The Role of Toll and Nonnuclear NF-κB Signaling in the Response to Alcohol
Abstract
1. Introduction
1.1. Toll-like Receptors
1.2. TLRs and Alcohol Responses
1.3. TLRs as Neurotrophin Receptors
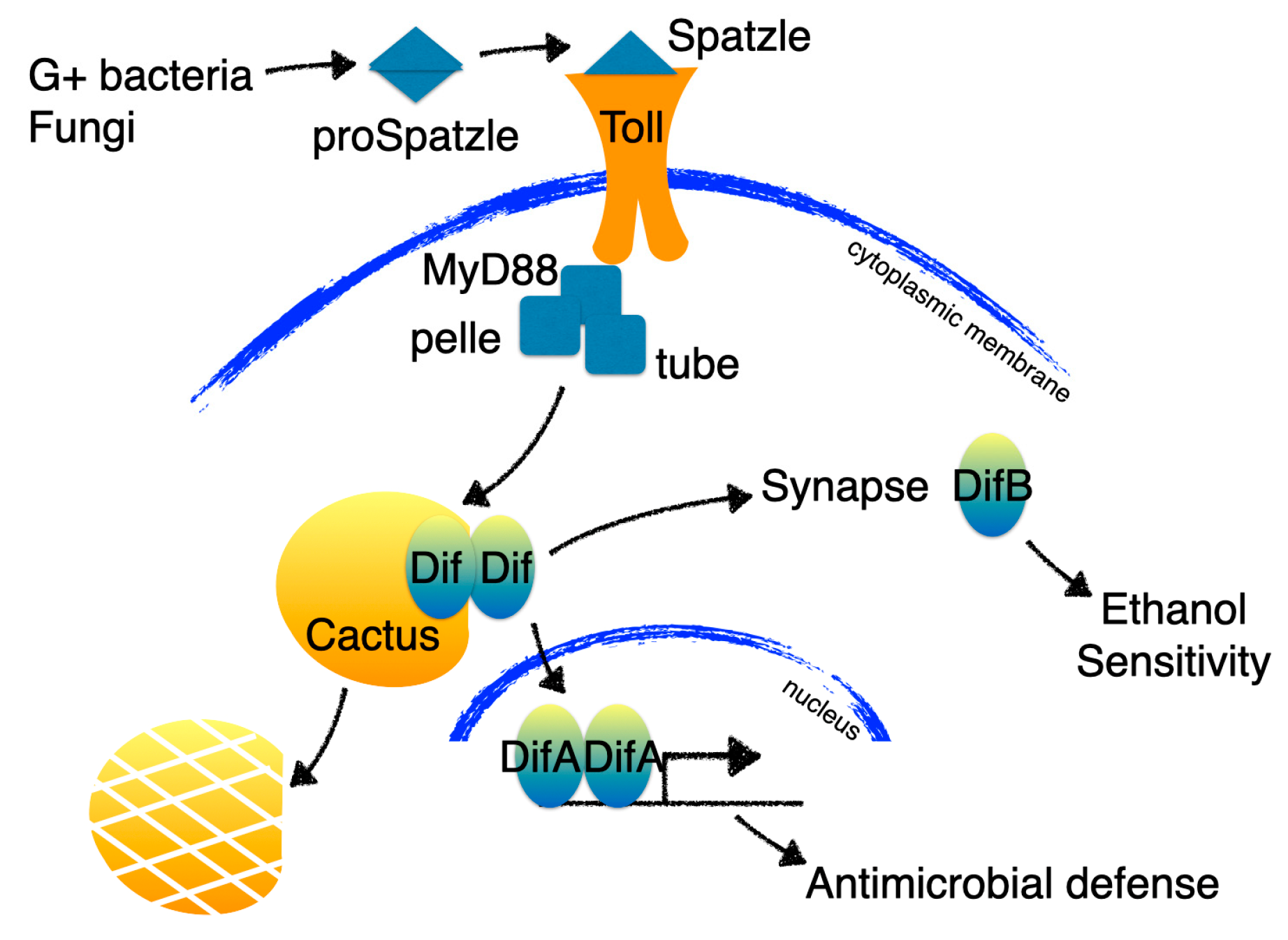
1.4. Synaptically Localized NF-κBs
1.5. Overlap of DifB and TLR Expression in the CNS
2. Closing
Funding
Acknowledgments
Conflicts of Interest
References
- Mokdad, A.H.; Marks, J.S.; Stroup, D.F.; Gerberding, J.L. Actual Causes of Death in the United States, 2000. JAMA 2004, 291, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. 2020; U.S. Department of Health and Human Services. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR1PDFW090120.pdf (accessed on 24 May 2023).
