Systematic Approaches to Study Eclipsed Targeting of Proteins Uncover a New Family of Mitochondrial Proteins
Abstract
1. Introduction
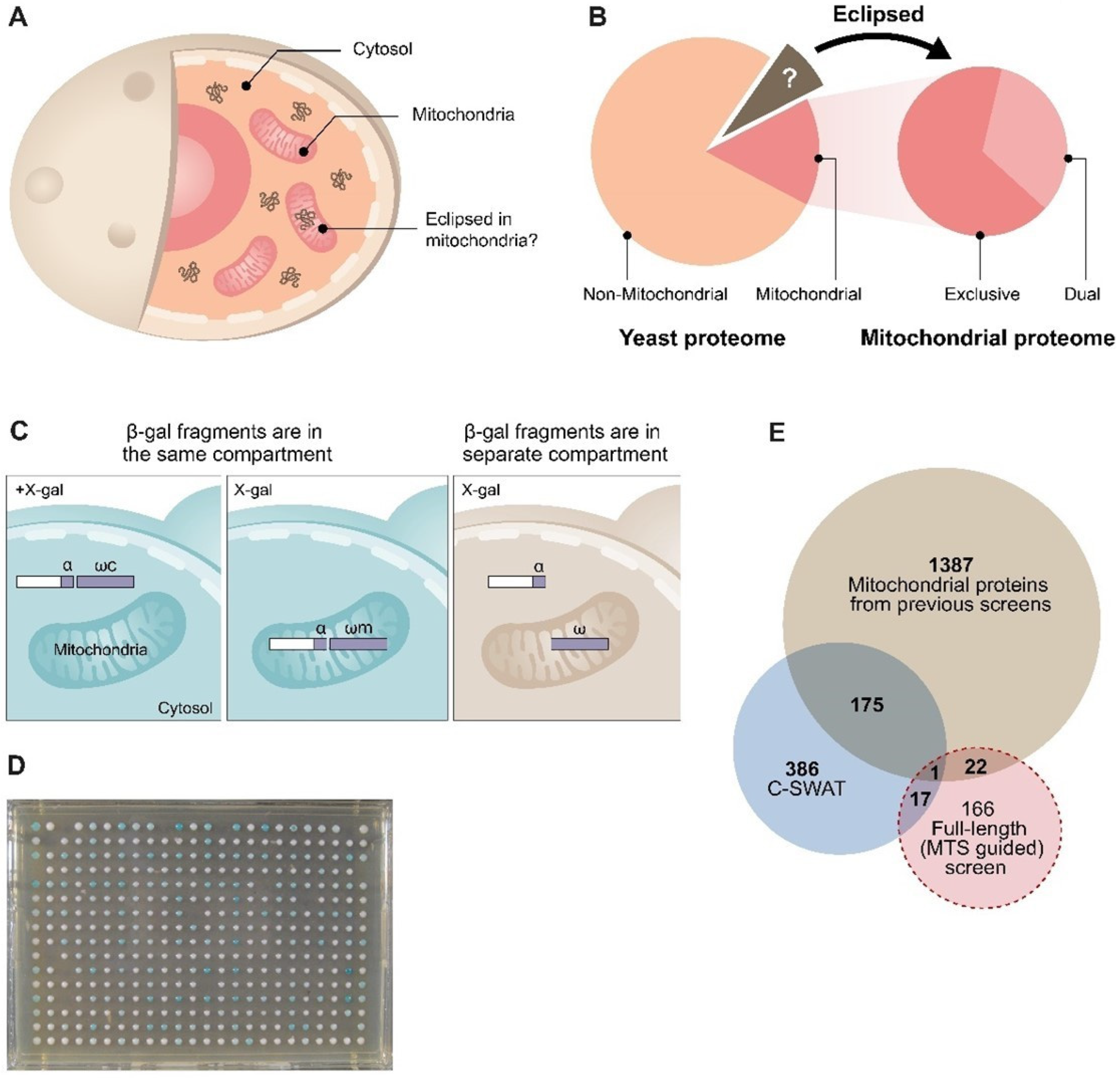
2. Results
2.1. Genome-Wide Analysis of the Yeast Proteome Reveals 137 Candidates of Eclipsed Mitochondrial Proteins
2.2. A Proteomics Approach to Uncover Eclipsed Mitochondrial Proteins
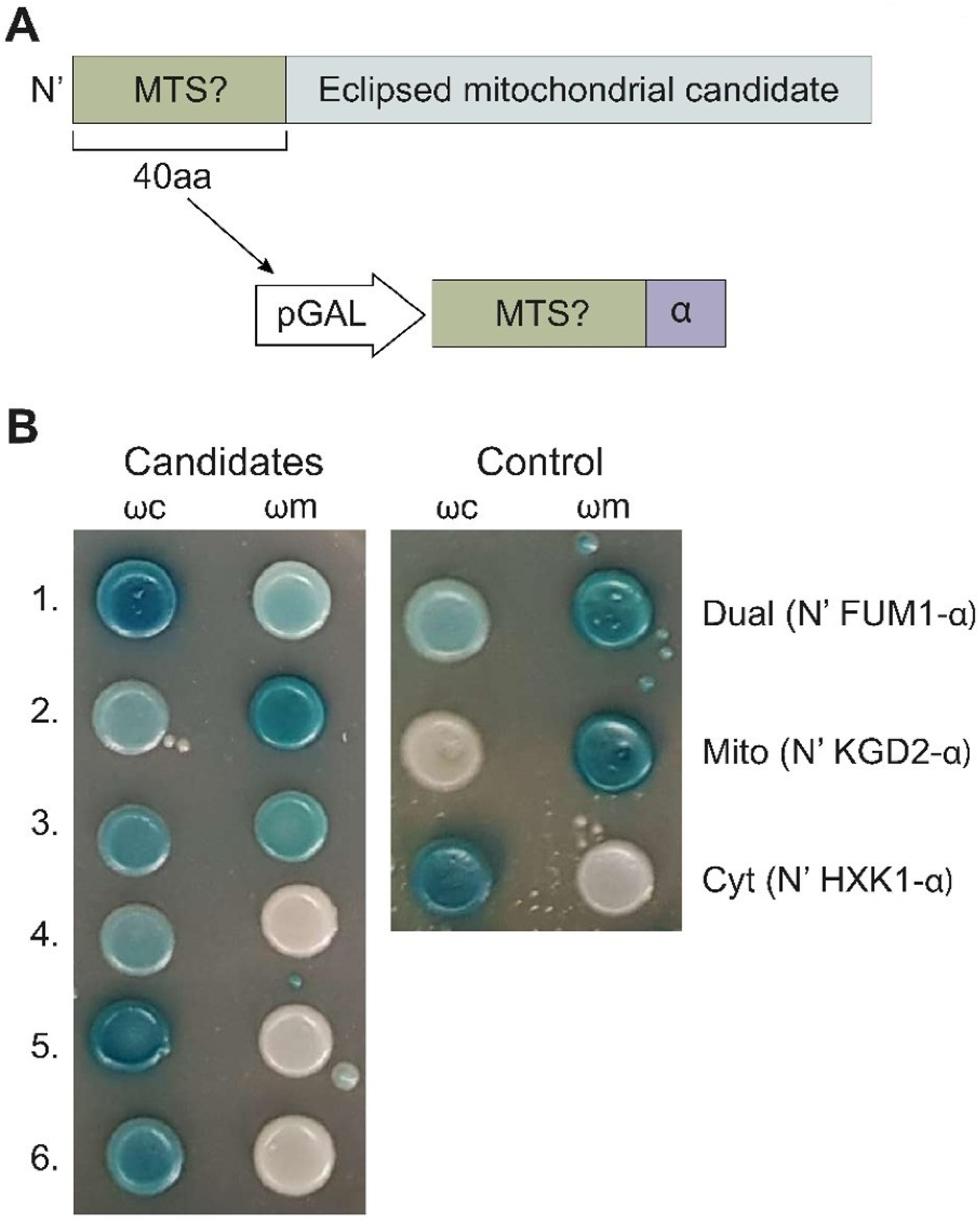
2.3. α-Complementation Analysis of Predicted MTSs
2.4. Analysis of Full-Length Proteins Harboring an MTS by α-Complementation
2.5. Bioinformatics Analysis of the Candidate Eclipsed Mitochondrial Proteins
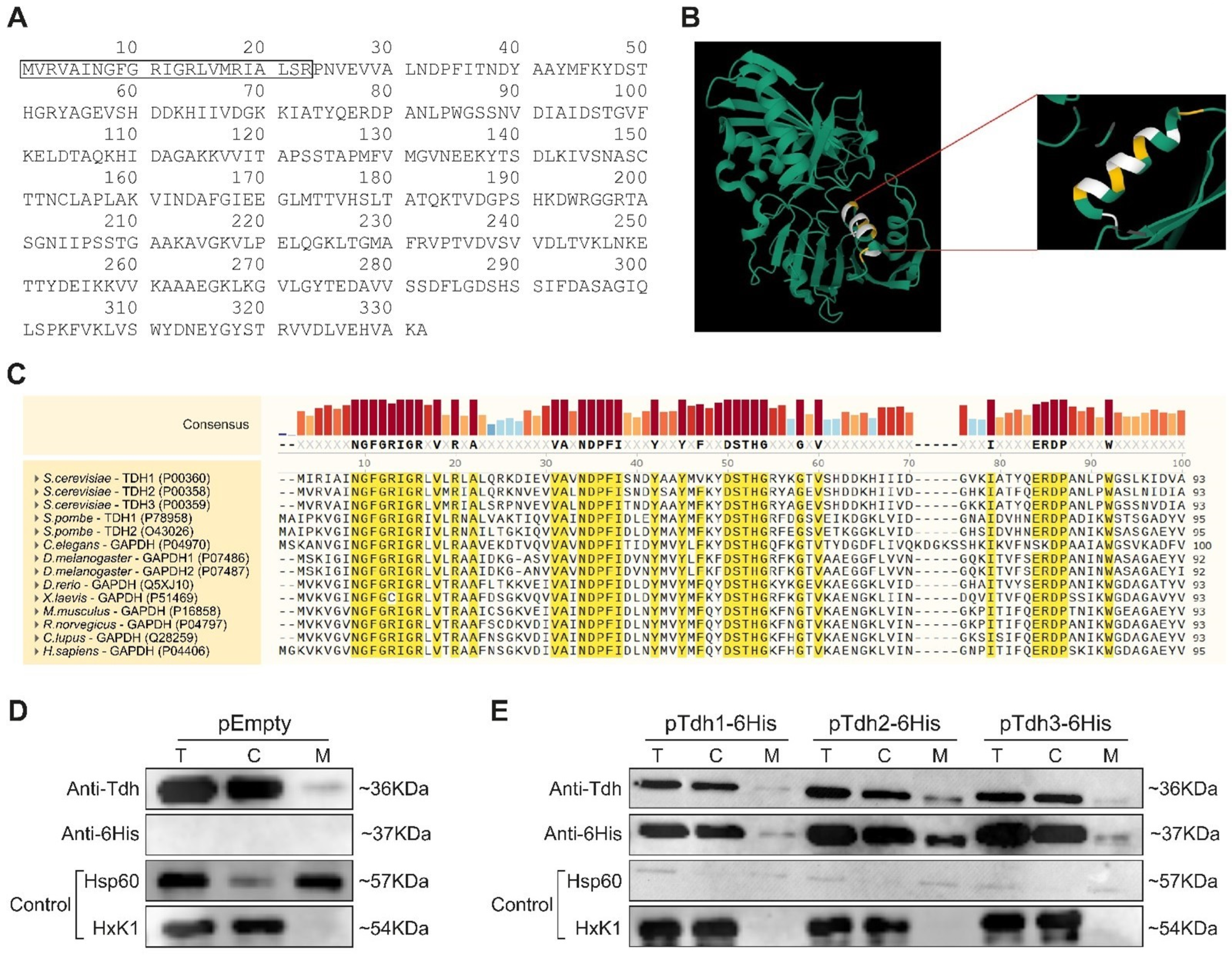
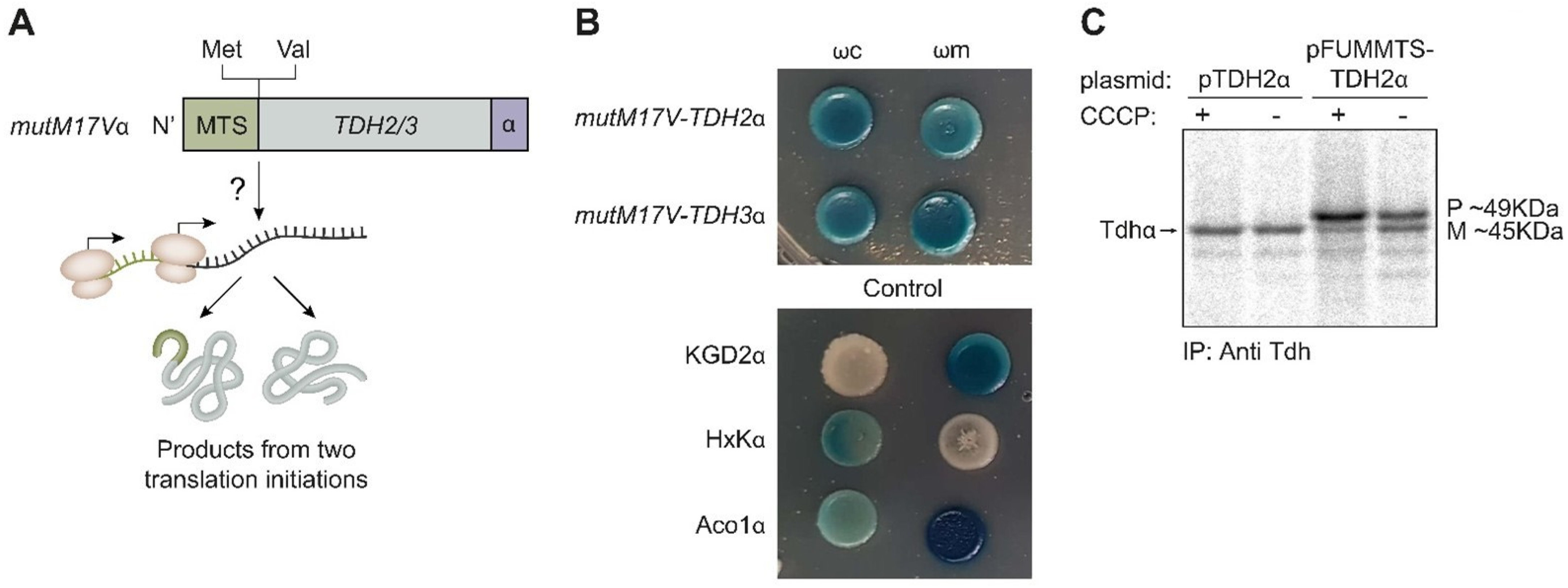
2.6. TDH Genes Are Eclipsed Mitochondrial Proteins
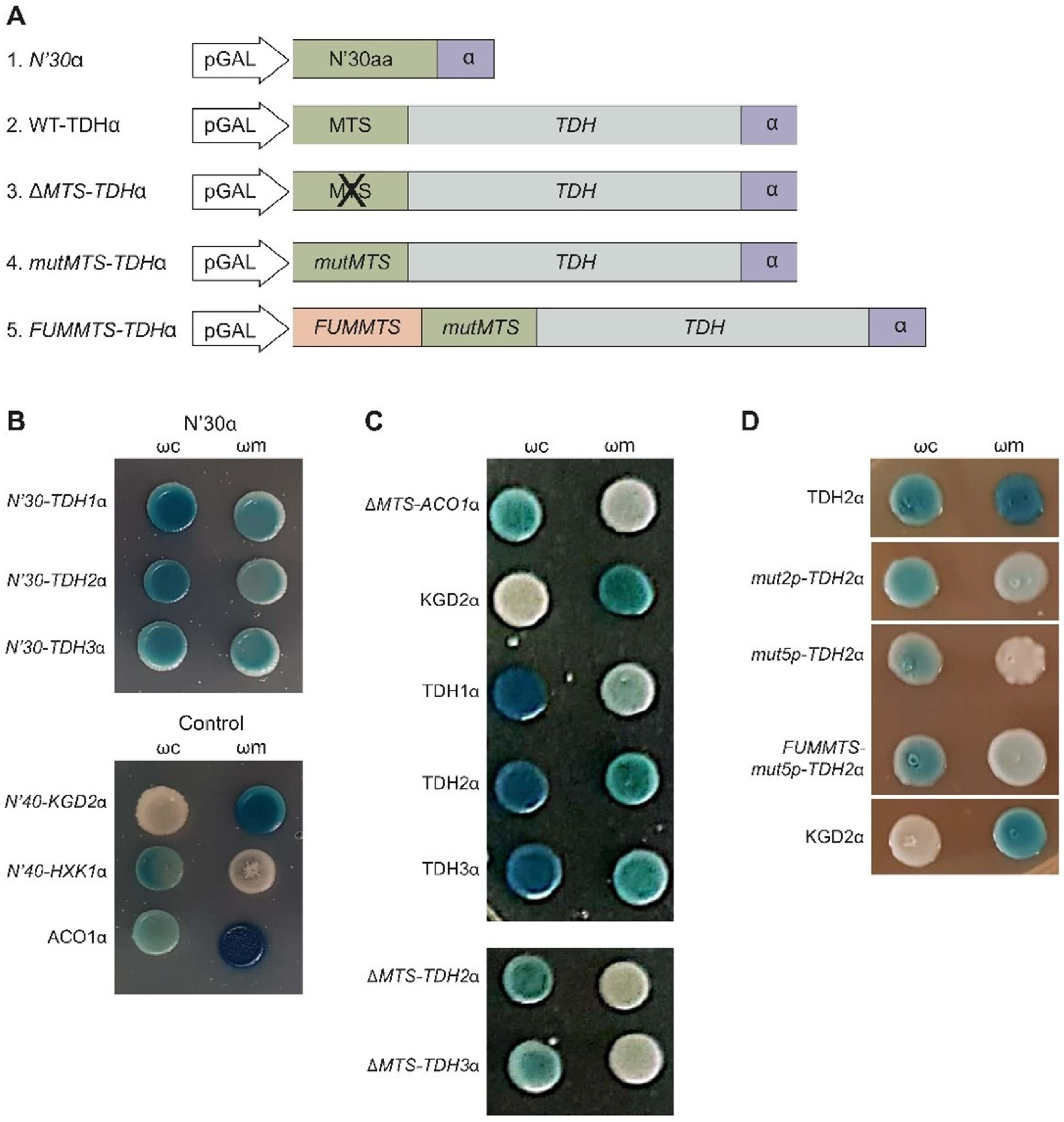
2.7. Tdh Proteins Rely on MTS-Like Signals for Their Eclipsed Distribution
2.8. Processing of Tdh2/3 Proteins
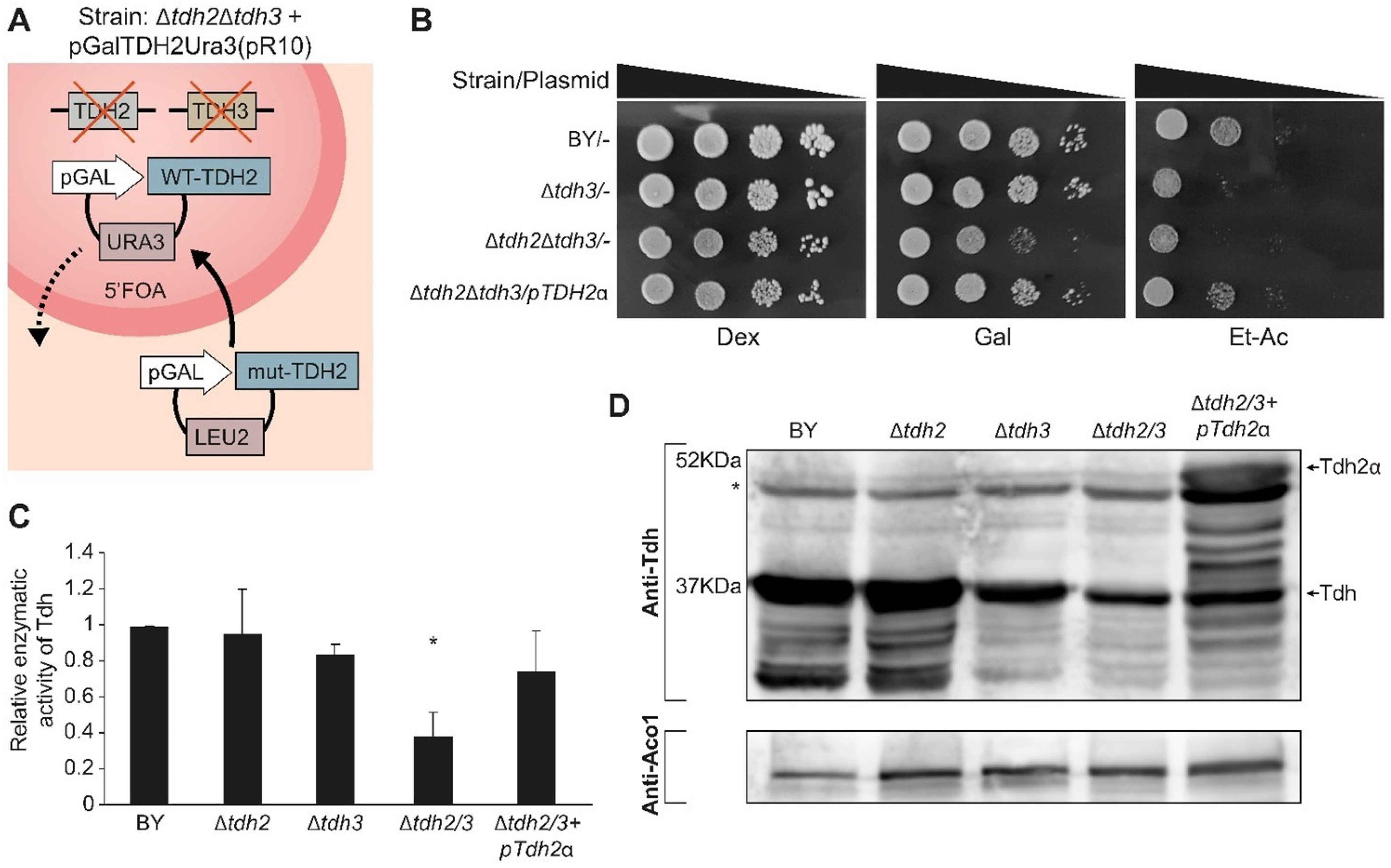
2.9. Tdh Proteins Are Important for Growth on Carbon Sources Requiring Mitochondria
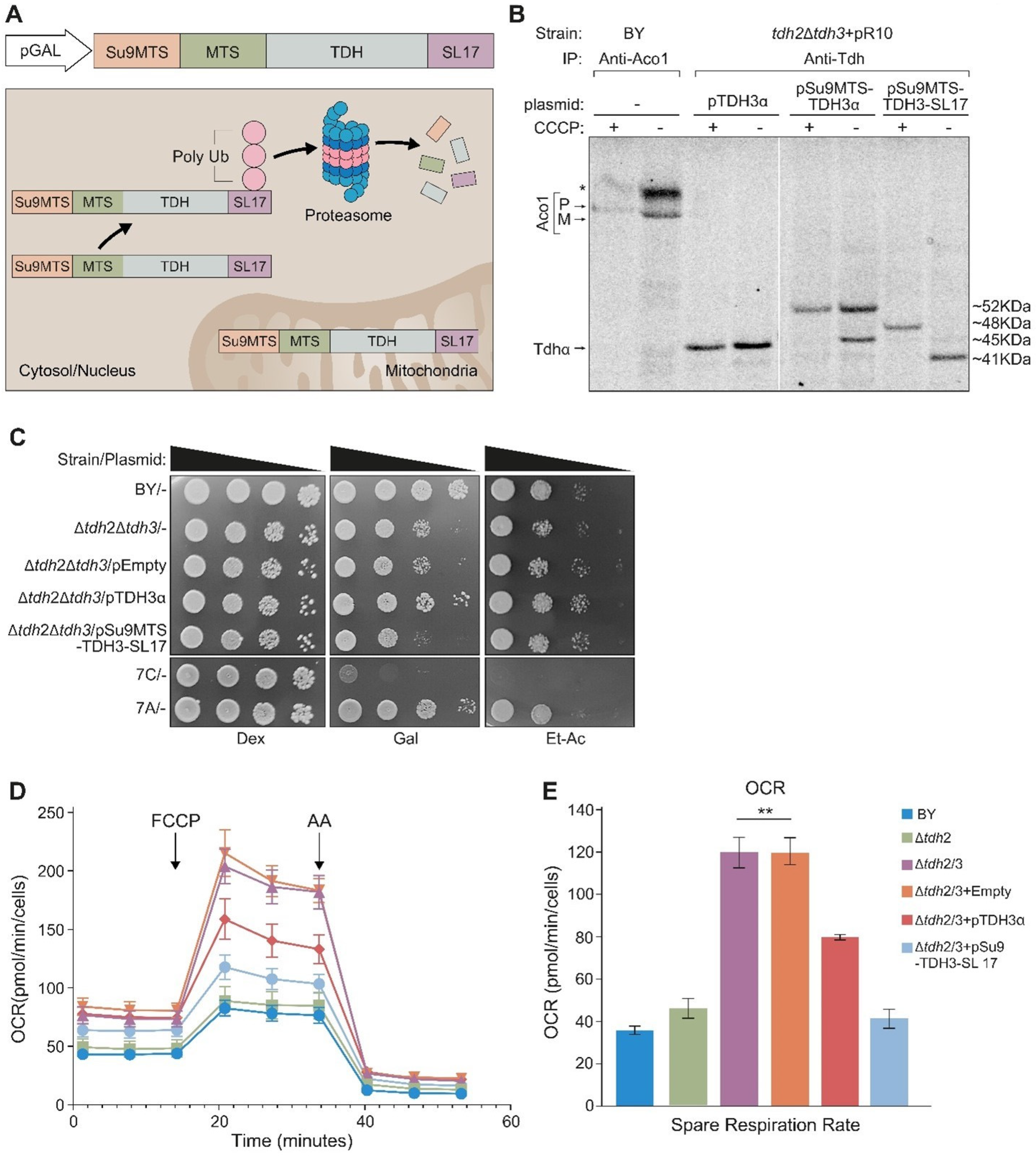
2.10. Tdh Proteins Affect Mitochondrial Activity When in the Mitochondrial Matrix
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Plasmids
4.3. Growth Conditions
4.4. β-Galactosidase α-Complementation Assay
4.5. C-SWAT Library
4.6. Metabolic Labeling
4.7. Subcellular Fractionation
4.8. Western Blot Analysis
4.9. Enzymatic Activity
4.10. Oxygen Consumption Evaluation by Seahorse
4.11. Microscopic Analysis of Mitochondria Phenotype
4.12. iTRAQ Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carrie, C.; Giraud, E.; Whelan, J. Protein transport in organelles: Dual targeting of proteins to mitochondria and chloroplasts. FEBS J. 2009, 276, 1187–1195. [Google Scholar] [CrossRef]
- Kalderon, B.; Pines, O. Protein folding as a driving force for dual protein targeting in eukaryotes. Front. Mol. Biosci. 2014, 1, 23. [Google Scholar] [CrossRef]
