The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis
Abstract
1. Introduction
2. T Cells
2.1. CD4+ T Cells
2.1.1. Th1 and Th2 Cells
2.1.2. Th9 Cells
2.1.3. Th17 Cells
2.1.4. Th22 Cells
2.1.5. TFH Cells
2.1.6. Treg Cells
2.2. CD8+ T Cells
3. B Cells
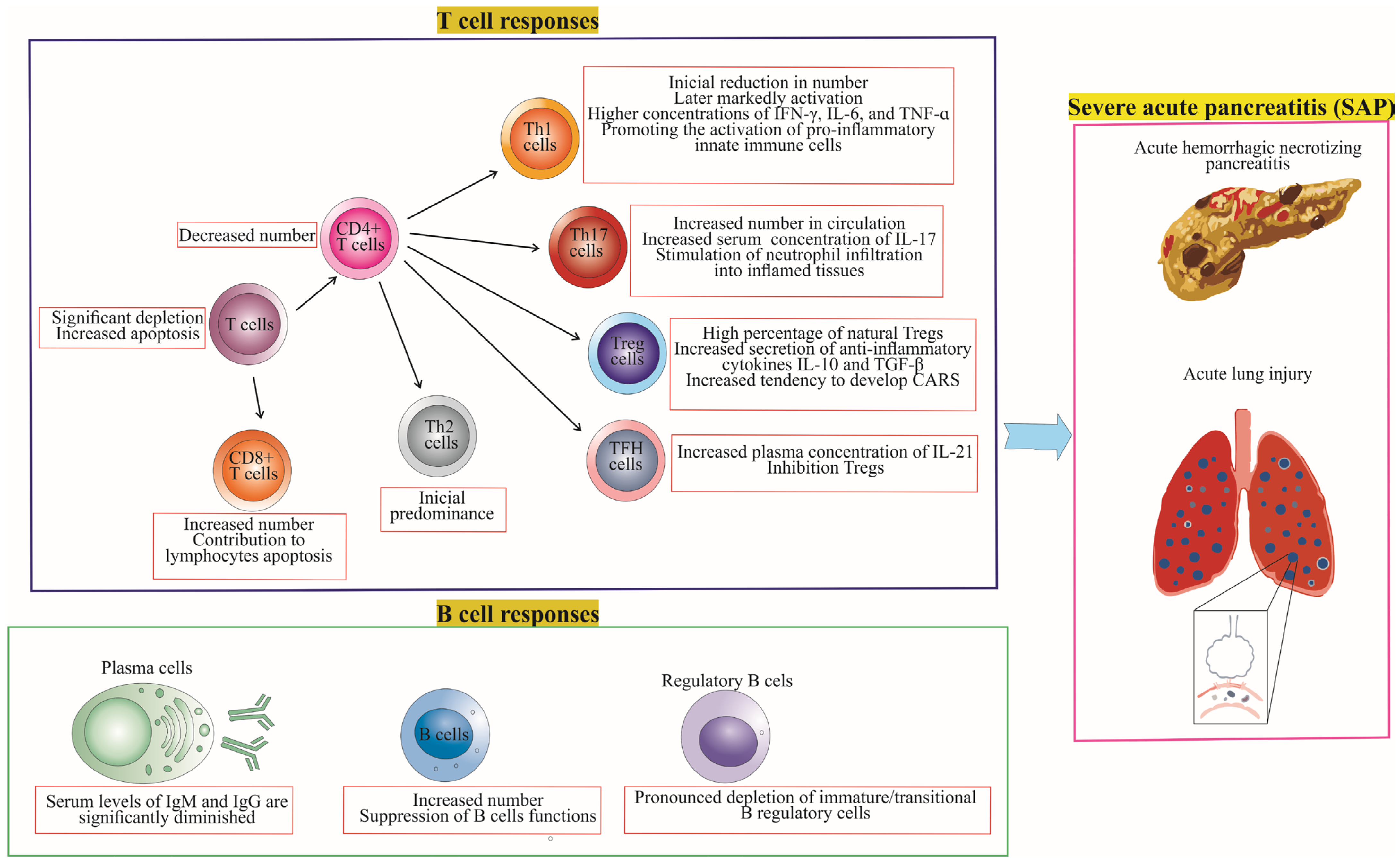
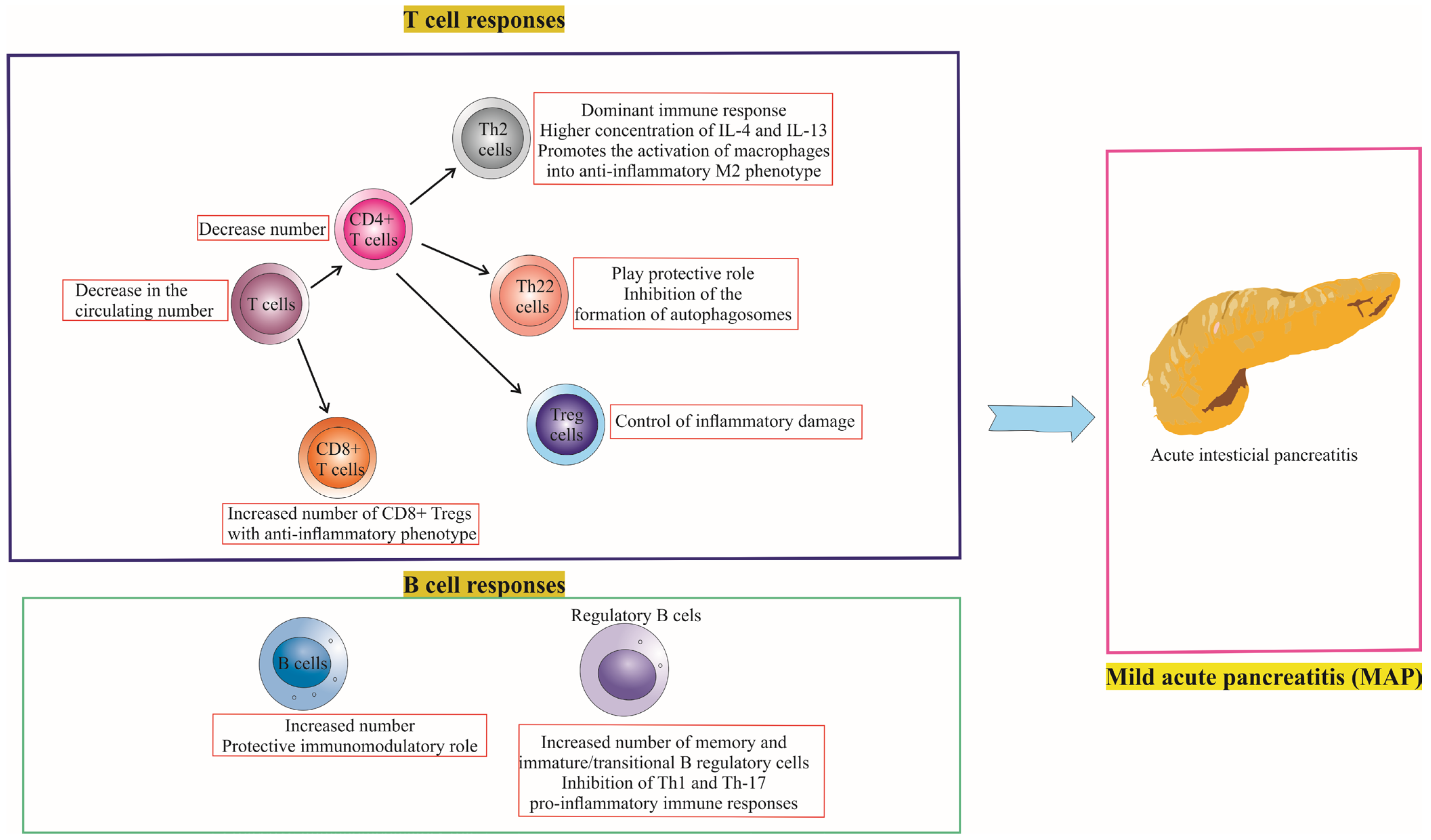
4. Differences in T Cell and B Cell Responses between Mild Acute Pancreatitis (MAP) and Severe Acute Pancreatitis (SAP)
4.1. T Cell Responses in MAP vs. SAP
4.2. B Cell Responses in MAP vs. SAP
5. Potential Therapeutic Strategies and Future Directions
5.1. Targeted Immunotherapies
5.2. Personalized Treatment Strategies for MAP and SAP
5.3. Combination Therapies and Immune Modulation
5.4. Addressing Knowledge Gaps and Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walkowska, J.; Zielinska, N.; Tubbs, R.S.; Podgórski, M.; Dłubek-Ruxer, J.; Olewnik, Ł. Diagnosis and Treatment of Acute Pancreatitis. Diagnostics 2022, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-J.; Ke, J.; Chen, F.; Zhang, J.-Y.; Zhang, C.; Chen, H.-Y. Effect of SNHG11/miR-7-5p/PLCB1 Axis on Acute Pancreatitis through Inhibiting p38MAPK Pathway. Cells 2023, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G. Acute pancreatitis. Semin. Diagn. Pathol. 2004, 21, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Leonard-Murali, S.; Lezotte, J.; Kalu, R.; Blyden, D.J.; Patton, J.H.; Johnson, J.L.; Gupta, A.H. Necrotizing Pancreatitis: A Review for the Acute Care Surgeon. Am. J. Surg. 2021, 221, 927–934. [Google Scholar] [CrossRef]
- Garg, P.K.; Singh, V.P. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology 2019, 156, 2008–2023. [Google Scholar] [CrossRef]
- Peng, C.; Li, Z.; Yu, X. The Role of Pancreatic Infiltrating Innate Immune Cells in Acute Pancreatitis. Int. J. Med. Sci. 2021, 18, 534–545. [Google Scholar] [CrossRef]
- Ding, L.; Yang, Y.; Li, H.; Wang, H.; Gao, P. Circulating Lymphocyte Subsets Induce Secondary Infection in Acute Pancreatitis. Front. Cell. Infect. Microbiol. 2020, 10, 128. [Google Scholar] [CrossRef]
- Kang, R.; Lotze, M.T.; Zeh, H.J.; Billiar, T.R.; Tang, D. Cell Death and DAMPs in Acute Pancreatitis. Mol. Med. 2014, 20, 466–477. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, X.; Xia, S.; Guo, F.; Pan, C.; Xiang, H.; Shang, D. T Lymphocytes: A Promising Immunotherapeutic Target for Pancreatitis and Pancreatic Cancer? Front. Oncol. 2020, 10, 382. [Google Scholar] [CrossRef]
- Brady, J.; Horie, S.; Laffey, J.G. Role of the adaptive immune response in sepsis. Intensive Care Med. Exp. 2020, 8, 20. [Google Scholar] [CrossRef]
