Fibroblast Activation Protein-Targeting Minibody-IRDye700DX for Ablation of the Cancer-Associated Fibroblast with Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
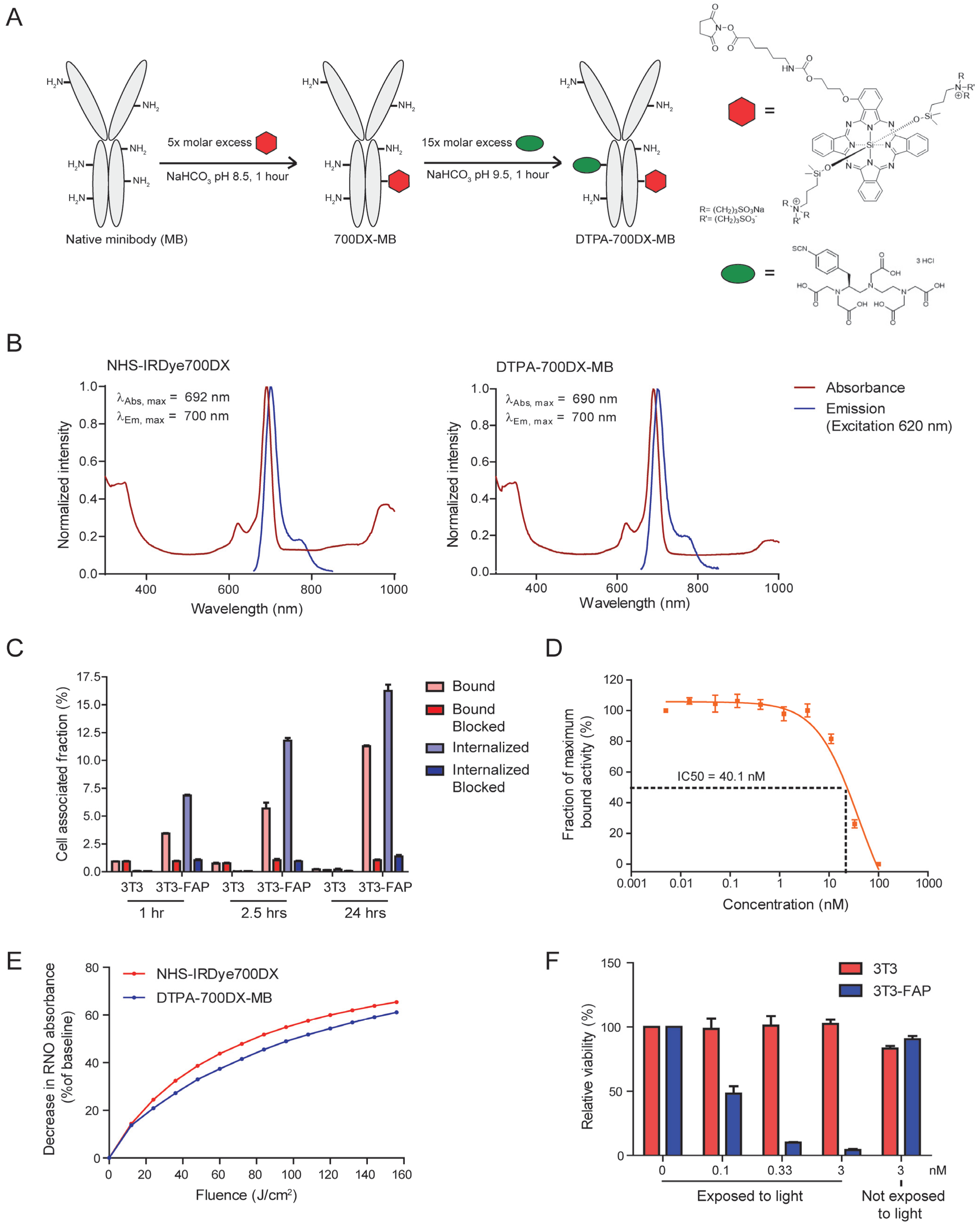
2.1. Minibody Conjugation and Characterization
2.2. Radiolabeling with 111In and Quality Control
2.3. Cell Culture
2.4. In Vitro Binding and Internalization of DTPA-700DX-MB
2.5. IC50 Determination
2.6. Singlet Oxygen Production
2.7. In Vitro Targeted Photodynamic Therapy with DTPA-700DX-MB
2.8. Animals
2.9. Biodistribution of 111In-Labelled DTPA-700DX-MB
2.10. MicroSPECT/CT
2.11. Autoradiography
2.12. In Vivo FAP-tPDT with DTPA-700DX-MB in the Subcutaneous PDAC299 Model
2.13. Histology and Immunohistochemistry
2.14. Automated Quantification Cleaved Caspase-3 IHC
2.15. Distribution Visualisation FAP IHC
2.16. Statistics
3. Results
3.1. DTPA-700DX-MB Binds to FAP-Expressing Cells and Causes Light-Induced Toxicity
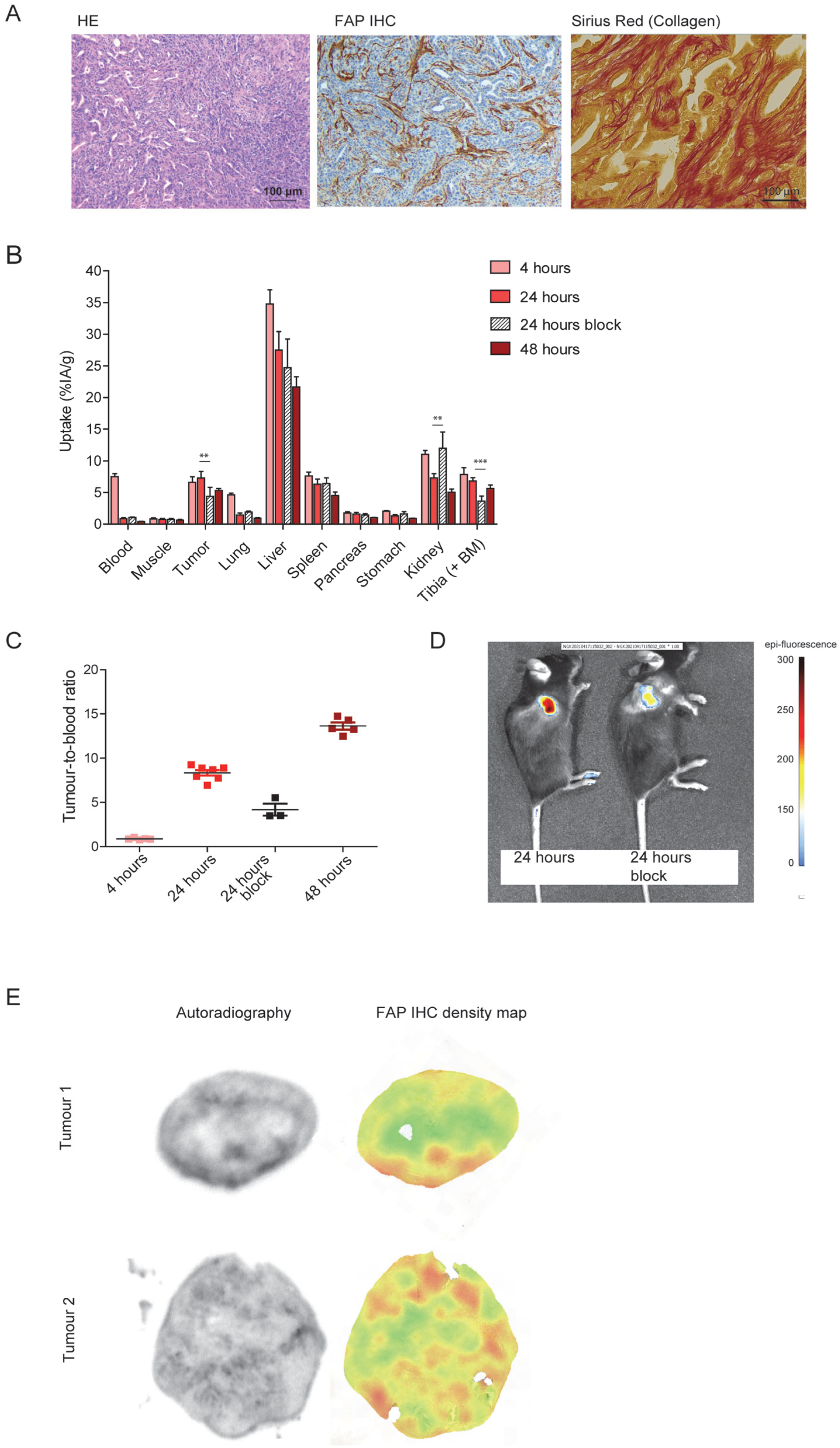
3.2. DTPA-700DX-MB Targets Subcutaneous PDAC299 Tumours In Vivo
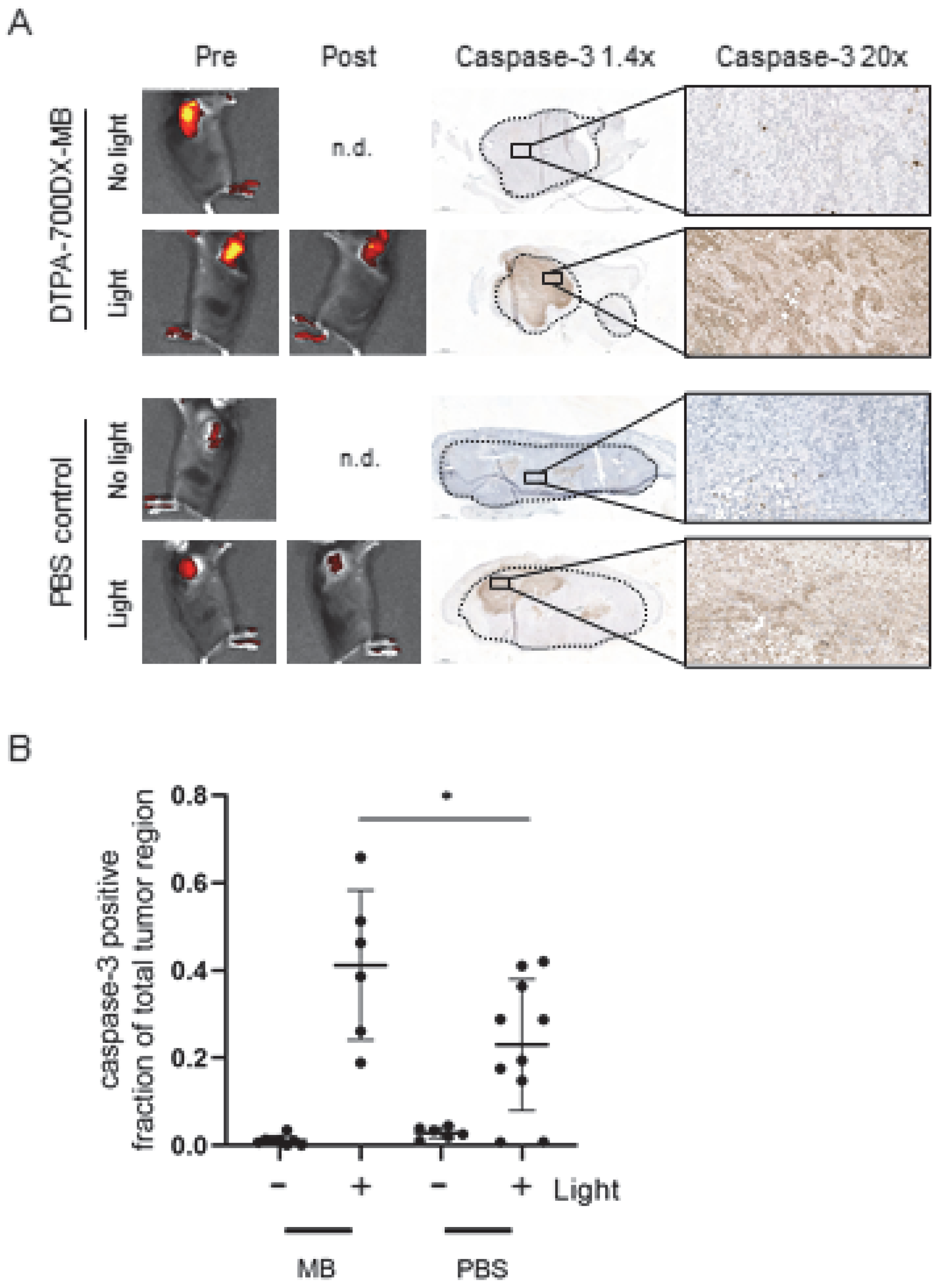
3.3. DTPA-700DX-MB Induces Cell Death in Subcutaneous PDAC299 Tumours In Vivo
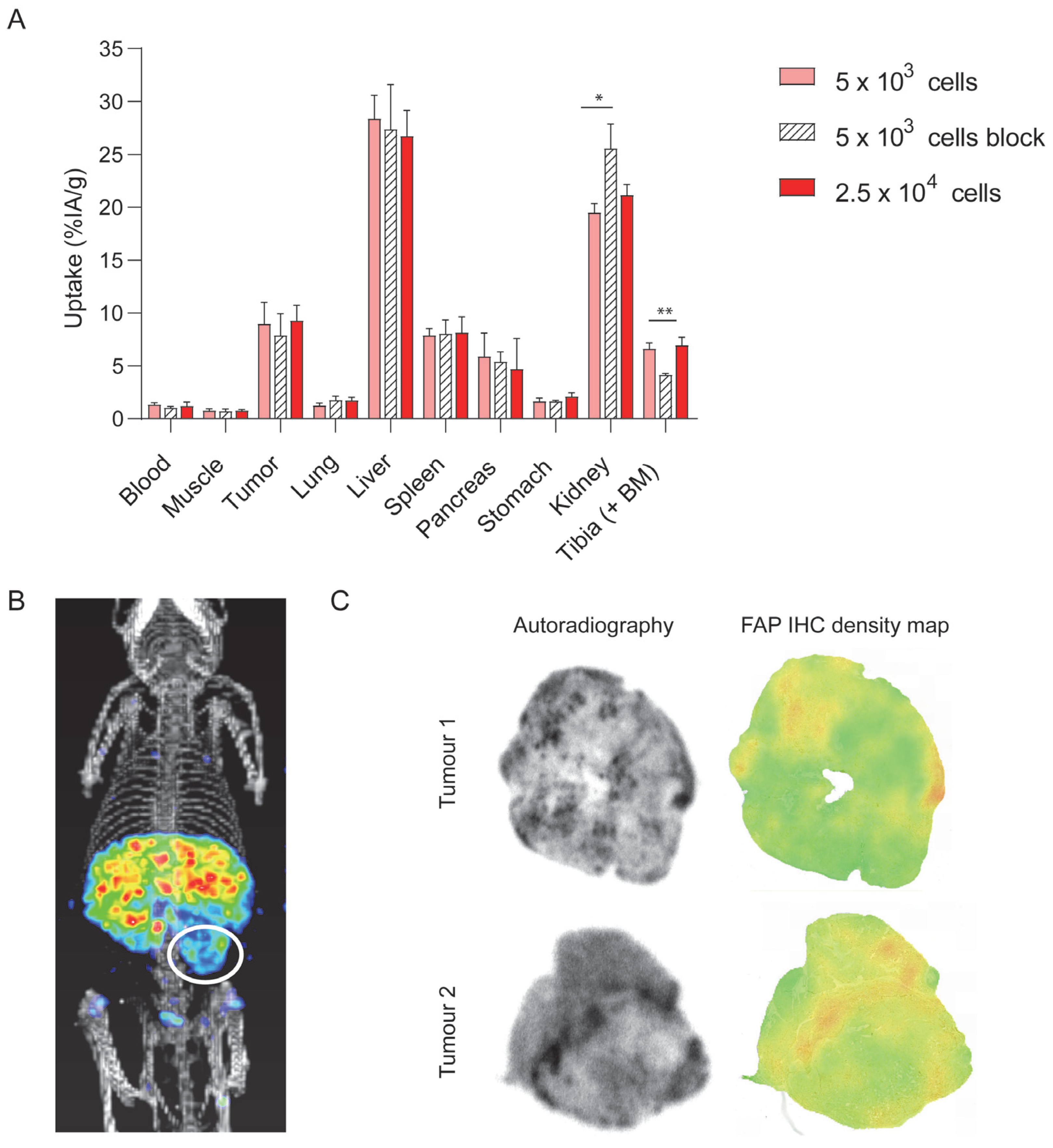
3.4. DTPA-700DX-MB Targets PDAC299 Orthotopic Tumours In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, T.; Huang, Y.; Simms, A.E.; Mazur, A. Fibroblast activation protein-alpha: A key modulator of the microenvironment in multiple pathologies. Int. Rev. Cell Mol. Biol. 2012, 297, 83–116. [Google Scholar]