- Regev-Rudzki, N.; Karniely, S.; Ben-Haim, N.N.; Pines, O. Yeast aconitase in two locations and two metabolic pathways: Seeing small amounts is believing. Mol. Biol. Cell 2005, 16, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Regev-Rudzki, N.; Pines, O. Eclipsed distribution: A phenomenon of dual targeting of protein and its significance. Bioessays 2007, 29, 772–782. [Google Scholar] [CrossRef]
- Silva-Filho, M.C. One ticket for multiple destinations: Dual targeting of proteins to distinct subcellular locations. Curr. Opin. Plant Biol. 2003, 6, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Weill, U.; Yofe, I.; Sass, E.; Stynen, B.; Davidi, D.; Natarajan, J.; Ben-Menachem, R.; Avihou, Z.; Goldman, O.; Harpaz, N.; et al. Genome-wide SWAp-Tag yeast libraries for proteome exploration. Nat. Methods 2018, 15, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Yofe, I.; Weill, U.; Meurer, M.; Chuartzman, S.; Zalckvar, E.; Goldman, O.; Ben-Dor, S.; Schütze, C.; Wiedemann, N.; Knop, M.; et al. One library to make them all: Streamlining the creation of yeast libraries via a SWAp-Tag strategy. Nat. Methods 2016, 13, 371–378. [Google Scholar] [CrossRef]
- Naamati, A.; Regev-Rudzki, N.; Galperin, S.; Lill, R.; Pines, O. Dual targeting of Nfs1 and discovery of its novel processing enzyme, Icp55. J. Biol. Chem. 2009, 284, 30200–30208. [Google Scholar] [CrossRef] [PubMed]
- Regev-Rudzki, N.; Battat, E.; Goldberg, I.; Pines, O. Dual localization of fumarase is dependent on the integrity of the glyoxylate shunt. Mol. Microbiol. 2009, 72, 297–306. [Google Scholar] [CrossRef]
- Yogev, O.; Naamati, A.; Pines, O. Fumarase: A paradigm of dual targeting and dual localized functions. FEBS J. 2011, 278, 4230–4242. [Google Scholar] [CrossRef]
- Yogev, O.; Pines, O. Dual targeting of mitochondrial proteins: Mechanism, regulation and function. Biochim. Biophys. Acta 2011, 1808, 1012–1020. [Google Scholar] [CrossRef]
- Avadhani, N.G. Targeting of the same proteins to multiple subcellular destinations: Mechanisms and physiological implications. FEBS J. 2011, 278, 4217. [Google Scholar] [CrossRef] [PubMed]
- Carrie, C.; Whelan, J. Widespread dual targeting of proteins in land plants: When, where, how and why. Plant Signal. Behav. 2013, 8, e25034. [Google Scholar] [CrossRef] [PubMed]
