Daphnetin, a Coumarin with Anticancer Potential against Human Melanoma: In Vitro Study of Its Effective Combination with Selected Cytostatic Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Malignant Melanoma Cell Culture
2.2. Tested Drugs
2.3. Cell Viability Assessment—MTT Test
2.4. Cell Proliferation Assessment—BrdU Test
2.5. Cell Cytotoxicity—LDH Test
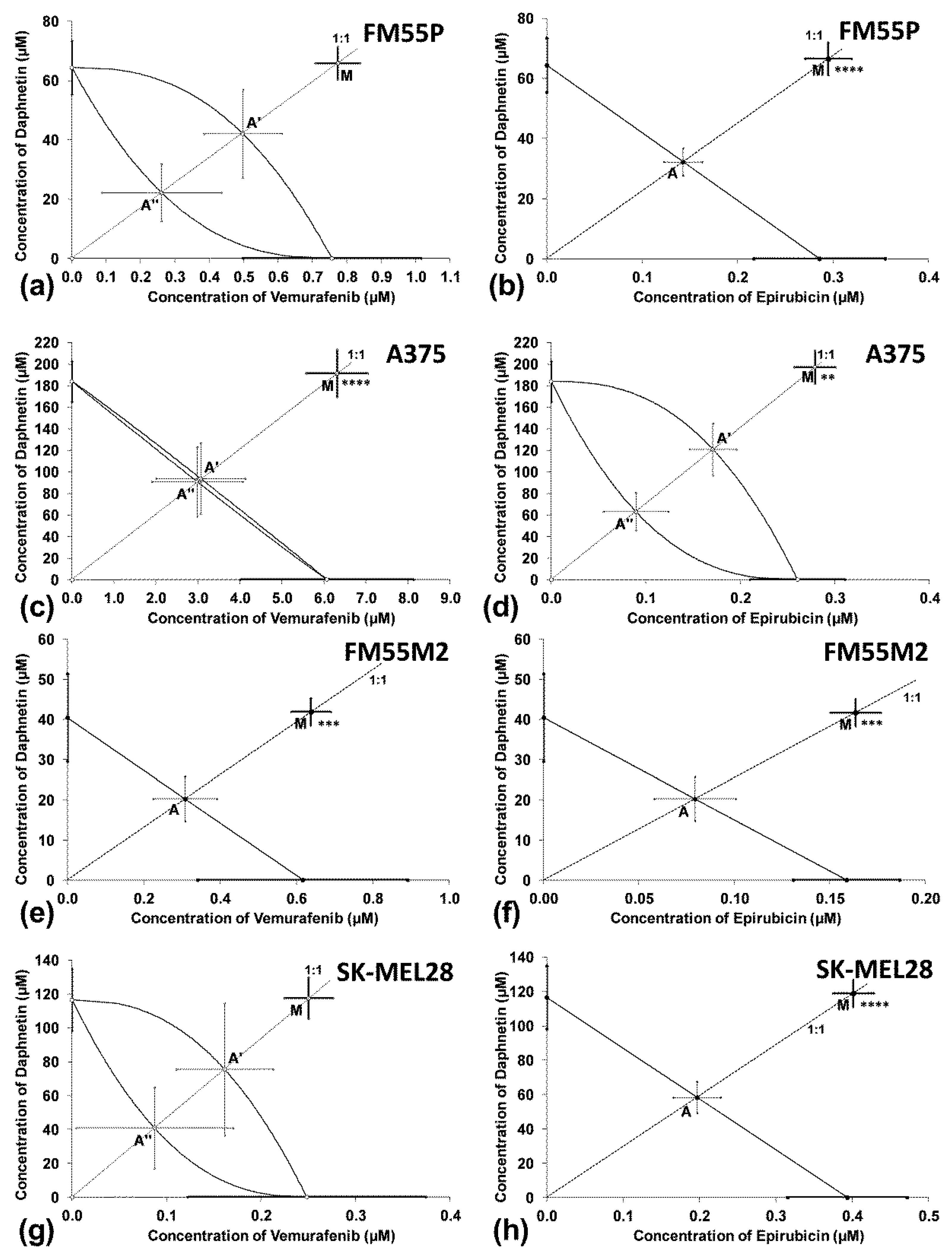
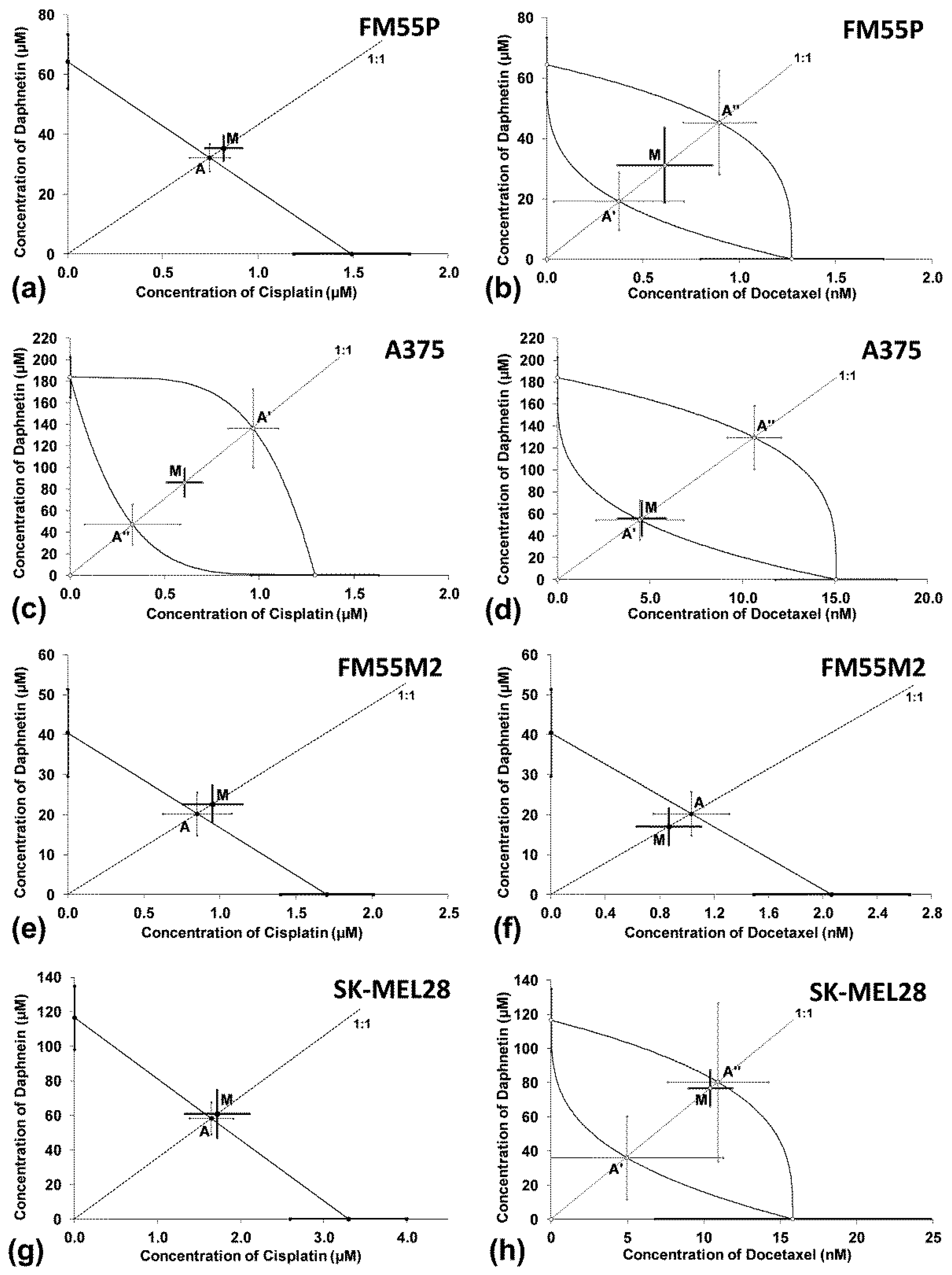
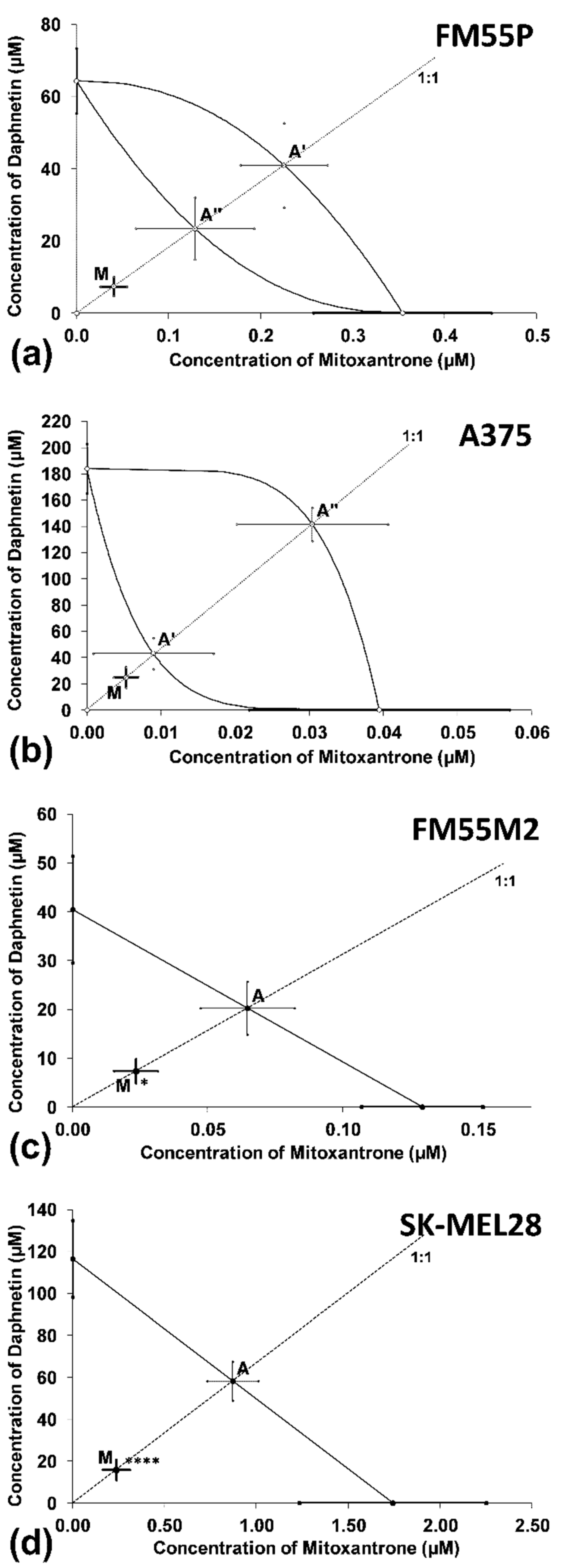
2.6. Isobolographic Analysis
2.7. Statistical Analysis
3. Results
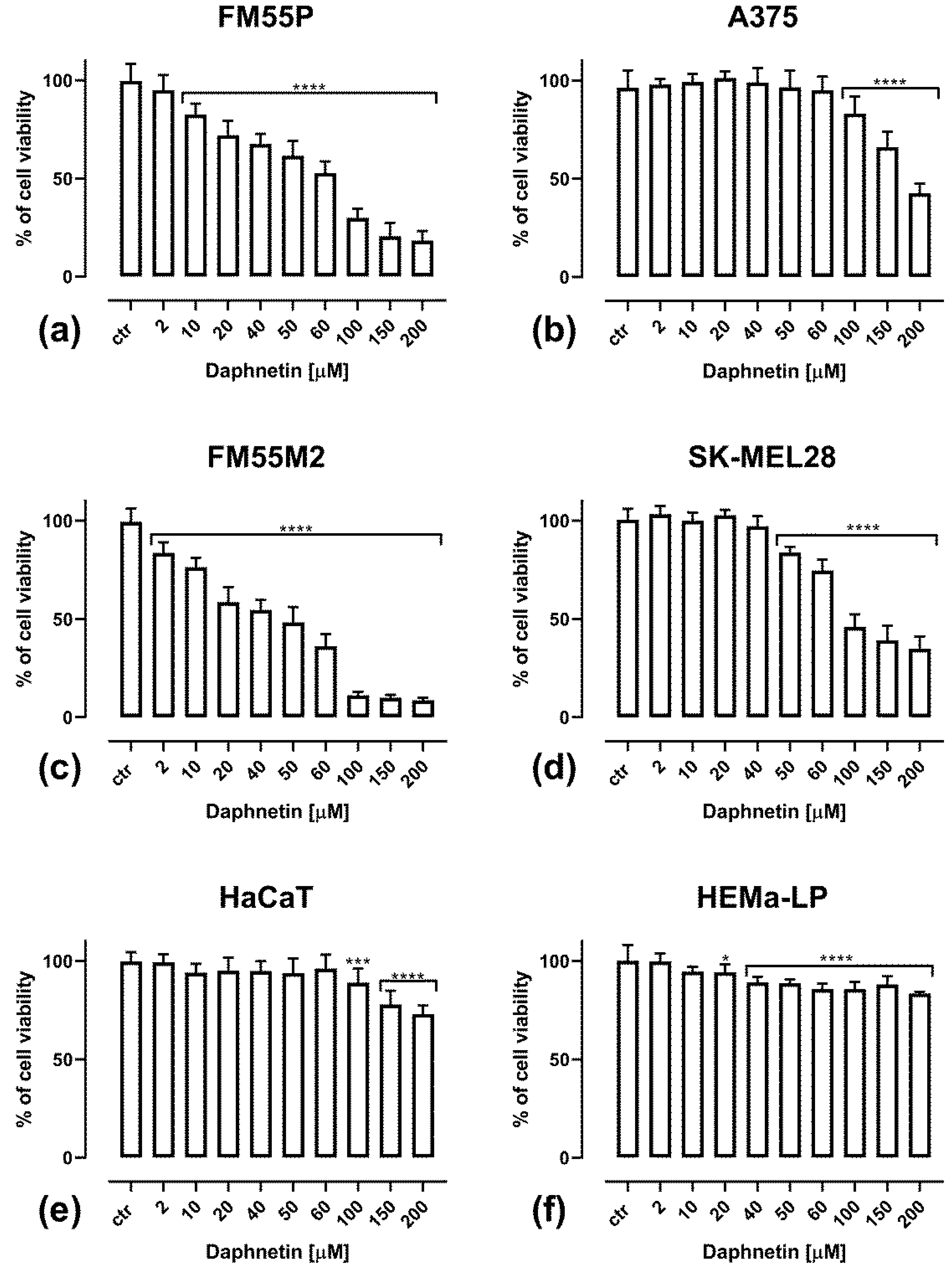
3.1. Cell Viability Assessment—MTT Assay Results
3.2. Cell Proliferation Assessment—BrdU Assay Results
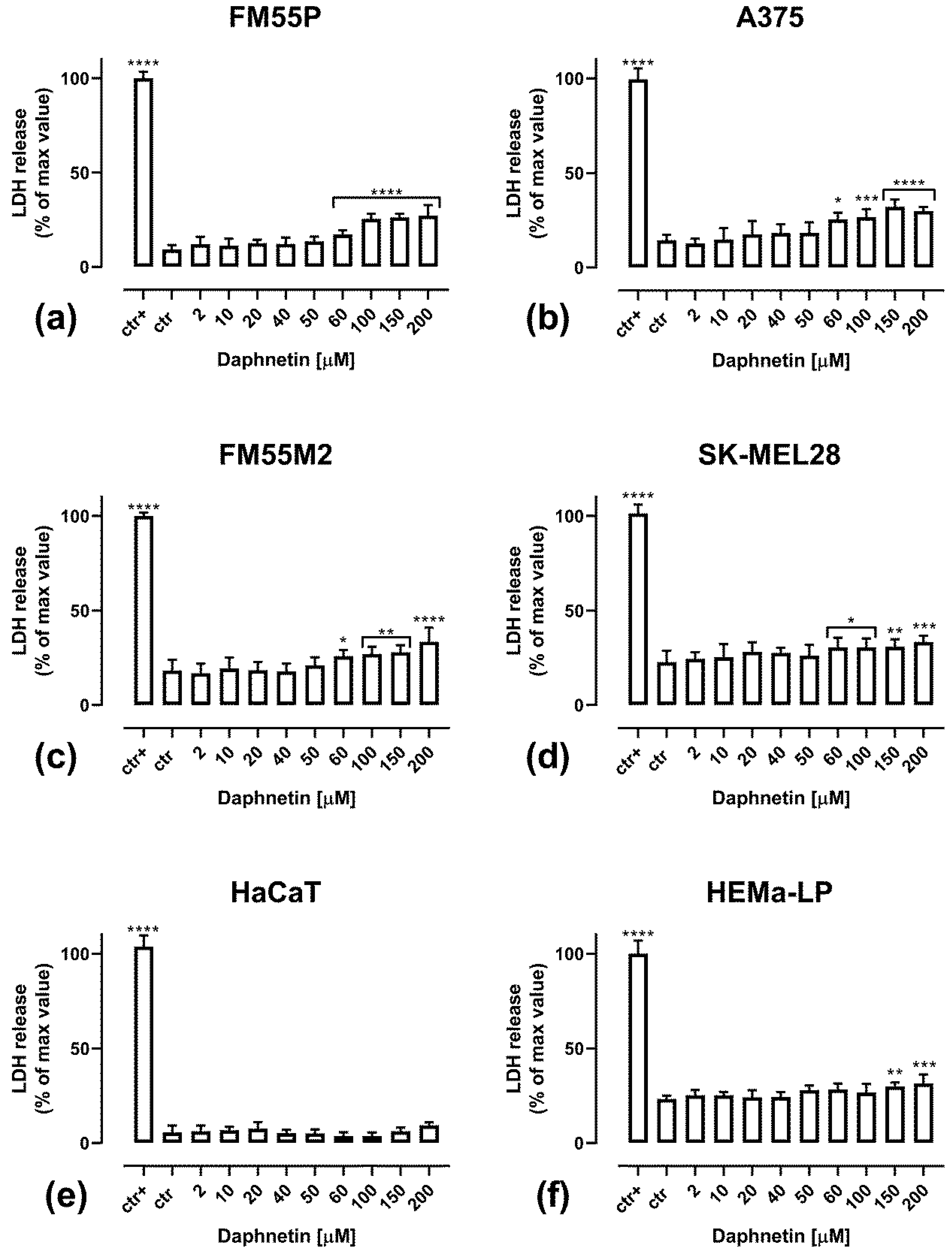
3.3. Cytotoxicity of Daphnetin Assessment—LDH Assay Results
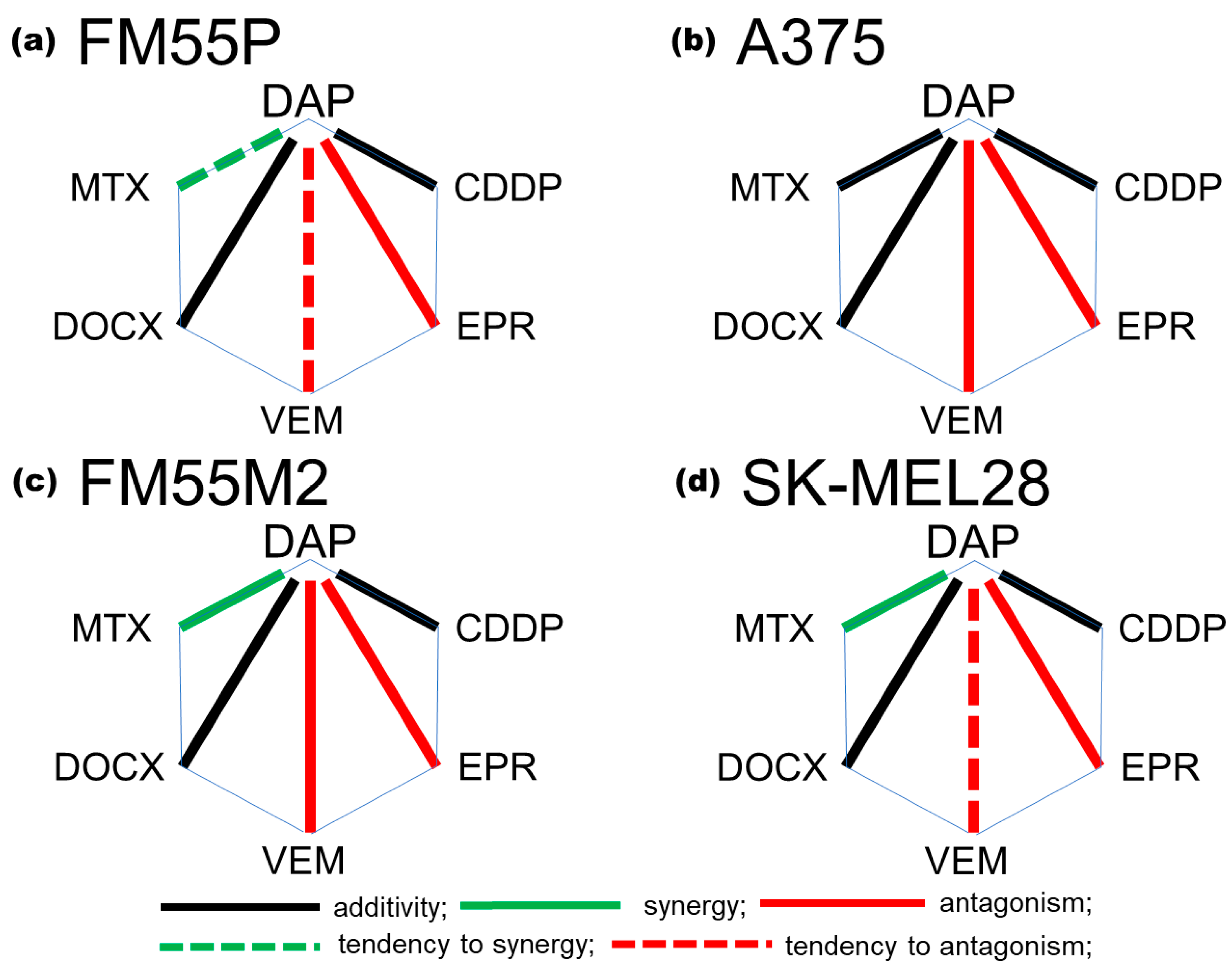
3.4. Interactions between Daphnetin and One Chemotheraputic: Mitoxantrone, Docetaxel, Vemurafenib, Epirubicin or Cisplatin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zob, D.L.; Augustin, I.; Caba, L.; Panzaru, M.C.; Popa, S.; Popa, A.D.; Florea, L.; Gorduza, E.V. Genomics and Epigenomics in the Molecular Biology of Melanoma-A Prerequisite for Biomarkers Studies. Int. J. Mol. Sci. 2022, 24, 716. [Google Scholar] [CrossRef]
- Lorenz, A.; Kozłowski, M.; Lenkiewicz, S.; Kwiatkowski, S.; Cymbaluk-Płoska, A. Cutaneous Melanoma versus Vulvovaginal Melanoma—Risk Factors, Pathogenesis and Comparison of Immunotherapy Efficacy. Cancers 2022, 14, 5123. [Google Scholar] [CrossRef] [PubMed]
