ioos, C.; Desguerre, I.; Lebre, A.S.; et al. Diagnostic approach to neurotransmitter monoamine disorders: Experience from clinical, biochemical, and genetic profiles. J. Inherit. Metab. Dis. 2018, 41, 129–139. [Google Scholar]
- Galiart, A.; Weber, P.; Datta, A.N. Infantile dystonia parkinsonism caused by mutations in SLC6A3: Case report of three siblings. Neuropediatrics 2017, 48, S1–S45. [Google Scholar] [CrossRef]
- Heidari, E.; Razmara, E.; Hosseinpour, S.; Tavasoli, A.R.; Garshasbi, M. Homozygous in-frame variant of SCL6A3 causes dopamine transporter deficiency syndrome in a consanguineous family. Ann. Hum. Genet. 2020, 84, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Baga, M.; Spagnioli, C.; Soliano, L.; Salerno, G.G.; Rizzi, S.; Frattini, D.; Pisani, F.; Fusco, C. Early-onset dopamine transporter deficiency syndrome: Long-term follow-up. Can. J. Neurol. Sci. 2021, 48, 285–286. [Google Scholar] [CrossRef]
- Nasehi, M.M.; Nikkah, A.; Salari, M.; Soltani, P.; Shirzadi, S. Dopamine transporter deficiency syndrome: A case with hyper- and hypokinetic extremes. Mov. Disord. Clin. Pract. 2020, 7, S57–S60. [Google Scholar] [CrossRef]
- Tehreem, B.; Kornitzer, J. Expanding the phenotypic spectrum of dopamine transporter deficiency syndrome with a novel mutation. Neurology 2020, 94 (Suppl. 15). [Google Scholar]
- Davletshina, R.; Kopishinskaia, S.; Vorontsova, E. The first case of a child with a dopamine transporter deficiency associated with SLC6A3 in Russia. Eur. J. Neurol. 2021, 28 (Suppl. 1), 907. [Google Scholar]
- Kurian, M.A. SLC6A3-Related Dopamine Transporter Deficiency Syndrome; GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 20 February 2023).
- Herborg, F.; Jensen, K.L.; Tolstoy, S.; Arends, N.V.; Posselt, L.P.; Shekar, A.; Aguilar, J.I.; Lund, V.K.; Erreger, K.; Rickhag, M.; et al. Identifying dominant-negative actions of a dopamine transporter variant in patients with parkinsonism and neuropsychiatric disease. JCI Insight 2021, 6, e151496. [Google Scholar] [CrossRef]
- Torres, G.E.; Carneiro, A.; Seamans, K.; Fiorentini, C.; Sweeney, A.; Yao, W.D.; Caron, M.G. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem. 2003, 278, 2731–2739. [Google Scholar] [CrossRef]
- Illiano, P.; Lanzo, A.; Leo, D.; Paglione, M.; Zampi, G.; Gainetdinov, R.R.; Di Schiavi, E. A Caenorhabditis elegans model to study dopamine transporter deficiency syndrome. Eur. J. Neurosci. 2017, 45, 207–214. [Google Scholar] [CrossRef]
- Asjad, H.M.M.; Kasture, A.; El-Kasaby, A.; Sackel, M.; Hummel, T.; Freissmuth, M.; Sucic, S. Pharmacochaperoning in a Drosophila model system rescues human dopamine transporter variants associated with infantile/juvenile parkinsonism. J. Biol. Chem. 2017, 292, 19250–19265. [Google Scholar] [CrossRef]
- Bhat, S.; Guthrie, D.A.; Kasture, A.; El-Kasaby, A.; Cao, J.; Bonifazi, A.; Ku, T.; Giancola, J.B.; Hummel, T.; Freissmuth, M.; et al. Tropane-Based Ibogaine Analog Rescues Folding-Deficient Serotonin and Dopamine Transporters. ACS Pharmacol. Transl. Sci. 2020, 4, 503–516. [Google Scholar] [CrossRef]
- Beerepoot, P.; Lam, V.M.; Salahpour, A. Pharmacological Chaperones of the Dopamine Transporter Rescue Dopamine Transporter Deficiency Syndrome Mutations in Heterologous Cells. J. Biol. Chem. 2016, 291, 22053–22062. [Google Scholar] [CrossRef]