- Duchene, A.M.; Giege, P. Dual localized mitochondrial and nuclear proteins as gene expression regulators in plants? Front. Plant Sci. 2012, 3, 221. [Google Scholar] [CrossRef] [PubMed]
- Karniely, S.; Pines, O. Single translation–dual destination: Mechanisms of dual protein targeting in eukaryotes. EMBO Rep. 2005, 6, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Monteuuis, G.; Miścicka, A.; Świrski, M.; Zenad, L.; Niemitalo, O.; Wrobel, L.; Alam, J.; Chacinska, A.; Kastaniotis, A.J.; Kufel, J. Non-canonical translation initiation in yeast generates a cryptic pool of mitochondrial proteins. Nucleic Acids Res. 2019, 47, 5777–5791. [Google Scholar] [CrossRef] [PubMed]
- Knox, C.; Sass, E.; Neupert, W.; Pines, O. Import into mitochondria, folding and retrograde movement of fumarase in yeast. J. Biol. Chem. 1998, 273, 25587–25593. [Google Scholar] [CrossRef]
- Yogev, O.; Singer, E.; Shaulian, E.; Goldberg, M.; Fox, T.D.; Pines, O. Fumarase: A mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol. 2010, 8, e1000328. [Google Scholar] [CrossRef]
- Danpure, C.J. How can the products of a single gene be localized to more than one intracellular compartment? Trends Cell Biol. 1995, 5, 230–238. [Google Scholar] [CrossRef]
- Di Bartolomeo, F.; Malina, C.; Campbell, K.; Mormino, M.; Fuchs, J.; Vorontsov, E.; Gustafsson, C.M.; Nielsen, J. Absolute yeast mitochondrial proteome quantification reveals trade-off between biosynthesis and energy generation during diauxic shift. Proc. Natl. Acad. Sci. USA 2020, 117, 7524–7535. [Google Scholar] [CrossRef]
- Prokisch, H.; Scharfe, C.; Camp, D.G.; Xiao, W.; David, L.; Andreoli, C.; Monroe, M.E.; Moore, R.J.; Gritsenko, M.A.; Kozany, C.; et al. Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol. 2004, 2, e160. [Google Scholar] [CrossRef] [PubMed]
- Reinders, J.; Zahedi, R.P.; Pfanner, N.; Meisinger, C.; Sickmann, A. Toward the complete yeast mitochondrial proteome: Multidimensional separation techniques for mitochondrial proteomics. J. Proteome Res. 2006, 5, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Sickmann, A.; Reinders, J.; Wagner, Y.; Joppich, C.; Zahedi, R.; Meyer, H.E.; Schönfisch, B.; Perschil, I.; Chacinska, A.; Guiard, B.; et al. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA 2003, 100, 13207–13212. [Google Scholar] [CrossRef]