- Dzwierzynski, W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021, 48, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Potestio, L.; Fabbrocini, G.; Troncone, G.; Malapelle, U.; Scalvenzi, M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022, 23, 6388. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, L.; Hang, S.; Wang, S.; Li, N.; Sun, X.; Wang, Z.; Sheng, R.; Wang, F.; Wu, W.; et al. Synthesis of Coumarin Derivatives: A New Class of Coumarin-Based G Protein-Coupled Receptor Activators and Inhibitors. Polymers 2022, 14, 2021. [Google Scholar] [CrossRef]
- Hang, S.; Wu, W.; Wang, Y.; Sheng, R.; Fang, Y.; Guo, R. Daphnetin, a Coumarin in Genus Stellera Chamaejasme Linn: Chemistry, Bioactivity and Therapeutic Potential. Chem. Biodivers. 2022, 19, e2022002612022. [Google Scholar] [CrossRef]
- Riveiro, M.E.; De Kimpe, N.; Moglioni, A.; Vazquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef]
- Javed, M.; Saleem, A.; Xaveria, A.; Akhtar, M.F. Daphnetin: A bioactive natural coumarin with diverse therapeutic potentials. Front. Pharmacol. 2022, 13, 993562. [Google Scholar] [CrossRef]
- Xu, K.; Guo, L.; Bu, H.; Wang, H. Daphnetin inhibits high glucose-induced extracellular matrix accumulation, oxidative stress and inflammation in human glomerular mesangial cells. J. Pharmacol. Sci. 2019, 139, 91–97. [Google Scholar] [CrossRef]
- Singh, L.; Singh, A.P.; Bhatti, R. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol. Rep. 2021, 73, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. 7,8-Dihydroxycoumarin (daphnetin) protects INS-1 pancreatic β-cells against streptozotocin-induced apoptosis. Phytomedicine 2017, 24, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Maurya, A.K.; Mishra, N.; Siddique, H.R. Virtual screening, ADME/T, and binding free energy analysis of anti-viral, anti-protease, and anti-infectious compounds against NSP10/NSP16 methyltransferase and main protease of