- Miller, W.R.; Walters, S.T.; Bennett, M.E. How Effective Is Alcoholism Treatment in the United States? J. Stud. Alcohol 2001, 62, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.A.; Goldstein, R.B.; Grant, B.F. Rates and Correlates of Relapse among Individuals in Remission from DSM-IV Alcohol Dependence: A 3-Year Follow-Up. Alcohol. Clin. Exp. Res. 2007, 31, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Calvo-Rodriguez, M.; Núñez, L.; Villalobos, C.; Ureña, J.; Guerri, C. Toll-like Receptors in Neuroinflammation, Neurodegeneration, and Alcohol-Induced Brain Damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Belvin, M.P.; Anderson, K.V. A Conserved Signaling Pathway: The Drosophila Toll-Dorsal Pathway. Annu. Rev. Cell Dev. Biol. 1996, 12, 393–416. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The Dorsoventral Regulatory Gene Cassette Spätzle/Toll/Cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A Human Homologue of the Drosophila Toll Protein Signals Activation of Adaptive Immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Anderson, K.V.; Jürgens, G.; Nüsslein-Volhard, C. Establishment of Dorsal-Ventral Polarity in the Drosophila Embryo: Genetic Studies on the Role of the Toll Gene Product. Cell 1985, 42, 779–789. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C. The Toll Gene in Drosophila Pattern Formation. Trends Genet. TIG 2022, 38, 231–245. [Google Scholar] [CrossRef]
- Pechmann, M.; Kenny, N.J.; Pott, L.; Heger, P.; Chen, Y.-T.; Buchta, T.; Özüak, O.; Lynch, J.; Roth, S. Striking Parallels between Dorsoventral Patterning in Drosophila and Gryllus Reveal a Complex Evolutionary History behind a Model Gene Regulatory Network. eLife 2021, 10, e68287. [Google Scholar] [CrossRef]
- Beutler, B.A. TLRs and Innate Immunity. Blood 2009, 113, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Toll-Like Receptors in Adaptive Immunity. Handb. Exp. Pharmacol. 2022, 276, 95–131. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Divanovic, S.; Trompette, A.; Atabani, S.F.; Madan, R.; Golenbock, D.T.; Visintin, A.; Finberg, R.W.; Tarakhovsky, A.; Vogel, S.N.; Belkaid, Y.; et al. Negative Regulation of Toll-like Receptor 4 Signaling by the Toll-like Receptor Homolog RP105. Nat. Immunol. 2005, 6, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.E.; Blumenthal, A. The RP105/MD-1 Complex: Molecular Signaling Mechanisms and Pathophysiological Implications. J. Leukoc. Biol. 2017, 101, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and Localization of Toll-like Receptor Signalling Complexes. Nat. Rev. Immunol. 2014, 14, 546–558. [Google Scholar] [CrossRef]