- Small, I.; Wintz, H.; Akashi, K.; Mireau, H. Two birds with one stone: Genes that encode products targeted to two or more compartments. Plant Mol. Biol. 1998, 38, 265–277. [Google Scholar] [CrossRef]
- Vögtle, F.-N.; Burkhart, J.M.; Gonczarowska-Jorge, H.; Kücükköse, C.; Taskin, A.A.; Kopczynski, D.; Ahrends, R.; Mossmann, D.; Sickmann, A.; Zahedi, R.P.; et al. Landscape of submitochondrial protein distribution. Nat. Commun. 2017, 8, 290. [Google Scholar] [CrossRef]
- Breker, M.; Gymrek, M.; Schuldiner, M. A novel single-cell screening platform reveals proteome plasticity during yeast stress responses. J. Cell Biol. 2013, 200, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Nakai, M.; Hayashi, H.; Kagamiyama, H. Nuclear localization of yeast Nfs1p is required for cell survival. J. Biol. Chem. 2001, 276, 8314–8320. [Google Scholar] [CrossRef]
- Shlevin, L.; Regev-Rudzki, N.; Karniely, S.; Pines, O. Location-specific depletion of a dual-localized protein. Traffic 2007, 8, 169–176. [Google Scholar] [CrossRef]
- Ullmann, A.; Jacob, F.; Monod, J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J. Mol. Biol. 1967, 24, 339–343. [Google Scholar] [CrossRef]
- Abbas-Terki, T.; Picard, D. Alpha-complemented beta-galactosidase. An in vivo model substrate for the molecular chaperone heat-shock protein 90 in yeast. Eur. J. Biochem 1999, 266, 517–523. [Google Scholar] [CrossRef]
- Ben-Menachem, R.; Tal, M.; Shadur, T.; Pines, O. A third of the yeast mitochondrial proteome is dual localized: A question of evolution. Proteomics 2011, 11, 4468–4476. [Google Scholar] [CrossRef] [PubMed]
- Dinur-Mills, M.; Tal, M.; Pines, O. Dual targeted mitochondrial proteins are characterized by lower MTS parameters and total net charge. PLoS ONE 2008, 3, e2161. [Google Scholar] [CrossRef]