SARS CoV-2. J. Recept. Signal Transduct. Res. 2020, 40, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pang, J.; Wang, G.; Hu, X.; Li, X.; Li, G.; Wang, X.; Yang, X.; Li, C.; You, X. Quantitative proteomics approach to investigate the antibacterial response of Helicobacter pylori to daphnetin, a traditional Chinese medicine monomer. RSC Adv. 2021, 11, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Kori, M.; Arga, K.Y.; Mardinoglu, A.; Turanli, B. Repositioning of Anti-Inflammatory Drugs for the Treatment of Cervical Cancer Sub-Types. Front. Pharmacol. 2022, 13, 884548. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Grabarska, A.; Florek-Łuszczki, M.; Plewa, Z.; Łuszczki, J.J. Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 537. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Romashin, D.D.; Rusanov, A.L.; Kozhin, P.M.; Karagyaur, M.N.; Tikhonova, O.V.; Zgoda, V.G.; Luzgina, N.G. Impact of p53 knockdown on protein dataset of HaCaT cells. Data Brief. 2022, 42, 108274. [Google Scholar] [CrossRef]
- Marzęda, P.; Wróblewska-Łuczka, P.; Drozd, M.; Florek-Łuszczki, M.; Załuska-Ogryzek, K.; Łuszczki, J.J. Cannabidiol Interacts Antagonistically with Cisplatin and Additively with Mitoxantrone in Various Melanoma Cell Lines—An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 6752. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Grabarska, A.; Góralczyk, A.; Marzęda, P.; Łuszczki, J.J. Fraxetin Interacts Additively with Cisplatin and Mitoxantrone, Antagonistically with Docetaxel in Various Human Melanoma Cell Lines—An Isobolographic Analysis. Int. J. Mol. Sci. 2023, 24, 212. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Luszczki, J.J. Isobolographic analysis of interaction between drugs with nonparallel dose-response relationship curves: A practical application. NaunynSchmiedebergs Arch. Pharmacol. 2007, 375, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Grabarska, A.; Wróblewska-Łuczka, P.; Kukula-Koch, W.; Łuszczki, J.J.; Kalpoutzakis, E.; Adamczuk, G.; Skaltsounis, A.L.; Stepulak, A. Palmatine, a Bioactive Protoberberine Alkaloid Isolated from Berberis cretica, Inhibits the Growth of Human Estrogen Receptor-Positive Breast Cancer Cells and Acts Synergistically and Additively with Doxorubicin. Molecules 2021, 26, 6253. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J.; Porreca, F.; Cowan, A. Statistical Analysis of Drug-Drug and Site-Site Interactions with Isobolograms. Life Sci. 1989, 45, 947–961. [Google Scholar] [CrossRef]
- Grabovsky, Y.; Tallarida, R.J. Isobolographic analysis for combinations of a full and partial agonist: Curved isoboles. J. Pharmacol. Exp. Ther. 2004, 310, 981–986. [Google Scholar] [CrossRef]
- Tallarida, R.J. Combination analysis. Adv. Exp. Med. Biol. 2010, 678, 133–137. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug Combinations: Tests and Analysis with Isoboles. Curr. Protoc. Pharmacol. 2016, 72, 9.19.1–9.19.19. [Google Scholar] [CrossRef] [Green Version]
- Krasowska, D.; Gerkowicz, A.; Wróblewska-Łuczka, P.; Grabarska, A.; Załuska-Ogryzek, K.; Krasowska, D.; Łuszczki, J.J. Anticancer Activity of Amantadine and Evaluation of Its Interactions with Selected Cytostatics in Relation to Human Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 7653. [Google Scholar] [CrossRef]
- Chou, T.C. Preclinical versus Clinical Drug Combination Studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef]