- Marongiu, L.; Gornati, L.; Artuso, I.; Zanoni, I.; Granucci, F. Below the Surface: The Inner Lives of TLR4 and TLR9. J. Leukoc. Biol. 2019, 106, 147–160. [Google Scholar] [CrossRef]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An Unconventional Role for MiRNA: Let-7 Activates Toll-like Receptor 7 and Causes Neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- Park, C.-K.; Xu, Z.-Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.-J.; Ji, R.-R. Extracellular MicroRNAs Activate Nociceptor Neurons to Elicit Pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef]
- Mielcarska, M.B.; Bossowska-Nowicka, M.; Toka, F.N. Cell Surface Expression of Endosomal Toll-Like Receptors—A Necessity or a Superfluous Duplication? Front. Immunol. 2021, 11, 620972. [Google Scholar] [CrossRef]
- Koblansky, A.A.; Jankovic, D.; Oh, H.; Hieny, S.; Sungnak, W.; Mathur, R.; Hayden, M.S.; Akira, S.; Sher, A.; Ghosh, S. Recognition of Profilin by Toll-like Receptor 12 Is Critical for Host Resistance to Toxoplasma Gondii. Immunity 2013, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR Signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.; Erickson, E.; Robinson, G.; Harris, R.A.; Mayfield, R.D. The Neuroimmune Transcriptome and Alcohol Dependence: Potential for Targeted Therapies. Pharmacogenomics 2016, 17, 2081–2096. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Walter, T.J.; Coleman, L.G.; Vetreno, R.P. Toll-like Receptor Signaling and Stages of Addiction. Psychopharmacology 2017, 234, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal Role of TLR4 Receptors in Alcohol-Induced Neuroinflammation and Brain Damage. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 8285–8295. [Google Scholar] [CrossRef]
- Pascual, M.; Baliño, P.; Alfonso-Loeches, S.; Aragón, C.M.G.; Guerri, C. Impact of TLR4 on Behavioral and Cognitive Dysfunctions Associated with Alcohol-Induced Neuroinflammatory Damage. Brain. Behav. Immun. 2011, 25 (Suppl. 1), S80–S91. [Google Scholar] [CrossRef]
- Meredith, L.R.; Burnette, E.M.; Grodin, E.N.; Irwin, M.R.; Ray, L.A. Immune Treatments for Alcohol Use Disorder: A Translational Framework. Brain. Behav. Immun. 2021, 97, 349–364. [Google Scholar] [CrossRef]
- Balan, I.; Warnock, K.T.; Puche, A.; Gondre-Lewis, M.C.; June, H.; Aurelian, L. The GABAA Receptor A2 Subunit Activates a Neuronal TLR4 Signal in the Ventral Tegmental Area That Regulates Alcohol and Nicotine Abuse. Brain Sci. 2018, 8, 72. [Google Scholar] [CrossRef]
