Clinical Significance of MicroRNAs, Long Non-Coding RNAs, and CircRNAs in Cardiovascular Diseases
Abstract
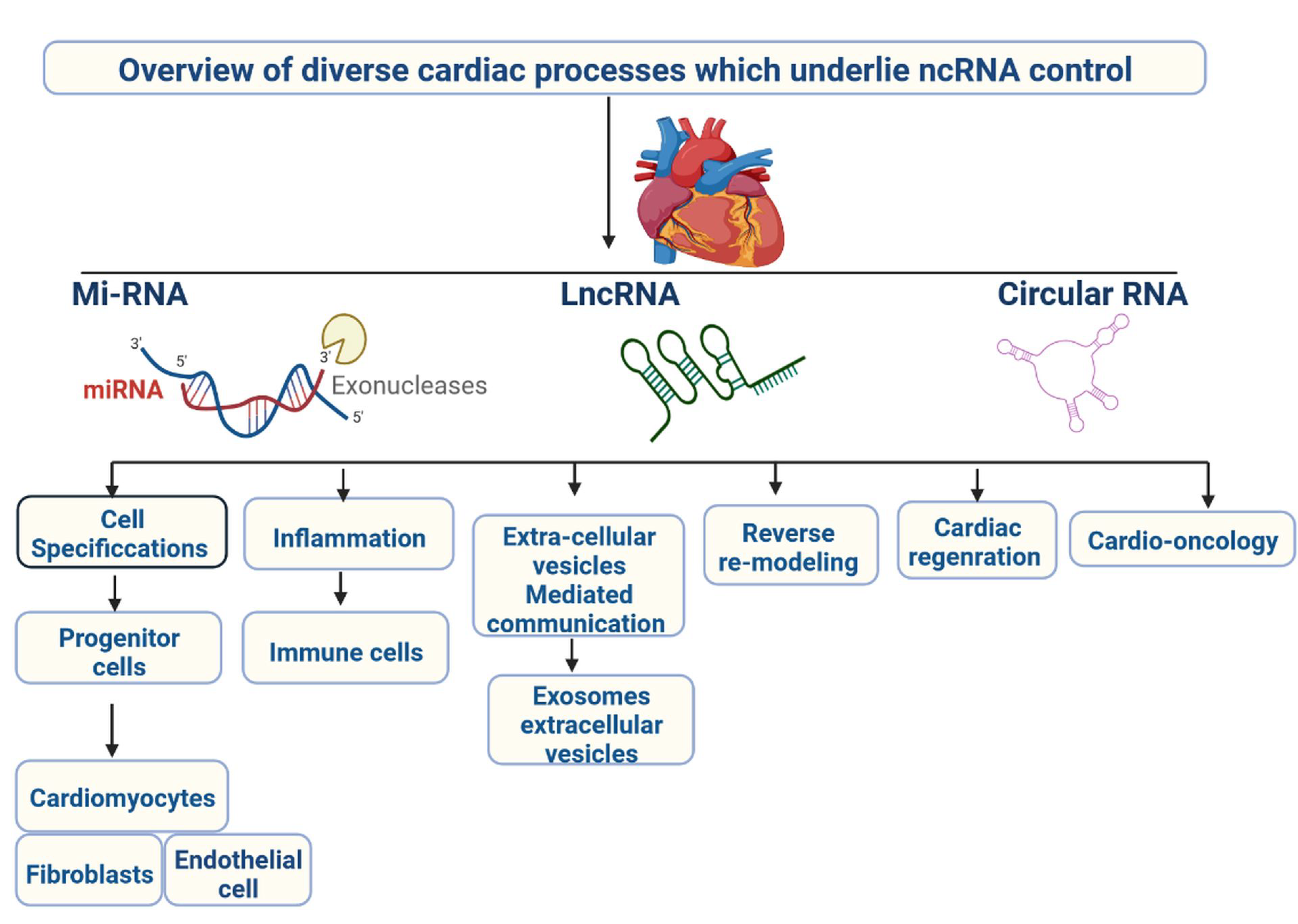
1. Introduction
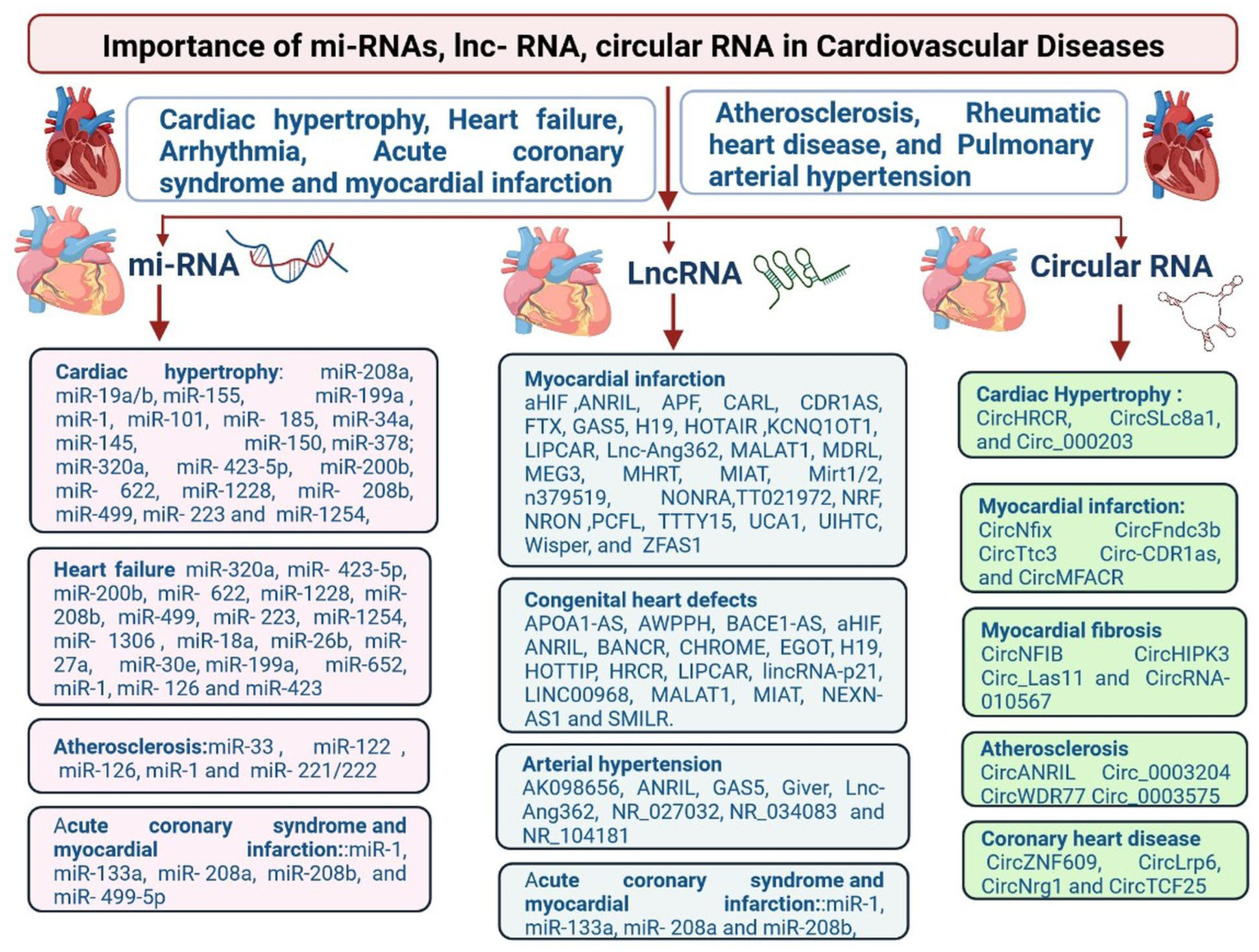
2. miRNAs and CVDs
2.1. miRNAs and HF
2.2. Arrhythmias
2.3. miRNAs and ACS and MI
2.4. miRNAs and Atherosclerosis
2.5. miRNAs and RHD
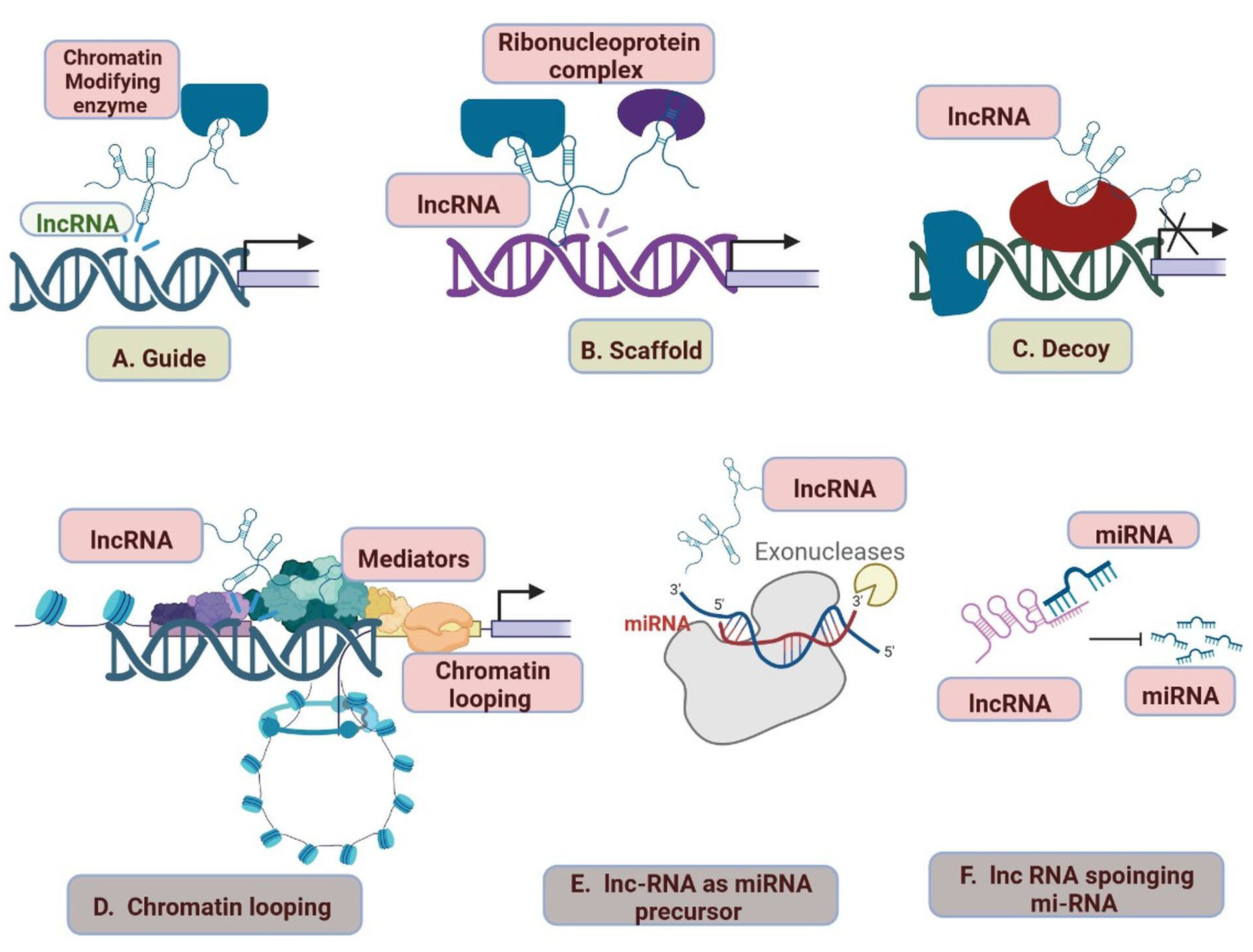
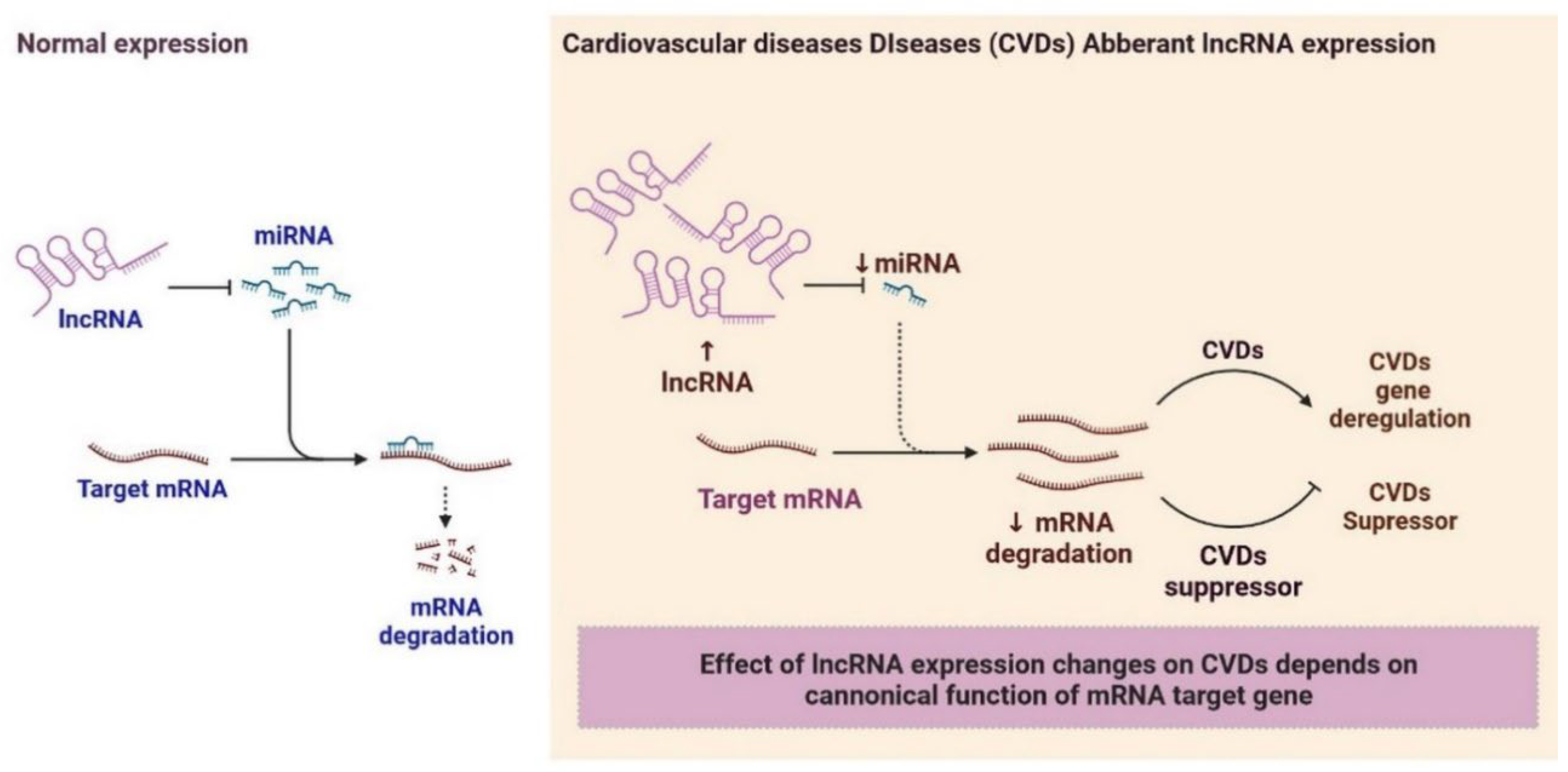
2.6. LncRNAs and Cardiovascular Diseases
3. Clinical Significance of lncRNAs in Cardiovascular Diseases
3.1. Arterial Hypertension
3.2. Coronary Heart Disease
3.3. Acute Myocardial Infarction
3.4. Heart Failure
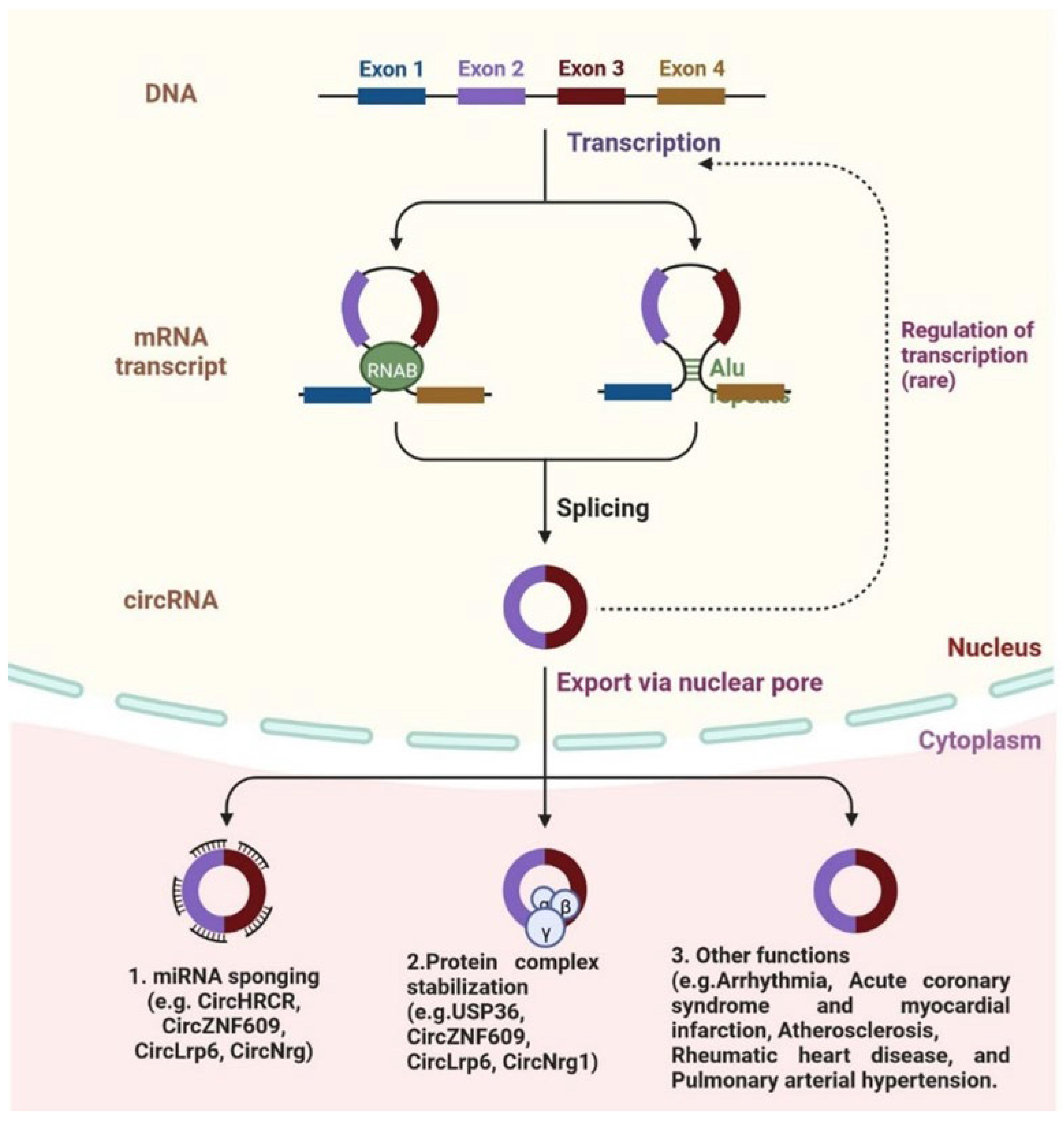
4. Circular RNA and Cardiovascular Diseases
4.1. circRNA in Atherosclerosis
4.2. Coronary Heart Disease (CHD)
4.3. Cardiac Ischaemia/Reperfusion (I/R) Injury and Myocardial Infarction (MI)
4.4. Heart Failure (HF)
4.5. Cardiomyopathies (CM)
4.6. circRNAS in Cardiac Regeneration
5. Clinical Investigations of Cardiovascular Non-Coding RNA Therapies
6. Future Prospective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Sreeniwas Kumar, A.; Sinha, N. Cardiovascular Disease in India: A 360 Degree Overview. Med. J. Armed Forces India 2020, 76, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Godia, E.; Ois, A.; Roquer, J. Heart Failure in Acute Ischemic Stroke. CCR 2010, 6, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Schwalm, J.D.; McKee, M.; Huffman, M.D.; Yusuf, S. Resource Effective Strategies to Prevent and Treat Cardiovascular Disease. Circulation 2016, 133, 742–755. [Google Scholar] [CrossRef]
- Sallam, T.; Sandhu, J.; Tontonoz, P. Long Noncoding RNA Discovery in Cardiovascular Disease: Decoding Form to Function. Circ. Res. 2018, 122, 155–166. [Google Scholar] [CrossRef]
- Zhang, C.; Han, B.; Xu, T.; Li, D. The Biological Function and Potential Mechanism of Long Non-coding RNAs in Cardiovascular Disease. J. Cell. Mol. Med. 2020, 24, 12900–12909. [Google Scholar] [CrossRef]
- Correia, C.C.M.; Rodrigues, L.F.; de Avila Pelozin, B.R.; Oliveira, E.M.; Fernandes, T. Long Non-Coding RNAs in Cardiovascular Diseases: Potential Function as Biomarkers and Therapeutic Targets of Exercise Training. ncRNA 2021, 7, 65. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.-M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-Coding RNAs in Cardiovascular Diseases: Diagnostic and Therapeutic Perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef]
- Lu, P.; Ding, F.; Xiang, Y.K.; Hao, L.; Zhao, M. Noncoding RNAs in Cardiac Hypertrophy and Heart Failure. Cells 2022, 11, 777. [Google Scholar] [CrossRef]
- Huang, C.-K.; Kafert-Kasting, S.; Thum, T. Preclinical and Clinical Development of Noncoding RNA Therapeutics for Cardiovascular Disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.-C.; Lazar, A.-L.; Marta, M.M.; Cozma, A.; Catana, C.-S. Non-Coding RNAs: Prevention, Diagnosis, and Treatment in Myocardial Ischemia–Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 2728. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-H.; Li, J.-L.; Li, X.-Y.; Wang, S.-X.; Jiao, Z.-H.; Li, S.-Q.; Liu, J.; Ding, J. MiR-208a in Cardiac Hypertrophy and Remodeling. Front. Cardiovasc. Med. 2021, 8, 773314. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hao, Q.; Wei, J.; Li, G.-H.; Wu, Y.; Zhao, Y.-F. MicroRNA-19a/b-3p Protect the Heart from Hypertension-Induced Pathological Cardiac Hypertrophy through PDE5A. J. Hypertens. 2018, 36, 1847–1857. [Google Scholar] [CrossRef]
- Seok, H.Y.; Chen, J.; Kataoka, M.; Huang, Z.-P.; Ding, J.; Yan, J.; Hu, X.; Wang, D.-Z. Loss of MicroRNA-155 Protects the Heart From Pathological Cardiac Hypertrophy. Circ. Res. 2014, 114, 1585–1595. [Google Scholar] [CrossRef]
- Yan, M.; Yang, S.; Meng, F.; Zhao, Z.; Tian, Z.; Yang, P. MicroRNA 199a-5p Induces Apoptosis by Targeting JunB. Sci. Rep. 2018, 8, 6699. [Google Scholar] [CrossRef]
- Wehbe, N.; Nasser, S.; Pintus, G.; Badran, A.; Eid, A.; Baydoun, E. MicroRNAs in Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 4714. [Google Scholar] [CrossRef]
- Wei, L.; Yuan, M.; Zhou, R.; Bai, Q.; Zhang, W.; Zhang, M.; Huang, Y.; Shi, L. MicroRNA-101 Inhibits Rat Cardiac Hypertrophy by Targeting Rab1a. J. Cardiovasc. Pharmacol. 2015, 65, 357–363. [Google Scholar] [CrossRef]
- Kim, J.O.; Song, D.W.; Kwon, E.J.; Hong, S.-E.; Song, H.K.; Min, C.K.; Kim, D.H. MiR-185 Plays an Anti-Hypertrophic Role in the Heart via Multiple Targets in the Calcium-Signaling Pathways. PLoS ONE 2015, 10, e0122509. [Google Scholar] [CrossRef]
- Huang, J.; Sun, W.; Huang, H.; Ye, J.; Pan, W.; Zhong, Y.; Cheng, C.; You, X.; Liu, B.; Xiong, L.; et al. MiR-34a Modulates Angiotensin II-Induced Myocardial Hypertrophy by Direct Inhibition of ATG9A Expression and Autophagic Activity. PLoS ONE 2014, 9, e94382. [Google Scholar] [CrossRef]
- Li, R.; Yan, G.; Zhang, Q.; Jiang, Y.; Sun, H.; Hu, Y.; Sun, J.; Xu, B. MiR-145 Inhibits Isoproterenol-induced Cardiomyocyte Hypertrophy by Targeting the Expression and Localization of GATA6. FEBS Lett. 2013, 587, 1754–1761. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhang, Y.; Zhu, X.; Zhang, R.; Guan, L.; Tang, Q.; Jiang, H.; Huang, C.; Huang, H. MicroRNA-150 Protects Against Pressure Overload-Induced Cardiac Hypertrophy: M ICRO RNA-150 M ODULATES C ARDIAC H YPERTROPHY. J. Cell. Biochem. 2015, 116, 2166–2176. [Google Scholar] [CrossRef]
- Ganesan, J.; Ramanujam, D.; Sassi, Y.; Ahles, A.; Jentzsch, C.; Werfel, S.; Leierseder, S.; Loyer, X.; Giacca, M.; Zentilin, L.; et al. MiR-378 Controls Cardiac Hypertrophy by Combined Repression of Mitogen-Activated Protein Kinase Pathway Factors. Circulation 2013, 127, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mao, S.; Liu, X.; Li, S.; Zhou, H.; Gu, Y.; Liu, W.; Fu, L.; Liao, C.; Wang, P. MiR-125b Inhibits Cardiomyocyte Apoptosis by Targeting BAK1 in Heart Failure. Mol. Med. 2021, 27, 72. [Google Scholar] [CrossRef]
- Huang, Z.-P.; Wang, D.-Z. MiR-22 in Cardiac Remodeling and Disease. Trends Cardiovasc. Med. 2014, 24, 267–272. [Google Scholar] [CrossRef]
- Li, F.; Li, S.-S.; Chen, H.; Zhao, J.-Z.; Hao, J.; Liu, J.-M.; Zu, X.-G.; Cui, W. MiR-320 Accelerates Chronic Heart Failure with Cardiac Fibrosis through Activation of the IL6/STAT3 Axis. Aging 2021, 13, 22516–22527. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p As a Circulating Biomarker for Heart Failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, N.; Du, J.; Zhang, H.; Zhang, C. MicroRNA-200b-3p Promotes Endothelial Cell Apoptosis by Targeting HDAC4 in Atherosclerosis. BMC Cardiovasc. Disord 2021, 21, 172. [Google Scholar] [CrossRef]
- Shen, N.-N.; Wang, J.-L.; Fu, Y. The MicroRNA Expression Profiling in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 856358. [Google Scholar] [CrossRef]
- Peterlin, A.; Počivavšek, K.; Petrovič, D.; Peterlin, B. The Role of MicroRNAs in Heart Failure: A Systematic Review. Front. Cardiovasc. Med. 2020, 7, 161. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Sun, X. The Functions of MicroRNA-208 in the Heart. Diabetes Res. Clin. Pract. 2020, 160, 108004. [Google Scholar] [CrossRef] [PubMed]
- Khanaghaei, M.; Tourkianvalashani, F.; Hekmatimoghaddam, S.; Ghasemi, N.; Rahaie, M.; Khorramshahi, V.; Sheikhpour, A.; Heydari, Z.; Pourrajab, F. Circulating MiR-126 and MiR-499 Reflect Progression of Cardiovascular Disease; Correlations with Uric Acid and Ejection Fraction. Heart Int. 2016, 11, heartint.500022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-W.; Shen, Y.-J.; Shi, J.; Yu, J.-G. MiR-223-3p in Cardiovascular Diseases: A Biomarker and Potential Therapeutic Target. Front. Cardiovasc. Med. 2021, 7, 610561. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Cediel, G.; Bär, C.; Núñez, J.; Revuelta-Lopez, E.; Gavara, J.; Ríos-Navarro, C.; Llorente-Cortes, V.; Bodí, V.; Thum, T.; et al. Circulating MiR-1254 Predicts Ventricular Remodeling in Patients with ST-Segment-Elevation Myocardial Infarction: A Cardiovascular Magnetic Resonance Study. Sci. Rep. 2018, 8, 15115. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Li, J.; Sheng, L.; Liu, X. Upregulation of MiR-1306-5p Decreases Cerebral Ischemia/Reperfusion Injury in Vitro by Targeting BIK. Biosci. Biotechnol. Biochem. 2019, 83, 2230–2237. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, C.; Li, D.; Yang, Z. MicroRNA-18a Expression in Female Coronary Heart Disease and Regulatory Mechanism on Endothelial Cell by Targeting Estrogen Receptor. J. Cardiovasc. Pharmacol. 2018, 72, 277–284. [Google Scholar] [CrossRef]
- Icli, B.; Dorbala, P.; Feinberg, M.W. An Emerging Role for the MiR-26 Family in Cardiovascular Disease. Trends Cardiovasc. Med. 2014, 24, 241–248. [Google Scholar] [CrossRef]
