Dependency of Tamoxifen Sensitive and Resistant ER+ Breast Cancer Cells on Semaphorin 3C (SEMA3C) for Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Antibodies, Chemicals and Reagents
2.3. External Datasets Utilized in This Study
2.4. RNA Extraction and qPCR
2.5. Immunoblotting
2.6. Cell Stimulation
2.7. Plexin B1 and SEMA3C Silencing
2.8. Cell Growth Assay
2.9. Sub-G0/G1 DNA Content Assay
2.10. Production of SEMA3C: FL and B1SP
2.11. Statistical Analysis and Software Used
3. Results
3.1. SEMA3C Is Significantly Upregulated in ER+ Breast Cancer, and Its Expression Is Positively Correlated with the Expression of Estrogen Receptor (ER)
3.2. SEMA3C Is an ER-Induced Gene That Is Highly Expressed in ER-Positive Breast Cancer Cell Lines
3.3. SEMA3C Activates RTK Signaling Pathways in Breast Cancer Cells via Plexin-B1
3.4. ER+ Breast Cancer Cell Lines Show Dependency on SEMA3C for Cell Growth, and Its Depletion Leads to the Induction of Pro Apoptotic Proteins
3.5. SEMA3C Pathway Inhibitor Showcases a Significant Impact on Endocrine Therapy Naive Cells Growth and Exhibits Potential Synergistic Behavior with Tamoxifen
3.6. Tamoxifen-Resistant ER+ Breast Cancer Remains Dependent on SEMA3C for Signaling and Growth
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, T.; Kehm, R.D.; Terry, M.B.; Argov, E.L.; Tehranifar, P. Incidence Trends of Breast Cancer Molecular Subtypes by Age and Race/Ethnicity in the US From 2010 to 2016. JAMA Netw. Open 2020, 3, e2013226. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.; Singhal, H.; Li, L.; Xiao, T.; Liu, W.; Pun, M.; Jeselsohn, R.; He, H.; Lim, E.; Vadhi, R.; et al. Estrogen receptor signaling is reprogrammed during breast tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 11437–11443. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef]
- Nardone, A.; De Angelis, C.; Trivedi, M.V.; Osborne, C.K.; Schiff, R. The changing role of ER in endocrine resistance. Breast 2015, 24 (Suppl. 2), S60–S66. [Google Scholar] [CrossRef] [PubMed]
- Shiino, S.; Kinoshita, T.; Yoshida, M.; Jimbo, K.; Asaga, S.; Takayama, S.; Tsuda, H. Prognostic Impact of Discordance in Hormone Receptor Status Between Primary and Recurrent Sites in Patients With Recurrent Breast Cancer. Clin. Breast Cancer 2016, 16, e133-40. [Google Scholar] [CrossRef] [PubMed]
- Hartkopf, A.D.; Grischke, E.-M.; Brucker, S.Y. Endocrine-Resistant Breast Cancer: Mechanisms and Treatment. Breast Care 2020, 15, 347–354. [Google Scholar] [CrossRef]
- Liu, R.; Shuai, Y.; Luo, J.; Zhang, Z. SEMA3C Promotes Cervical Cancer Growth and Is Associated With Poor Prognosis. Front. Oncol. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Hui, D.H.F.; Tam, K.J.; Jiao, I.Z.F.; Ong, C.J. Semaphorin 3C as a Therapeutic Target in Prostate and Other Cancers. Int. J. Mol. Sci. 2019, 20, 774. [Google Scholar] [CrossRef]
- Zhang, D.; Lindstrom, A.; Kim, E.J.; Hwang, C.-I.; Hall, M.L.; Lin, T.-Y.; Li, Y. SEMA3C Supports Pancreatic Cancer Progression by Regulating the Autophagy Process and Tumor Immune Microenvironment. Front. Oncol. 2022, 12, 890154. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.W.; Takeuchi, A.; Hayashi, N.; Liu, L.; Tam, K.J.; Al Nakouzi, N.; Khazamipour, N.; Tombe, T.; Dejima, T.; Lee, K.C.; et al. SEMA3C drives cancer growth by transactivating multiple receptor tyrosine kinases via Plexin B1. EMBO Mol. Med. 2018, 10, 219–238. [Google Scholar] [CrossRef]
- Malik, M.F.A.; Satherley, L.K.; Davies, E.L.; Ye, L.; Jiang, W.G. Expression of Semaphorin 3C in Breast Cancer and its Impact on Adhesion and Invasion of Breast Cancer Cells. Anticancer. Res. 2016, 36, 1281–1286. [Google Scholar] [PubMed]
- Cole-Healy, Z.; Vergani, P.; Hunter, K.; Brown, N.J.; Reed, M.W.R.; Staton, C.A. The relationship between semaphorin 3C and microvessel density in the progression of breast and oral neoplasia. Exp. Mol. Pathol. 2015, 99, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, l1. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2, an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Pereira, B.; Chin, S.-F.; Rueda, O.M.; Vollan, H.-K.M.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.J.; et al. The somatic mutation profiles of 2433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Index of /runs/stddata__2016_01_28/data/BRCA/20160128. Available online: https://gdac.broadinstitute.org/runs/stddata__2016_01_28/data/BRCA/20160128/ (accessed on 18 March 2023).