- June, H.L.; Liu, J.; Warnock, K.T.; Bell, K.A.; Balan, I.; Bollino, D.; Puche, A.; Aurelian, L. CRF-Amplified Neuronal TLR4/MCP-1 Signaling Regulates Alcohol Self-Administration. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 1549–1559. [Google Scholar] [CrossRef]
- Harris, R.A.; Bajo, M.; Bell, R.L.; Blednov, Y.A.; Varodayan, F.P.; Truitt, J.M.; de Guglielmo, G.; Lasek, A.W.; Logrip, M.L.; Vendruscolo, L.F.; et al. Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. J. Neurosci. 2017, 37, 1139–1155. [Google Scholar] [CrossRef]
- Rasmusson, A.J.; Gallwitz, M.; Soltanabadi, B.; Ciuculete, D.M.; Mengel-From, J.; Christensen, K.; Nygaard, M.; Soerensen, M.; Boström, A.E.; Fredriksson, R.; et al. Toll-like Receptor 4 Methylation Grade Is Linked to Depressive Symptom Severity. Transl. Psychiatry 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Blednov, Y.A.; Black, M.; Chernis, J.; Da Costa, A.; Mayfield, J.; Harris, R.A. Ethanol Consumption in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcohol. Clin. Exp. Res. 2017, 41, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.; Wu, Y.; Tuke, J.; Coller, J.K.; Rice, K.C.; Diener, K.R.; Hayball, J.D.; Watkins, L.R.; Somogyi, A.A.; Hutchinson, M.R. Alcohol-Induced Sedation and Synergistic Interactions between Alcohol and Morphine: A Key Mechanistic Role for Toll-like Receptors and MyD88-Dependent Signaling. Brain. Behav. Immun. 2015, 45, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. Ethanol Induces TLR4/TLR2 Association, Triggering an Inflammatory Response in Microglial Cells. J. Neurochem. 2013, 126, 261–273. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, G.M.; Warden, A.S.; Bridges, C.R.; Blednov, Y.A.; Harris, R.A. Chronic Ethanol Consumption: Role of TLR3/TRIF-Dependent Signaling. Addict. Biol. 2018, 23, 889–903. [Google Scholar] [CrossRef]
- Warden, A.S.; Azzam, M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Messing, R.O.; Mayfield, R.D.; Harris, R.A. Toll-like Receptor 3 Dynamics in Female C57BL/6J Mice: Regulation of Alcohol Intake. Brain. Behav. Immun. 2019, 77, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.S.; Azzam, M.; DaCosta, A.; Mason, S.; Blednov, Y.A.; Messing, R.O.; Mayfield, R.D.; Harris, R.A. Toll-like Receptor 3 Activation Increases Voluntary Alcohol Intake in C57BL/6J Male Mice. Brain. Behav. Immun. 2019, 77, 55–65. [Google Scholar] [CrossRef]
- Gano, A.; Lebonville, C.L.; Becker, H.C. TLR3 Activation with Poly I:C Exacerbates Escalated Alcohol Consumption in Dependent Male C57BL/6J Mice. Am. J. Drug Alcohol Abuse 2022, 1–12. [Google Scholar] [CrossRef]
