Monocytes in Tumorigenesis and Tumor Immunotherapy
Abstract
1. Introduction
2. The Global Effects of TME on Monocytes
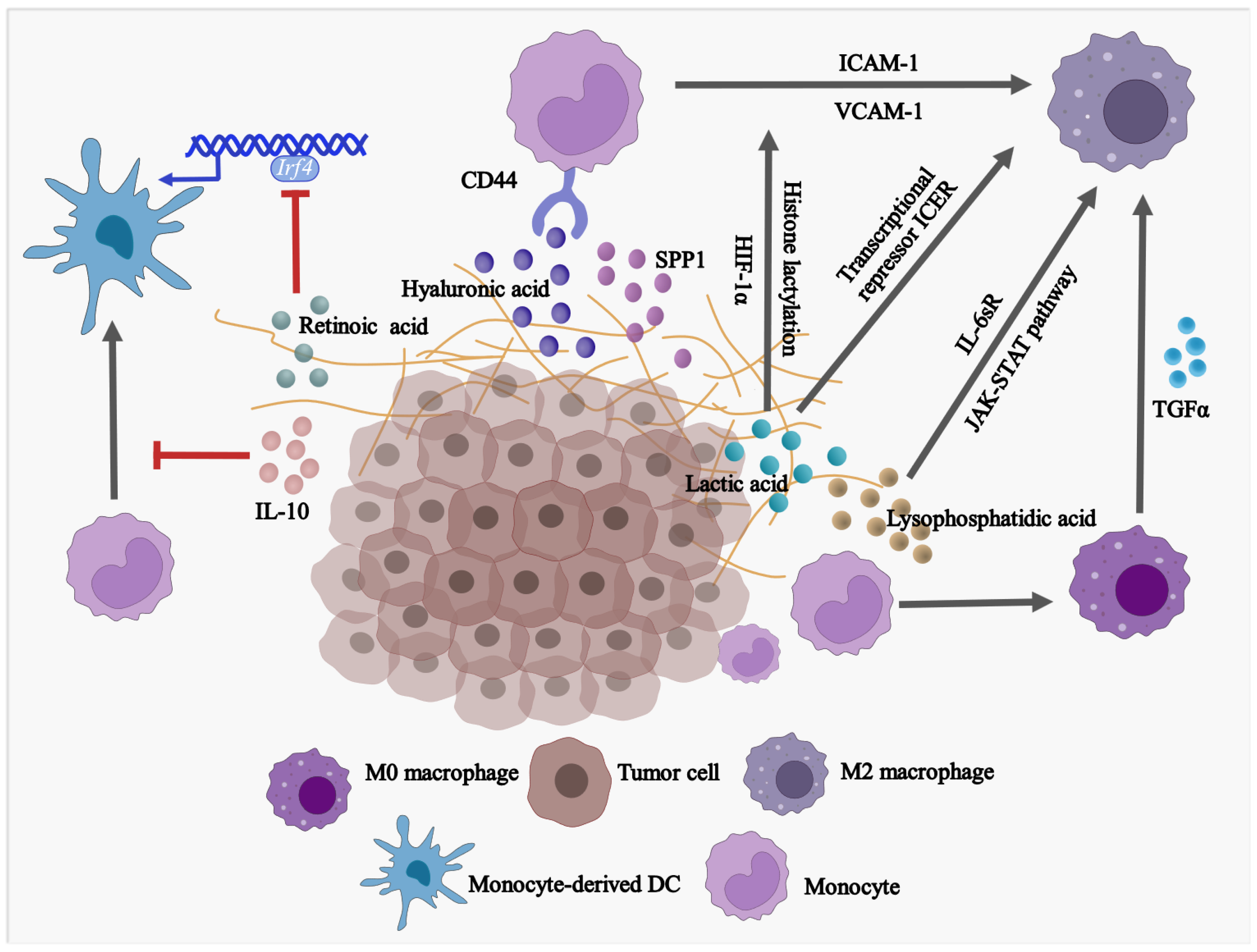
2.1. The Effect of TME on the Population and Phenotype of Monocytes
2.2. The Effect of TME on the Differentiation of Monocytes
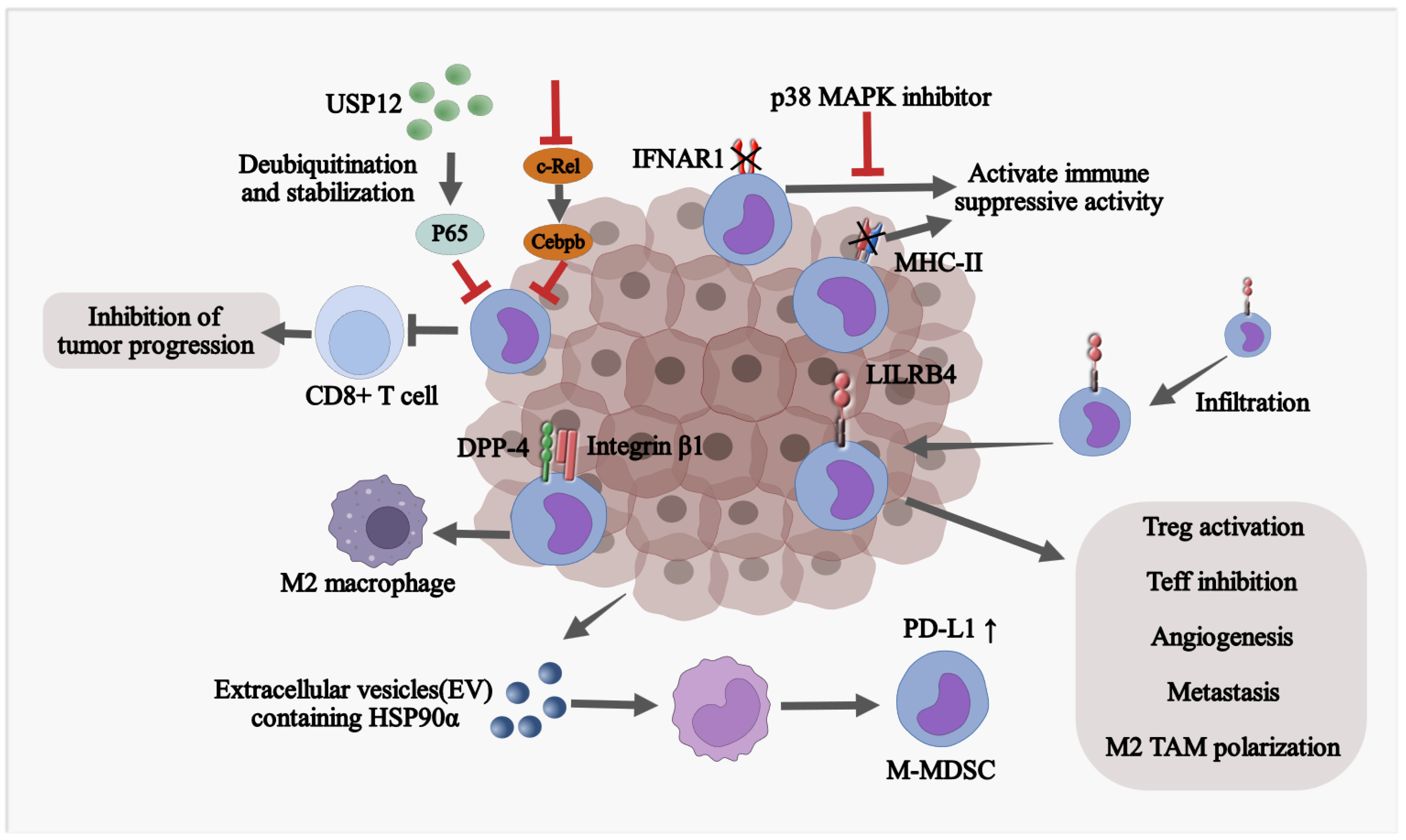
2.3. The Effect of TME on the Fate of Monocytic MDSCs
3. Monocytes and Monocyte-Derived Cells in Tumorigenesis
3.1. Monocyte-Derived TAMs
3.2. Monocytic MDSCs
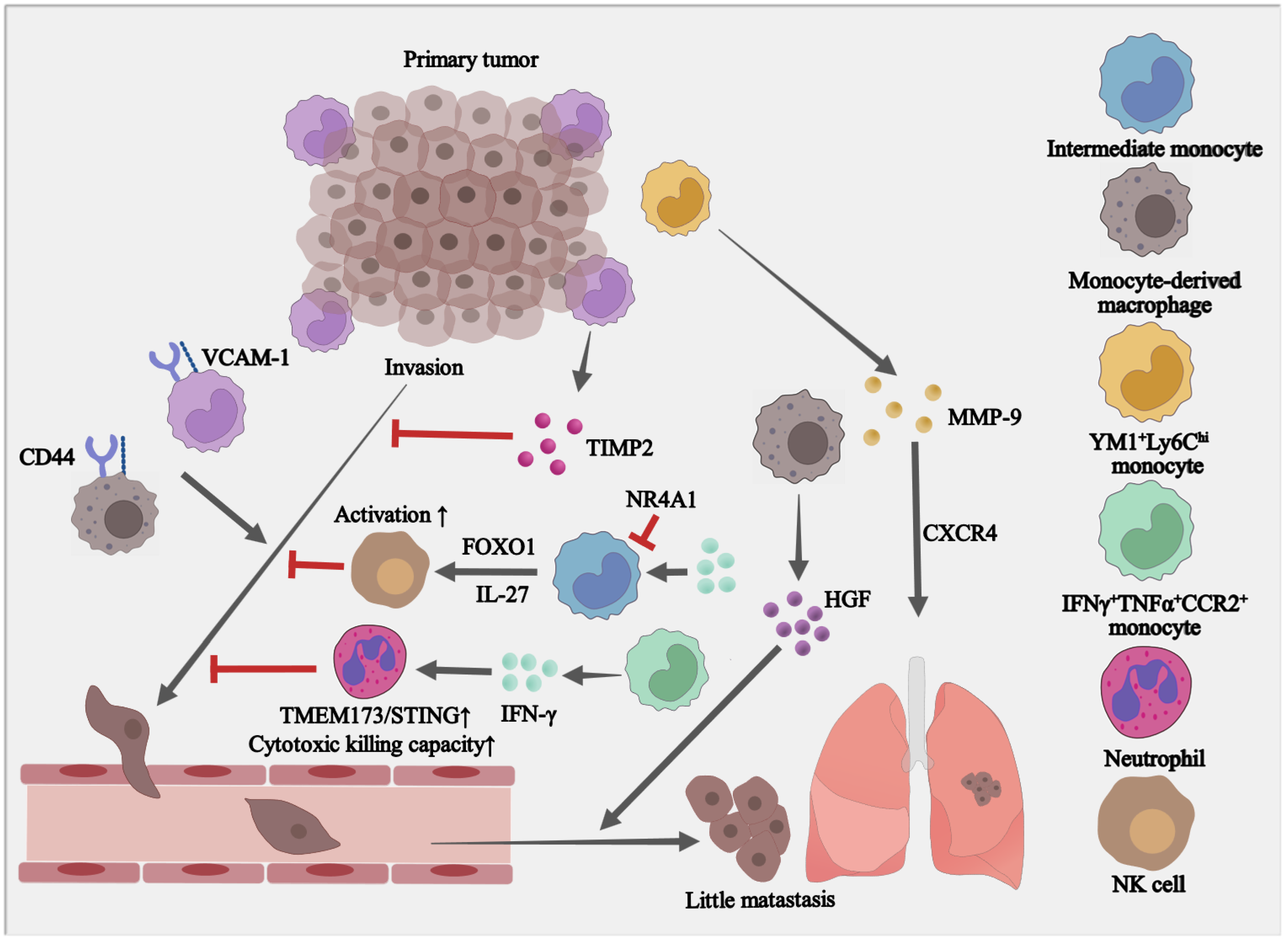
4. The Paradoxical Roles of Monocytes in Tumor Metastasis
5. Therapeutic Strategies Targeting Tumor-Related Monocytes
5.1. Targeting Monocyte Differentiation and Reprogramming
5.2. Targeting Monocyte Recruitment and Adhesion to Tumor Sites
5.3. Monocytes as Carriers to Deliver Antigens and Drugs
5.4. Additional Therapeutic Strategies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ugel, S.; Canè, S.; De Sanctis, F.; Bronte, V. Monocytes in the Tumor Microenvironment. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Caro, A.A.; Raes, G.; Laoui, D. Systemic Reprogramming of Monocytes in Cancer. Front. Oncol. 2020, 10, 1399. [Google Scholar] [CrossRef]
- Kwiecień, I.; Rutkowska, E.; Polubiec-Kownacka, M.; Raniszewska, A.; Rzepecki, P.; Domagała-Kulawik, J. Blood Monocyte Subsets with Activation Markers in Relation with Macrophages in Non-Small Cell Lung Cancer. Cancers 2020, 12, 2513. [Google Scholar] [CrossRef]
- Patysheva, M.; Larionova, I.; Stakheyeva, M.; Grigoryeva, E.; Iamshchikov, P.; Tarabanovskaya, N.; Weiss, C.; Kardashova, J.; Frolova, A.; Rakina, M.; et al. Effect of Early-Stage Human Breast Carcinoma on Monocyte Programming. Front. Oncol. 2021, 11, 800235. [Google Scholar] [CrossRef]
- Sakakura, K.; Takahashi, H.; Motegi, S.-I.; Yokobori-Kuwabara, Y.; Oyama, T.; Chikamatsu, K. Immunological Features of Circulating Monocyte Subsets in Patients with Squamous Cell Carcinoma of the Head and Neck. Clin. Immunol. 2021, 225, 108677. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef]
- Martín-Sierra, C.; Martins, R.; Coucelo, M.; Abrantes, A.M.; Oliveira, R.C.; Tralhão, J.G.; Botelho, M.F.; Furtado, E.; Domingues, M.R.; Paiva, A.; et al. Elevated Soluble TNFα Levels and Upregulated TNFα MRNA Expression in Purified Peripheral Blood Monocyte Subsets Associated with High-Grade Hepatocellular Carcinoma. J. Inflamm. 2020, 17, 14. [Google Scholar] [CrossRef]
- Edwards, C.V.; Hassan, H.; Yildirim, C.; Ferri, G.; Verma, K.P.; Murray Horwitz, M.E.; Fillmore, N.R.; Munshi, N.C. Peripheral Blood Monocyte Count Is a Dynamic Prognostic Biomarker in Multiple Myeloma. Blood Adv. 2022, 7, 482–490. [Google Scholar] [CrossRef]
- Barclay, A.N.; Van den Berg, T.K. The Interaction between Signal Regulatory Protein Alpha (SIRPα) and CD47: Structure, Function, and Therapeutic Target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Kim, H.J.; Wu, H.; Price-Troska, T.; Villasboas, J.C.; Jalali, S.; Feldman, A.L.; Novak, A.J.; Yang, Z.-Z.; Ansell, S.M. SIRPα Expression Delineates Subsets of Intratumoral Monocyte/Macrophages with Different Functional and Prognostic Impact in Follicular Lymphoma. Blood Cancer J. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Lv, J.; Yao, Y.; Zhao, Y.; He, Z.; Wang, Q.; Cui, L.; Dai, H. Elevations of Monocyte and Neutrophils, and Higher Levels of Granulocyte Colony-Stimulating Factor in Peripheral Blood in Lung Cancer Patients. Thorac. Cancer 2021, 12, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Juusola, M.; Kuuliala, K.; Kuuliala, A.; Mustonen, H.; Vähä-Koskela, M.; Puolakkainen, P.; Seppänen, H. Pancreatic Cancer Is Associated with Aberrant Monocyte Function and Successive Differentiation into Macrophages with Inferior Anti-Tumour Characteristics. Pancreatology 2021, 21, 397–405. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, S.Y.; Jeong, H.; Han, J.; Chae, H.Y.; Yang, H.; Sung, C.O.; Choi, Y.-L.; Shin, Y.K.; Kwon, M.J. Matrix Metalloproteinase 11 (MMP11) in Macrophages Promotes the Migration of HER2-Positive Breast Cancer Cells and Monocyte Recruitment through CCL2-CCR2 Signaling. Lab. Investig. 2022, 102, 376–390. [Google Scholar] [CrossRef]
- Cui, R.; Yue, W.; Lattime, E.C.; Stein, M.N.; Xu, Q.; Tan, X.-L. Targeting Tumor-Associated Macrophages to Combat Pancreatic Cancer. Oncotarget 2016, 7, 50735–50754. [Google Scholar] [CrossRef]