- Cohen, S.J.; Alpaugh, R.K.; Palazzo, I.; Meropol, N.J.; Rogatko, A.; Xu, Z.; Hoffman, J.P.; Weiner, L.M.; Cheng, J.D. Fibroblast Activation Protein and Its Relationship to Clinical Outcome in Pancreatic Adenocarcinoma. Pancreas 2008, 37, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yu, D.-H.; Chen, Y.; Zhao, C.-Y.; Zhang, J.; Liu, Q.-H.; Ni, C.-R.; Zhu, M.-H. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J. Gastroenterol. 2012, 18, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.O.; Mullins, S.R.; Franco-Barraza, J.; Valianou, M.; Cukierman, E.; Cheng, J.D. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 2011, 11, 245. [Google Scholar] [CrossRef]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330, 827–830. [Google Scholar] [CrossRef]
- Altmann, A.; Haberkorn, U.A.; Siveke, J. The Latest Developments in Imaging of Fibroblast Activation Protein. J. Nucl. Med. 2020, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Imlimthan, S.; Moon, E.S.; Rathke, H.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals 2021, 14, 1023. [Google Scholar] [CrossRef]
- Santos, A.M.; Jung, J.; Aziz, N.; Kissil, J.L.; Puré, E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Investig. 2009, 119, 3613–3625. [Google Scholar] [CrossRef]
- Lee, J.; Fassnacht, M.; Nair, S.; Boczkowski, D.; Gilboa, E. Tumor Immunotherapy Targeting Fibroblast Activation Protein, a Product Expressed in Tumor-Associated Fibroblasts. Cancer Res. 2005, 65, 11156–11163. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, C.-T.; Ma, T.-T.; Li, Z.-Y.; Zhou, L.-N.; Mu, B.; Leng, F.; Shi, H.-S.; Li, Y.-O.; Wei, Y.-Q. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010, 101, 2325–2332. [Google Scholar] [CrossRef]
- Loeffler, M.; Krüger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64Cu- and 225Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Shon, Y.; Kim, J.; Oh, Y.-K. Selective Activation of Anticancer Chemotherapy by Cancer-Associated Fibroblasts in the Tumor Microenvironment. J. Natl. Cancer Inst. 2017, 109, djw186. [Google Scholar] [CrossRef] [PubMed]