- Lovelock, D.F.; Randall, P.A.; Van Voorhies, K.; Vetreno, R.P.; Crews, F.T.; Besheer, J. Increased Alcohol Self-Administration Following Repeated Toll-like Receptor 3 Agonist Treatment in Male and Female Rats. Pharmacol. Biochem. Behav. 2022, 216, 173379. [Google Scholar] [CrossRef]
- Grantham, E.K.; Warden, A.S.; McCarthy, G.S.; DaCosta, A.; Mason, S.; Blednov, Y.; Mayfield, R.D.; Harris, R.A. Role of Toll-like Receptor 7 (TLR7) in Voluntary Alcohol Consumption. Brain. Behav. Immun. 2020, 89, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, D.F.; Liu, W.; Langston, S.E.; Liu, J.; Van Voorhies, K.; Giffin, K.A.; Vetreno, R.P.; Crews, F.T.; Besheer, J. The Toll-like Receptor 7 Agonist Imiquimod Increases Ethanol Self-Administration and Induces Expression of Toll-like Receptor Related Genes. Addict. Biol. 2022, 27, e13176. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Qin, L.; Sheedy, D.; Vetreno, R.P.; Zou, J. High Mobility Group Box 1/Toll-like Receptor Danger Signaling Increases Brain Neuroimmune Activation in Alcohol Dependence. Biol. Psychiatry 2013, 73, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila Melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Hidalgo, A.; Learte, A.R.; McQuilton, P.; Pennack, J.; Zhu, B. Neurotrophic and Gliatrophic Contexts in Drosophila. Brain. Behav. Evol. 2006, 68, 173–180. [Google Scholar] [CrossRef]
- McIlroy, G.; Foldi, I.; Aurikko, J.; Wentzell, J.S.; Lim, M.A.; Fenton, J.C.; Gay, N.J.; Hidalgo, A. Toll-6 and Toll-7 Function as Neurotrophin Receptors in the Drosophila Melanogaster CNS. Nat. Neurosci. 2013, 16, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Pennack, J.A.; McQuilton, P.; Forero, M.G.; Mizuguchi, K.; Sutcliffe, B.; Gu, C.-J.; Fenton, J.C.; Hidalgo, A. Drosophila Neurotrophins Reveal a Common Mechanism for Nervous System Formation. PLoS Biol. 2008, 6, e284. [Google Scholar] [CrossRef]
- Li, G.; Forero, M.G.; Wentzell, J.S.; Durmus, I.; Wolf, R.; Anthoney, N.C.; Parker, M.; Jiang, R.; Hasenauer, J.; Strausfeld, N.J.; et al. A Toll-Receptor Map Underlies Structural Brain Plasticity. eLife 2020, 9, e52743. [Google Scholar] [CrossRef]
- Ballard, S.L.; Miller, D.L.; Ganetzky, B. Retrograde Neurotrophin Signaling through Tollo Regulates Synaptic Growth in Drosophila. J. Cell Biol. 2014, 204, 1157–1172. [Google Scholar] [CrossRef]
- Sutcliffe, B.; Forero, M.G.; Zhu, B.; Robinson, I.M.; Hidalgo, A. Neuron-Type Specific Functions of DNT1, DNT2 and Spz at the Drosophila Neuromuscular Junction. PLoS ONE 2013, 8, e75902. [Google Scholar] [CrossRef]