- Fang, P.; Li, X.; Dai, J.; Cole, L.; Camacho, J.A.; Zhang, Y.; Ji, Y.; Wang, J.; Yang, X.-F.; Wang, H. Immune cell subset differentiation and tissue inflammation. J. Hematol. Oncol. 2018, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Maldini, M.; Morselli-Labate, A.M.; Barakat, B.; Romboli, E.; Beltrandi, E.; Migliori, M.; Tomassetti, P.; Corinaldesi, R. Early activation of peripheral lymphocytes in human acute pancreatitis. J. Clin. Gastroenterol. 2003, 36, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, Y.; Takas, K.; Ueda, T.; Hori, Y.; Goshima, M.; Kuroda, Y. Peripheral lymphocyte reduction in severe acute pancreatitis is caused by apoptotic cell death. J. Gastrointest. Surg. 2000, 4, 379–387. [Google Scholar] [CrossRef]
- Wei, X.; Yao, W.; Li, H.; Qian, J.; Xie, Y.; Zhang, Z.; Lu, H.; Shi, L.; Lin, X. B and NK Cells Closely Correlate with the Condition of Patients with Acute Pancreatitis. Gastroenterol. Res. Pract. 2019, 2019, 7568410. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takeyama, Y.; Yasuda, T.; Shinzeki, M.; Sawa, H.; Nakajima, T.; Ajiki, T.; Fujino, Y.; Suzuki, Y.; Kuroda, Y. Immunosuppression in patients with severe acute pancreatitis. J. Gastroenterol. 2006, 41, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.J.; Kell, M.R.; Coates, C.; Murphy, T.; Reynolds, J.V. Serum antigen(s) drive the proinflammatory T cell response in acute pancreatitis. Br. J. Surg. 2003, 90, 313–319. [Google Scholar] [CrossRef]
- Pietruczuk, M.; Dabrowska, M.I.; Wereszczynska-Siemiatkowska, U.; Dabrowski, A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J. Gastroenterol. 2006, 12, 5344–5351. [Google Scholar] [CrossRef]
- Zhang, X.P.; Chen, H.Q.; Liu, F.; Zhang, J. Advances in researches on the immune dysregulation and therapy of severe acute pancreatitis. J. Zhejiang Univ. Sci. B 2009, 10, 493–498. [Google Scholar] [CrossRef]
- Zheng, L.; Xue, J.; Jaffee, E.M.; Habtezion, A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013, 144, 1230–1240. [Google Scholar] [CrossRef]
- Blom, B.; Spits, H. Development of human lymphoid cells. Annu. Rev. Immunol. 2006, 24, 287–320. [Google Scholar] [CrossRef]
- Curley, P.J.; McMahon, M.J.; Lancaster, F.; Banks, R.E.; Barclay, G.R.; Shefta, J.; Boylston, A.W.; Whicher, J.T. Reduction in circulating levels of CD4-positive lymphocytes in acute pancreatitis: Relationship to endotoxin, interleukin 6 and disease severity. Br. J. Surg. 1993, 80, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Demols, A.; Le Moine, O.; Desalle, F.; Quertinmont, E.; Van Laethem, J.L.; Devière, J. CD4(+) T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology 2000, 118, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yang, F.; Huang, H.; Du, Y.; Chen, Y.; Wang, M.; Zhu, D.; Yue, X.; Wang, L. A reduced lymphocyte ratio as an early marker for predicting acute pancreatitis. Sci. Rep. 2017, 7, 44087. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, J.; Ke, L.; Zou, L.; Li, B.; Tong, Z.; Li, W.; Li, N.; Li, J. Reduced lymphocyte count as an early marker for predicting infected pancreatic necrosis. BMC Gastroenterol. 2015, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Pinhu, L.; Qin, Y.; Xiong, B.; You, Y.; Li, J.; Sooranna, S.R. Overexpression of Fas and FasL is associated with infectious complications and severity of experimental severe acute pancreatitis by promoting apoptosis of lymphocytes. Inflammation 2014, 37, 1202–1212. [Google Scholar] [CrossRef]
- Qin, Y.; Pinhu, L.; You, Y.; Sooranna, S.; Huang, Z.; Zhou, X.; Yin, Y.; Song, S. The role of Fas expression on the occurrence of immunosuppression in severe acute pancreatitis. Dig. Dis. Sci. 2013, 58, 3300–3307. [Google Scholar] [CrossRef]
- Krummel, M.F.; Bartumeus, F.; Gérard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 2016, 16, 193–201. [Google Scholar] [CrossRef]
- Takeda, A.; Sasaki, N.; Miyasaka, M. The molecular cues regulating immune cell trafficking. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 183–195. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Shi, C.; Hou, C.; Zhu, X.; Peng, Y.; Guo, F.; Zhang, K.; Huang, D.; Li, Q.; Miao, Y. New Predictor of Organ Failure in Acute Pancreatitis: CD4+ T Lymphocytes and CD19+ B Lymphocytes. Biomed. Res. Int. 2018, 2018, 1012584. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Dong, L.; Yang, C.; Gou, S.; Yin, T.; Wu, H.; Wang, C. The Reduction of Peripheral Blood CD4+ T Cell Indicates Persistent Organ Failure in Acute Pancreatitis. PLoS ONE 2015, 10, e0125529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Cai, Z.; Zhao, P.; Peng, C.; Zhao, L.; Wan, C. The Decrease of Peripheral Blood CD4+ T Cells Indicates Abdominal Compartment Syndrome in Severe Acute Pancreatitis. PLoS ONE 2015, 10, e0135768. [Google Scholar] [CrossRef] [PubMed]
- Papavramidis, T.S.; Marinis, A.D.; Pliakos, I.; Kesisoglou, I.; Papavramidou, N. Abdominal compartment syndrome—Intra-abdominal hypertension: Defining, diagnosing, and managing. J. Emergencies Trauma Shock 2011, 4, 279–291. [Google Scholar] [CrossRef]
- Zarnescu, N.O.; Dumitrascu, I.; Zarnescu, E.C.; Costea, R. Abdominal Compartment Syndrome in Acute Pancreatitis: A Narrative Review. Diagnostics 2023, 13, 1. [Google Scholar] [CrossRef]