- Aguilar, J.I.; Cheng, M.H.; Font, J.; Schwartz, A.C.; Ledwitch, K.; Duran, A.; Mabry, S.J.; Belovich, A.N.; Zhu, Y.; Carter, A.M.; et al. Psychomotor impairments and therapeutic implications revealed by a mutation associated with infantile Parkinsonism-Dystonia. eLife 2021, 10, e68039. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Barral, S.; De La Fuente Barrigon, C.; Lignani, G.; Erdem, F.A.; Wallings, R.; Privolizzi, R.; Rossignoli, G.; Alrashidi, H.; Heasman, S.; et al. Gene therapy restores dopamine transporter expression and ameliorates pathology in iPSC and mouse models of infantile parkinsonism. Sci. Transl. Med. 2021, 13, eaaw1564. [Google Scholar] [CrossRef] [PubMed]
- Cartier, E.; Garcia-Olivares, J.; Janezic, E.; Viana, J.; Moore, M.; Lin, M.L.; Caplan, J.L.; Torres, G.; Kim, Y.H. The SUMO-Conjugase Ubc9 Prevents the Degradation of the Dopamine Transporter, Enhancing Its Cell Surface Level and Dopamine Uptake. Front. Cell. Neurosci. 2019, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Privolizzi, R.; Chu, W.S.; Tijani, M.; Ng, J. Viral gene therapy for paediatric neurological diseases: Progress to clinical reality. Dev. Med. Child Neurol. 2021, 63, 1019–1029. [Google Scholar] [CrossRef]
- Illiano, P.; Bass, C.E.; Fichera, L.; Mus, L.; Budygin, E.A.; Sotnikova, T.D.; Leo, D.; Espinoza, S.; Gainetdinov, R.R. Recombinant Adeno-Associated Virus-mediated rescue of function in a mouse model of Dopamine Transporter Deficiency Syndrome. Sci Rep. 2017, 7, 46280. [Google Scholar] [CrossRef]
| Cases | Neuropsychiatric Diagnosis | SLC6A3 Variant | Protein Effect | Functional Alteration hDAT | Vmax |
|---|---|---|---|---|---|
| 1 [14] | ASD | Heterozygous c.1205C > T | Thr356Met | Outward bias: AMPH-DA efflux ↓efflux but constitutive effluxer | 42 34 |
| 1 [14] | ADHD | Heterozygous c.1981C > T | Arg615Cys | Accelerated endocytosis and recycling Altered hDAT microdomain localisation | 62 102 |
| 2 unrelated 21 patient cohort [14] | ASD + atypical DTDS, bipolar, depression schizophrenia | Heterozygous c.1995C > T | Lys619Asn | AMPH-DA efflux ↓ Mature ↓, surface ↓ | 69 |
| 5 unrelated [14] | Bipolar, ADHD, ASD | Heterozygous c.1814C > T | Arg559Val | AMPH-DA efflux ↓ Constitutive effluxer | 100 104 |
| 2 siblings [14] | ASD | c.289C > T | Arg51W | AMPH-DA efflux ↓ Syntaxin interaction ↓ | 94 |
| Case | Clinical | SLC6A3 Variant | Protein Effect | Functional Effects on hDAT (In Vitro Modelling) | DAT Uptake %WT |
|---|---|---|---|---|---|
| 1,2 [8,9] | Classical Homozygous | c.1103T→A | Leu368Gln | Mature ↓↓, surface ↓ | 0 |
| 3 [8,9] | Classical Homozygous | c.1184C→T | Pro395Leu | Mature ↓↓, surface ↓ | 0 |
| 4 [8,9] | Classical Homozygous | c.1156 + 5delG | Unknown— presumed splicing defect | N.D. | N.D. |
| 5 [9] | Classical Compound heterozygous | c.472G→T | Val158Phe | Mature ↓↓, surface ↓ | 0 |
| c.1161C→T | Pro554Leu | Mature ↓↓, surface ↓ | 0 | ||
| 6 [9] | Classical Homozygous | c.1031+1G→A | Unknown— presumed splicing defect | N.D. | N.D. |
| 7 [9] | Classical Homozygous | c.399delG | Ile134SerfX5 | N.D. | N.D. |
| 8 [9] | Classical Homozygous | c.1499_179del | Gly500GlufsX13 | N.D. | N.D. |
| 9 [9] | Classical Compound Heterozygous (3 variants) | c.979G→A | Gly327Arg | Surface ↓ | 0 |