- Zhang, B.; Ye, H.; Ren, X.; Zheng, S.; Zhou, Q.; Chen, C.; Lin, Q.; Li, G.; Wei, L.; Fu, Z.; et al. Macrophage-Expressed CD51 Promotes Cancer Stem Cell Properties via the TGF-Β1/Smad2/3 Axis in Pancreatic Cancer. Cancer Lett. 2019, 459, 204–215. [Google Scholar] [CrossRef]
- Devalaraja, S.; To, T.K.J.; Folkert, I.W.; Natesan, R.; Alam, M.Z.; Li, M.; Tada, Y.; Budagyan, K.; Dang, M.T.; Zhai, L.; et al. Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression. Cell 2020, 180, 1098–1114.e16. [Google Scholar] [CrossRef]
- Michielon, E.; López González, M.; Burm, J.L.A.; Waaijman, T.; Jordanova, E.S.; de Gruijl, T.D.; Gibbs, S. Micro-Environmental Cross-Talk in an Organotypic Human Melanoma-in-Skin Model Directs M2-like Monocyte Differentiation via IL-10. Cancer Immunol. Immunother. 2020, 69, 2319–2331. [Google Scholar] [CrossRef]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical Relevance of Tumour-Associated Macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421. [Google Scholar] [CrossRef]
- Fogg, K.C.; Miller, A.E.; Li, Y.; Flanigan, W.; Walker, A.; O’Shea, A.; Kendziorski, C.; Kreeger, P.K. Ovarian Cancer Cells Direct Monocyte Differentiation through a Non-Canonical Pathway. BMC Cancer 2020, 20, 1008. [Google Scholar] [CrossRef] [PubMed]
- Kultti, A.; Li, X.; Jiang, P.; Thompson, C.B.; Frost, G.I.; Shepard, H.M. Therapeutic Targeting of Hyaluronan in the Tumor Stroma. Cancers 2012, 4, 873–903. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional Regulation of Macrophage Polarization: Enabling Diversity with Identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Kim, H.; Cha, J.; Jang, M.; Kim, P. Hyaluronic Acid-Based Extracellular Matrix Triggers Spontaneous M2-like Polarity of Monocyte/Macrophage. Biomater. Sci. 2019, 7, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-K.; Huang, B.-R.; Yeh, W.-L.; Chen, C.-W.; Liu, Y.-S.; Lai, S.-W.; Tseng, W.-P.; Lu, D.-Y.; Tsai, C.-F. Regulatory Effects of IL-1β in the Interaction of GBM and Tumor-Associated Monocyte through VCAM-1 and ICAM-1. Eur. J. Pharmacol. 2021, 905, 174216. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, M.; Cao, G.; Liu, H.; Li, Y.; Wang, S.; Zijtveld, S.; Delvoux, B.; Xanthoulea, S.; Romano, A.; et al. Human Monocytes Differentiate into Tumor-Associated Macrophages upon SKOV3 Cells Coculture and/or Lysophosphatidic Acid Stimulation. J. Inflamm. 2022, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Stadanlick, J.; Annunziata, M.J.; Rao, A.S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Cantu, E.; Danet-Desnoyers, G.; Ra, H.-J.; et al. Human Tumor-Associated Monocytes/Macrophages and Their Regulation of T Cell Responses in Early-Stage Lung Cancer. Sci. Transl. Med. 2019, 11, eaat1500. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Bohn, T.; Rapp, S.; Luther, N.; Klein, M.; Bruehl, T.-J.; Kojima, N.; Aranda Lopez, P.; Hahlbrock, J.; Muth, S.; Endo, S.; et al. Tumor Immunoevasion via Acidosis-Dependent Induction of Regulatory Tumor-Associated Macrophages. Nat. Immunol. 2018, 19, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Broz, M.L.; Krummel, M.F. The emerging understanding of myeloid cells as partners and targets in tumor rejection. Cancer Immunol. Res. 2015, 3, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bruger, A.M.; Dorhoi, A.; Esendagli, G.; Barczyk-Kahlert, K.; van der Bruggen, P.; Lipoldova, M.; Perecko, T.; Santibanez, J.; Saraiva, M.; Van Ginderachter, J.A.; et al. How to Measure the Immunosuppressive Activity of MDSC: Assays, Problems and Potential Solutions. Cancer Immunol. Immunother. 2019, 68, 631–644. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Zamani, A.; Wang, W.; Lee, C.-N.; Li, M.; Luo, G.; Eiler, E.; Sun, H.; Ghosh, S.; et al. C-Rel Is a Myeloid Checkpoint for Cancer Immunotherapy. Nat. Cancer 2020, 1, 507–517. [Google Scholar] [CrossRef]
- Waight, J.D.; Netherby, C.; Hensen, M.L.; Miller, A.; Hu, Q.; Liu, S.; Bogner, P.N.; Farren, M.R.; Lee, K.P.; Liu, K.; et al. Myeloid-Derived Suppressor Cell Development Is Regulated by a STAT/IRF-8 Axis. J. Clin. Investig. 2013, 123, 4464–4478. [Google Scholar] [CrossRef]
- Sinha, P.; Okoro, C.; Foell, D.; Freeze, H.H.; Ostrand-Rosenberg, S.; Srikrishna, G. Proinflammatory S100 Proteins Regulate the Accumulation of Myeloid-Derived Suppressor Cells. J. Immunol. 2008, 181, 4666–4675. [Google Scholar] [CrossRef]