- Tian, C.; Hu, G.; Gao, L.; Hackfort, B.T.; Zucker, I.H. Extracellular Vesicular MicroRNA-27a* Contributes to Cardiac Hypertrophy in Chronic Heart Failure. J. Mol. Cell. Cardiol. 2020, 143, 120–131. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.-S.; Fan, S.-W.; Zhao, X.-Y.; Li, C.; Zhao, Z.-Y.; Pei, H.-J.; Qiu, L.; Zhuang, X.; Yang, C.-H. Prognostic Value of MicroRNAs in Heart Failure: A Meta-Analysis. Medicine 2021, 100, e27744. [Google Scholar] [CrossRef]
- Guan, X.; Wang, L.; Liu, Z.; Guo, X.; Jiang, Y.; Lu, Y.; Peng, Y.; Liu, T.; Yang, B.; Shan, H.; et al. MiR-106a Promotes Cardiac Hypertrophy by Targeting Mitofusin 2. J. Mol. Cell. Cardiol. 2016, 99, 207–217. [Google Scholar] [CrossRef]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA Therapy Stimulates Uncontrolled Cardiac Repair after Myocardial Infarction in Pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef]
- Chi, X.; Jiang, Y.; Chen, Y.; Lv, L.; Chen, J.; Yang, F.; Zhang, X.; Pan, F.; Cai, Q. Upregulation of MicroRNA MiR-652-3p Is a Prognostic Risk Factor for Hepatocellular Carcinoma and Regulates Cell Proliferation, Migration, and Invasion. Bioengineered 2021, 12, 7519–7528. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Kalocayova, B.; Devaux, Y.; Bartekova, M. Potential Clinical Implications of MiR-1 and MiR-21 in Heart Disease and Cardioprotection. Int. J. Mol. Sci. 2020, 21, 700. [Google Scholar] [CrossRef]
- Wang, X.; Lian, Y.; Wen, X.; Guo, J.; Wang, Z.; Jiang, S.; Hu, Y. Expression of MiR-126 and Its Potential Function in Coronary Artery Disease. Afr. Health Sci. 2017, 17, 474. [Google Scholar] [CrossRef] [PubMed]
- Rizzacasa, B.; Morini, E.; Mango, R.; Vancheri, C.; Budassi, S.; Massaro, G.; Maletta, S.; Macrini, M.; D’Annibale, S.; Romeo, F.; et al. MiR-423 Is Differentially Expressed in Patients with Stable and Unstable Coronary Artery Disease: A Pilot Study. PLoS ONE 2019, 14, e0216363. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Gharakhanlou, R.; Rezaei, R. The Changes Of Heart MiR-1 And MiR-133 Expressions Following Physiological Hypertrophy Due To Endurance Training. Cell J. 2020, 22, 133–140. [Google Scholar] [CrossRef]
- Luo, X.; Pan, Z.; Shan, H.; Xiao, J.; Sun, X.; Wang, N.; Lin, H.; Xiao, L.; Maguy, A.; Qi, X.-Y.; et al. MicroRNA-26 Governs Profibrillatory Inward-Rectifier Potassium Current Changes in Atrial Fibrillation. J. Clin. Investig. 2013, 123, 1939–1951. [Google Scholar] [CrossRef]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.-D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B.; et al. Cardiac Myocyte MiR-29 Promotes Pathological Remodeling of the Heart by Activating Wnt Signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Tang, Q. MiR-133: A Suppressor of Cardiac Remodeling? Front. Pharmacol. 2018, 9, 903. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Liang, X.; Chen, X.; Chen, X.; Chen, C. Upregulated MiR-328-3p and Its High Risk in Atrial Fibrillation: A Systematic Review and Meta-Analysis with Meta-Regression. Medicine 2022, 101, e28980. [Google Scholar] [CrossRef]
- Ling, T.-Y.; Wang, X.-L.; Chai, Q.; Lau, T.-W.; Koestler, C.M.; Park, S.J.; Daly, R.C.; Greason, K.L.; Jen, J.; Wu, L.-Q.; et al. Regulation of the SK3 Channel by MicroRNA-499—Potential Role in Atrial Fibrillation. Heart Rhythm. 2013, 10, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Cardin, S.; Guasch, E.; Luo, X.; Naud, P.; Le Quang, K.; Shi, Y.; Tardif, J.-C.; Comtois, P.; Nattel, S. Role for MicroRNA-21 in Atrial Profibrillatory Fibrotic Remodeling Associated With Experimental Postinfarction Heart Failure. Circ. Arrhythmia Electrophysiol. 2012, 5, 1027–1035. [Google Scholar] [CrossRef]
- Girmatsion, Z.; Biliczki, P.; Bonauer, A.; Wimmer-Greinecker, G.; Scherer, M.; Moritz, A.; Bukowska, A.; Goette, A.; Nattel, S.; Hohnloser, S.H.; et al. Changes in MicroRNA-1 Expression and IK1 up-Regulation in Human Atrial Fibrillation. Heart Rhythm. 2009, 6, 1802–1809. [Google Scholar] [CrossRef]
- Wexler, Y.; Nussinovitch, U. The Diagnostic Value of Mir-133a in ST Elevation and Non-ST Elevation Myocardial Infarction: A Meta-Analysis. Cells 2020, 9, 793. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Tian, L.; Sun, Q. Circulating MicroRNA-208 Family as Early Diagnostic Biomarkers for Acute Myocardial Infarction: A Meta-Analysis. Medicine 2021, 100, e27779. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M. MicroRNA-499-5p: A Therapeutic Target in the Context of Cardiovascular Disease. Ann. Transl. Med. 2016, 4, 539. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Guo, Z.; Shi, Y.; Zhang, L.; Song, C. Serum Exosomal MicroRNA-21, MicroRNA-126, and PTEN Are Novel Biomarkers for Diagnosis of Acute Coronary Syndrome. Front. Physiol. 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, J.; Song, M.; Zhang, L.; Li, Y.; Han, L.; Tang, M.; Zhang, W.; Zhong, M.; Wang, Z. Associations of Circulating MicroRNA-221 and 222 With the Severity of Coronary Artery Lesions in Acute Coronary Syndrome Patients. Angiology 2022, 73, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Rusu-Nastase, E.G.; Lupan, A.-M.; Marinescu, C.I.; Neculachi, C.A.; Preda, M.B.; Burlacu, A. MiR-29a Increase in Aging May Function as a Compensatory Mechanism Against Cardiac Fibrosis Through SERPINH1 Downregulation. Front. Cardiovasc. Med. 2022, 8, 810241. [Google Scholar] [CrossRef]
- Caruso, P.; Dempsie, Y.; Stevens, H.C.; McDonald, R.A.; Long, L.; Lu, R.; White, K.; Mair, K.M.; McClure, J.D.; Southwood, M.; et al. A Role for MiR-145 in Pulmonary Arterial Hypertension: Evidence From Mouse Models and Patient Samples. Circ. Res. 2012, 111, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.N.; Jin, R.C.; Rabello, S.; Gulbahce, N.; White, K.; Hale, A.; Cottrill, K.A.; Shaik, R.S.; Waxman, A.B.; Zhang, Y.-Y.; et al. MicroRNA-21 Integrates Pathogenic Signaling to Control Pulmonary Hypertension: Results of a Network Bioinformatics Approach. Circulation 2012, 125, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Ramanathan, G.K.; Parthasarathy, P.T.; Aljubran, S.; Galam, L.; Yunus, A.; Garcia, S.; Cox, R.R.; Lockey, R.F.; Kolliputi, N. Mir-206 Regulates Pulmonary Artery Smooth Muscle Cell Proliferation and Differentiation. PLoS ONE 2012, 7, e46808. [Google Scholar] [CrossRef]
- Guo, L.; Qiu, Z.; Wei, L.; Yu, X.; Gao, X.; Jiang, S.; Tian, H.; Jiang, C.; Zhu, D. The MicroRNA-328 Regulates Hypoxic Pulmonary Hypertension by Targeting at Insulin Growth Factor 1 Receptor and L-Type Calcium Channel-A1C. Hypertension 2012, 59, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Courboulin, A.; Paulin, R.; Giguère, N.J.; Saksouk, N.; Perreault, T.; Meloche, J.; Paquet, E.R.; Biardel, S.; Provencher, S.; Côté, J.; et al. Role for MiR-204 in Human Pulmonary Arterial Hypertension. J. Exp. Med. 2011, 208, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Ediriweera, H.; Afonso, M.S.; Ramkhelawon, B.; Singaravelu, R.; Liao, X.; Bandler, R.C.; Rahman, K.; Fisher, E.A.; Rayner, K.J.; et al. MicroRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. ATVB 2017, 37, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Du, X.; Yang, Y.; Liu, X.; Liu, X.; Zhang, N.; Li, Y.; Jiang, X.; Jiang, Y.; Yang, Z. Inhibition of MiR-122 Reduced Atherosclerotic Lesion Formation by Regulating NPAS3-Mediated Endothelial to Mesenchymal Transition. Life Sci. 2021, 265, 118816. [Google Scholar] [CrossRef]
- Boon, R.A.; Dimmeler, S. MicroRNA-126 in Atherosclerosis. ATVB 2014, 34, 449–454. [Google Scholar] [CrossRef]
- Šatrauskienė, A.; Navickas, R.; Laucevičius, A.; Krilavičius, T.; Užupytė, R.; Zdanytė, M.; Ryliškytė, L.; Jucevičienė, A.; Holvoet, P. Mir-1, MiR-122, MiR-132, and MiR-133 Are Related to Subclinical Aortic Atherosclerosis Associated with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 1483. [Google Scholar] [CrossRef]
- Song, J.; Ouyang, Y.; Che, J.; Li, X.; Zhao, Y.; Yang, K.; Zhao, X.; Chen, Y.; Fan, C.; Yuan, W. Potential Value of MiR-221/222 as Diagnostic, Prognostic, and Therapeutic Biomarkers for Diseases. Front. Immunol. 2017, 8, 56. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of MiRNAs and SiRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, N.; Sun, L.; Zheng, D.; Shao, G. Non-Coding RNAs: The Key Detectors and Regulators in Cardiovascular Disease. Genomics 2021, 113, 1233–1246. [Google Scholar] [CrossRef]
- Saheera, S.; Krishnamurthy, P. Cardiovascular Changes Associated with Hypertensive Heart Disease and Aging. Cell Transpl. 2020, 29, 096368972092083. [Google Scholar] [CrossRef]
- Vavassori, C.; Cipriani, E.; Colombo, G.I. Circulating MicroRNAs as Novel Biomarkers in Risk Assessment and Prognosis of Coronary Artery Disease. Eur. Cardiol. 2022, 17, e06. [Google Scholar] [CrossRef]