- Kisslov, I.; Naamati, A.; Shakarchy, N.; Pines, O. Dual-targeted proteins tend to be more evolutionarily conserved. Mol. Biol. Evol. 2014, 31, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Karniely, S.; Rayzner, A.; Sass, E.; Pines, O. Alpha-complementation as a probe for dual localization of mitochondrial proteins. Exp. Cell Res. 2006, 312, 3835–3846. [Google Scholar] [CrossRef]
- Meurer, M.; Duan, Y.; Sass, E.; Kats, I.; Herbst, K.; Buchmuller, B.C.; Dederer, V.; Huber, F.; Kirrmaier, D.; Štefl, M.; et al. Genome-wide C-SWAT library for high-throughput yeast genome tagging. Nat. Methods 2018, 15, 598–600. [Google Scholar] [CrossRef]
- Velez-Bermudez, I.C.; Wen, T.N.; Lan, P.; Schmidt, W. Isobaric Tag for Relative and Absolute Quantitation (iTRAQ)-Based Protein Profiling in Plants. Methods Mol. Biol. 2016, 1450, 213–221. [Google Scholar] [PubMed]
- Zhang, Y.; Karmon, O.; Das, K.; Wiener, R.; Lehming, N.; Pines, O. Ubiquitination occurs in the mitochondrial matrix by eclipsed targeted components of the ubiquitin machinery. Cells 2022, 11, 4109. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C. MitoP2, an integrated database on mitochondrial proteins in yeast and man. Nucleic Acids Res. 2004, 32, D459–D462. [Google Scholar] [CrossRef]
- Elbaz, A.M. Study of Mitochondrial Eclipsed Dually Targeted Proteins in Yeast, in Microbiology and Molecular Genetics; The Hebrew University of Jerusalem: Jerusalem, Israel, 2021. [Google Scholar]
- Breker, M.; Gymrek, M.; Moldavski, O.; Schuldiner, M. LoQAtE–Localization and Quantitation ATlas of the yeast proteomE. A new tool for multiparametric dissection of single-protein behavior in response to biological perturbations in yeast. Nucleic Acids Res. 2014, 42, D726–D730. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Saitou, N. Exploration for functional nucleotide sequence candidates within coding regions of mammalian genes. DNA Res. 2011, 18, 177–187. [Google Scholar] [CrossRef]