- Yan, D.; Wang, J.; Sun, H.; Zamani, A.; Zhang, H.; Chen, W.; Tang, A.; Ruan, Q.; Yang, X.; Chen, Y.H.; et al. TIPE2 Specifies the Functional Polarization of Myeloid-Derived Suppressor Cells during Tumorigenesis. J. Exp. Med. 2020, 217, e20182005. [Google Scholar] [CrossRef]
- Fultang, N.; Li, X.; Li, T.; Chen, Y.H. Myeloid-Derived Suppressor Cell Differentiation in Cancer: Transcriptional Regulators and Enhanceosome-Mediated Mechanisms. Front. Immunol. 2021, 11, 619253. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Gerondakis, S. The C-Rel Transcription Factor in Development and Disease. Genes Cancer 2011, 2, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bou-Dargham, M.J.; Fultang, N.; Li, X.; Pear, W.S.; Sun, H.; Chen, Y.H. C-Rel-Dependent Monocytes Are Potent Immune Suppressor Cells in Cancer. J. Leukoc. Biol. 2022, 112, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Horzum, U.; Yoyen-Ermis, D.; Taskiran, E.Z.; Yilmaz, K.B.; Hamaloglu, E.; Karakoc, D.; Esendagli, G. CD66b+ Monocytes Represent a Proinflammatory Myeloid Subpopulation in Cancer. Cancer Immunol. Immunother. 2021, 70, 75–87. [Google Scholar] [CrossRef]
- Qu, Y.; Wen, J.; Thomas, G.; Yang, W.; Prior, W.; He, W.; Sundar, P.; Wang, X.; Potluri, S.; Salek-Ardakani, S. Baseline Frequency of Inflammatory Cxcl9-Expressing Tumor-Associated Macrophages Predicts Response to Avelumab Treatment. Cell Rep. 2020, 32, 107873. [Google Scholar] [CrossRef]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, M.; Guo, H.; Hou, J.; Zhang, Y.; Li, M.; Wu, X.; Chen, X.; Wang, L. Integrated Analysis Highlights the Immunosuppressive Role of TREM2+ Macrophages in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 848367. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Quintanal-Villalonga, Á.; Gao, V.R.; Xie, Y.; Allaj, V.; Chaudhary, O.; Masilionis, I.; Egger, J.; Chow, A.; Walle, T.; et al. Signatures of Plasticity, Metastasis, and Immunosuppression in an Atlas of Human Small Cell Lung Cancer. Cancer Cell 2021, 39, 1479–1496.e18. [Google Scholar] [CrossRef]
- Li, H.; van der Leun, A.M.; Yofe, I.; Lubling, Y.; Gelbard-Solodkin, D.; van Akkooi, A.C.J.; van den Braber, M.; Rozeman, E.A.; Haanen, J.B.A.G.; Blank, C.U.; et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 2019, 176, 775–789.e18. [Google Scholar] [CrossRef]
- Alshetaiwi, H.; Pervolarakis, N.; McIntyre, L.L.; Ma, D.; Nguyen, Q.; Rath, J.A.; Nee, K.; Hernandez, G.; Evans, K.; Torosian, L.; et al. Defining the Emergence of Myeloid-Derived Suppressor Cells in Breast Cancer Using Single-Cell Transcriptomics. Sci. Immunol. 2020, 5, eaay6017. [Google Scholar] [CrossRef]
- Stenzel, A.E.; Abrams, S.I.; Joseph, J.M.; Goode, E.L.; Tario, J.D.; Wallace, P.K.; Kaur, D.; Adamson, A.-K.; Buas, M.F.; Lugade, A.A.; et al. Circulating CD14+ HLA-DRlo/− Monocytic Cells as a Biomarker for Epithelial Ovarian Cancer Progression. Am. J. Reprod. Immunol. 2021, 85, e13343. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, X.; Li, Z.; Zhu, B. Metabolic Regulatory Crosstalk between Tumor Microenvironment and Tumor-Associated Macrophages. Theranostics 2021, 11, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-Induced Chemokine Cascade Promotes Breast Cancer Metastasis by Enhancing Retention of Metastasis-Associated Macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Tabu, K.; Taga, T. Cancer Ego-System in Glioma: An Iron-Replenishing Niche Network Systemically Self-Organized by Cancer Stem Cells. Inflamm. Regen. 2022, 42, 54. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.T.; Stein, U.; El Tayebi, H.M. The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer. Cancers 2022, 14, 5460. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Irvine, K.M.; Hume, D.A. Functions of Macrophage Colony-Stimulating Factor (CSF1) in Development, Homeostasis, and Tissue Repair. Semin. Immunol. 2021, 54, 101509. [Google Scholar] [CrossRef] [PubMed]
- Hamerman, J.A.; Jarjoura, J.R.; Humphrey, M.B.; Nakamura, M.C.; Seaman, W.E.; Lanier, L.L. Cutting Edge: Inhibition of TLR and FcR Responses in Macrophages by Triggering Receptor Expressed on Myeloid Cells (TREM)-2 and DAP12. J. Immunol. 2006, 177, 2051–2055. [Google Scholar] [CrossRef]
- Ma, R.-Y.; Zhang, H.; Li, X.-F.; Zhang, C.-B.; Selli, C.; Tagliavini, G.; Lam, A.D.; Prost, S.; Sims, A.H.; Hu, H.-Y.; et al. Monocyte-Derived Macrophages Promote Breast Cancer Bone Metastasis Outgrowth. J. Exp. Med. 2020, 217, e20191820. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the Mechanisms That May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front. Immunol. 2017, 8, 1970. [Google Scholar] [CrossRef]