- Knezevic, I.; Patel, A.; Sundaresan, N.R.; Gupta, M.P.; Solaro, R.J.; Nagalingam, R.S.; Gupta, M. A Novel Cardiomyocyte-Enriched MicroRNA, MiR-378, Targets Insulin-like Growth Factor 1 Receptor. J. Biol. Chem. 2012, 287, 12913–12926. [Google Scholar] [CrossRef]
- Gozuacik, D.; Akkoc, Y.; Ozturk, D.G.; Kocak, M. Autophagy-Regulating MicroRNAs and Cancer. Front. Oncol. 2017, 7, 65. [Google Scholar] [CrossRef]
- Ikeda, S.; He, A.; Kong, S.W.; Lu, J.; Bejar, R.; Bodyak, N.; Lee, K.-H.; Ma, Q.; Kang, P.M.; Golub, T.R.; et al. MicroRNA-1 Negatively Regulates Expression of the Hypertrophy-Associated Calmodulin and Mef2a Genes. Mol. Cell Biol. 2009, 29, 2193–2204. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure With Preserved Ejection Fraction In Perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef]
- Wong, L.; Wang, J.; Liew, O.; Richards, A.; Chen, Y.-T. MicroRNA and Heart Failure. Int. J. Mol. Sci. 2016, 17, 502. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C. Diagnostic and Prognostic Value of Circulating MicroRNAs in Heart Failure with Preserved and Reduced Ejection Fraction. WJC 2015, 7, 843. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Nishida, K.; Kato, T.; Nattel, S. Atrial Fibrillation Pathophysiology: Implications for Management. Circulation 2011, 124, 2264–2274. [Google Scholar] [CrossRef]
- Ultimo, S.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Capitani, S.; Neri, L.M. Cardiovascular Disease-Related MiRNAs Expression: Potential Role as Biomarkers and Effects of Training Exercise. Oncotarget 2018, 9, 17238–17254. [Google Scholar] [CrossRef]
- Osbourne, A.; Calway, T.; Broman, M.; McSharry, S.; Earley, J.; Kim, G.H. Downregulation of Connexin43 by MicroRNA-130a in Cardiomyocytes Results in Cardiac Arrhythmias. J. Mol. Cell. Cardiol. 2014, 74, 53–63. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Sayed, A.S.M.; Xia, K.; Yang, T.-L.; Peng, J. Circulating MicroRNAs: A Potential Role in Diagnosis and Prognosis of Acute Myocardial Infarction. Dis. Markers 2013, 35, 561–566. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, J.; Wang, J.; Zhang, Z.; Freedman, J.H.; Zheng, Y.; Cai, L. MiRNAS in Cardiovascular Diseases: Potential Biomarkers, Therapeutic Targets and Challenges. Acta Pharm. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Halushka, P.V.; Goodwin, A.J.; Halushka, M.K. Opportunities for MicroRNAs in the Crowded Field of Cardiovascular Biomarkers. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 211–238. [Google Scholar] [CrossRef] [PubMed]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef]
- Ali Sheikh, M.S.; Alduraywish, A.; Almaeen, A.; Alruwali, M.; Alruwaili, R.; Alomair, B.M.; Salma, U.; Hedeab, G.M.; Bugti, N.; A.M.Abdulhabeeb, I. Therapeutic Value of MiRNAs in Coronary Artery Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8853748. [Google Scholar] [CrossRef] [PubMed]
- Andreou, I.; Sun, X.; Stone, P.H.; Edelman, E.R.; Feinberg, M.W. MiRNAs in Atherosclerotic Plaque Initiation, Progression, and Rupture. Trends Mol. Med. 2015, 21, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Uray, K.; Major, E.; Lontay, B. MicroRNA Regulatory Pathways in the Control of the Actin–Myosin Cytoskeleton. Cells 2020, 9, 1649. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S.; Chello, M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. Int. J. Mol. Sci. 2021, 22, 12132. [Google Scholar] [CrossRef] [PubMed]
- Bielska, A.; Niemira, M.; Kretowski, A. Recent Highlights of Research on MiRNAs as Early Potential Biomarkers for Cardiovascular Complications of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3153. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xu, Y.; Wang, R.; Hu, L.; Guo, D.; Xue, F.; Guo, W.; Zhang, D.; Hu, J.; Li, Y.; et al. Recent Advances on the Roles of LncRNAs in Cardiovascular Disease. J. Cell. Mol. Med. 2020, 24, 12246–12257. [Google Scholar] [CrossRef] [PubMed]
- Bär, C.; Chatterjee, S.; Thum, T. Long Noncoding RNAs in Cardiovascular Pathology, Diagnosis, and Therapy. Circulation 2016, 134, 1484–1499. [Google Scholar] [CrossRef]
- Uchida, S.; Dimmeler, S. Long Noncoding RNAs in Cardiovascular Diseases. Circ. Res. 2015, 116, 737–750. [Google Scholar] [CrossRef]
- Ounzain, S.; Pedrazzini, T. Super-Enhancer Lncs to Cardiovascular Development and Disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1953–1960. [Google Scholar] [CrossRef]
- Su, W.; Huo, Q.; Wu, H.; Wang, L.; Ding, X.; Liang, L.; Zhou, L.; Zhao, Y.; Dan, J.; Zhang, H. The Function of LncRNA-H19 in Cardiac Hypertrophy. Cell Biosci. 2021, 11, 153. [Google Scholar] [CrossRef]
- Wolska, M.; Jarosz-Popek, J.; Junger, E.; Wicik, Z.; Porshoor, T.; Sharif, L.; Czajka, P.; Postula, M.; Mirowska-Guzel, D.; Czlonkowska, A.; et al. Long Non-Coding RNAs as Promising Therapeutic Approach in Ischemic Stroke: A Comprehensive Review. Mol. Neurobiol. 2021, 58, 1664–1682. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Q.; Mao, J.; Zhang, J.; Li, L. The Roles of LncRNA in Myocardial Infarction: Molecular Mechanisms, Diagnosis Biomarkers, and Therapeutic Perspectives. Front. Cell Dev. Biol. 2021, 9, 680713. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X.; Hu, F.; Fu, X.; Jiang, Z.; Chen, K. LncRNA ANRIL Knockdown Relieves Myocardial Cell Apoptosis in Acute Myocardial Infarction by Regulating IL-33/ST2. Cell Cycle 2019, 18, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Li, N.; Xu, X.-X.; Li, X.-X.; Xu, X.-J.; Guo, D.; Zhang, D.; Wu, Z.-H.; Zhang, S.-Y. Long Noncoding RNA FTX Regulates Cardiomyocyte Apoptosis by Targeting MiR-29b-1-5p and Bcl2l2. Biochem. Biophys. Res. Commun. 2018, 495, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cantile, M.; Di Bonito, M.; Tracey De Bellis, M.; Botti, G. Functional Interaction among LncRNA HOTAIR and MicroRNAs in Cancer and Other Human Diseases. Cancers 2021, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, Y.; Yan, S.; Shi, Y.; Han, B.; Li, J.; Cha, L.; Mu, J. Down-Regulation of LncRNA KCNQ1OT1 Protects against Myocardial Ischemia/Reperfusion Injury Following Acute Myocardial Infarction. Biochem. Biophys. Res. Commun. 2017, 491, 1026–1033. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients With Heart Failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, S.; Song, F.; Zhou, Y.; He, X. Lnc-Ang362 Is a pro-Fibrotic Long Non-Coding RNA Promoting Cardiac Fibrosis after Myocardial Infarction by Suppressing Smad7. Arch. Biochem. Biophys. 2020, 685, 108354. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Singh, K.K. Epigenetic Regulation of Autophagy in Cardiovascular Pathobiology. Int. J. Mol. Sci. 2021, 22, 6544. [Google Scholar] [CrossRef]
- Nukala, S.B.; Jousma, J.; Cho, Y.; Lee, W.H.; Ong, S.-G. Long Non-Coding RNAs and MicroRNAs as Crucial Regulators in Cardio-Oncology. Cell Biosci. 2022, 12, 24. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, Z.-A.; Liu, J.; Hao, K.; Yu, Y.; Han, X.; Li, J.; Wang, Y.; Lei, W.; Dong, N.; et al. Long Noncoding RNA Meg3 Regulates Cardiomyocyte Apoptosis in Myocardial Infarction. Gene Ther. 2018, 25, 511–523. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, C.; Meng, M.; Tang, H. Long Noncoding RNA MHRT Protects Cardiomyocytes against H2O2-Induced Apoptosis. Biomol. Ther. 2016, 24, 19–24. [Google Scholar] [CrossRef]
- Wang, X.; Yong, C.; Yu, K.; Yu, R.; Zhang, R.; Yu, L.; Li, S.; Cai, S. Long Noncoding RNA (LncRNA) N379519 Promotes Cardiac Fibrosis in Post-Infarct Myocardium by Targeting MiR-30. Med. Sci. Monit. 2018, 24, 3958–3965. [Google Scholar] [CrossRef]
- Magadum, A.; Singh, N.; Kurian, A.A.; Munir, I.; Mehmood, T.; Brown, K.; Sharkar, M.T.K.; Chepurko, E.; Sassi, Y.; Oh, J.G.; et al. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation 2020, 141, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, F.; Liu, C.-Y.; An, T.; Zhang, J.; Zhou, L.-Y.; Wang, M.; Dong, Y.-H.; Li, N.; Gao, J.-N.; et al. The Long Noncoding RNA NRF Regulates Programmed Necrosis and Myocardial Injury during Ischemia and Reperfusion by Targeting MiR-873. Cell Death Differ. 2016, 23, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, R.; Plaisance, I.; Abraham, B.J.; Sarre, A.; Ting, C.-C.; Alexanian, M.; Maric, D.; Maison, D.; Nemir, M.; Young, R.A.; et al. The Long Noncoding RNA Wisper Controls Cardiac Fibrosis and Remodeling. Sci. Transl. Med. 2017, 9, eaai9118. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, M.; Shao, Y.; Zhang, Y.; Gong, M.; Yang, X.; Wang, Y.; Tan, Z.; Sun, L.; Xuan, L.; et al. LncRNA-ZFAS1 Induces Mitochondria-Mediated Apoptosis by Causing Cytosolic Ca2+ Overload in Myocardial Infarction Mice Model. Cell Death Dis. 2019, 10, 942. [Google Scholar] [CrossRef]