- Elias, D.; Vever, H.; Lænkholm, A.-V.; Gjerstorff, M.F.; Yde, C.W.; Lykkesfeldt, A.E.; Ditzel, H.J. Gene expression profiling identifies FYN as an important molecule in tamoxifen resistance and a predictor of early recurrence in patients treated with endocrine therapy. Oncogene 2015, 34, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.S.; Meyer, C.A.; Song, J.; Li, W.; Geistlinger, T.R.; Eeckhoute, J.; Brodsky, A.S.; Keeton, E.K.; Fertuck, K.C.; Hall, G.F.; et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006, 38, 1289–1297. [Google Scholar] [CrossRef]
- Al Saleh, S.; Al Mulla, F.; Luqmani, Y.A. Estrogen receptor silencing induces epithelial to mesenchymal transition in human breast cancer cells. PLoS ONE 2011, 6, e20610. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.W.; Palmer, J.; Fink, D.; Ip, S.; Pietras, E.M.; Mui, A.L.-F.; Chung, S.W.; Gleave, M.E.; Cox, M.E.; Parsons, R.; et al. PTEN loss promotes mitochondrially dependent type II Fas-induced apoptosis via PEA-15. Mol. Cell. Biol. 2009, 29, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357. [Google Scholar] [CrossRef]
- Cheng, R.; Qi, L.; Kong, X.; Wang, Z.; Fang, Y.; Wang, J. Identification of the Significant Genes Regulated by Estrogen Receptor in Estrogen Receptor-Positive Breast Cancer and Their Expression Pattern Changes When Tamoxifen or Fulvestrant Resistance Occurs. Front. Genet. 2020, 11, 538734. [Google Scholar] [CrossRef]