- Qiao, S.F.; Lu, T.J.; Sun, J.B.; Li, F. Alterations of intestinal immune function and regulatory effects of L-arginine in experimental severe acute pancreatitis rats. World J. Gastroenterol. 2005, 11, 6216–6218. [Google Scholar] [CrossRef]
- Lee, G.R. Molecular Mechanisms of T Helper Cell Differentiation and Functional Specialization. Immune Netw. 2023, 23, e4. [Google Scholar] [CrossRef]
- Khantakova, J.N.; Bulygin, A.S.; Sennikov, S.V. The Regulatory-T-Cell Memory Phenotype: What We Know. Cells 2022, 11, 1687. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect Biol. 2019, 11, a028480. [Google Scholar] [CrossRef]
- Allen, J.E.; Sutherland, T.E. Host protective roles of type 2 immunity: Parasite killing and tissue repair, flip sides of the same coin. Semin. Immunol. 2014, 26, 329–340. [Google Scholar] [CrossRef]
- Jiang, D.L.; Yang, J.; Jiang, S.Y.; Yuan, F.L.; Gu, Y.L.; Li, J.P.; Pei, Z.J. Modified Da Chengqi granules improvement in immune function in early severe acute pancreatitis patients. Genet. Mol. Res. 2016, 15, gmr.15028787. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nicolas, A.; Martínez-Chamorro, A.; Jiménez, P.; Matas-Cobos, A.M.; Redondo-Cerezo, E.; Ruiz-Cabello, F. TH1 and TH2 Cytokine Profiles as Predictors of Severity in Acute Pancreatitis. Pancreas 2018, 47, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sendler, M.; van den Brandt, C.; Glaubitz, J.; Wilden, A.; Golchert, J.; Weiss, F.U.; Homuth, G.; De Freitas Chama, L.L.; Mishra, N.; Mahajan, U.M.; et al. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology 2020, 158, 253–269.e214. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. How diverse—CD4 effector T cells and their functions. J. Mol. Cell. Biol. 2009, 1, 20–36. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Hufford, M.M.; Olson, M.R. The development and in vivo function of T helper 9 cells. Nat. Rev. Immunol. 2015, 15, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Verma, A.K.; Venkateshaiah, S.U.; Mishra, A. Role of eosinophils in the initiation and progression of pancreatitis pathogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G211–G222. [Google Scholar] [CrossRef] [PubMed]
- Meriläinen, S.; Mäkelä, J.; Jensen, H.A.; Dahlbacka, S.; Lehtonen, S.; Karhu, T.; Herzig, K.H.; Kröger, M.; Koivukangas, V.; Koskenkari, J.; et al. Portal vein cytokines in the early phase of acute experimental oedematous and necrotizing porcine pancreatitis. Scand. J. Gastroenterol. 2012, 47, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.M. Biology of Interleukin-17 and Its Pathophysiological Significance in Sepsis. Front. Immunol. 2020, 11, 1558. [Google Scholar] [CrossRef]