- Tumino, N.; Fiore, P.F.; Pelosi, A.; Moretta, L.; Vacca, P. Myeloid Derived Suppressor Cells in Tumor Microenvironment: Interaction with Innate Lymphoid Cells. Semin. Immunol. 2022, 61–64, 101668. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-Derived Suppressor Cells: An Emerging Target for Anticancer Immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xiao, J.; Zhang, W.; Wang, F.; Yan, Y.; Wu, X.; Zeng, Z.; He, Y.; Yang, W.; Liao, W.; et al. Inhibition of CCL7 Derived from Mo-MDSCs Prevents Metastatic Progression from Latency in Colorectal Cancer. Cell Death Dis. 2021, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Siemińska, I.; Węglarczyk, K.; Walczak, M.; Czerwińska, A.; Pach, R.; Rubinkiewicz, M.; Szczepanik, A.; Siedlar, M.; Baran, J. Mo-MDSCs Are Pivotal Players in Colorectal Cancer and May Be Associated with Tumor Recurrence after Surgery. Transl. Oncol. 2022, 17, 101346. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tao, Q.; Wang, H.; Zhang, Q.; Zhou, M.; Liu, L.; Zhai, Z. Monocytic Myeloid-Derived Suppressor Cells but Not Monocytes Predict Poor Prognosis of Acute Myeloid Leukemia. Turk. J. Haematol. 2022, 39, 230–236. [Google Scholar] [CrossRef]
- Boral, B.; Ballı, H.T.; Sözütok, S.; Pehlivan, U.A.; Aikimbaev, K. Clinical and Prognostic Significance of CD14 (+) HLA-DR (−/Low) Myeloid-Derived Suppressor Cells in Patients with Hepatocellular Carcinoma Received Transarterial Radioembolization with Yttrium-90. Scand. J. Immunol. 2022, 95, e13132. [Google Scholar] [CrossRef]
- Kajihara, N.; Kobayashi, T.; Otsuka, R.; Nio-Kobayashi, J.; Oshino, T.; Takahashi, M.; Imanishi, S.; Hashimoto, A.; Wada, H.; Seino, K.-I. Tumor-Derived Interleukin-34 Creates an Immunosuppressive and Chemoresistant Tumor Microenvironment by Modulating Myeloid-Derived Suppressor Cells in Triple-Negative Breast Cancer. Cancer Immunol. Immunother. 2022, 72, 851–864. [Google Scholar] [CrossRef]
- Bronte, G.; Petracci, E.; De Matteis, S.; Canale, M.; Zampiva, I.; Priano, I.; Cravero, P.; Andrikou, K.; Burgio, M.A.; Ulivi, P.; et al. High Levels of Circulating Monocytic Myeloid-Derived Suppressive-Like Cells Are Associated with the Primary Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer: An Exploratory Analysis. Front. Immunol. 2022, 13, 866561. [Google Scholar] [CrossRef]
- Hopkins, R.; Xiang, W.; Marlier, D.; Au, V.B.; Ching, Q.; Wu, L.X.; Guan, R.; Lee, B.; Chia, W.-K.; Wang, W.-W.; et al. Monocytic Myeloid-Derived Suppressor Cells Underpin Resistance to Adoptive T Cell Therapy in Nasopharyngeal Carcinoma. Mol. Ther. 2021, 29, 734–743. [Google Scholar] [CrossRef]
- Jain, M.D.; Zhao, H.; Wang, X.; Atkins, R.; Menges, M.; Reid, K.; Spitler, K.; Faramand, R.; Bachmeier, C.; Dean, E.A.; et al. Tumor Interferon Signaling and Suppressive Myeloid Cells Are Associated with CAR T-Cell Failure in Large B-Cell Lymphoma. Blood 2021, 137, 2621–2633. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, L.; Qin, G.; Qiao, Y.; Ren, F.; Shen, C.; Wang, S.; Liu, S.; Lian, J.; Wang, D.; et al. Cancer-Associated Fibroblasts Induce Monocytic Myeloid-Derived Suppressor Cell Generation via IL-6/Exosomal MiR-21-Activated STAT3 Signaling to Promote Cisplatin Resistance in Esophageal Squamous Cell Carcinoma. Cancer Lett. 2021, 518, 35–48. [Google Scholar] [CrossRef]
- Bayik, D.; Bartels, C.F.; Lovrenert, K.; Watson, D.C.; Zhang, D.; Kay, K.; Lee, J.; Lauko, A.; Johnson, S.; Lo, A.; et al. Distinct Cell Adhesion Signature Defines Glioblastoma Myeloid-Derived Suppressor Cell Subsets. Cancer Res. 2022, 82, 4274–4287. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Muise, E.S.; Bhattacharya, A.; Grein, J.; Javaid, S.; Stivers, P.; Zhang, J.; Qu, Y.; Joyce-Shaikh, B.; Loboda, A.; et al. ILT3 (LILRB4) Promotes the Immunosuppressive Function of Tumor-Educated Human Monocytic Myeloid-Derived Suppressor Cells. Mol. Cancer Res. 2021, 19, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Su, M.-T.; Kumata, S.; Endo, S.; Okada, Y.; Takai, T. LILRB4 Promotes Tumor Metastasis by Regulating MDSCs and Inhibiting MiR-1 Family MiRNAs. Oncoimmunology 2022, 11, 2060907. [Google Scholar] [CrossRef] [PubMed]