- Oki, S.; Ohta, T. ChIP-Atlas. In Practical Guide to Life Science Databases; Abugessaisa, I., Kasukawa, T., Eds.; Springer Nature Singapore: Singapore, 2021; pp. 95–116. [Google Scholar]
- Oki, S.; Ohta, T.; Shioi, G.; Hatanaka, H.; Ogasawara, O.; Okuda, Y.; Kawaji, H.; Nakaki, R.; Sese, J.; Meno, C. ChIP-Atlas: A data-mining suite powered by full integration of public ChIP-seq data. EMBO Rep. 2018, 19, e46255. [Google Scholar] [CrossRef]
- Tam, K.J.; Dalal, K.; Hsing, M.; Cheng, C.W.; Khosravi, S.; Yenki, P.; Tse, C.; Peacock, J.W.; Sharma, A.; Chiang, Y.T.; et al. Androgen receptor transcriptionally regulates semaphorin 3C in a GATA2-dependent manner. Oncotarget 2017, 8, 9617–9633. [Google Scholar] [CrossRef]
- ENCODE Project Consortium; Moore, J.E.; Purcaro, M.J.; Pratt, H.E.; Epstein, C.B.; Shoresh, N.; Adrian, J.; Kawli, T.; Davis, C.A.; Dobin, A.; et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 2020, 583, 699–710. [Google Scholar]
- Castro-Mondragon, J.A.; Riudavets-Puig, R.; Rauluseviciute, I.; Lemma, R.B.; Turchi, L.; Blanc-Mathieu, R.; Lucas, J.; Boddie, P.; Khan, A.; Pérez, N.M.; et al. JASPAR 2022, the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022, 50, D165–D173. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.T.; Ortona, E.; Dupuis, M.L. A Role for Estrogen Receptor alpha36 in Cancer Progression. Front. Endocrinol. 2020, 11, 506. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0, an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.; Kannan, N.; Krissansen, G.W.; Findlay, M.P.; Baguley, B.C. MCF-7 breast cancer cells selected for tamoxifen resistance acquire new phenotypes differing in DNA content, phospho-HER2 and PAX2 expression, and rapamycin sensitivity. Cancer Biol. Ther. 2010, 9, 717–724. [Google Scholar] [CrossRef]
- Zhang, X.; Klamer, B.; Li, J.; Fernandez, S.; Li, L. A pan-cancer study of class-3 semaphorins as therapeutic targets in cancer. BMC Med. Genom. 2020, 13, 45. [Google Scholar] [CrossRef]
- Cheng, G.J.; Leung, E.Y.; Singleton, D.C. In vitro breast cancer models for studying mechanisms of resistance to endocrine therapy. Explor. Target. Antitumor Ther. 2022, 3, 297–320. [Google Scholar] [CrossRef]
- Cortés, J.; Im, S.-A.; Holgado, E.; Perez-Garcia, J.M.; Schmid, P.; Chavez-MacGregor, M. The next era of treatment for hormone receptor-positive, HER2-negative advanced breast cancer: Triplet combination-based endocrine therapies. Cancer Treat. Rev. 2017, 61, 53–60. [Google Scholar] [CrossRef]