- Tallarida, R.J. Quantitative methods for assessing drug synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 6, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Łuczka, P.; Góralczyk, A.; Łuszczki, J.J. Synergy, Additivity and Antagonism between Esculetin and Six Commonly Used Chemotherapeutics in Various Malignant Melanoma Cell Lines—An Isobolographic Analysis. Molecules 2023, 28, 3889. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Nakamura, S.; Chisaki, Y.; Takada, T.; Toda, Y.; Murata, H.; Itoh, K.; Yano, Y.; Takata, K.; Ashihara, E. Daphnetin inhibits invasion and migration of LM8 murine osteosarcoma cells by decreasing RhoA and Cdc42 expression. Biochem. Biophys. Res. Commun. 2016, 471, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.B.; Zhao, Y.N.; Zhang, K.; Mack, P. Daphnetin, one of coumarin derivatives, is a protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1999, 260, 682–685. [Google Scholar] [CrossRef]
- Yao, B.; Yang, Q.; Yang, Y.; Li, Y.; Peng, H.; Wu, S.; Wang, L.; Zhang, S.; Huang, M.; Wang, E.; et al. Screening for Active Compounds Targeting Human Natural Killer Cell Activation Identifying Daphnetin as an Enhancer for IFN-γ Production and Direct Cytotoxicity. Front. Immunol. 2021, 12, 680611. [Google Scholar] [CrossRef]
- Kumar, A.; Sunita, P.; Jha, S.; Pattanayak, S.P. Daphnetin inhibits TNF-α and VEGF-induced angiogenesis through inhibition of the IKKs/IκBα/NF-κB, Src/FAK/ERK1/2 and Akt signalling pathways. Clin. Exp. Pharmacol. Physiol. 2016, 43, 939–950. [Google Scholar] [CrossRef]
- Fan, X.; Xie, M.; Zhao, F.; Li, J.; Fan, C.; Zheng, H.; Wei, Z.; Ci, X.; Zhang, S. Daphnetin triggers ROS-induced cell death and induces cytoprotective autophagy by modulating the AMPK/Akt/mTOR pathway in ovarian cancer. Phytomedicine 2021, 82, 153465. [Google Scholar] [CrossRef]