- Jia, R.; Tang, M.; Qiu, L.; Sun, R.; Cheng, L.; Ma, X.; Yin, G.; Hu, G.; Wang, X.; Zhao, Y. Increased interleukin-23/17 axis and C-reactive protein are associated with severity of acute pancreatitis in patients. Pancreas 2015, 44, 321–325. [Google Scholar] [CrossRef]
- Ni, J.; Hu, G.; Xiong, J.; Shen, J.; Shen, J.; Yang, L.; Tang, M.; Zhao, Y.; Ying, G.; Yu, G.; et al. Involvement of interleukin-17A in pancreatic damage in rat experimental acute necrotizing pancreatitis. Inflammation 2013, 36, 53–65. [Google Scholar] [CrossRef]
- Wang, D.; Tang, M.; Zong, P.; Liu, H.; Zhang, T.; Liu, Y.; Zhao, Y. MiRNA-155 Regulates the Th17/Treg Ratio by Targeting SOCS1 in Severe Acute Pancreatitis. Front. Physiol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Z.; Tang, D.; Zhang, J. Th17/Treg imbalance in patients with severe acute pancreatitis: Attenuated by high-volume hemofiltration treatment. Medicine 2020, 99, e21491. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, H.; Liu, L.; Xiao, P.; Xie, Y.; Geng, X.; Zhang, T.; Zhang, Y.; Lu, T.; Tan, H.; et al. Role of Interleukin-17 in Acute Pancreatitis. Front. Immunol. 2021, 12, 674803. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Xiao, F.; Xie, J.; Wang, S.; Lu, L.; Cui, D. Role of Th22 Cells in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 688066. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, C. The biology and functions of Th22 cells. Adv. Exp. Med. Biol. 2014, 841, 209–230. [Google Scholar] [CrossRef]
- Vasseur, P.; Devaure, I.; Sellier, J.; Delwail, A.; Chagneau-Derrode, C.; Charier, F.; Tougeron, D.; Tasu, J.P.; Rabeony, H.; Lecron, J.C.; et al. High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology 2014, 14, 465–469. [Google Scholar] [CrossRef]
- Feng, D.; Park, O.; Radaeva, S.; Wang, H.; Yin, S.; Kong, X.; Zheng, M.; Zakhari, S.; Kolls, J.K.; Gao, B. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int. J. Biol. Sci. 2012, 8, 249–257. [Google Scholar] [CrossRef]
- Huai, J.P.; Sun, X.C.; Chen, M.J.; Jin, Y.; Ye, X.H.; Wu, J.S.; Huang, Z.M. Melatonin attenuates acute pancreatitis-associated lung injury in rats by modulating interleukin 22. World J. Gastroenterol. 2012, 18, 5122–5128. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Qiao, Y.Y.; Liu, X.Q.; Xu, C.Q.; Zhang, Z.; Xu, H.W. Interleukin-22 ameliorates acute severe pancreatitis-associated lung injury in mice. World J. Gastroenterol. 2016, 22, 5023–5032. [Google Scholar] [CrossRef]