- LeBeau, A.M.; Brennen, W.N.; Aggarwal, S.; Denmeade, S.R. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol. Cancer Ther. 2009, 8, 1378–1386. [Google Scholar] [CrossRef]
- Roy, J.; Hettiarachchi, S.U.; Kaake, M.; Mukkamala, R.; Low, P.S. Design and validation of fibroblast activation protein alpha targeted imaging and therapeutic agents. Theranostics 2020, 10, 5778–5789. [Google Scholar] [CrossRef]
- Fischer, E.; Chaitanya, K.; Wuest, T.; Wadle, A.; Scott, A.M.; van den Broek, M.; Schibli, R.; Bauer, S.; Renner, C. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin. Cancer Res. 2012, 18, 6208–6218. [Google Scholar] [CrossRef]
- Tran, E.; Chinnasamy, D.; Yu, Z.; Morgan, R.A.; Lee, C.-C.R.; Restifo, N.P.; Rosenberg, S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013, 210, 1125–1135. [Google Scholar] [CrossRef]
- Roberts, E.W.; Deonarine, A.; Jones, J.O.; Denton, A.E.; Feig, C.; Lyons, S.K.; Espeli, M.; Kraman, M.; McKenna, B.; Wells, R.J.B.; et al. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013, 210, 1137–1151. [Google Scholar] [CrossRef]
- Dolznig, H.; Schweifer, N.; Puri, C.; Kraut, N.; Rettig, W.J.; Kerjaschki, D.; Garin-Chesa, P. Characterization of cancer stroma markers: In silico analysis of an mRNA expression database for fibroblast activation protein and endosialin. Cancer Immun. 2005, 5, 10. [Google Scholar]