- Liu, C.; Pan, J.; Liu, H.; Lin, R.; Chen, Y.; Zhang, C. Daphnetin inhibits the survival of hepatocellular carcinoma cells through regulating Wnt/β-catenin signaling pathway. Drug Dev. Res. 2022, 83, 952–960. [Google Scholar] [CrossRef]
- Finn, G.J.; Creaven, B.S.; Egan, D.A. Daphnetin induced differentiation of human renal carcinoma cells and its mediation by p38 mitogen-activated protein kinase. Biochem. Pharmacol. 2004, 67, 1779–1788. [Google Scholar] [CrossRef]
- Li, T.; Yang, G.; Hao, Q.; Zhang, X. Daphnetin Ameliorates the Expansion of Chemically Induced Hepatocellular Carcinoma via Reduction of Inflammation and Oxidative Stress. J. Oleo Sci. 2022, 71, 575–585. [Google Scholar] [CrossRef]
- Pei, Q.; Hu, P.; Zhang, H.; Li, H.; Yang, T.; Liu, R. Daphnetin exerts an anticancer effect by attenuating the pro-inflammatory cytokines. J. Biochem. Mol. Toxicol. 2021, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Chen, J.; Dong, C.; Yan, X.; Zhu, Z.; Lu, P.; Song, Z.; Liu, H.; Chen, S. Daphnetin ameliorates glucocorticoid-induced osteoporosis via activation of Wnt/GSK-3β/β-catenin signaling. Toxicol. Appl. Pharmacol. 2020, 409, 115333. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jha, S.; Pattanayak, S.P. Daphnetin ameliorates 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis through Nrf-2-Keap1 and NF-κB pathways. Biomed. Pharmacother. 2016, 82, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sunita, P.; Jha, S.; Pattanayak, S.P. 7,8-Dihydroxycoumarin exerts antitumor potential on DMBA-induced mammary carcinogenesis by inhibiting ERα, PR, EGFR, and IGF1R: Involvement of MAPK1/2-JNK1/2-Akt pathway. J. Physiol. Biochem. 2018, 74, 223–234. [Google Scholar] [CrossRef]