- Xue, J.; Nguyen, D.T.; Habtezion, A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology 2012, 143, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Song, W.; Craft, J. T follicular helper cell heterogeneity: Time, space, and function. Immunol. Rev. 2019, 288, 85–96. [Google Scholar] [CrossRef]
- Shi, J.; Hou, S.; Fang, Q.; Liu, X.; Liu, X.; Qi, H. PD-1 Controls Follicular T Helper Cell Positioning and Function. Immunity 2018, 49, 264–274.e264. [Google Scholar] [CrossRef] [PubMed]
- Law, H.; Venturi, V.; Kelleher, A.; Munier, C.M.L. Tfh Cells in Health and Immunity: Potential Targets for Systems Biology Approaches to Vaccination. Int. J. Mol. Sci. 2020, 21, 8524. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, M.P.; Salek-Ardakani, S.; Fousteri, G. Editorial: Follicular Helper T Cells in Immunity and Autoimmunity. Front. Immunol. 2020, 11, 1042. [Google Scholar] [CrossRef]
- Thomson, J.E.; Nweke, E.E.; Brand, M.; Nel, M.; Candy, G.P.; Fonteh, P.N. Transient Expression of Interleukin-21 in the Second Hit of Acute Pancreatitis May Potentiate Immune Paresis in Severe Acute Pancreatitis. Pancreas 2019, 48, 107–112. [Google Scholar] [CrossRef]
- Linnebacher, A.; Mayer, P.; Marnet, N.; Bergmann, F.; Herpel, E.; Revia, S.; Yin, L.; Liu, L.; Hackert, T.; Giese, T.; et al. Interleukin 21 Receptor/Ligand Interaction Is Linked to Disease Progression in Pancreatic Cancer. Cells 2019, 8, 1104. [Google Scholar] [CrossRef]
- Vogelzang, A.; McGuire, H.M.; Liu, S.M.; Gloss, B.; Mercado, K.; Earls, P.; Dinger, M.E.; Batten, M.; Sprent, J.; King, C. IL-21 contributes to fatal inflammatory disease in the absence of Foxp3+ T regulatory cells. J. Immunol. 2014, 192, 1404–1414. [Google Scholar] [CrossRef]
- Rocamora-Reverte, L.; Melzer, F.L.; Würzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020, 11, 616949. [Google Scholar] [CrossRef]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccines Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kudo, M.; Strober, W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017, 10, 283–298. [Google Scholar] [CrossRef]
- Li, J.P.; Yang, J.; Huang, J.R.; Jiang, D.L.; Zhang, F.; Liu, M.F.; Qiang, Y.; Gu, Y.L. Immunosuppression and the infection caused by gut mucosal barrier dysfunction in patients with early severe acute pancreatitis. Front. Biosci. (Landmark Ed.) 2013, 18, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, Q.X.; Shan, H.W.; Li, W.F.; Lin, Z.F. Prognostic value of CD4(+)CD25(+) Tregs as a valuable biomarker for patients with sepsis in ICU. World J. Emerg. Med. 