- Dueñas, A.; Expósito, A.; Aranega, A.; Franco, D. The Role of Non-Coding RNA in Congenital Heart Diseases. JCDD 2019, 6, 15. [Google Scholar] [CrossRef]
- Lu, M.; Lu, Q.; Zhang, Y.; Tian, G. ApoB/ApoA1 Is an Effective Predictor of Coronary Heart Disease Risk in Overweight and Obesity. J. Biomed. Res. 2011, 25, 266–273. [Google Scholar] [CrossRef]
- Li, X.; Song, F.; Sun, H. Long Non-coding RNA AWPPH Interacts with ROCK2 and Regulates the Proliferation and Apoptosis of Cancer Cells in Pediatric T-cell Acute Lymphoblastic Leukemia. Oncol. Lett. 2020, 20, 239. [Google Scholar] [CrossRef]
- Li, Y.; Fang, J.; Zhou, Z.; Zhou, Q.; Sun, S.; Jin, Z.; Xi, Z.; Wei, J. Downregulation of LncRNA BACE1-AS Improves Dopamine-Dependent Oxidative Stress in Rats with Parkinson’s Disease by Upregulating MicroRNA-34b-5p and Downregulating BACE1. Cell Cycle 2020, 19, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhou, Y.; Lu, L.; Zhang, P.; Ren, R.; Wang, Y.; Wang, J. Identifying a Serum Exosomal-Associated LncRNA/CircRNA-MiRNA-MRNA Network in Coronary Heart Disease. Cardiol. Res. Pract. 2021, 2021, 6682183. [Google Scholar] [CrossRef]
- Hennessy, E.J.; van Solingen, C.; Scacalossi, K.R.; Ouimet, M.; Afonso, M.S.; Prins, J.; Koelwyn, G.J.; Sharma, M.; Ramkhelawon, B.; Carpenter, S.; et al. The Long Noncoding RNA CHROME Regulates Cholesterol Homeostasis in Primates. Nat. Metab. 2019, 1, 98–110. [Google Scholar] [CrossRef]
- Guo, F.; Sha, Y.; Hu, B.; Li, G. Correlation of Long Non-Coding RNA LncRNA-FA2H-2 With Inflammatory Markers in the Peripheral Blood of Patients With Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 682959. [Google Scholar] [CrossRef]
- Toni, L.; Hailu, F.; Sucharov, C.C. Dysregulated Micro-RNAs and Long Noncoding RNAs in Cardiac Development and Pediatric Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2020, 318, H1308–H1315. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Mao, Y.; Nan, G. Long Noncoding RNA-H19 Contributes to Atherosclerosis and Induces Ischemic Stroke via the Upregulation of Acid Phosphatase 5. Front. Neurol. 2019, 10, 32. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, S.; Wan, C.; Ruan, Q.; Xie, X.; Wei, D.; Li, G.; Lin, S.; Li, H.; Wu, S. Knockdown of LncRNA ENST00000609755.1 Confers Protection Against Early OxLDL-Induced Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 650212. [Google Scholar] [CrossRef]
- Wang, F.; Cai, X.; Jiao, P.; Liu, Y.; Yuan, B.; Zhang, P.; Liu, H.; Ma, L. Relationship between Long Non-Coding RNA and Prognosis of Patients with Coronary Heart Disease after Percutaneous Coronary Intervention: A Protocol for Systematic Review and Meta-Analysis. Medicine 2020, 99, e23525. [Google Scholar] [CrossRef]
- Wu, G.; Cai, J.; Han, Y.; Chen, J.; Huang, Z.-P.; Chen, C.; Cai, Y.; Huang, H.; Yang, Y.; Liu, Y.; et al. LincRNA-P21 Regulates Neointima Formation, Vascular Smooth Muscle Cell Proliferation, Apoptosis, and Atherosclerosis by Enhancing P53 Activity. Circulation 2014, 130, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-C.; Wang, Z.-Y.; Xu, Q.; Chen, X.-L.; Shi, R.-Z. LncRNA Expression Profiles and Associated CeRNA Network Analyses in Epicardial Adipose Tissue of Patients with Coronary Artery Disease. Sci. Rep. 2021, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhen, W.; Yu, H.; Zhang, L.; Liu, Y. LncRNA MALAT1/MiR-143 Axis Is a Potential Biomarker for in-Stent Restenosis and Is Involved in the Multiplication of Vascular Smooth Muscle Cells. Open Life Sci. 2021, 16, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Saygili, H.; Bozgeyik, I.; Yumrutas, O.; Akturk, E.; Bagis, H. Differential Expression of Long Noncoding RNAs in Patients with Coronary Artery Disease. Mol. Syndr. 2021, 12, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-W.; Guo, F.-X.; Xu, Y.-J.; Li, P.; Lu, Z.-F.; McVey, D.G.; Zheng, L.; Wang, Q.; Ye, J.H.; Kang, C.-M.; et al. Long Noncoding RNA NEXN-AS1 Mitigates Atherosclerosis by Regulating the Actin-Binding Protein NEXN. J. Clin. Investig. 2019, 129, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, J.; Liu, Y.; Li, J.; Duan, L. Transcriptome Sequencing of LncRNA, MiRNA, MRNA and Interaction Network Constructing in Coronary Heart Disease. BMC Med. Genom. 2019, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Lin, X.; Yang, L.; Fan, X.; Wang, W.; Li, S.; Li, J.; Liu, X.; Bao, M.; Cui, X.; et al. AK098656, a Novel Vascular Smooth Muscle Cell–Dominant Long Noncoding RNA, Promotes Hypertension. Hypertension 2018, 71, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Ghafouri-Fard, S.; Mirzajani, S.; Arsang-Jang, S.; Taheri, M.; Dehbani, Z.; Dehghani, S.; Houshmand, B.; Amid, R.; Sayad, A.; et al. The LncRNA ANRIL Is Down-Regulated in Peripheral Blood of Patients with Periodontitis. Non-Coding RNA Res. 2020, 5, 60–66. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, J.; Xu, P.; Gui, R. Long Non-Coding RNA GAS5 Maintains Insulin Secretion by Regulating Multiple MiRNAs in INS-1 832/13 Cells. Front. Mol. Biosci. 2020, 7, 559267. [Google Scholar] [CrossRef]
- Das, S.; Zhang, E.; Senapati, P.; Amaram, V.; Reddy, M.A.; Stapleton, K.; Leung, A.; Lanting, L.; Wang, M.; Chen, Z.; et al. A Novel Angiotensin II–Induced Long Noncoding RNA Giver Regulates Oxidative Stress, Inflammation, and Proliferation in Vascular Smooth Muscle Cells. Circ. Res. 2018, 123, 1298–1312. [Google Scholar] [CrossRef]
- Yu, B.; Wang, S. Angio-LncRs: LncRNAs That Regulate Angiogenesis and Vascular Disease. Theranostics 2018, 8, 3654–3675. [Google Scholar] [CrossRef]
- Jusic, A.; Devaux, Y. On behalf of the EU-CardioRNA COST Action (CA17129) Noncoding RNAs in Hypertension. Hypertension 2019, 74, 477–492. [Google Scholar] [CrossRef]
- Han, Y.; Ali, M.K.; Dua, K.; Spiekerkoetter, E.; Mao, Y. Role of Long Non-Coding RNAs in Pulmonary Arterial Hypertension. Cells 2021, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- El Azzouzi, H.; Doevendans, P.A.; Sluijter, J.P.G. Long Non-Coding RNAs in Heart Failure: An Obvious Lnc. Ann. Transl. Med. 2016, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Zaccagnini, G.; Fuschi, P.; Voellenkle, C.; Carrara, M.; Sadeghi, I.; Bearzi, C.; Maimone, B.; Castelvecchio, S.; Stellos, K.; et al. Increased BACE1-AS Long Noncoding RNA and β-Amyloid Levels in Heart Failure. Cardiovasc. Res. 2017, 113, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, L.; Martins, P.A.D.C. Non-coding RNAs in cardiac hypertrophy. J. Physiol. 2017, 595, 4037–4050. [Google Scholar] [CrossRef]
- Gomes, C.P.d.C.; Schroen, B.; Kuster, G.M.; Robinson, E.L.; Ford, K.; Squire, I.B.; Heymans, S.; Martelli, F.; Emanueli, C.; Devaux, Y.; et al. Regulatory RNAs in Heart Failure. Circulation 2020, 141, 313–328. [Google Scholar] [CrossRef]
- Fan, J.; Li, H.; Xie, R.; Zhang, X.; Nie, X.; Shi, X.; Zhan, J.; Yin, Z.; Zhao, Y.; Dai, B.; et al. LncRNA ZNF593-AS Alleviates Contractile Dysfunction in Dilated Cardiomyopathy. Circ. Res. 2021, 128, 1708–1723. [Google Scholar] [CrossRef]
- Wang, S.; Lv, T.; Chen, Q.; Yang, Y.; Xu, L.; Zhang, X.; Wang, E.; Hu, X.; Liu, Y. Transcriptome Sequencing and LncRNA-MiRNA-MRNA Network Construction in Cardiac Fibrosis and Heart Failure. Bioengineered 2022, 13, 7118–7133. [Google Scholar] [CrossRef]
- Greco, S.; Zaccagnini, G.; Perfetti, A.; Fuschi, P.; Valaperta, R.; Voellenkle, C.; Castelvecchio, S.; Gaetano, C.; Finato, N.; Beltrami, A.P.; et al. Long Noncoding RNA Dysregulation in Ischemic Heart Failure. J. Transl. Med. 2016, 14, 183. [Google Scholar] [CrossRef]
- Santer, L.; López, B.; Ravassa, S.; Baer, C.; Riedel, I.; Chatterjee, S.; Moreno, M.U.; González, A.; Querejeta, R.; Pinet, F.; et al. Circulating Long Noncoding RNA LIPCAR Predicts Heart Failure Outcomes in Patients Without Chronic Kidney Disease. Hypertension 2019, 73, 820–828. [Google Scholar] [CrossRef]
- Sato, M.; Kadomatsu, T.; Miyata, K.; Warren, J.S.; Tian, Z.; Zhu, S.; Horiguchi, H.; Makaju, A.; Bakhtina, A.; Morinaga, J.; et al. The LncRNA Caren Antagonizes Heart Failure by Inactivating DNA Damage Response and Activating Mitochondrial Biogenesis. Nat. Commun. 2021, 12, 2529. [Google Scholar] [CrossRef]
- Pinheiro, A.; Naya, F.J. The Key Lnc (RNA)s in Cardiac and Skeletal Muscle Development, Regeneration, and Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 84. [Google Scholar] [CrossRef]
- Han, P.; Chang, C.-P. Long Non-Coding RNA and Chromatin Remodeling. RNA Biol. 2015, 12, 1094–1098. [Google Scholar] [CrossRef]