- Nam, G.; An, S.K.; Park, I.C.; Bae, S.; Lee, J.H. Daphnetin inhibits α-MSH-induced melanogenesis via PKA and ERK signaling pathways in B16F10 melanoma cells. Biosci. Biotechnol. Biochem. 2022, 86, 596–609. [Google Scholar] [CrossRef]
- Jiménez-Orozco, F.A.; Ranđelović, I.; Hegedüs, Z.; Vega-Lopez, A.; Martínez-Flores, F.; Tóvarí, J. In vitro anti-proliferative effect and in vivo antitumor action of daphnetin in different tumor cells. Cir. Cir. 2020, 88, 765–771. [Google Scholar] [CrossRef]
- Liston, D.R.; Davis, M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Wei, W.; Huang, J.; Peng, L.; Ci, X. Daphnetin Attenuated Cisplatin-Induced Acute Nephrotoxicity With Enhancing Antitumor Activity of Cisplatin by Upregulating SIRT1/SIRT6-Nrf2 Pathway. Front. Pharmacol. 2020, 11, 579178. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, L.; Li, Y.; Zou, L. Chemoprotective effect of daphnetin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse. Dokl. Biochem. Biophys. 2021, 499, 273–281. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug. Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]

| Type of Cancer | Cell Line | Dose of Daphnetin | Mechanism of Action | References |
|---|---|---|---|---|
| Murine metastatic osteosarcoma | LM8 | ≥100 μM | reduced levels of the proteins that regulate actin polymerization, RhoA and Cdc42 (which are responsible for the production of stress fibers, filopodia and lamellipodia), thereby inhibiting cell invasion and migration | [33] |
| Ovarian cancer | A2780, SKOV3, OVCAR8 | 5–40 µg/mL (≈28–224 µM) | increased amounts of reactive oxygen species (ROS); increased expression of pro-apoptotic proteins (Bax, PARP and caspase-3) and reduced expression of Bcl2 (anti-apoptotic protein); | [37] |