- Foldi, I.; Anthoney, N.; Harrison, N.; Gangloff, M.; Verstak, B.; Nallasivan, M.P.; AlAhmed, S.; Zhu, B.; Phizacklea, M.; Losada-Perez, M.; et al. Three-Tier Regulation of Cell Number Plasticity by Neurotrophins and Tolls in Drosophila. J. Cell Biol. 2017, 216, 1421–1438. [Google Scholar] [CrossRef]
- Chowdhury, M.; Li, C.-F.; He, Z.; Lu, Y.; Liu, X.-S.; Wang, Y.-F.; Ip, Y.T.; Strand, M.R.; Yu, X.-Q. Toll Family Members Bind Multiple Spätzle Proteins and Activate Antimicrobial Peptide Gene Expression in Drosophila. J. Biol. Chem. 2019, 294, 10172–10181. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hidalgo, A. The Toll Route to Structural Brain Plasticity. Front. Physiol. 2021, 12, 679766. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF Signaling in Context: From Synaptic Regulation to Psychiatric Disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Liran, M.; Rahamim, N.; Ron, D.; Barak, S. Growth Factors and Alcohol Use Disorder. Cold Spring Harb. Perspect. Med. 2020, 10, a039271. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A.; Shechter, R.; London, A.; Ziv, Y.; Ronen, A.; Levy, R.; Schwartz, M. Toll-like Receptors Modulate Adult Hippocampal Neurogenesis. Nat. Cell Biol. 2007, 9, 1081–1088. [Google Scholar] [CrossRef]
- Squillace, S.; Salvemini, D. Toll-like Receptor-Mediated Neuroinflammation: Relevance for Cognitive Dysfunctions. Trends Pharmacol. Sci. 2022, 43, 726–739. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Chen, O.; Ji, R.-R. How Do Sensory Neurons Sense Danger Signals? Trends Neurosci. 2020, 43, 822–838. [Google Scholar] [CrossRef]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-Kappa B Functions in Synaptic Signaling and Behavior. Nat. Neurosci. 2003, 6, 1072–1078. [Google Scholar] [CrossRef]
- Salles, A.; Boccia, M.; Blake, M.; Corbi, N.; Passananti, C.; Baratti, C.M.; Romano, A.; Freudenthal, R. Hippocampal Dynamics of Synaptic NF-Kappa B during Inhibitory Avoidance Long-Term Memory Consolidation in Mice. Neuroscience 2015, 291, 70–80. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-ΚB Function in the Nervous System. Front. Immunol. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Crews, F.T.; Lawrimore, C.J.; Walter, T.J.; Coleman, L.G. The Role of Neuroimmune Signaling in Alcoholism. Neuropharmacology 2017, 122, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J.; Xuei, X.; Wetherill, L.F.; Bierut, L.; Bucholz, K.; Dick, D.M.; Hesselbrock, V.; Kuperman, S.; Porjesz, B.; Schuckit, M.A.; et al. Association of NFKB1, Which Encodes a Subunit of the Transcription Factor NF-KappaB, with Alcohol Dependence. Hum. Mol. Genet. 