- Yang, L.; Deng, J.; Ma, W.; Qiao, A.; Xu, S.; Yu, Y.; Boriboun, C.; Kang, X.; Han, D.; Ernst, P.; et al. Ablation of LncRNA Miat Attenuates Pathological Hypertrophy and Heart Failure. Theranostics 2021, 11, 7995–8007. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Zhang, X.; Dang, Y.; Cheng, Y.; Hua, W.; Teng, M.; Wang, S.; Lu, X. Novel LncRNA-MiRNA-MRNA Competing Endogenous RNA Triple Networks Associated Programmed Cell Death in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 747449. [Google Scholar] [CrossRef]
- Garcia-Padilla, C.; Lozano-Velasco, E.; Garcia-Lopez, V.; Aranega, A.; Franco, D.; Garcia-Martinez, V.; Lopez-Sanchez, C. Comparative Analysis of Non-Coding RNA Transcriptomics in Heart Failure. Biomedicines 2022, 10, 3076. [Google Scholar] [CrossRef]
- Ou, Y.; Liao, C.; Li, H.; Yu, G. LncRNA SOX2OT/Smad3 Feedback Loop Promotes Myocardial Fibrosis in Heart Failure. IUBMB Life 2020, 72, 2469–2480. [Google Scholar] [CrossRef]
- Di Salvo, T.G.; Guo, Y.; Su, Y.R.; Clark, T.; Brittain, E.; Absi, T.; Maltais, S.; Hemnes, A. Right Ventricular Long Noncoding RNA Expression in Human Heart Failure. Pulm. Circ. 2015, 5, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Jose, P.A.; Zeng, C. Noncoding RNAs in the Regulatory Network of Hypertension. Hypertension 2018, 72, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, B.; Yang, Y.; Jia, Q.; Zhang, A.; Qi, Z.; Zhang, J. Long Noncoding RNAs in Pathological Cardiac Remodeling: A Review of the Update Literature. BioMed Res. Int. 2019, 2019, 7159592. [Google Scholar] [CrossRef]
- Lu, B.-H.; Liu, H.-B.; Guo, S.-X.; Zhang, J.; Li, D.-X.; Chen, Z.-G.; Lin, F.; Zhao, G.-A. Long Non-Coding RNAs: Modulators of Phenotypic Transformation in Vascular Smooth Muscle Cells. Front. Cardiovasc. Med. 2022, 9, 959955. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Archer, K.; Broskova, Z.; Bayoumi, A.S.; Teoh, J.-p.; Davila, A.; Tang, Y.; Su, H.; Kim, I.-m. Long Non-Coding RNAs as Master Regulators in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 23651–23667. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Kim, J.; Moore, J.B., IV; Uchida, S. Macrophage Long Non-Coding RNAs in Pathogenesis of Cardiovascular Disease. Non-Coding RNA 2020, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Vausort, M.; Wagner, D.R.; Devaux, Y. Long Noncoding RNAs in Patients With Acute Myocardial Infarction. Circ. Res. 2014, 115, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Hermans-Beijnsberger, S.; van Bilsen, M.; Schroen, B. Long Non-Coding RNAs in the Failing Heart and Vasculature. Non-Coding RNA Res. 2018, 3, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Beijnsberger, S. Beijnsberger Emerging Roles of Small and Long Non-Coding RNAs in Cardiac Disease; Maastricht University: Maastricht, The Netherlands, 2019. [Google Scholar]
- Fan, X.; Weng, X.; Zhao, Y.; Chen, W.; Gan, T.; Xu, D. Circular RNAs in Cardiovascular Disease: An Overview. BioMed Res. Int. 2017, 2017, 5135781. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, J.; Hu, J.; Liu, L.; Xu, D. Circular RNA in Cardiovascular Disease: Expression, Mechanisms and Clinical Prospects. J. Cell. Mol. Med. 2021, 25, 1817–1824. [Google Scholar] [CrossRef]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Molecular Functions and Specific Roles of CircRNAs in the Cardiovascular System. Non-Coding RNA Res. 2018, 3, 75–98. [Google Scholar] [CrossRef]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular Non-Coding RNA ANRIL Modulates Ribosomal RNA Maturation and Atherosclerosis in Humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Sitkiewicz, D. Involvement of CircRNAs in the Development of Heart Failure. Int. J. Mol. Sci. 2022, 23, 14129. [Google Scholar] [CrossRef]
- Sun, C.; Ni, M.; Song, B.; Cao, L. Circulating Circular RNAs: Novel Biomarkers for Heart Failure. Front. Pharmacol. 2020, 11, 560537. [Google Scholar] [CrossRef]
- Prestes, P.R.; Maier, M.C.; Woods, B.A.; Charchar, F.J. A Guide to the Short, Long and Circular RNAs in Hypertension and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 3666. [Google Scholar] [CrossRef]
- Wu, S.; Chen, L.; Zhou, X. Circular RNAs in the Regulation of Cardiac Hypertrophy. Mol. Ther.-Nucleic Acids 2022, 27, 484–490. [Google Scholar] [CrossRef]
- Lim, T.B.; Aliwarga, E.; Luu, T.D.A.; Li, Y.P.; Ng, S.L.; Annadoray, L.; Sian, S.; Ackers-Johnson, M.A.; Foo, R.S.-Y. Targeting the Highly Abundant Circular RNA CircSlc8a1 in Cardiomyocytes Attenuates Pressure Overload Induced Hypertrophy. Cardiovasc. Res. 2019, 115, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, J.-D.; Fang, X.-H.; Zhu, J.-N.; Yang, J.; Pan, R.; Yuan, S.-J.; Zeng, N.; Yang, Z.-Z.; Yang, H.; et al. Circular RNA CircRNA_000203 Aggravates Cardiac Hypertrophy via Suppressing MiR-26b-5p and MiR-140-3p Binding to Gata4. Cardiovasc. Res. 2020, 116, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, Z.; Guo, G.; Xu, C.; Song, Z.; Li, K.; Zhong, K.; Wang, D. Circ_nuclear Factor I X (CircNfix) Attenuates Pressure Overload-Induced Cardiac Hypertrophy via Regulating MiR-145-5p/ATF3 Axis. Bioengineered 2021, 12, 5373–5385. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, Y.; Wang, S.; Wu, X.; Cao, J.; Sun, T. Circular RNAs: Biogenesis, Biological Functions, and Roles in Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 4233. [Google Scholar] [CrossRef]
- Cai, L.; Qi, B.; Wu, X.; Peng, S.; Zhou, G.; Wei, Y.; Xu, J.; Chen, S.; Liu, S. Circular RNA Ttc3 Regulates Cardiac Function after Myocardial Infarction by Sponging MiR-15b. J. Mol. Cell. Cardiol. 2019, 130, 10–22. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B. Circular RNA in Diseased Heart. Cells 2020, 9, 1240. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wei, Z.; Cheng, Y.; Tian, G.; Quan, Y.; Kong, Q.; Wu, W.; Liu, X. Identification of Circular RNAs in Cardiac Hypertrophy and Cardiac Fibrosis. Front. Pharmacol. 2022, 13, 940768. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, X.; Zhan, X.; Kang, S.; Liu, H.; Luo, Y.; Lin, L. Advance in Circular RNA Modulation Effects of Heart Failure. Gene 2020, 763, 100036. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yu, F.; Li, X.; Gao, H.; Li, P. CircHIPK3 Plays Vital Roles in Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 733248. [Google Scholar] [CrossRef]
- Tang, L.; Li, P.; Jang, M.; Zhu, W. Circular RNAs and Cardiovascular Regeneration. Front. Cardiovasc. Med. 2021, 8, 672600. [Google Scholar] [CrossRef]
- Wen, Z.-J.; Xin, H.; Wang, Y.-C.; Liu, H.-W.; Gao, Y.-Y.; Zhang, Y.-F. Emerging Roles of CircRNAs in the Pathological Process of Myocardial Infarction. Mol. Ther.-Nucleic Acids 2021, 26, 828–848. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Ji, H.; Zhang, H.; Yang, J.; Guo, R.; Wang, J. CircANRIL Reduces Vascular Endothelial Injury, Oxidative Stress and Inflammation in Rats with Coronary Atherosclerosis. Exp. Ther. Med. 2020, 3, 2245–2251. [Google Scholar] [CrossRef]
- Gao, X.; Tian, X.; Huang, Y.; Fang, R.; Wang, G.; Li, D.; Zhang, J.; Li, T.; Yuan, R. Role of Circular RNA in Myocardial Ischemia and Ageing-Related Diseases. Cytokine Growth Factor Rev. 2022, 65, 1–11. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.-K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 Circular RNA Promotes Cardiac Senescence by Modulating Multiple Factors Associated with Stress and Senescence Responses. Eur. Heart J. 2016, 38, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Long, T.; Du, T.; Chen, Y.; Dong, Y.; Huang, Z.-P. Circle the Cardiac Remodeling With CircRNAs. Front. Cardiovasc. Med. 2021, 8, 702586. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, M.; Liu, D.; Min, X.; Shao, T.; Xu, Z.; Jing, X.; Cai, M.; Xu, S.; Liang, X.; et al. CircGNAQ, a Circular RNA Enriched in Vascular Endothelium, Inhibits Endothelial Cell Senescence and Atherosclerosis Progression. Mol. Ther.-Nucleic Acids 2021, 26, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b Modulates Cardiac Repair after Myocardial Infarction via FUS/VEGF-A Axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Liu, D.; Xiong, X. Circular RNAs as Competing Endogenous RNAs in Cardiovascular and Cerebrovascular Diseases: Molecular Mechanisms and Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 682357. [Google Scholar] [CrossRef]
- Pan, L.; Lian, W.; Zhang, X.; Han, S.; Cao, C.; Li, X.; Li, M. Human Circular RNA-0054633 Regulates High Glucose-induced Vascular Endothelial Cell Dysfunction through the MicroRNA-218/Roundabout 1 and MicroRNA-218/Heme Oxygenase-1 Axes. Int. J. Mol. Med. 2018, 42, 597–606. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Yuan, J.; Zhang, Y.; Sang, H. Circular RNA WDR77 Target FGF-2 to Regulate Vascular Smooth Muscle Cells Proliferation and Migration by Sponging MiR-124. Biochem. Biophys. Res. Commun. 2017, 494, 126–132. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Yang, S. Circ_RUSC2 Upregulates the Expression of MiR-661 Target Gene SYK and Regulates the Function of Vascular Smooth Muscle Cells. Biochem. Cell Biol. 2019, 97, 709–714. [Google Scholar] [CrossRef]