- Arkhypov, I.; Özbay Kurt, F.G.; Bitsch, R.; Novak, D.; Petrova, V.; Lasser, S.; Hielscher, T.; Groth, C.; Lepper, A.; Hu, X.; et al. HSP90α Induces Immunosuppressive Myeloid Cells in Melanoma via TLR4 Signaling. J. Immunother. Cancer 2022, 10, e005551. [Google Scholar] [CrossRef] [PubMed]
- Alicea-Torres, K.; Sanseviero, E.; Gui, J.; Chen, J.; Veglia, F.; Yu, Q.; Donthireddy, L.; Kossenkov, A.; Lin, C.; Fu, S.; et al. Immune Suppressive Activity of Myeloid-Derived Suppressor Cells in Cancer Requires Inactivation of the Type I Interferon Pathway. Nat. Commun. 2021, 12, 1717. [Google Scholar] [CrossRef]
- Zhan, X.; He, Q.; Sheng, J.; Jiang, X.; Lin, L.; Huang, Y.; He, S.; Chen, Y.; Li, L.; Zeng, Z.; et al. USP12 Positively Regulates M-MDSC Function to Inhibit Antitumour Immunity through Deubiquitinating and Stabilizing P65. Immunology 2022, 167, 544–557. [Google Scholar] [CrossRef]
- Alissafi, T.; Hatzioannou, A.; Mintzas, K.; Barouni, R.M.; Banos, A.; Sormendi, S.; Polyzos, A.; Xilouri, M.; Wielockx, B.; Gogas, H.; et al. Autophagy Orchestrates the Regulatory Program of Tumor-Associated Myeloid-Derived Suppressor Cells. J. Clin. Investig. 2018, 128, 3840–3852. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, J.; Liu, X.; Feng, Y.; Yang, W.; Wu, F.; Cheung, O.K.-W.; Sun, H.; Zeng, X.; Tang, W.; et al. Targeting Monocyte-Intrinsic Enhancer Reprogramming Improves Immunotherapy Efficacy in Hepatocellular Carcinoma. Gut 2020, 69, 365–379. [Google Scholar] [CrossRef]
- Arihara, F.; Mizukoshi, E.; Kitahara, M.; Takata, Y.; Arai, K.; Yamashita, T.; Nakamoto, Y.; Kaneko, S. Increase in CD14+HLA-DR−/Low Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients and Its Impact on Prognosis. Cancer Immunol. Immunother. 2013, 62, 1421–1430. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Ikeda, N.; Asano, K.; Kikuchi, K.; Uchida, Y.; Ikegami, H.; Takagi, R.; Yotsumoto, S.; Shibuya, T.; Makino-Okamura, C.; Fukuyama, H.; et al. Emergence of Immunoregulatory Ym1+Ly6Chi Monocytes during Recovery Phase of Tissue Injury. Sci. Immunol. 2018, 3, eaat0207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.H.-F.; Massagué, J. Macrophage Binding to Receptor VCAM-1 Transmits Survival Signals in Breast Cancer Cells That Invade the Lungs. Cancer Cell 2011, 20, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-C.; Weng, C.-C.; Hou, Y.-S.; Jian, S.-F.; Fang, K.-T.; Hou, M.-F.; Cheng, K.-H. Activation of VCAM-1 and Its Associated Molecule CD44 Leads to Increased Malignant Potential of Breast Cancer Cells. Int. J. Mol. Sci. 2014, 15, 3560–3579. [Google Scholar] [CrossRef]
- Gomez, K.E.; Wu, F.; Keysar, S.B.; Morton, J.J.; Miller, B.; Chimed, T.-S.; Le, P.N.; Nieto, C.; Chowdhury, F.N.; Tyagi, A.; et al. Cancer Cell CD44 Mediates Macrophage/Monocyte-Driven Regulation of Head and Neck Cancer Stem Cells. Cancer Res. 2020, 80, 4185–4198. [Google Scholar] [CrossRef]
- Arif, A.A.; Huang, Y.-H.; Freeman, S.A.; Atif, J.; Dean, P.; Lai, J.C.Y.; Blanchet, M.-R.; Wiegand, K.C.; McNagny, K.M.; Underhill, T.M.; et al. Inflammation-Induced Metastatic Colonization of the Lung Is Facilitated by Hepatocyte Growth Factor-Secreting Monocyte-Derived Macrophages. Mol. Cancer Res. 2021, 19, 2096–2109. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Yan, F.; Li, H.; Ren, X. Response to Comment on “Myeloid-Derived Suppressor Cells Suppress Antitumor Immune Responses through IDO Expression and Correlate with Lymph Node Metastasis in Patients with Breast Cancer”. J. Immunol. 2013, 190, 5341–5342. [Google Scholar] [CrossRef]
- Benzing, C.; Lam, H.; Tsang, C.M.; Rimmer, A.; Arroyo-Berdugo, Y.; Calle, Y.; Wells, C.M. TIMP-2 Secreted by Monocyte-like Cells Is a Potent Suppressor of Invadopodia Formation in Pancreatic Cancer Cells. BMC Cancer 2019, 19, 1214. [Google Scholar] [CrossRef] [PubMed]
- Hagerling, C.; Gonzalez, H.; Salari, K.; Wang, C.-Y.; Lin, C.; Robles, I.; van Gogh, M.; Dejmek, A.; Jirström, K.; Werb, Z. Immune Effector Monocyte-Neutrophil Cooperation Induced by the Primary Tumor Prevents Metastatic Progression of Breast Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 21704–21714. [Google Scholar] [CrossRef]
- Wang, R.; Bao, W.; Pal, M.; Liu, Y.; Yazdanbakhsh, K.; Zhong, H. Intermediate Monocytes Induced by IFN-γ Inhibit Cancer Metastasis by Promoting NK Cell Activation through FOXO1 and Interleukin-27. J. Immunother. Cancer 2022, 10, e003539. [Google Scholar] [CrossRef]