| c.1315C→T | Gln439X | N.D. | N.D. | ||
| c.1586C→T | Pro529Leu | Mature ↓↓, surface ↓ | 6.2 | ||
| Gly327Arg + Gln439X + Pro529Leu | N.D. | 1.7 | |||
| 10 [9] | Classical Homozygous | c.671T→C | Leu224Pro | Mature ↓↓, surface ↓ | 0 |
| 11 [9] | Classical Homozygous | c.1561→T | Arg521Trp | Mature ↓, surface ↓ Syntaxin interaction ↓ | 26.9 |
| 12–14 [15] | Atypical Homozygous | c.941C→T | Ala314Val | Mature ↓, surface ↓ | 8.8 |
| 15 [15] | Classical Homozygous | c.1269 + 1G→A | Unknown— presumed splicing defect | N.D. | N.D. |
| 16 [15] | Classical Homozygous | c.1269 + 1G→A | Unknown— presumed splicing defect | N.D. | N.D. |
| 17 [15] | Classical Compound heterozygous | c.287-5_287-2delinsAAC | Unknown— presumed splicing defect | N.D. | N.D. |
| c.1156G→A | Gly386Arg | Mature ↓↓ | 0 | ||
| 18 [15] | Classical Homozygous | c.1408_1409delins AG | Try470Ser | Mature ↓↓, surface ↓ | 0 |
| 19 [15] | Classical Compound heterozygous | c.254G→T | Arg85Leu | Mature ↓, surface ↓ | 0.5 |
| c.1333C→T | Arg445Cys | ↓ Surface and block of N terminus release | 5.6 | ||
| Arg85Leu + Arg445Cys | 1.8 | ||||
| 20 [16] | Atypical Compound heterozygous | c.934 A→T | Ile312Phe | Outward bias | 56 (Vmax) |
| c.1261 G→A | Asp421Asn | Inward bias: loss of Na+ Cl− binding; constitute efflux | 10 | ||
| Ile312Phe + Asp421Asn | 30 | ||||
| 21 [17] | Classical Homozygous | c.418_3800_653+3058del | Unknown— presumed splicing defect | N.D. | N.D. |
| 22 [17] | Classical Homozygous | c.1156G→A | Gly386Arg | Mature ↓↓ | 0 |
| 23 [18] | Classical Homozygous | 1139–1150del | Unknown— presumed splicing defect | N.D. | N.D. |
| 24 [19] | Classical Homozygous | c.655G→A | Arg219Gly | In silico predicted damaged function | 0 predicted |
| 25 [20] | Classical Homozygous | c.655C→A | Arg219Ser | In silico predicted damaged function | 0 predicted |
| 26 [21] | Classical Homozygous | c.1639dupC | His547ProfsX56 | N.D. | N.D. |
| 27–29 [22] | Classical Homozygous | c.1333C→T | Arg445Cys | ↓Surface and block of N terminus release | 5.6 |
| 30 [23] | Classical Homozygous | c.1029→G | Tyr343X | N.D. | N.D. |
| 31 [24] | Classical Homozygous | c.1297G→A | Gly433Arg | In silico predicted damaged function | 0 predicted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, J.; Barral, S.; Waddington, S.N.; Kurian, M.A. Dopamine Transporter Deficiency Syndrome (DTDS): Expanding the Clinical Phenotype and Precision Medicine Approaches. Cells 2023, 12, 1737. https://doi.org/10.3390/cells12131737
Ng J, Barral S, Waddington SN, Kurian MA. Dopamine Transporter Deficiency Syndrome (DTDS): Expanding the Clinical Phenotype and Precision Medicine Approaches. Cells. 2023; 12(13):1737. https://doi.org/10.3390/cells12131737
Chicago/Turabian StyleNg, Joanne, Serena Barral, Simon N. Waddington, and Manju A. Kurian. 2023. "Dopamine Transporter Deficiency Syndrome (DTDS): Expanding the Clinical Phenotype and Precision Medicine Approaches" Cells 12, no. 13: 1737. https://doi.org/10.3390/cells12131737
APA StyleNg, J., Barral, S., Waddington, S. N., & Kurian, M. A. (2023). Dopamine Transporter Deficiency Syndrome (DTDS): Expanding the Clinical Phenotype and Precision Medicine Approaches. Cells, 12(13), 1737. https://doi.org/10.3390/cells12131737