- Turer, A.T.; Hill, J.A. Pathogenesis of Myocardial Ischemia-Reperfusion Injury and Rationale for Therapy. Am. J. Cardiol. 2010, 106, 360–368. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, W.; Pan, W.; Wang, Z. CircRNA 010567 Plays a Significant Role in Myocardial Infarction via the Regulation of the MiRNA-141/DAPK1 Axis. J. Thorac. Dis. 2021, 13, 2447–2459. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Wu, X.; Zhou, X. Regulatory Roles of Circular RNAs in Coronary Artery Disease. Mol. Ther.-Nucleic Acids 2020, 21, 172–179. [Google Scholar] [CrossRef]
- Morbach, C.; Wagner, M.; Güntner, S.; Malsch, C.; Oezkur, M.; Wood, D.; Kotseva, K.; Leyh, R.; Ertl, G.; Karmann, W.; et al. Heart Failure in Patients with Coronary Heart Disease: Prevalence, Characteristics and Guideline Implementation—Results from the German EuroAspire IV Cohort. BMC Cardiovasc. Disord. 2017, 17, 108. [Google Scholar] [CrossRef]
- Si, X.; Zheng, H.; Wei, G.; Li, M.; Li, W.; Wang, H.; Guo, H.; Sun, J.; Li, C.; Zhong, S.; et al. CircRNA Hipk3 Induces Cardiac Regeneration after Myocardial Infarction in Mice by Binding to Notch1 and MiR-133a. Mol. Ther.-Nucleic Acids 2020, 21, 636–655. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated Cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef]
- Long, Q.; Lv, B.; Jiang, S.; Lin, J. The Landscape of Circular RNAs in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 4571. [Google Scholar] [CrossRef]
- Mester-Tonczar, J.; Hašimbegović, E.; Spannbauer, A.; Traxler, D.; Kastner, N.; Zlabinger, K.; Einzinger, P.; Pavo, N.; Goliasch, G.; Gyöngyösi, M. Circular RNAs in Cardiac Regeneration: Cardiac Cell Proliferation, Differentiation, Survival, and Reprogramming. Front. Physiol. 2020, 11, 580465. [Google Scholar] [CrossRef]
- Jiapaer, Z.; Li, C.; Yang, X.; Sun, L.; Chatterjee, E.; Zhang, L.; Lei, J.; Li, G. Extracellular Non-Coding RNAs in Cardiovascular Diseases. Pharmaceutics 2023, 15, 155. [Google Scholar] [CrossRef]
- Shah, A.M.; Giacca, M. Small non-coding RNA therapeutics for cardiovascular disease. Eur. Heart J. 2022, 43, 4548–4561. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Giacca, M. Non-coding RNA therapeutics for cardiac regeneration. Non-coding RNA therapeutics for cardiac regeneration. Cardiovasc. Res. 2021, 117, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Tréguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a regulates cardiac ageing and function. Nature 2013, 495, 107–110. [Google Scholar] [CrossRef]
- Gandhi, S.; Ruehle, F.; Stoll, M. Evolutionary Patterns of Non-Coding RNA in Cardiovascular Biology. Non-Coding RNA 2019, 5, 15. [Google Scholar] [CrossRef]

| Name of the Product | Name of the Manufacturer | Functionalization | Route of Administration | Indication | Target Delivery Organ | Target mRNA or miRNA | Phase | Clinical Trial Identification Number |
|---|---|---|---|---|---|---|---|---|
| Vupanorsen | Ionis Pharmaceuticals, Pfizer | ASO (Antisense oligonucleotides) (GalNAc-conjugated) | Subcutaneous | Familial Chylomicronaemia Syndrome (FCS) | Liver | ANGPTL3 (angiopoietin-like 3) mRNA | Phase 2 | NCT03371355 |
| Olpasiran | Amgen | siRNA (Small interfering RNA) (GalNAc-conjugated) | Subcutaneous | Elevated Lp(a) | Liver | LPA (Lipoprotein A) mRNA | Phase 2 | NCT04270760 |
| SLN360 | Silence Therapeutics | siRNA (GalNAc-conjugated) | Subcutaneous | Elevated Lp(a) | Liver | LPA mRNA | Phase 1 | NCT04606602 |
| MRG-110 | miRagen, Servier | ASO (LNA) | Intradermal | Neovascularization, wound healing | Vasculature | miR-92a-3p | Phase 1 | NCT03494712 |
| ARO-ANG3 | Arrowhead Pharmaceuticals | siRNA (liver targeted) | Subcutaneous | Mixed dyslipidaemia | Liver | ANGPTL3 mRNA | Phase 2 | NCT04832971 |
| MRG-110 | miRagen, Servier | ASO (LNA) | Intradermal | Neovascularization, wound healing | Vasculature | miR-92a-3p | Phase 1 | NCT03494712 |
| CDR132L | Cardior | ASO (LNA) | Intravenous | Heart failure | Heart | miR-132-3p | Phase 1b | NCT04045405 |
| elacarsen | Lonis Pharmaceuticals | ASO (PS, 2′-MOE, GalNAc-conjugated) | Subcutaneous | Elevated Lp(a) | Liver | LPA mRNA | Phase 3 | NCT04023552 |
| Vutrisiran | Anylam Pharmaceuticals | siRNA (GalNAc-conjugated) | Subcutaneous | TT-amyloid with cardiomyopathy | Liver | Transthyretin mRNA | Phase 3 | NCT03759379 |
| Phase 3 | NCT04153149 | |||||||
| Teprasiran | Quark Pharmaceuticals | siRNA | Intravenous | Acute kidney injury | Kidney | p53 mRNA | Phase 2 | NCT02610283 |
| Phase 3 | NCT03510897 |
| S.N. | Type of Disease | miRNA | Regulation | Importance | Reference |
|---|---|---|---|---|---|
| 1 | Cardiac hypertrophy (CH) | miR-208a | Up-regulation | Cardiac remodelling | [13] |
| 2 | CH | miR-19a/b | Up-regulation | Cardiac remodelling in response to angiotensin II infusion | [14] |
| 3 | CH | miR-155 | Up-regulation | Cardiac remodelling | [15] |
| 4 | CH | miR-199a | Up-regulation | Maintenance of cell size in cardiomyocytes | [16] |
| 5 | CH | miR-1, | Down-regulation | Induces cardiac hypertrophy. | [17] |
| 6 | CH | miR-101 | Down-regulation | Inhibit cardiac hypertrophy signalling | [18] |
| 7 | CH | miR-185 | Down-regulation | Inhibit CH hypertrophy signalling | [19] |
| 8 | CH | miR-34a | Down-regulation | Regulation of Ang II-induced cardi myocyte hypertrophy | [20] |
| 9 | CH | miR-145 | Down-regulation | Inhibits isoproterenol-induced cardiomyocyte hypertrophy | [21] |
| 10 | CH | miR-150 | Down-regulation | Reduces the immunosuppression function of Myeloid-derived suppressor cells (MDSCs) | [22] |
| 11 | CH | miR-378 | Down-regulation | Act as negative regulator for CH | [23] |
| 12 | Heart failure (HF) | miR-125b | Up-regulation | Conduction of Cardiac fibrosis (CF) | [24] |
| 13 | HF | miR-22, | Up-regulation | Regulator for cardiac remodelling | |
| 14 | HF | miR-92b | Up-regulation | Related to the left atrium diameter, left ventricular end-diastolic dimension | [25] |
| 15 | HF | miR-320a | Up-regulation | CF through activation of the IL6/STAT3 axis. | [26] |
| 16 | HF | miR-423-5p | Up-regulation | Upregulated in human failing myocardium | [27] |
| 17 | HF | miR-200b | Up-regulation | Regulation of multiple cellular pathways in HF | [28] |
| 18 | HF | miR-622 | Up-regulation | Improves blood vessel growth | [29] |
| 19 | HF | miR-1228 | Up-regulation | Marker for systolic HF | [30] |
| 20 | HF | miR-208b | Up-regulation | pathogenesis of DCM | [31] |
| 21 | HF | miR-499 | Up-regulation | Cardiac development | [32] |
| 22 | HF | miR-223 | Up-regulation | Altered in post-MI HF in humans | [33] |
| 23 | HF | miR-1254 | Up-regulation | Altered in post-MI HF in humans | [34] |
| 24 | HF | miR-1306 | Up-regulation | Elected to explore novel circulating markers for HF | [35] |
| 25 | HF | miR-18a | Down-regulation | CF through the Notch2 pathway. | [36] |
| 26 | HF | miR-26b | Down-regulation | Controlling critical signalling pathways, such as BMP/ SMAD1 signalling | [37] |
| 27 | HF | miR-27a | Down-regulation | Inhibiting miR-27a-3p mitigated CH phenotype induced by Ang II (Angiotensin -II) | [38] |
| 28 | HF | miR-30e | Down-regulation | The overexpression of miR-30c reduces the level of connective tissue growth | [39] |
| 29 | HF | miR-106a | Down-regulation | Notch 3 pathway in ischemic heart injury. | [40] |
| 30 | HF | miR-199a | Down-regulation | Improves contractile function | [41] |
| 31 | HF | miR-652 | Down-regulation | Marker for predicting acute coronary syndrome | [42] |
| 32 | HF | miR-1 | Down-regulation | Systolic HF | [43] |
| 33 | HF | miR-126 | Down-regulation | Activation of the vascular endothelial growth factor (VEGM) signalling pathway in the endothelium. | [44] |
| 34 | HF | miR-423 | Down-regulation | It is a circulating biomarker for heart failure. | [45] |
| 35 | Cardiac electrical and structural remodelling (CE and SR) | miR-1 | Down-regulated | Increased altered conduction Increased CF | [46] |
| 36 | CE and SR | miR-26 | Down-regulated | Increase inwardly rectifying channel | [47] |
| 37 | CE and SR | miR-29 | Down-regulated | Increased CF | [48] |
| 38 | CE and SR | miR-30 | Down-regulated | Increased CF | [49] |
| 39 | CE and SR | miR-133 | Down-regulated | Increased CF | [50] |
| 40 | CE and SR | miR-328 | Up-regulated | Shortened atrial action potential duration by targeting | [51] |
| 41 | CE and SR | miR-499 | Up-regulated | Altered conduction by targeting | [52] |