| induced cell autophagy through abnormalities in the functioning of the AMPK/Akt/mTOR pathway | ||||
| Hepatocellular carcinoma | Huh7, SK-HEP-1 | ≥100 μM | cell cycle arrest in the G1 phase | [38] |
| inhibition of Wnt/β-catenin signaling | ||||
| Human renal adenocarcinoma | A-498 | ≥500 μM | cell cycle arrest in the S phase (at low dose, 48 h and 72 h incubation) or G1 phase (250 and 500 µM, 96 h); | [39] |
| activation of mitogen-activated protein kinase p38 MAPK and inhibition of ERK1/ERK2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska-Łuczka, P.; Góralczyk, A.; Łuszczki, J.J. Daphnetin, a Coumarin with Anticancer Potential against Human Melanoma: In Vitro Study of Its Effective Combination with Selected Cytostatic Drugs. Cells 2023, 12, 1593. https://doi.org/10.3390/cells12121593
Wróblewska-Łuczka P, Góralczyk A, Łuszczki JJ. Daphnetin, a Coumarin with Anticancer Potential against Human Melanoma: In Vitro Study of Its Effective Combination with Selected Cytostatic Drugs. Cells. 2023; 12(12):1593. https://doi.org/10.3390/cells12121593
Chicago/Turabian StyleWróblewska-Łuczka, Paula, Agnieszka Góralczyk, and Jarogniew J. Łuszczki. 2023. "Daphnetin, a Coumarin with Anticancer Potential against Human Melanoma: In Vitro Study of Its Effective Combination with Selected Cytostatic Drugs" Cells 12, no. 12: 1593. https://doi.org/10.3390/cells12121593
APA StyleWróblewska-Łuczka, P., Góralczyk, A., & Łuszczki, J. J. (2023). Daphnetin, a Coumarin with Anticancer Potential against Human Melanoma: In Vitro Study of Its Effective Combination with Selected Cytostatic Drugs. Cells, 12(12), 1593. https://doi.org/10.3390/cells12121593