- Feiner, L.; Webber, A.L.; Brown, C.B.; Lu, M.M.; Jia, L.; Feinstein, P.; Mombaerts, P.; Epstein, J.A.; Raper, J.A. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 2001, 128, 3061–3070. [Google Scholar] [CrossRef]
- Cremers, C.G.; Nguyen, L.K. Network rewiring, adaptive resistance and combating strategies in breast cancer. Cancer Drug Resist. 2019, 2, 1106–1126. [Google Scholar] [CrossRef]
- Kinnel, B.; Singh, S.K.; Oprea-Ilies, G.; Singh, R. Targeted Therapy and Mechanisms of Drug Resistance in Breast Cancer. Cancers 2023, 15, 1320. [Google Scholar] [CrossRef] [PubMed]
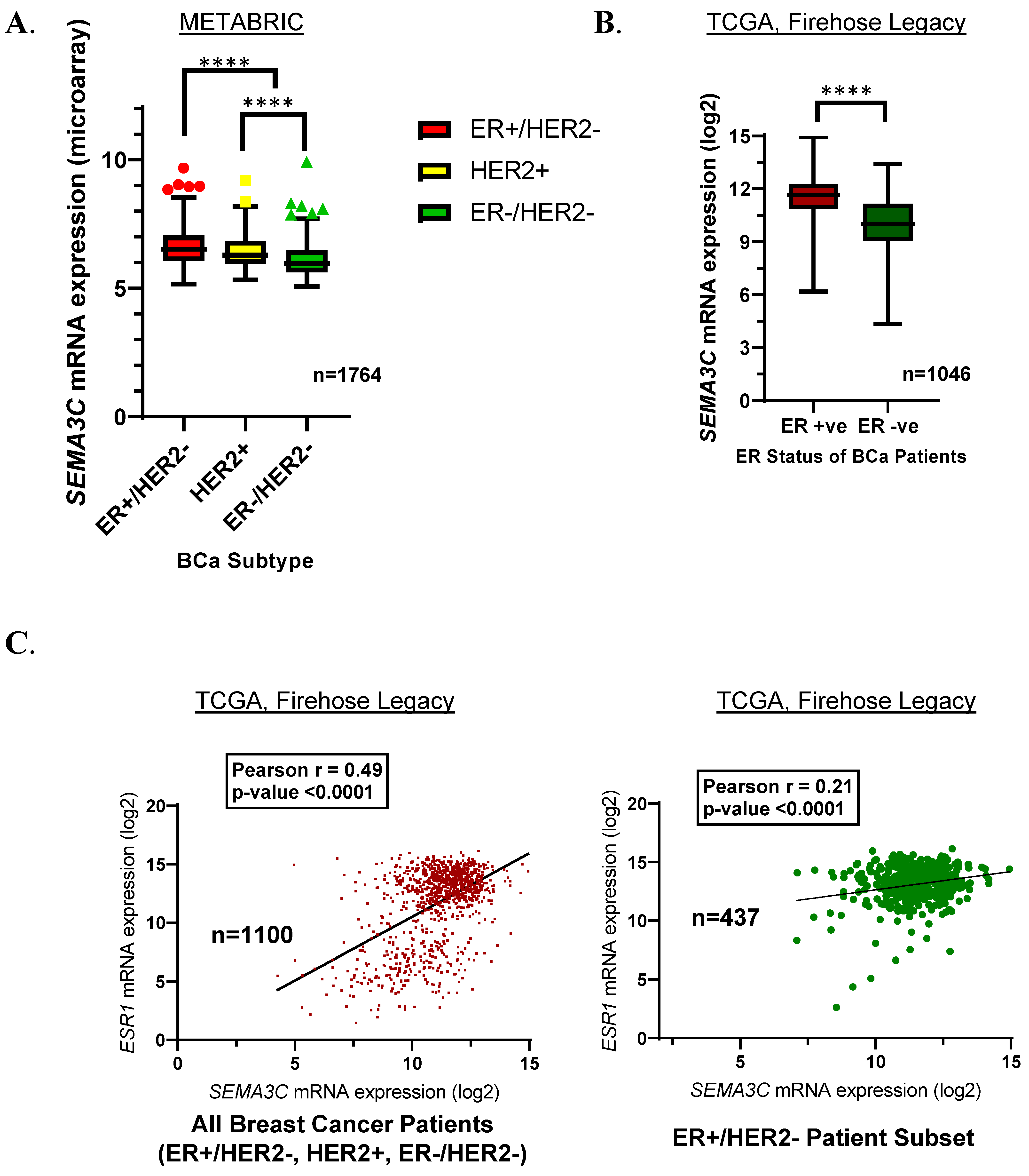

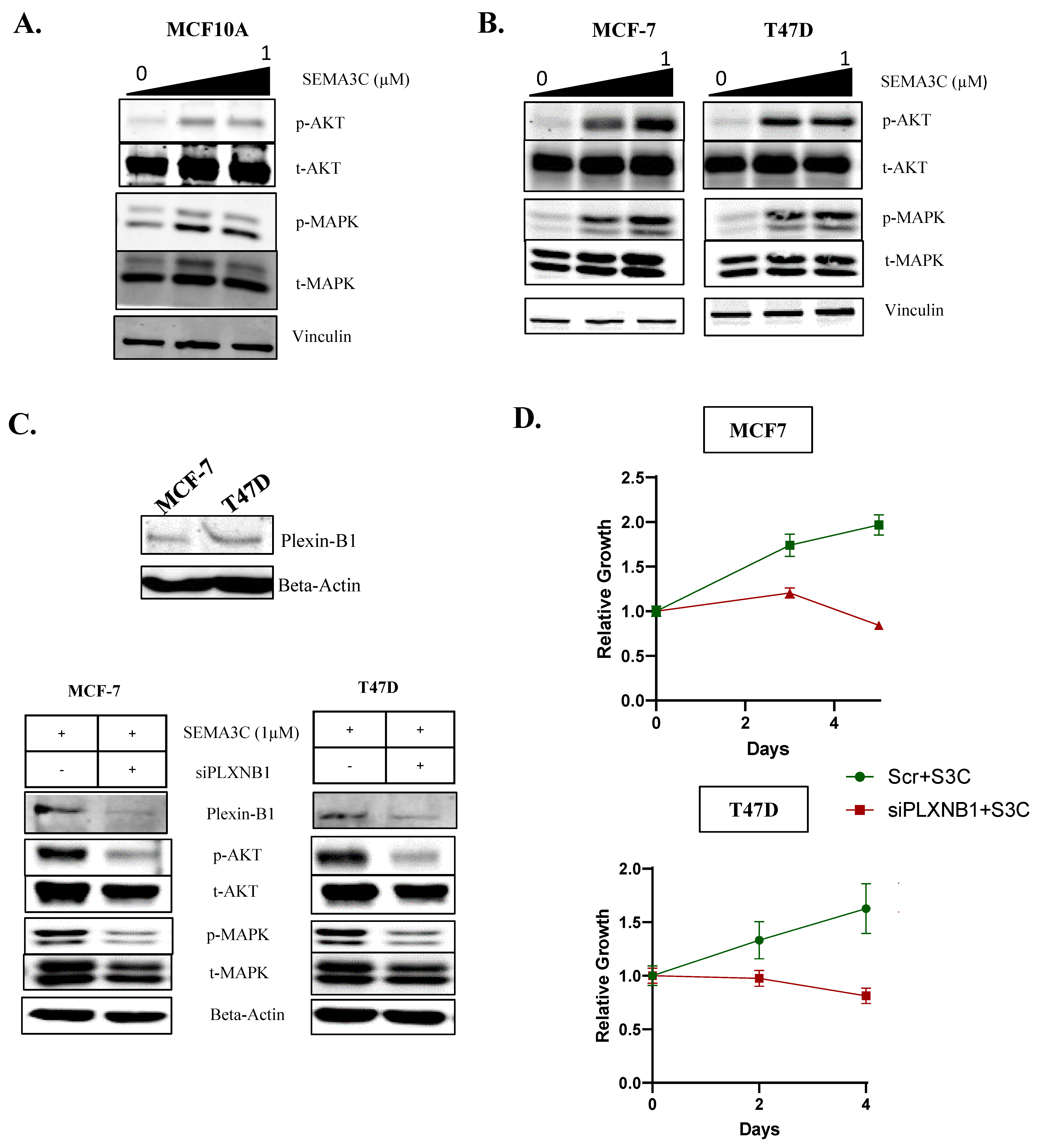


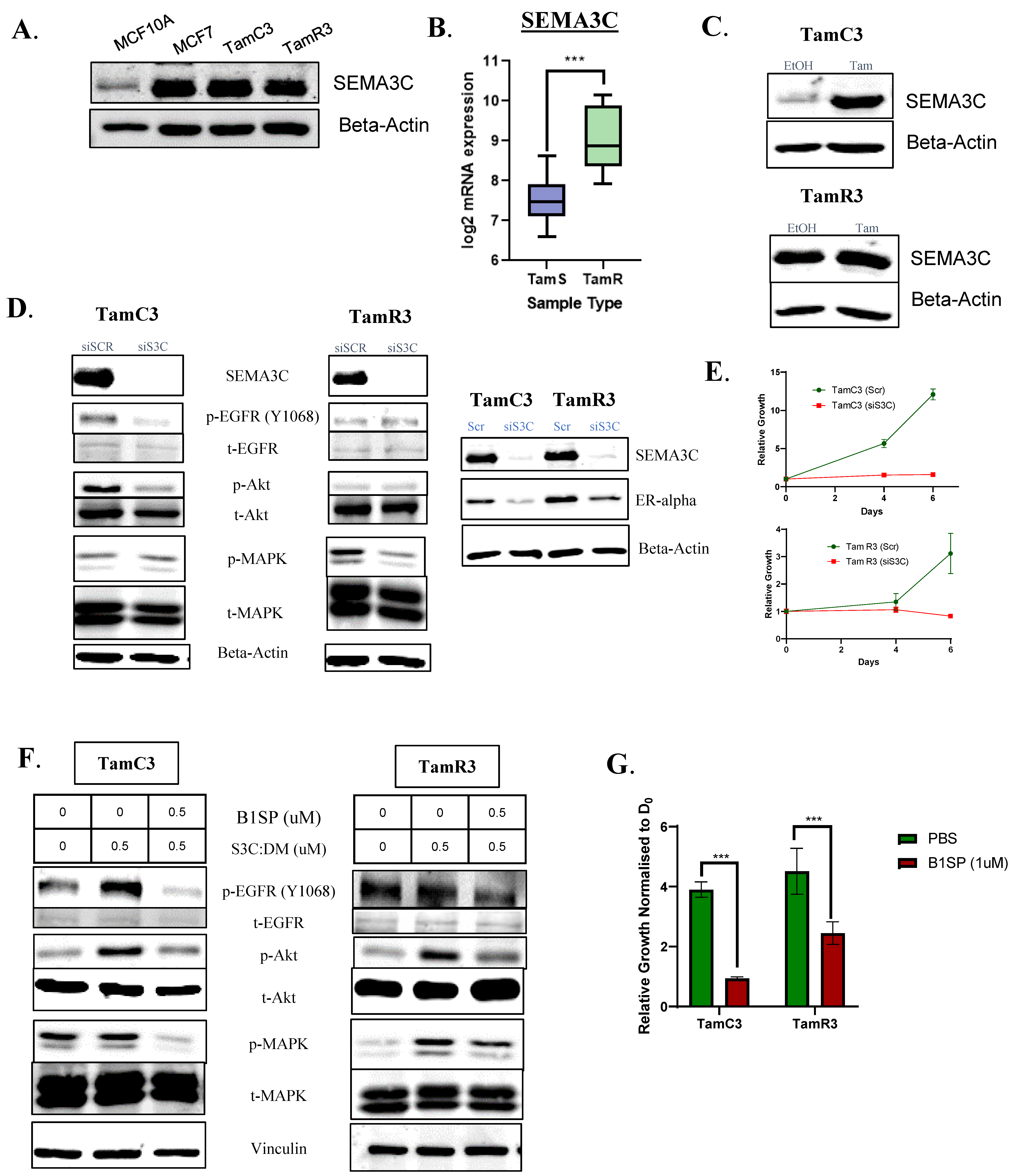
| STUDY NAME | Number of Samples Utilized for Analysis | Database | Sample Grouping |
|---|---|---|---|
| Breast Cancer (METABRIC) [20,21] | 1764 | Cbioportal | ER+/HER2 − (n = 1257); HER2+ (n = 198); ER−/HER2 − (n = 309) |
| Breast Invasive Carcinoma (TCGA, Firehose Legacy) [22] | 1101 | Cbioportal | ER+/HER2 − (n = 437); All samples (n = 1101) |
| GSE67916 [23] | 18 | GEO | TamR1 (3 replicates); TamR4 (3 replicates); TamR7 (2 replicates); TamR8 (2 replicates); TamS (8 replicates) |
| GSE11324 [24] | 12 | GEO | 0hr (3 replicates); 3hr (3 replicates); 6hr (3 replicates); 12hr (3 replicates) |