| 42 | CE and SR | miR-21 | Up-regulated | Inhibition of fibroblast proliferation | [53] |
| 43 | Acute coronary syndrome (ACS) and myocardial infarction (MI) | miR-1 | Up-regulated | marker of cardiomyocyte injury | [54] |
| 44 | ACS and MI | miR-133a | Up-regulated | Development of VF (Ventricular fibrillation) | [55] |
| 45 | ACS and MI | miR-208a | Up-regulated | Regulates the cardiac stress response. | [56] |
| 46 | ACS and MI | miR-499-5p | Up-regulated | Associated with cardiac injury and also with cardio protection | [57] |
| 47 | ACS and MI | miR-126, | Down-regulated | downregulated in the region adjacent to MI areas | [58] |
| 48 | ACS and MI | miR-221/222 | Down-regulated | Severity of the coronary artery lesions | [59] |
| 49 | ACS and MI | miR-29 | Dysregulation | Involved in CF multiple collagens, fibrillin’s, and elastin | [60] |
| 50 | ACS and MI | miR-145 | Up-regulated | Significantly upregulated in mice in response to chronic hypoxia and that genetic ablation | [61] |
| 51 | ACS and MI | miR-21 | Up-regulated | Up-regulated in the hypoxia | [62] |
| 52 | ACS and MI | miR-206 | Up-regulated | Normal and hypertensive mouse PASMCs. | [63] |
| 53 | ACS and MI | miR-328 | Down-regulated | Regulates Hypoxic Pulmonary Hypertension | [64] |
| 54 | Pulmonary arterial hypertension (PAH) | miR-204 | Down-regulated | Hypoxia related to pulmonary arterial hypertension | [65] |
| 55 | Atherosclerosis (AS) | miR-33 | Dysregulation | Promising strategy to reverse autophagy dysfunction in atherosclerosis. | [66] |
| 56 | (AS) | miR-122 | Up | Significantly up-regulated in patients with atherosclerotic lesion | [67] |
| 57 | (AS) | miR-126 | Down-regulated | [68] | |
| 58 | (AS) | miR-1 | Down-regulated | Downregulation of miR-10a enhances IκB/NF-κB activation | [69] |
| 59 | (AS) | miR-221/222 | Down-regulated | Suppression of PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) in the progression of atherosclerosis | [70] |
| 60 | Congenital heart diseases (CHDs) | miR-1275, miR-27b, miR-421 | Up-regulated | usually developing hearts | [13] |
| 61 | CHD | miR-122, miR-1201 | Down-regulated | developing hearts | [14] |
| 62 | CHD | miR-222, miR-337-5p, miR-363, miR-424, miR-424, miR-660, miR-708, miR-421, miR-19a, miR-130b, miR-146b-5p, miR-154, miR-155, miR-181c, miR-181d and miR-192, | Up-regulated | tetralogy of Fallot | [15] |
| 63 | CHDs | miR-181a, miR-720, miR-29c and miR-940 | Down-regulated | tetralogy of Fallot | [16] |
| 64 | CHD | miR-181c | Up-regulated | ventricular septal defect | [17] |
| CHD | miR-1-1 | Down-regulated | ventricular septal defect | [18] | |
| 65 | CHD | miR-106a, miR-144, miR-451, miR-486-3p, miR-486-5p, hsa-let-7e, miR-16, miR-18a, miR-25, miR-93, and miR-505 | Up-regulated | transposition of the great arteries | [19] |
| 66 | CHD | miR-873 | Up-regulated | Cyanotic CHD | [20] |
| 67 | CHD | miR-182 | Down-regulated | Cyanotic CHD | [21] |
| 68 | CHD | miR-498 | Up-regulated | Ventricular septal defect | |
| 69 | CHD | miR-379-5p, miR-409-3p, miR-433, hsa-let-7e-5p, miR-155-5p, miR-222-3p, and miR-487b | Down-regulated | Ventricular septal defect | [22] |
| 70 | CHD | hsa-let-7b, hsa-let-7a, and miR-486 | Up-regulated | Atrioventricular septal defect and atrial septal defect | [23] |
| 71 | CHD | miR-19b, miR-22, miR-29c, miR-375 | Up-regulated | Atrioventricular septal defect and atrial septal defect | [22,23] |
| 72 | Myocrdial infraction (I) | miR-1 | Cardiomyocyte Downstream Targets: Ncx-1; KCNJ2, GJA1; IGF-1 | [55] | |
| 73 | MI | miR-15 | Up-regulated | Cardiomyocyte Downstream Targets: Pdk4, Sgk1 | [56] |
| 74 | MI | miR-21 | Down-regulated | Fibroblast, Downstream Targets Pten; Sprouty-1, collagens | [57] |
| 75 | MI | miR-24 | Up-regulated | Anti-apoptosis in Cardiomyocyte, fibroblast, endothelial cell; Downstream Targets Bim; Furin; Gata2, Pak4 | [58] |
| 76 | MI | miR-29 | Down-regulated | Cardiomyocyte, fibroblast Downstream Targets: Mcl-1; Collagens | [59] |
| 77 | MI | miR-92a | Up-regulated | Endothelial cell Downstream Targets: Itga5 | [60] |
| 78 | MI | miR-101 | Down-regulated | Cardiac remodelling Downstream Targets: Collagens | [61] |
| 79 | MI | miR-126 | Down-regulated | Protects against myocardial ischemia-reperfusion injury | [62] |
| Type of Disease | lncRNA | Regulations | Importance | References | |
|---|---|---|---|---|---|
| 1 | Myocardial infraction (MI) | aHIF | Regulations of the angiogenesis process and a biomarker | Inhibits the autophagy of cardiac cells during MI | [102] |
| 2 | MI | ANRIL | Regulates myocardial cell apoptosis in AMI | Protection of cardiomyocytes | [103] |
| 3 | MI | APF | APF lncRNA regulates autophagy | Acting as a sponge for miRNA- 188-3p. | [102] |
| 4 | MI | CARL | Regulates mitochondrial fission and apoptosis | Acting as a sponge for miRNA-539. | [102] |
| 5 | MI | CDR1AS | Inhibiting the autophagy of cardiac cells during MI | Biomarker. | [102] |
| 6 | MI | FTX | Regulates cardiomyocytes | Act as a sponge for miRNA- 29b-1-5. | [104] |
| 7 | MI | GAS5 | Regulates the protection of cardiomyocytes against hypoxic injury | Act as a sponge for miRNA-142; improves apoptosis by negatively regulating sema3a. | [102] |
| 8 | MI | H19 | Regulates autophagy | Induction of cardiac remodeling, autophagy, and biomarker. | [102] |
| 9 | MI | HOTAIR | Regulates cardioprotective | Act as a sponge for miRNA-1 and as a biomarker. | [105] |
| 10 | MI | KCNQ1OT1 | Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury | Biomarker for left ventricular dysfunction. | [106] |
| 11 | MI | LIPCAR | Down-regulated | Biomarker for cardiac remodelling. | [107] |
| 12 | MI | Lnc-Ang362 | Upregulation | Promotion of CF | [108] |
| MI | MALAT1 | Down-regulation | Regulation of cardiomyocytes apoptosis and autophagy through miRNA- 558; and biomarker. | [109] | |
| 13 | MI | MDRL | Regulates mitochondrial fission | Reduction of mitochondrial fission and apoptosis acting as a sponge for miRNA-361. | [110] |
| 14 | MI | MEG3 | Regulates cardiomyocytes | Regulation of cardiomyocytes apoptosis. | [111] |
| 15 | MI | MHRT | Regulates cardiomyocytes | Regulation of cardiomyocytes apoptosis and biomarker. | [112] |
| 16 | MI | MIAT | Regulates CF | Regulation of cardiac hypertrophy and fibrosis acting as a sponge for miRNA-150 and -93. | [102] |
| 17 | MI | Mirt1/2 | Regulates cardiomyocytes | Regulation of cardiac remodelling. | [102] |
| 18 | MI | n379519 | Regulates CF | Promotion of cardiac fibrosis through miRNA-30. | [113] |
| 19 | MI | NONRATT021972 | Regulates cardiomyocytes | Promotion of cardiac function. | [114] |
| 20 | MI | NRF | Regulates cardiomyocyte necrosis. | Regulation of cardiomyocyte necrosis. | [115] |
| 21 | MI | NRON | Up-regulated | Wisper in cardiac fibroblast; Biomarker | [102] |
| 22 | MI | PCFL | Up-regulated | Promotion of cardiac fibrosis through miRNA-378. | [102] |
| 23 | MI | TTTY15 | Up-regulated | Induction of cardiomyocyte injury by hypoxia targeting miRNA-455. | [102] |
| 24 | MI | UCA1 | Regulates cardiomyocytes | Regulated ischemia and hypoxia of cardiomyocytes; Biomarker. | [115] |
| 25 | MI | UIHTC | Regulates cardiomyocytes against MI | Promotion of mitochondrial function. | [102] |
| 26 | MI | Wisper | Regulates cardiac fibroblast | MI-induced fibrosis and cardiac dysfunction | [116] |
| 27 | MI | ZFAS1 | Regulates cardiomyocyte | Induction of cardiomyocyte apoptosis, cardiac contractility reduction, and biomarker. | [117] |
| 28 | Coronary heart disease | aHIF | Up-regulated | Biomarker. | [118] |
| 29 | Coronary heart disease | ANRIL | Down-regulates | Diagnostic and prognostic indicator for CHD | [118] |
| 30 | Coronary heart disease | APOA1-AS | Up-regulations increase the risk of CHD | Biomarker. | [119] |
| 31 | Coronary heart disease | AWPPH | Regulates apoptosis. | Promotion of ECs apoptosis. | [120] |
| 32 | Coronary heart disease | BACE1-AS | dysregulation | dysregulation of the BACE1/BACE1-AS/Aβ axis is associated with HF. | [121] |
| 33 | Coronary heart disease | BANCR | Differentially expressed | Promotion of VSMCs proliferation and migration. | [122] |
| 34 | Coronary heart disease | CHROME | Up-regulated | Regulation of cellular cholesterol homeostasis | [123] |
| 35 | Coronary heart disease | CoroMarker | Differentially expressed | novel biomarker for the diagnosis | [124] |
| 36 | Coronary heart disease | EGOT | Differentially expressed | Biomarker. | [125] |
| 37 | Coronary heart disease | H19 | Differentially expressed | Biomarker. | [126] |
| 38 | Coronary heart disease | HOTTIP | Up-regulates | Promotes ECs proliferation and migration | [127] |