- Zhang, F.; Broughton, R.E. Mitochondrial-nuclear interactions: Compensatory evolution or variable functional constraint among vertebrate oxidative phosphorylation genes? Genome Biol. Evol. 2013, 5, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Holland, M.J. The primary structure of a glyceraldehyde-3-phosphate dehydrogenase gene from Saccharomyces cerevisiae. J. Biol. Chem. 1979, 254, 9839–9845. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Holland, M.J. Structural comparison of two nontandemly repeated yeast glyceraldehyde-3-phosphate dehydrogenase genes. J. Biol. Chem. 1980, 255, 2596–2605. [Google Scholar] [CrossRef]
- McAlister, L.; Holland, M.J. Isolation and characterization of yeast strains carrying mutations in the glyceraldehyde-3-phosphate dehydrogenase genes. J. Biol. Chem. 1985, 260, 15013–15018. [Google Scholar] [CrossRef] [PubMed]
- McAlister, L.; Holland, M.J. Differential expression of the three yeast glyceraldehyde-3-phosphate dehydrogenase genes. J. Biol. Chem. 1985, 260, 15019–15027. [Google Scholar] [CrossRef]
- Martin, W.F.; Cerff, R. Physiology, phylogeny, early evolution, and GAPDH. Protoplasma 2017, 254, 1823–1834. [Google Scholar] [CrossRef]
- Almeida, B.; Büttner, S.; Ohlmeier, S.; Silva, A.; Mesquita, A.M.M.; Sampaio-Marques, B.; Osório, N.S.; Kollau, A.; Mayer, B.; Leão, C.; et al. NO-mediated apoptosis in yeast. J. Cell Sci. 2007, 120 Pt 18, 3279–3288. [Google Scholar] [CrossRef]
- Boucherié, H.; Bataille, N.; Fitch, I.T.; Perrot, M.; Tuite, M.F. Differential synthesis of glyceraldehyde-3-phosphate dehydrogenase polypeptides in stressed yeast cells. FEMS Microbiol. Lett. 1995, 125, 127–133. [Google Scholar] [CrossRef]
- Delgado, M.L.; Gil, M.L.; Gozalbo, D. Starvation and temperature upshift cause an increase in the enzymatically active cell wall-associated glyceraldehyde-3-phosphate dehydrogenase protein in yeast. FEMS Yeast Res. 2003, 4, 297–303. [Google Scholar] [CrossRef]
- Delgado, M.L.; O’connor, J.E.; Azorín, I.; Renau-Piqueras, J.; Gil, M.L.; Gozalbo, D. The glyceraldehyde-3-phosphate dehydrogenase polypeptides encoded by the Saccharomyces cerevisiae TDH1, TDH2 and TDH3 genes are also cell wall proteins. Microbiology 2001, 147 Pt 2, 411–417. [Google Scholar] [CrossRef]