- Niedermeyer, J.; Garin-Chesa, P.; Kriz, M.; Hilberg, F.; Mueller, E.; Bamberger, U.; Schnapp, A. Expression of the fibroblast activation protein during mouse embryo development. Int. J. Dev. Biol. 2001, 45, 445–447. [Google Scholar]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell–selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Bernardo, M.; Saito, K.; Koyasu, S.; Mitchell, J.B.; Choyke, P.L.; Krishna, M.C. Evaluation of oxygen dependence on in vitro and in vivo cytotoxicity of photoimmunotherapy using IR-700–antibody conjugates. Free Radic. Biol. Med. 2015, 85, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Draney, D.R.; Volcheck, W.M.; Bashford, G.R.; Lamb, D.T.; Grone, D.L.; Zhang, Y.; Johnson, C.M. Phthalocyanine dye as an extremely photostable and highly fluorescent near-infrared labeling reagent. Opt. Mol. Probes Biomed. Appl. 2006, 6097, 113–124. [Google Scholar] [CrossRef]
- Smeets, E.; Dorst, D.; van Lith, S.; Freimoser-Grundschober, A.; Klein, C.; Trajkovic-Arsic, M.; Gotthardt, M.; Siveke, J.; Aarntzen, E. A dual-labeled anti-FAP antibody for imaging and targeted photodynamic therapy of cancer associated fibroblasts in a pancreatic cancer mouse model. Eur. J. Nucl. Med. Mol. I 2019, 46 (Suppl. S1), S665–S666. [Google Scholar]
- Katsube, R.; Noma, K.; Ohara, T.; Nishiwaki, N.; Kobayashi, T.; Komoto, S.; Sato, H.; Kashima, H.; Kato, T.; Kikuchi, S.; et al. Fibroblast activation protein targeted near infrared photoimmunotherapy (NIR PIT) overcomes therapeutic resistance in human esophageal cancer. Sci. Rep. 2021, 11, 1693. [Google Scholar] [CrossRef]
- Watanabe, S.; Noma, K.; Ohara, T.; Kashima, H.; Sato, H.; Kato, T.; Urano, S.; Katsube, R.; Hashimoto, Y.; Tazawa, H.; et al. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol. Ther. 2019, 20, 1234–1248. [Google Scholar] [CrossRef]