| 39 | Coronary heart disease | HRCR | Regulates hypertrophic Ca2+ signaling pathway | Regulation of cardiomyocytes apoptosis and proliferation. | [128] |
| 40 | Coronary heart disease | LIPCAR | Differentially expressed | Biomarker. | [107] |
| 41 | Coronary heart disease | lincRNA-p21 | Regulates cardiac remodelling and heart failure | Regulation of cardiomyocytes apoptosis and proliferation. | [129] |
| 42 | Coronary heart disease | LINC00968 | Up-regulated | Promotion of ECs proliferation and migration acting as a sponge for miRNA-9 | [130] |
| 43 | Coronary heart disease | MALAT1 | Differentially expressed | Biomarker. | [131] |
| 44 | Coronary heart disease | MIAT | Differentially expressed | Biomarker | [132] |
| 45 | Coronary heart disease | NEXN-AS1 | Differentially expressed | Mitigation of atherosclerosis. | [133] |
| 46 | Coronary heart disease | SMILR | Differentially expressed | Biomarker. | [134] |
| 47 | Arterial Hypertension | AK098656 | Up-regulated | Regulation of arteries of resistance and a biomarker | [135] |
| 48 | Arterial Hypertension | ANRIL | Regulates endothelial cell activities | Increase of susceptibility to higher systolic blood pressure conferred by polymorphisms. | [136] |
| 49 | Arterial Hypertension | GAS5 | Regulates ECs and VSMCs function | Regulation of ECs and VSMCs function acting as endogenous RNA competing of miRNA-21; and a biomarker. GAS5 Targets miR-194-3p. miR-194-3 | [137] |
| 50 | Arterial Hypertension | Giver | Regulates VSMCs dysfunction. | Promotion of VSMCs dysfunction. | [138] |
| 51 | Arterial Hypertension | Lnc-Ang362 | Regulates VSMCs | Regulation of VSMCs proliferation through miRNA-221 and -222. | [139] |
| 52 | Arterial Hypertension | NR_027032 | Differentially expressed | Biomarker. | [140] |
| 53 | Arterial Hypertension | NR_034083 | Differentially expressed | Biomarker. | [141] |
| 54 | Arterial Hypertension | NR_104181 | Differentially expressed | Biomarker. | [140] |
| 55 | Heart failure | ANRIL | Differentially expressed | Biomarker. | [142] |
| 56 | Heart failure | BACE1-AS | Regulates apoptosis. | Promotion of ECs apoptosis. | [143] |
| 57 | Heart failure | Chaer | Dysregulation | Induction of Pathological cardiac remodelling. | [144] |
| 58 | Heart failure | Chast | Down-regulates | Induction of Pathological cardiac remodelling. | [145] |
| 59 | Heart failure | CHRF | Up-regulated | Endogenous sponge to miRNA-489 activity. | [146] |
| 60 | Heart failure | HEAT2 | Up-regulated | Biomarker. | [147] |
| 61 | Heart failure | HOTAIR | Up-regulated | LncRNA HOTAIR may function as a miR-19-sponge to modulate PTEN levels Biomarker. | [148] |
| 62 | Heart failure | LIPCAR | Up-regulated | Biomarker. | [149] |
| 63 | Heart failure | lincRNA-ROR | Regulates CH | Regulation of cardiac hypertrophy acting as a sponge for miRNA-133. | [150] |
| 64 | Heart failure | LOC285194 | Up-regulated | overexpression suppressed MKN45 and HGC-27 cell proliferation and promoted cell apoptosis; Biomarker. | [148] |
| 65 | Heart failure | MEG3 | Regulates CF | Regulation of cardiac fibrosis and diastolic dysfunction | [151] |
| 66 | Heart failure | MHRT | Regulates of chromatin re-modellers | Regulation of chromatin remodels and biomarker. | [152] |
| 67 | Heart failure | MIAT | Regulates CH | Regulation of cardiac hypertrophy acting as a sponge for miRNA-150. | [153] |
| 68 | Heart failure | NRON | Upregulated | Biomarker. | [154] |
| 69 | Heart failure | RNY5 | Dysregulation | Biomarker. | [155] |
| 70 | Heart failure | SOX2-OT | Dysregulation | Biomarker. | [156] |
| 71 | Heart failure | SRA1 | Dysregulation | Biomarker. | [157] |
| S.N. | Type of Disease | CircRNA | Regulation | Importance | References |
|---|---|---|---|---|---|
| 1 | Cardiac Hypertrophy (CH) | CircHRCR | Down-regulated attenuates cardiac hypertrophy | Down-regulated attenuates CH | [175] |
| 2 | CH | CircSLc8a1 | Up-regulated | Knockdown reduces CH caused by pressure overload | [176] |
| 3 | CH | Circ_000203 | Up-regulated aggravates cardiac hypertrophy | Increased CH | [177] |
| 4 | Myocardial Infarction (MI) | CircNfix | Down-regulated | Promotes cardiomyocyte proliferation and angiogenesis | [178] |
| 5 | MI | CircFndc3b | Down-regulated | Overexpression increases ECF (endothelial cell function) | [179] |
| 6 | MI | CircTtc3 | Up-regulated | Inhibits apoptosis of cardiomyocytes | [180] |
| 7 | MI | Circ-CDR1as | Up-regulated | Promotes cell apoptosis | [181] |
| 8 | MI | CircMFACR | Up-regulated | Induces autophagy and cell death in cardiomyocytes | [182] |
| 9 | Myocardial fibrosis (MF) | CircNFIB | Down-regulated | Increases proliferation and differentiation of myocardial fibroblasts (MF) | [183] |
| 10 | MF | CircHIPK3 | Up-regulated | Overexpression attenuates the proliferation and migration of CF | [184] |
| 11 | MF | Circ_Las1l | Down-regulated | Regulates proliferation and migration, and apoptosis | [185] |
| 12 | MF | CircRNA-010567 | Up-regulated | Silencing inhibits the CF | [186] |
| 13 | Atherosclerosis (AS) | CircANRIL | Down-regulated monocytic | Regulates increase in a apoptosis and decrease in proliferation | [187] |
| 14 | AS | Circ_0003204 | Up-regulated | Ectopic expression inhibits proliferation, migration, and endothelial cells | [172] |
| 15 | AS | CircWDR77 | Up-regulated | Silencing inhibits VSMC proliferation and migration | [181] |
| 16 | AS | Circ_0003575 | Unchanged | Increase proliferation and angiogenesis | [186] |
| 17 | Cardiac senescence | CircAmotl1 | Down-regulated during aging | Ectopic expression induces primary cardiomyocyte proliferation | [188] |
| 18 | Cardiac senescence | CircFOXO3 | Up-regulated | Ectopic expression induces senescence | [189] |
| 19 | Coronary heart disease (CHD) | CircZNF60 | Up-regulated | Silencing increases, proliferation, and migration | [190] |
| 20 | CHD | CircLrp6 | Unchanged | Silencing prevents intimal | [168] |
| 21 | CHD | CircNrg1 | Down-regulated | Knockdown inhibits the apoptosis | [191] |
| 22 | CHD | CircTCF25 | Down-regulated | Down-regulated expression in coronary heart disease | [192] |
| Target mRNA or miRNA | Type (Modifications) | Route of Administration | Target mRNA or miRNA | Product (Developer/Manufacturer) | Indication | Phase | Latest Clinical Studies |
|---|---|---|---|---|---|---|---|
| LPA mRNA | Antisense oligonucleotide (ASO) (PS, 2′-MOE, GalNAc-conjugated | Subcutaneous | LPA mRNA | elacarsen (Ionis Pharmaceuticals) | Elevated Lp(a) | Phase 3 | NCT0402355 |
| LPA mRNA | siRNA (GalNAc-conjugated) | Subcutaneous | LPA mRNA | Olpasiran (Amgen) | Elevated Lp(a) | Phase 2 | NCT04270760 |
| LPA mRNA | siRNA (GalNAc-conjugated) | Subcutaneou | LPA mRNA | SLN360 (Silence Therapeutics) | Elevated Lp(a) | Phase 1 | NCT046066 |
| ANGPTL3 mRNA | ASO (GalNAc-conjugated) | Subcutaneous | ANGPTL3 mRNA | Vupanorsen (Ionis Pharmaceuticals, Pfizer) | Hypertriglyceridemia; Familial Chylomicronemia Syndrome (FCS) | Phase 2 | NCT0337135 NCT04516291 |
| ANGPTL3 mRNA | siRNA (liver targeted) | Subcutaneous | ANGPTL3 mRNA | ARO-ANG3 (Arrowhead Pharmaceuticals) | Mixed dyslipidaemia | Phase 2 | NCT04832971 |
| miR-92a-3p | ASO (LNA) | Intradermal | miR-92a-3p | MRG-110 (S95010) (miRagen, Servier) | Neovascularization, wound healing | Phase 1 | NCT03494712; NCT03603431 |
| miR-132-3p | ASO (LNA) | Intravenous | miR-132-3p | CDR132L (Cardior) | Heart failure | Phase 1 | NCT0404540 |
| p53 mRNA | siRNA (short interfering RNA) | Intravenous | p53 mRNA | Teprasiran (Quark Pharmaceuticals) | Acute kidney injury | Phase 3 | NCT03510897 |
| Transthyretin mRNA | siRNA (GalNAc-conjugated) | Subcutaneous | Transthyretin mRNA | Vutrisiran (Anylam Pharmaceuticals) | HTT-amyloid with polyneuropathy; TT-amyloid with cardiomyopathy | Phase 3 | NCT04153149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.D.; Kim, Y.; Choi, S.A.; Han, I.; Yadav, D.K. Clinical Significance of MicroRNAs, Long Non-Coding RNAs, and CircRNAs in Cardiovascular Diseases. Cells 2023, 12, 1629. https://doi.org/10.3390/cells12121629
Singh DD, Kim Y, Choi SA, Han I, Yadav DK. Clinical Significance of MicroRNAs, Long Non-Coding RNAs, and CircRNAs in Cardiovascular Diseases. Cells. 2023; 12(12):1629. https://doi.org/10.3390/cells12121629
Chicago/Turabian StyleSingh, Desh Deepak, Youngsun Kim, Seung Ah Choi, Ihn Han, and Dharmendra Kumar Yadav. 2023. "Clinical Significance of MicroRNAs, Long Non-Coding RNAs, and CircRNAs in Cardiovascular Diseases" Cells 12, no. 12: 1629. https://doi.org/10.3390/cells12121629
APA StyleSingh, D. D., Kim, Y., Choi, S. A., Han, I., & Yadav, D. K. (2023). Clinical Significance of MicroRNAs, Long Non-Coding RNAs, and CircRNAs in Cardiovascular Diseases. Cells, 12(12), 1629. https://doi.org/10.3390/cells12121629