- Ringel, A.E.; Ryznar, R.; Picariello, H.; Huang, K.-L.; Lazarus, A.G.; Holmes, S.G. Yeast Tdh3 (glyceraldehyde 3-phosphate dehydrogenase) is a Sir2-interacting factor that regulates transcriptional silencing and rDNA recombination. PLoS Genet. 2013, 9, e1003871. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Almeida, B.; Sampaio-Marques, B.; Reis, M.; Ohlmeier, S.; Rodrigues, F.; Vale, A.D.; Ludovico, P. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a specific substrate of yeast metacaspase. Biochim. Biophys. Acta 2011, 1813, 2044–2049. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Liu, Q.; Liu, H.; Teng, M.; Li, X. Structural insights into RNA recognition properties of glyceraldehyde-3-phosphate dehydrogenase 3 from Saccharomyces cerevisiae. IUBMB Life 2014, 66, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, H.; Liu, H.; Teng, M.; Li, X. Preliminary crystallographic analysis of glyceraldehyde-3-phosphate dehydrogenase 3 from Saccharomyces cerevisiae. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 978–980. [Google Scholar] [CrossRef]
- Brandina, I.; Graham, J.; Lemaitre-Guillier, C.; Entelis, N.; Krasheninnikov, I.; Sweetlove, L.; Tarassov, I.; Martin, R.P. Enolase takes part in a macromolecular complex associated to mitochondria in yeast. Biochim. Biophys. Acta 2006, 1757, 1217–1228. [Google Scholar] [CrossRef]
- Eisenberg, A.R.; Higdon, A.L.; Hollerer, I.; Fields, A.P.; Jungreis, I.; Diamond, P.D.; Kellis, M.; Jovanovic, M.; Brar, G.A. Translation Initiation Site Profiling Reveals Widespread Synthesis of Non-AUG-Initiated Protein Isoforms in Yeast. Cell Syst. 2020, 11, 145–160.e5. [Google Scholar] [CrossRef] [PubMed]
- Backes, S.; Hess, S.; Boos, F.; Woellhaf, M.W.; Gödel, S.; Jung, M.; Mühlhaus, T.; Herrmann, J.M. Tom70 enhances mitochondrial preprotein import efficiency by binding to internal targeting sequences. J. Cell Biol. 2018, 217, 1369–1382. [Google Scholar] [CrossRef]
- Schneider, K.; Zimmer, D.; Nielsen, H.; Herrmann, J.M.; Mühlhaus, T. iMLP, a predictor for internal matrix targeting-like sequences in mitochondrial proteins. Biol. Chem. 2021, 402, 937–943. [Google Scholar] [CrossRef]