- Hanna, R.N.; Cekic, C.; Sag, D.; Tacke, R.; Thomas, G.D.; Nowyhed, H.; Herrley, E.; Rasquinha, N.; McArdle, S.; Wu, R.; et al. Patrolling Monocytes Control Tumor Metastasis to the Lung. Science 2015, 350, 985–990. [Google Scholar] [CrossRef]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R Blockade Reprograms Tumor-Infiltrating Macrophages and Improves Response to T-Cell Checkpoint Immunotherapy in Pancreatic Cancer Models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, J.; Xu, D.; Gao, X.-M.; Zhang, Z.; Hsu, J.L.; Li, C.-W.; Lim, S.-O.; Sheng, Y.-Y.; Zhang, Y.; et al. Disruption of Tumour-Associated Macrophage Trafficking by the Osteopontin-Induced Colony-Stimulating Factor-1 Signalling Sensitises Hepatocellular Carcinoma to Anti-PD-L1 Blockade. Gut 2019, 68, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Moughon, D.L.; He, H.; Schokrpur, S.; Jiang, Z.K.; Yaqoob, M.; David, J.; Lin, C.; Iruela-Arispe, M.L.; Dorigo, O.; Wu, L. Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res. 2015, 75, 4742–4752. [Google Scholar] [CrossRef]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.A.; Le Tourneau, C.; Toulmonde, M.; Cannarile, M.A.; Ries, C.; Brillouet, A.; Müller, C.; Jegg, A.-M.; et al. CSF1R Inhibition with Emactuzumab in Locally Advanced Diffuse-Type Tenosynovial Giant Cell Tumours of the Soft Tissue: A Dose-Escalation and Dose-Expansion Phase 1 Study. Lancet Oncol. 2015, 16, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Vogelzang, A.; Miyajima, M.; Sugiura, Y.; Wu, Y.; Chamoto, K.; Nakano, R.; Hatae, R.; Menzies, R.J.; Sonomura, K.; et al. B Cell-Derived GABA Elicits IL-10+ Macrophages to Limit Anti-Tumour Immunity. Nature 2021, 599, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Boelaars, K.; Brown, K.; Eveline Li, R.J.; Kruijssen, L.; Bruijns, S.C.M.; van Ee, T.; Schetters, S.T.T.; Crommentuijn, M.H.W.; van der Horst, J.C.; et al. Sialic Acids in Pancreatic Cancer Cells Drive Tumour-Associated Macrophage Differentiation via the Siglec Receptors Siglec-7 and Siglec-9. Nat. Commun. 2021, 12, 1270. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and Macrophage Heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.I.; Olalla Saad, S.T.; Azambuja, J.H. Artesunate Switches Monocytes to an Inflammatory Phenotype with the Ability to Kill Leukemic Cells. Int. J. Mol. Sci. 2021, 22, 608. [Google Scholar] [CrossRef]
- Holtzhausen, A.; Harris, W.; Ubil, E.; Hunter, D.M.; Zhao, J.; Zhang, Y.; Zhang, D.; Liu, Q.; Wang, X.; Graham, D.K.; et al. TAM Family Receptor Kinase Inhibition Reverses MDSC-Mediated Suppression and Augments Anti-PD-1 Therapy in Melanoma. Cancer Immunol. Res. 2019, 7, 1672–1686. [Google Scholar] [CrossRef]
- Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; Van den Bossche, J.; Mack, M.; Pipeleers, D.; In’t Veld, P.; De Baetselier, P.; et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived from Ly6C(High) Monocytes. Cancer Res. 2010, 70, 5728–5739. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, S.; Yang, Y.; Hong, T.; Xiang, Z.; Zhao, J.; Zhu, C.; Zeng, L.; Zhang, L. TAM-Targeted Reeducation for Enhanced Cancer Immunotherapy: Mechanism and Recent Progress. Front. Oncol. 2022, 12, 1034842. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct Cell Reprogramming: Approaches, Mechanisms and Progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [CrossRef]
- Chaintreuil, P.; Laplane, L.; Esnault, F.; Ghesquier, V.; Savy, C.; Furstoss, N.; Arcangeli, M.-L.; Cluzeau, T.; Robert, G.; Droin, N.; et al. Reprogramming Monocyte-Derived Macrophages through Caspase Inhibition. Oncoimmunology 2022, 11, 2015859. [Google Scholar] [CrossRef]
- Liao, J.; Zeng, D.-N.; Li, J.-Z.; Hua, Q.-M.; Huang, C.-X.; Xu, J.; Wu, C.; Zheng, L.; Wen, W.-P.; Wu, Y. Type I IFNs Repolarized a CD169+ Macrophage Population with Anti-Tumor Potentials in Hepatocellular Carcinoma. Mol. Ther. 2022, 30, 632–643. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte Heterogeneity and Functions in Cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Minopoli, M.; Sarno, S.; Di Carluccio, G.; Azzaro, R.; Costantini, S.; Fazioli, F.; Gallo, M.; Apice, G.; Cannella, L.; Rea, D.; et al. Inhibiting Monocyte Recruitment to Prevent the Pro-Tumoral Activity of Tumor-Associated Macrophages in Chondrosarcoma. Cells 2020, 9, 1062. [Google Scholar] [CrossRef]