- Dorst, D.N.; van Caam, A.P.M.; Vitters, E.L.; Walgreen, B.; Helsen, M.M.A.; Klein, C.; Gudi, S.; Wubs, T.; Kumari, J.; Vonk, M.C.; et al. Fibroblast Activation Protein Targeted Photodynamic Therapy Selectively Kills Activated Skin Fibroblasts from Systemic Sclerosis Patients and Prevents Tissue Contraction. Int. J. Mol. Sci. 2021, 22, 12681. [Google Scholar] [CrossRef]
- Dorst, D.N.; Rijpkema, M.; Buitinga, M.; Walgreen, B.; Helsen, M.M.A.; Brennan, E.; Klein, C.; Laverman, P.; Ramming, A.; Schmidkonz, C.; et al. Targeting of fibroblast activation protein in rheumatoid arthritis patients: Imaging and ex vivo photodynamic therapy. Rheumatology 2021, 61, 2999–3009. [Google Scholar] [CrossRef]
- Jin, J.; Barnett, J.D.; Krishnamachary, B.; Mironchik, Y.; Luo, C.K.; Kobayashi, H.; Bhujwalla, Z.M. Evaluating near-infrared photoimmunotherapy for targeting fibroblast activation protein-α expressing cells in vitro and in vivo. Cancer Sci. 2022, 114, 236–246. [Google Scholar] [CrossRef]
- Sato, H.; Noma, K.; Ohara, T.; Kawasaki, K.; Akai, M.; Kobayashi, T.; Nishiwaki, N.; Narusaka, T.; Komoto, S.; Kashima, H.; et al. Dual-targeted near-infrared photoimmunotherapy for esophageal cancer and cancer-associated fibroblasts in the tumor microenvironment. Sci. Rep. 2022, 12, 20152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhen, Z.; Paschall, A.V.; Xue, L.; Yang, X.; Blackwell, A.B.; Cao, Z.; Zhang, W.; Wang, M.; Teng, Y.; et al. FAP-Targeted Photodynamic Therapy Mediated by Ferritin Nanoparticles Elicits an Immune Response against Cancer Cells and Cancer Associated Fibroblasts. Adv. Funct. Mater. 2020, 31, 2007017. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Wang, M.; Zhou, S.; Wang, H.; Wu, Z.; Hao, Z.; Li, Z.; Liu, L.; Xie, J. Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy to Enhance Cytotoxic T Cell Infiltration and Tumor Control. Nano Lett. 2017, 17, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Hanaoka, H.; Sato, K.; Nagaya, T.; Harada, T.; Mitsunaga, M.; Kim, I.; Paik, C.H.; Wu, A.M.; Choyke, P.L.; et al. Photoimmunotherapy targeting prostate-specific membrane antigen: Are antibody fragments as effective as antibodies? J. Nucl. Med. 2015, 56, 140–144. [Google Scholar] [CrossRef]
- Van Driel, P.B.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.A.; De Bruijn, H.S.; Van Diest, P.J.; Vahrmeijer, A.L.; van Bergen en Henegouwen, P.M.P.; et al. EGFR targeted nanobody–photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control. Release 2016, 229, 93–105. [Google Scholar] [CrossRef]
- Maresca, K.P.; Chen, J.; Mathur, D.; Giddabasappa, A.; Root, A.; Narula, J.; King, L.; Schaer, D.; Golas, J.; Kobylarz, K.; et al. Preclinical Evaluation of 89Zr-Df-IAB22M2C PET as an Imaging Biomarker for the Development of the GUCY2C-CD3 Bispecific PF-07062119 as a T Cell Engaging Therapy. Mol. Imaging Biol. 2021, 23, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Nagle, V.L.; Henry, K.E.; Hertz, C.A.J.; Graham, M.S.; Campos, C.; Parada, L.F.; Pandit-Taskar, N.; Schietinger, A.; Mellinghoff, I.K.; Lewis, J.S. Imaging Tumor-Infiltrating Lymphocytes in Brain Tumors with [(64)Cu]Cu-NOTA-anti-CD8 PET. Clin. Cancer Res. 2021, 27, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-Humans Imaging with (89)Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies: Preliminary Pharmacokinetics, Biodistribution, and Lesion Targeting. J. Nucl. Med. 2020, 61, 512–519. [Google Scholar] [CrossRef]