- Morgenstern, M.; Stiller, S.B.; Lübbert, P.; Peikert, C.D.; Dannenmaier, S.; Drepper, F.; Weill, U.; Höß, P.; Feuerstein, R.; Gebert, M.; et al. Definition of a High-Confidence Mitochondrial Proteome at Quantitative Scale. Cell Rep. 2017, 19, 2836–2852. [Google Scholar] [CrossRef]
- Beadle, G.W.; Tatum, E.L. Genetic Control of Biochemical Reactions in Neurospora. Proc. Natl. Acad. Sci. USA 1941, 27, 499–506. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [PubMed]
- Bradley, M.C.; Yang, K.; Fernández-Del-Río, L.; Ngo, J.; Ayer, A.; Tsui, H.S.; Novales, N.A.; Stocker, R.; Shirihai, O.S.; Barros, M.H.; et al. COQ11 deletion mitigates respiratory deficiency caused by mutations in the gene encoding the coenzyme Q chaperone protein Coq10. J. Biol. Chem. 2020, 295, 6023–6042. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, 419–426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mark, M.; Klein, O.; Zhang, Y.; Das, K.; Elbaz, A.; Hazan, R.N.; Lichtenstein, M.; Lehming, N.; Schuldiner, M.; Pines, O. Systematic Approaches to Study Eclipsed Targeting of Proteins Uncover a New Family of Mitochondrial Proteins. Cells 2023, 12, 1550. https://doi.org/10.3390/cells12111550
Mark M, Klein O, Zhang Y, Das K, Elbaz A, Hazan RN, Lichtenstein M, Lehming N, Schuldiner M, Pines O. Systematic Approaches to Study Eclipsed Targeting of Proteins Uncover a New Family of Mitochondrial Proteins. Cells. 2023; 12(11):1550. https://doi.org/10.3390/cells12111550
Chicago/Turabian StyleMark, Maayan, Ofir Klein, Yu Zhang, Koyeli Das, Adi Elbaz, Reut Noa Hazan, Michal Lichtenstein, Norbert Lehming, Maya Schuldiner, and Ophry Pines. 2023. "Systematic Approaches to Study Eclipsed Targeting of Proteins Uncover a New Family of Mitochondrial Proteins" Cells 12, no. 11: 1550. https://doi.org/10.3390/cells12111550
APA StyleMark, M., Klein, O., Zhang, Y., Das, K., Elbaz, A., Hazan, R. N., Lichtenstein, M., Lehming, N., Schuldiner, M., & Pines, O. (2023). Systematic Approaches to Study Eclipsed Targeting of Proteins Uncover a New Family of Mitochondrial Proteins. Cells, 12(11), 1550. https://doi.org/10.3390/cells12111550