- Minopoli, M.; Polo, A.; Ragone, C.; Ingangi, V.; Ciliberto, G.; Pessi, A.; Sarno, S.; Budillon, A.; Costantini, S.; Carriero, M.V. Structure-Function Relationship of an Urokinase Receptor-Derived Peptide Which Inhibits the Formyl Peptide Receptor Type 1 Activity. Sci. Rep. 2019, 9, 12169. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Xia, R.; Wei, Y.; Wei, X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell. Prolif. 2021, 54, e13115. [Google Scholar] [CrossRef]
- Regan, D.P.; Coy, J.W.; Chahal, K.K.; Chow, L.; Kurihara, J.N.; Guth, A.M.; Kufareva, I.; Dow, S.W. The Angiotensin Receptor Blocker Losartan Suppresses Growth of Pulmonary Metastases via AT1R-Independent Inhibition of CCR2 Signaling and Monocyte Recruitment. J. Immunol. 2019, 202, 3087–3102. [Google Scholar] [CrossRef]
- Regan, D.P.; Chow, L.; Das, S.; Haines, L.; Palmer, E.; Kurihara, J.N.; Coy, J.W.; Mathias, A.; Thamm, D.H.; Gustafson, D.L.; et al. Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined with the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma. Clin. Cancer Res. 2022, 28, 662–676. [Google Scholar] [CrossRef]
- Bess, S.N.; Greening, G.J.; Rajaram, N.; Muldoon, T.J. Macrophage-Targeted Anti-CCL2 Immunotherapy Enhances Tumor Sensitivity to 5-Fluorouracil in a Balb/c-CT26 Murine Colon Carcinoma Model Measured Using Diffuse Reflectance Spectroscopy. BMC Immunol. 2022, 23, 20. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the Immune System in Cancer: From Tumor Initiation to Metastatic Progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Singleton, D.C.; Macann, A.; Wilson, W.R. Therapeutic Targeting of the Hypoxic Tumour Microenvironment. Nat. Rev. Clin. Oncol. 2021, 18, 751–772. [Google Scholar] [CrossRef]
- Arora, L.; Patra, D.; Roy, S.; Nanda, S.; Singh, N.; Verma, A.K.; Chakraborti, A.; Dasgupta, S.; Pal, D. Hypoxia-Induced MiR-210-3p Expression in Lung Adenocarcinoma Potentiates Tumor Development by Regulating CCL2-Mediated Monocyte Infiltration. Mol. Oncol. 2022. [Google Scholar] [CrossRef]
- Kleindienst, P.; Brocker, T. Endogenous Dendritic Cells Are Required for Amplification of T Cell Responses Induced by Dendritic Cell Vaccines in Vivo. J. Immunol. 2003, 170, 2817–2823. [Google Scholar] [CrossRef]
- Yewdall, A.W.; Drutman, S.B.; Jinwala, F.; Bahjat, K.S.; Bhardwaj, N. CD8+ T Cell Priming by Dendritic Cell Vaccines Requires Antigen Transfer to Endogenous Antigen Presenting Cells. PLoS ONE 2010, 5, e11144. [Google Scholar] [CrossRef]
- Randolph, G.J.; Jakubzick, C.; Qu, C. Antigen Presentation by Monocytes and Monocyte-Derived Cells. Curr. Opin. Immunol. 2008, 20, 52–60. [Google Scholar] [CrossRef]
- Huang, M.-N.; Nicholson, L.T.; Batich, K.A.; Swartz, A.M.; Kopin, D.; Wellford, S.; Prabhakar, V.K.; Woroniecka, K.; Nair, S.K.; Fecci, P.E.; et al. Antigen-Loaded Monocyte Administration Induces Potent Therapeutic Antitumor T Cell Responses. J. Clin. Investig. 2020, 130, 774–788. [Google Scholar] [CrossRef]
- Huang, M.-N.; D’Anniballe, V.M.; Gunn, M.D. Monocytes as a Cellular Vaccine Platform to Induce Antitumor Immunity. Methods Mol. Biol. 2022, 2410, 627–647. [Google Scholar] [CrossRef]
- Allavena, P.; Germano, G.; Marchesi, F.; Mantovani, A. Chemokines in Cancer Related Inflammation. Exp. Cell Res. 2011, 317, 664–673. [Google Scholar] [CrossRef]
- Huang, W.-C.; Shen, M.-Y.; Chen, H.-H.; Lin, S.-C.; Chiang, W.-H.; Wu, P.-H.; Chang, C.-W.; Chiang, C.-S.; Chiu, H.-C. Monocytic Delivery of Therapeutic Oxygen Bubbles for Dual-Modality Treatment of Tumor Hypoxia. J. Control. Release 2015, 220, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Palmioli, A.; Avigni, R.; Sironi, M.; La Ferla, B.; Maeda, A. PLGA Based Nanoparticles for the Monocyte-Mediated Anti-Tumor Drug Delivery System. J. Biomed. Nanotechnol. 2020, 16, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L.E.; Beaugé, L.; Arias-Ramos, N.; Rivarola, V.A.; Chesta, C.A.; López-Larrubia, P.; Palacios, R.E. Trojan Horse Monocyte-Mediated Delivery of Conjugated Polymer Nanoparticles for Improved Photodynamic Therapy of Glioblastoma. Nanomedicine 2020, 15, 1687–1707. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hu, X.; Wu, H.; Mo, L.; Xie, S.; Li, J.; Peng, C.; Xu, S.; Qiu, L.; Tan, W. In Vivo Monocyte/Macrophage-Hitchhiked Intratumoral Accumulation of Nanomedicines for Enhanced Tumor Therapy. J. Am. Chem. Soc. 2020, 142, 382–391. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of Phagocytosis in Macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte Recruitment during Infection and Inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Martner, A.; Aydin, E.; Hellstrand, K. NOX2 in Autoimmunity, Tumor Growth and Metastasis. J. Pathol. 2019, 247, 151–154. [Google Scholar] [CrossRef]