| GSE27473 [25] | 6 | GEO | MCF7-Scr (3 replicates); MCF7-silenced ER (3 replicates) |
| BRCA | 1376 | GEPIA2 | Tumor (n = 1085); Normal (n = 291) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhasin, S.; Dusek, C.; Peacock, J.W.; Cherkasov, A.; Wang, Y.; Gleave, M.; Ong, C.J. Dependency of Tamoxifen Sensitive and Resistant ER+ Breast Cancer Cells on Semaphorin 3C (SEMA3C) for Growth. Cells 2023, 12, 1715. https://doi.org/10.3390/cells12131715
Bhasin S, Dusek C, Peacock JW, Cherkasov A, Wang Y, Gleave M, Ong CJ. Dependency of Tamoxifen Sensitive and Resistant ER+ Breast Cancer Cells on Semaphorin 3C (SEMA3C) for Growth. Cells. 2023; 12(13):1715. https://doi.org/10.3390/cells12131715
Chicago/Turabian StyleBhasin, Satyam, Christopher Dusek, James W. Peacock, Artem Cherkasov, Yuzhuo Wang, Martin Gleave, and Christopher J. Ong. 2023. "Dependency of Tamoxifen Sensitive and Resistant ER+ Breast Cancer Cells on Semaphorin 3C (SEMA3C) for Growth" Cells 12, no. 13: 1715. https://doi.org/10.3390/cells12131715
APA StyleBhasin, S., Dusek, C., Peacock, J. W., Cherkasov, A., Wang, Y., Gleave, M., & Ong, C. J. (2023). Dependency of Tamoxifen Sensitive and Resistant ER+ Breast Cancer Cells on Semaphorin 3C (SEMA3C) for Growth. Cells, 12(13), 1715. https://doi.org/10.3390/cells12131715