2008, 17, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Truitt, J.M.; Blednov, Y.A.; Benavidez, J.M.; Black, M.; Ponomareva, O.; Law, J.; Merriman, M.; Horani, S.; Jameson, K.; Lasek, A.W.; et al. Inhibition of IKKβ Reduces Ethanol Consumption in C57BL/6J Mice. eNeuro 2016, 3, ENEURO.0256-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Nennig, S.E.; Schank, J.R. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Ghezzi, A.; Pietrzykowski, A.Z.; Atkinson, N.S. Alcohol Resistance in Drosophila Is Modulated by the Toll Innate Immune Pathway. Genes Brain Behav. 2016, 15, 382–394. [Google Scholar] [CrossRef]
- López-Pedrajas, R.; Almansa, I.; Sánchez-Villarejo, M.V.; Muriach, B.; Barcia, J.M.; Romero, F.J.; Muriach, M. Role of Hippocampal NF-ΚB and GluN2B in the Memory Acquisition Impairment of Experiences Gathered Prior to Cocaine Administration in Rats. Sci. Rep. 2021, 11, 20033. [Google Scholar] [CrossRef]
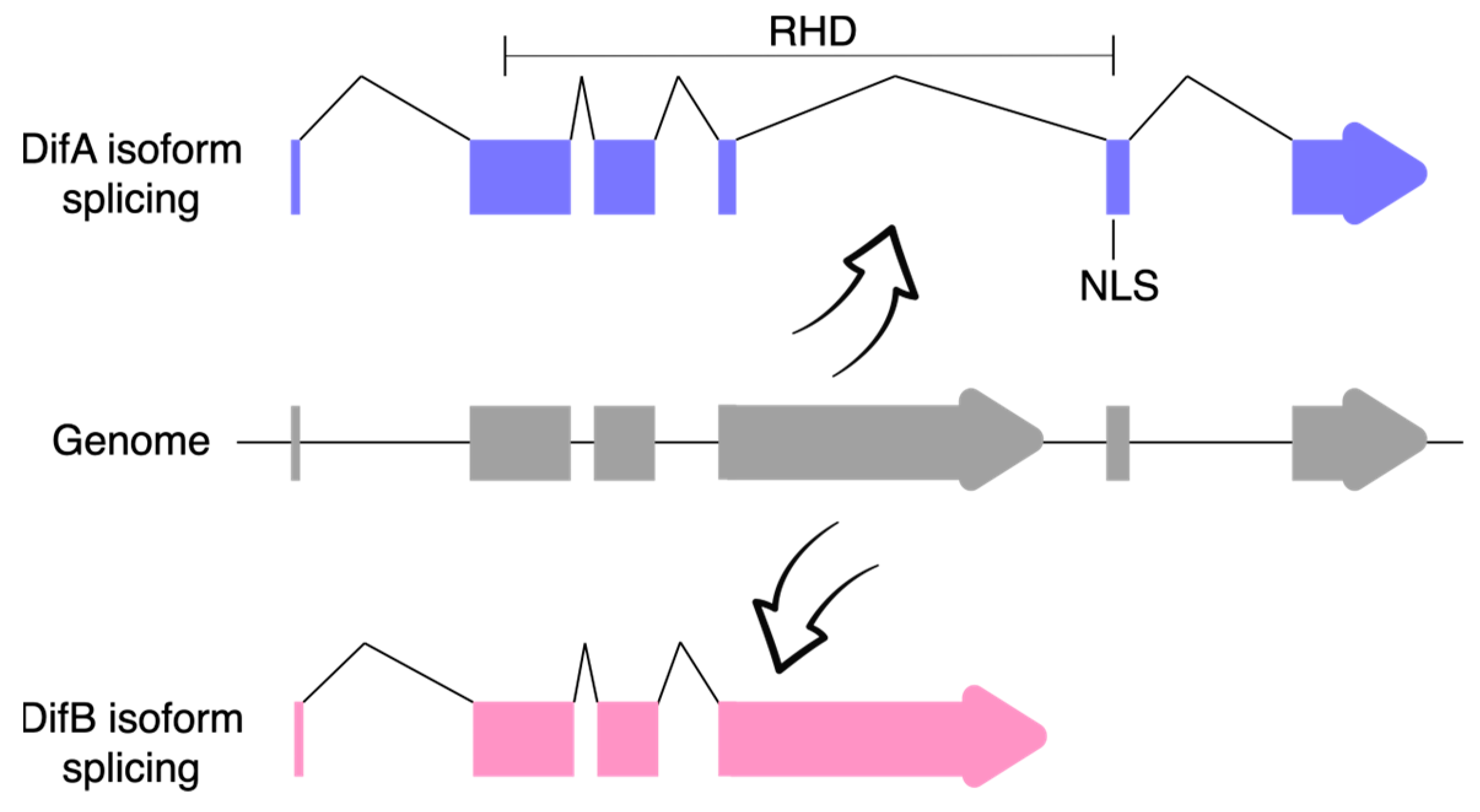
- Zhou, B.; Lindsay, S.A.; Wasserman, S.A. Alternative NF-ΚB Isoforms in the Drosophila Neuromuscular Junction and Brain. PLoS ONE 2015, 10, e0132793. [Google Scholar] [CrossRef] [PubMed]
- Heckscher, E.S.; Fetter, R.D.; Marek, K.W.; Albin, S.D.; Davis, G.W. NF-KappaB, IkappaB, and IRAK Control Glutamate Receptor Density at the Drosophila NMJ. Neuron 2007, 55, 859–873. [Google Scholar] [CrossRef]
- Guan, B.; Hartmann, B.; Kho, Y.-H.; Gorczyca, M.; Budnik, V. The Drosophila Tumor Suppressor Gene, Dlg, Is Involved in Structural Plasticity at a Glutamatergic Synapse. Curr. Biol. CB 1996, 6, 695–706. [Google Scholar] [CrossRef]
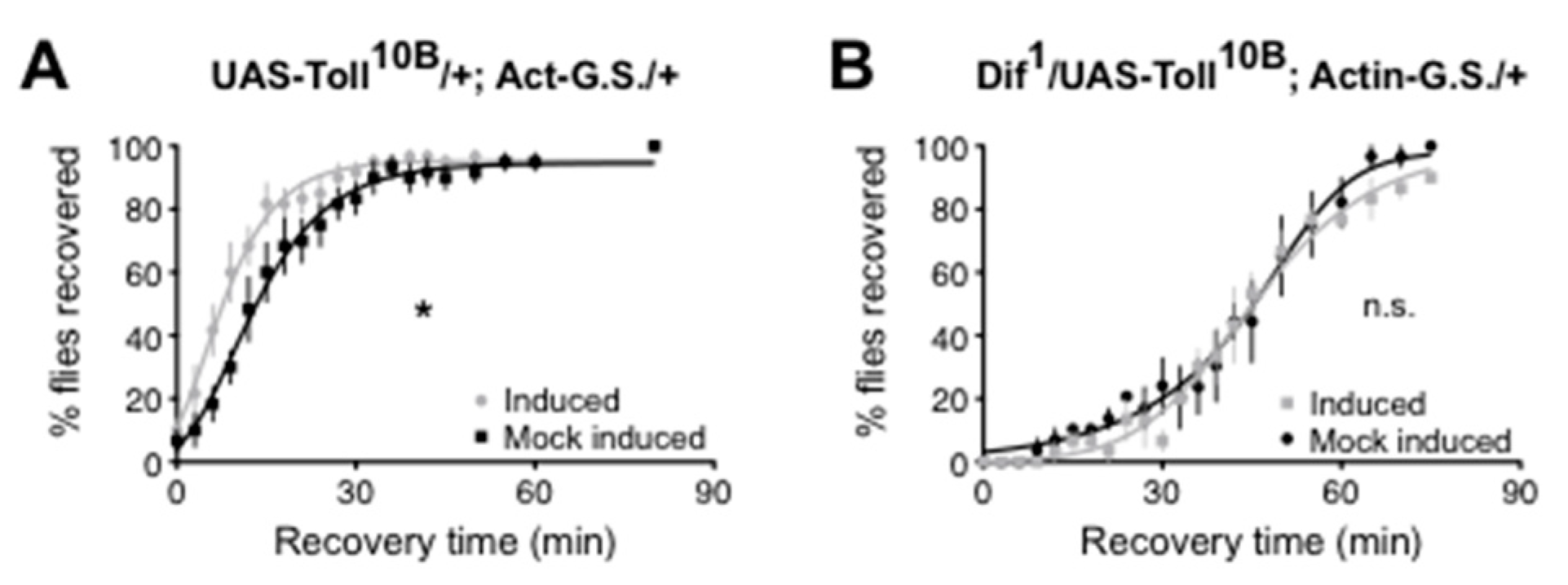
- Wijesekera, T.P.; Wu, Z.; Stephens, N.P.; Godula, R.; Lew, L.K.; Atkinson, N.S. A Non-Nuclear NF-ΚB Modulates Alcohol Sensitivity But Not Immunity. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 3329–3343. [Google Scholar] [CrossRef]
- Bland, M.L. Regulating Metabolism to Shape Immune Function: Lessons from Drosophila. Semin. Cell Dev. Biol. 2022, 138, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Khanuja, B.S.; Ip, Y.T. Toll Receptor-Mediated Drosophila Immune Response Requires Dif, an NF-ΚB Factor. Genes Dev. 1999, 13, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Nighorn, A.; Healy, M.; Davis, R. The Cyclic AMP Phosphodiesterase Encoded by the Drosophila Dunce Gene Is Concentrated in the Mushroom Body Neuropil. Neuron 1991, 6, 455–467. [Google Scholar] [CrossRef]
- Park, A.; Tran, T.; Atkinson, N.S. Monitoring Food Preference in Drosophila by Oligonucleotide Tagging. Proc. Natl. Acad. Sci. USA 2018, 115, 9020–9025. [Google Scholar] [CrossRef]
- Park, A.; Ghezzi, A.; Wijesekera, T.P.; Atkinson, N.S. Genetics and Genomics of Alcohol Responses in Drosophila. Neuropharmacology 2017, 122, 22–35. [Google Scholar] [CrossRef]