- Koyasu, S. The Role of PI3K in Immune Cells. Nat. Immunol. 2003, 4, 313–319. [Google Scholar] [CrossRef]
- Condliffe, A.M.; Davidson, K.; Anderson, K.E.; Ellson, C.D.; Crabbe, T.; Okkenhaug, K.; Vanhaesebroeck, B.; Turner, M.; Webb, L.; Wymann, M.P.; et al. Sequential Activation of Class IB and Class IA PI3K Is Important for the Primed Respiratory Burst of Human but Not Murine Neutrophils. Blood 2005, 106, 1432–1440. [Google Scholar] [CrossRef]
- Hansson, M.; Asea, A.; Ersson, U.; Hermodsson, S.; Hellstrand, K. Induction of Apoptosis in NK Cells by Monocyte-Derived Reactive Oxygen Metabolites. J. Immunol. 1996, 156, 42–47. [Google Scholar] [CrossRef]
- Schmielau, J.; Finn, O.J. Activated Granulocytes and Granulocyte-Derived Hydrogen Peroxide Are the Underlying Mechanism of Suppression of t-Cell Function in Advanced Cancer Patients. Cancer Res. 2001, 61, 4756–4760. [Google Scholar] [PubMed]
- Akhiani, A.A.; Hallner, A.; Kiffin, R.; Aydin, E.; Werlenius, O.; Aurelius, J.; Martner, A.; Thorén, F.B.; Hellstrand, K. Idelalisib Rescues Natural Killer Cells from Monocyte-Induced Immunosuppression by Inhibiting NOX2-Derived Reactive Oxygen Species. Cancer Immunol. Res. 2020, 8, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The Microbiome and Human Cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopès, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota Triggers STING-Type I IFN-Dependent Monocyte Reprogramming of the Tumor Microenvironment. Cell 2021, 184, 5338–5356.e21. [Google Scholar] [CrossRef]
| Subset | Cellular Origin | Function | Methods | Cancer Type | |
|---|---|---|---|---|---|
| CD66b+CD14+CD33hiCD16−/+HLA-DR+/hi monocytes | CD33hiCD14+ monocytes | Anti-tumoral | Display high phagocytic activity, matrix adhesion, and migration, and provide co-stimulation for T cell proliferation and interferon-γ (IFN-γ) secretion. | RNA-seq and flow cytometry | Breast cancer and colorectal cancer [43] |
| CXCL9+CXCL10+ CCL5+MHCII+ CD40+STAT1+ macrophages | - | Anti-tumoral | Secrete CXCL9 to facilitate recruitment of protective T cells. | scRNA-seq | Lung cancer [44,45] |
| CSFR1+CCR2−CD68+ CD163+SIGLEC1− macrophages; CSFR1+CCR2−CD68+CD163+SIGLEC1+ macrophages; CSFR1+CCR2−CD68+CD163−SIGLEC1+ macrophages | CD14++CD16−CCR2+ classical monocytes | Pro-tumoral | Engage in a tumor cell-TAM auto-stimulatory loop, increase tumor cell motility, and increase monocyte infiltration into the tumor site to generate more pro-tumoral TAMs. | RNA-seq | Breast cancer [8] |
| TREM2+FOLR2+CD163+ macrophages | S100A8+ monocytes | Pro-tumoral | Recruit suppressive regulatory T cells (Treg) and MDSCs to facilitate immunosuppressive microenvironment. | scRNA-seq | Hepatocellular carcinoma [46] |
| CD14+CD16+(FCGR3A) CD81+ITGAX+CSF1R+ monocytes/macrophages | - | Pro-tumoral | Secrete specific profibrotic, pro-metastatic growth factors involved ECM deposition and remodeling. | scRNA-seq | Small cell lung cancer [47] |
| CD11b+CCR2+IL-1βhiArg1− M-MDSCs | CD11b+CCR2+ monocytes | Pro-tumoral | Promote tumor growth, suppress T cell function, and maintain suppressive TME. | scRNA-seq | Melanoma [42,48] |
| CD84+CD11b+/CD14+ M-MDSCs | PBMC | Pro-tumoral | Exhibit T cell suppression and increase ROS production. | scRNA-seq | Breast cancer [49] |
| CD14+HLA-DRlo/− monocytes/MDSCs | CD14+HLA-DRlo/− monocytes | Pro-tumoral | Inhibit T cell responses. | Flow cytometry | Epithelial ovarian cancer [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, Y.; Xia, H.; Chen, Y.H. Monocytes in Tumorigenesis and Tumor Immunotherapy. Cells 2023, 12, 1673. https://doi.org/10.3390/cells12131673
Chen X, Li Y, Xia H, Chen YH. Monocytes in Tumorigenesis and Tumor Immunotherapy. Cells. 2023; 12(13):1673. https://doi.org/10.3390/cells12131673
Chicago/Turabian StyleChen, Xiaodie, Yunqing Li, Houjun Xia, and Youhai H. Chen. 2023. "Monocytes in Tumorigenesis and Tumor Immunotherapy" Cells 12, no. 13: 1673. https://doi.org/10.3390/cells12131673
APA StyleChen, X., Li, Y., Xia, H., & Chen, Y. H. (2023). Monocytes in Tumorigenesis and Tumor Immunotherapy. Cells, 12(13), 1673. https://doi.org/10.3390/cells12131673