2015, 6, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.S.; Wu, Z.S.; Zhang, L.Y.; Ke, L.; Li, W.Q.; Li, N.; Li, J.S. Nicotine ameliorates experimental severe acute pancreatitis via enhancing immunoregulation of CD4+ CD25+ regulatory T cells. Pancreas 2015, 44, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Minkov, G.A.; Yovtchev, Y.P.; Halacheva, K.S. Increased Circulating CD4+CD25+CD127low/neg Regulatory T-cells as a Prognostic Biomarker in Acute Pancreatitis. Pancreas 2017, 46, 1003–1010. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, H.P.; Wang, H.P.; Zhu, L.X.; Geng, X.P. CD4+CD25+CD127 high cells as a negative predictor of multiple organ failure in acute pancreatitis. World J. Emerg. Surg. 2017, 12, 7. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.; Stojanovic, B.S.; Stojanovic, M.D.; Gajovic, N.; Radosavljevic, G.; Pantic, J.; Arsenijevic, N.; Lukic, M.L. Deletion of Galectin-3 attenuates acute pancreatitis in mice by affecting activation of innate inflammatory cells. Eur. J. Immunol. 2019, 49, 940–946. [Google Scholar] [CrossRef]
- Glaubitz, J.; Wilden, A.; Frost, F.; Ameling, S.; Homuth, G.; Mazloum, H.; Rühlemann, M.C.; Bang, C.; Aghdassi, A.A.; Budde, C.; et al. Activated regulatory T-cells promote duodenal bacterial translocation into necrotic areas in severe acute pancreatitis. Gut 2023, gutjnl-2022-327448. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Uehara, S.; Gothoh, K.; Handa, H.; Tomita, H.; Tomita, Y. Immune function in patients with acute pancreatitis. J. Gastroenterol. Hepatol. 2003, 18, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, A.; Osada, J.; Dabrowska, M.I.; Wereszczynska-Siemiatkowska, U. Monocyte subsets and natural killer cells in acute pancreatitis. Pancreatology 2008, 8, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Churlaud, G.; Pitoiset, F.; Jebbawi, F.; Lorenzon, R.; Bellier, B.; Rosenzwajg, M.; Klatzmann, D. Human and Mouse CD8(+)CD25(+)FOXP3(+) Regulatory T Cells at Steady State and during Interleukin-2 Therapy. Front. Immunol. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Vieyra-Lobato, M.R.; Vela-Ojeda, J.; Montiel-Cervantes, L.; López-Santiago, R.; Moreno-Lafont, M.C. Description of CD8(+) Regulatory T Lymphocytes and Their Specific Intervention in Graft-versus-Host and Infectious Diseases, Autoimmunity, and Cancer. J. Immunol. Res. 2018, 2018, 3758713. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sachdev, V.; Singh, N.; Bhardwaj, P.; Pal, A.; Kapur, S.; Saraya, A. Alterations in intestinal permeability and endotoxemia in severe acute pancreatitis. Trop. Gastroenterol. 2012, 33, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Laine, V.J.O.; Rau, B.; Hotz, H.G.; Foitzik, T.; Nevalainen, T.J.; Beger, H.G. Systemic Lymphocyte Activation Modulates the Severity of Diet-Induced Acute Pancreatitis in Mice. Pancreas 1999, 19, 62–68. [Google Scholar] [CrossRef]