- Farwell, M.D.; Gamache, R.F.; Babazada, H.; Hellmann, M.D.; Harding, J.J.; Korn, R.; Mascioni, A.; Le, W.; Wilson, I.; Gordon, M.S.; et al. CD8-targeted PET Imaging of Tumor Infiltrating T cells in Patients with Cancer: A Phase I First-in-Human Study of (89)Zr-Df-IAB22M2C, a Radiolabeled anti-CD8 Minibody. J. Nucl. Med. 2021, 63, 720–726. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef]
- de Boer, E.; Warram, J.M.; Hartmans, E.; Bremer, P.J.; Bijl, B.; Crane, L.M.; Nagengast, W.B.; Rosenthal, E.L.; van Dam, G.M. A Standardized Light-Emitting Diode Device for Photoimmunotherapy. J. Nucl. Med. 2014, 55, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Geijs, D.J.; Intezar, M.; Litjens, G.J.S.; van der Laak, J.A.W.M. Automatic color unmixing of IHC stained whole slide images. In Medical Imaging 2018: Digital Pathology; SPIE: Bellingham, WA USA, 2018; Volume 10581, pp. 165–171. [Google Scholar] [CrossRef]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar]
- Okuyama, S.; Nagaya, T.; Ogata, F.; Maruoka, Y.; Sato, K.; Nakamura, Y.; Choyke, P.L.; Kobayashi, H. Avoiding thermal injury during near-infrared photoimmunotherapy (NIR-PIT): The importance of NIR light power density. Oncotarget 2017, 8, 113194–113201. [Google Scholar] [CrossRef]
- Fu, H.M.; Huang, J.; Sun, L.M.; Wu, H.M.; Chen, H.M. FAP-Targeted Radionuclide Therapy of Advanced Radioiodine-Refractory Differentiated Thyroid Cancer with Multiple Cycles of 177Lu-FAPI-46. Clin. Nucl. Med. 2022, 47, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Pang, Y.; Zhao, L.; Lin, L.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. FAP-targeted radionuclide therapy with [177Lu]Lu-FAPI-46 in metastatic nasopharyngeal carcinoma. Eur. J. Nucl. Med. 2021, 49, 1767–1769. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Almaguel, F.; Zboralski, D.; et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using (177)Lu-FAP-2286: First-in-Humans Results. J. Nucl. Med. 2022, 63, 415–423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smeets, E.M.M.; Dorst, D.N.; Franssen, G.M.; van Essen, M.S.; Frielink, C.; Stommel, M.W.J.; Trajkovic-Arsic, M.; Cheung, P.F.; Siveke, J.T.; Wilson, I.; et al. Fibroblast Activation Protein-Targeting Minibody-IRDye700DX for Ablation of the Cancer-Associated Fibroblast with Photodynamic Therapy. Cells 2023, 12, 1420. https://doi.org/10.3390/cells12101420
Smeets EMM, Dorst DN, Franssen GM, van Essen MS, Frielink C, Stommel MWJ, Trajkovic-Arsic M, Cheung PF, Siveke JT, Wilson I, et al. Fibroblast Activation Protein-Targeting Minibody-IRDye700DX for Ablation of the Cancer-Associated Fibroblast with Photodynamic Therapy. Cells. 2023; 12(10):1420. https://doi.org/10.3390/cells12101420
Chicago/Turabian StyleSmeets, Esther M. M., Daphne N. Dorst, Gerben M. Franssen, Merijn S. van Essen, Cathelijne Frielink, Martijn W. J. Stommel, Marija Trajkovic-Arsic, Phyllis F. Cheung, Jens T. Siveke, Ian Wilson, and et al. 2023. "Fibroblast Activation Protein-Targeting Minibody-IRDye700DX for Ablation of the Cancer-Associated Fibroblast with Photodynamic Therapy" Cells 12, no. 10: 1420. https://doi.org/10.3390/cells12101420
APA StyleSmeets, E. M. M., Dorst, D. N., Franssen, G. M., van Essen, M. S., Frielink, C., Stommel, M. W. J., Trajkovic-Arsic, M., Cheung, P. F., Siveke, J. T., Wilson, I., Mascioni, A., Aarntzen, E. H. J. G., & van Lith, S. A. M. (2023). Fibroblast Activation Protein-Targeting Minibody-IRDye700DX for Ablation of the Cancer-Associated Fibroblast with Photodynamic Therapy. Cells, 12(10), 1420. https://doi.org/10.3390/cells12101420