- Scaplen, K.M.; Talay, M.; Nunez, K.M.; Salamon, S.; Waterman, A.G.; Gang, S.; Song, S.L.; Barnea, G.; Kaun, K.R. Circuits That Encode and Guide Alcohol-Associated Preference. eLife 2020, 9, e48730. [Google Scholar] [CrossRef]
- Kaun, K.R.; Azanchi, R.; Maung, Z.; Hirsh, J.; Heberlein, U. A Drosophila Model for Alcohol Reward. Nat. Neurosci. 2011, 14, 612–619. [Google Scholar] [CrossRef]
- Butts, A.R.; Ojelade, S.A.; Pronovost, E.D.; Seguin, A.; Merrill, C.B.; Rodan, A.R.; Rothenfluh, A. Altered Actin Filament Dynamics in the Drosophila Mushroom Bodies Lead to Fast Acquisition of Alcohol Consumption Preference. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 8877–8884. [Google Scholar] [CrossRef]
- Engel, G.L.; Marella, S.; Kaun, K.R.; Wu, J.; Adhikari, P.; Kong, E.C.; Wolf, F.W. Sir2/Sirt1 Links Acute Inebriation to Presynaptic Changes and the Development of Alcohol Tolerance, Preference, and Reward. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 5241–5251. [Google Scholar] [CrossRef]
- Davie, K.; Janssens, J.; Koldere, D.; De Waegeneer, M.; Pech, U.; Kreft, Ł.; Aibar, S.; Makhzami, S.; Christiaens, V.; Bravo González-Blas, C.; et al. A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell 2018, 174, 982–998.e20. [Google Scholar] [CrossRef]
- Strausfeld, N.J. Atlas of an Insect Brain; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1976. [Google Scholar]
- Bullock, T.; Horridge, G.A. Structure and Function in the Nervous Systems of Invertebrates; W. H. Freeman a. Co., Ltd.: New York, NY, USA; San Francisco, CA, USA; London, UK, 1965; Volume II. [Google Scholar]
- Xie, M.-X.; Zhang, X.-L.; Xu, J.; Zeng, W.-A.; Li, D.; Xu, T.; Pang, R.-P.; Ma, K.; Liu, X.-G. Nuclear Factor-KappaB Gates Nav1.7 Channels in DRG Neurons via Protein-Protein Interaction. iScience 2019, 19, 623–633. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkinson, N.S. The Role of Toll and Nonnuclear NF-κB Signaling in the Response to Alcohol. Cells 2023, 12, 1508. https://doi.org/10.3390/cells12111508
Atkinson NS. The Role of Toll and Nonnuclear NF-κB Signaling in the Response to Alcohol. Cells. 2023; 12(11):1508. https://doi.org/10.3390/cells12111508
Chicago/Turabian StyleAtkinson, Nigel S. 2023. "The Role of Toll and Nonnuclear NF-κB Signaling in the Response to Alcohol" Cells 12, no. 11: 1508. https://doi.org/10.3390/cells12111508
APA StyleAtkinson, N. S. (2023). The Role of Toll and Nonnuclear NF-κB Signaling in the Response to Alcohol. Cells, 12(11), 1508. https://doi.org/10.3390/cells12111508