- Chekol Abebe, E.; Asmamaw Dejenie, T.; Mengie Ayele, T.; Dagnew Baye, N.; Agegnehu Teshome, A.; Tilahun Muche, Z. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J. Inflamm. Res. 2021, 14, 75–84. [Google Scholar] [CrossRef]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013, 5, 173ra123. [Google Scholar] [CrossRef]
- Iwata, Y.; Matsushita, T.; Horikawa, M.; Dilillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011, 117, 530–541. [Google Scholar] [CrossRef]
- Oleinika, K.; Mauri, C.; Salama, A.D. Effector and regulatory B cells in immune-mediated kidney disease. Nat. Rev. Nephrol. 2019, 15, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhou, Y.; Yu, Q.; Yu, J.; Li, Q.; Sun, R. Decreased levels of regulatory B cells in patients with acute pancreatitis: Association with the severity of the disease. Oncotarget 2018, 9, 36067–36082. [Google Scholar] [CrossRef] [PubMed]
- Pongratz, G.; Hochrinner, H.; Straub, R.H.; Lang, S.; Brünnler, T. B cell activating factor of the tumor necrosis factor family (BAFF) behaves as an acute phase reactant in acute pancreatitis. PLoS ONE 2013, 8, e54297. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, B.; Jovanovic, I.P.; Stojanovic, M.D.; Jovanovic, M.; Vekic, B.; Milosevic, B.; Cvetkovic, A.; Spasic, M.; Stojanovic, B.S. The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis. Cells 2023, 12, 1495. https://doi.org/10.3390/cells12111495
Stojanovic B, Jovanovic IP, Stojanovic MD, Jovanovic M, Vekic B, Milosevic B, Cvetkovic A, Spasic M, Stojanovic BS. The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis. Cells. 2023; 12(11):1495. https://doi.org/10.3390/cells12111495
Chicago/Turabian StyleStojanovic, Bojan, Ivan P. Jovanovic, Milica Dimitrijevic Stojanovic, Marina Jovanovic, Berislav Vekic, Bojan Milosevic, Aleksandar Cvetkovic, Marko Spasic, and Bojana S. Stojanovic. 2023. "The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis" Cells 12, no. 11: 1495. https://doi.org/10.3390/cells12111495
APA StyleStojanovic, B., Jovanovic, I. P., Stojanovic, M. D., Jovanovic, M., Vekic, B., Milosevic, B., Cvetkovic, A., Spasic, M., & Stojanovic, B. S. (2023). The Emerging Roles of the Adaptive Immune Response in Acute Pancreatitis. Cells, 12(11), 1495. https://doi.org/10.3390/cells12111495





