A Plethora of Functions Condensed into Tiny Phospholipids: The Story of PI4P and PI(4,5)P2
Abstract
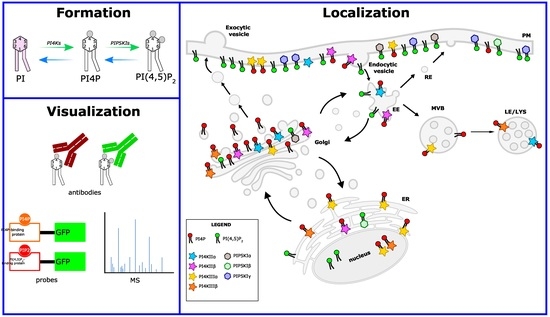
1. Introduction
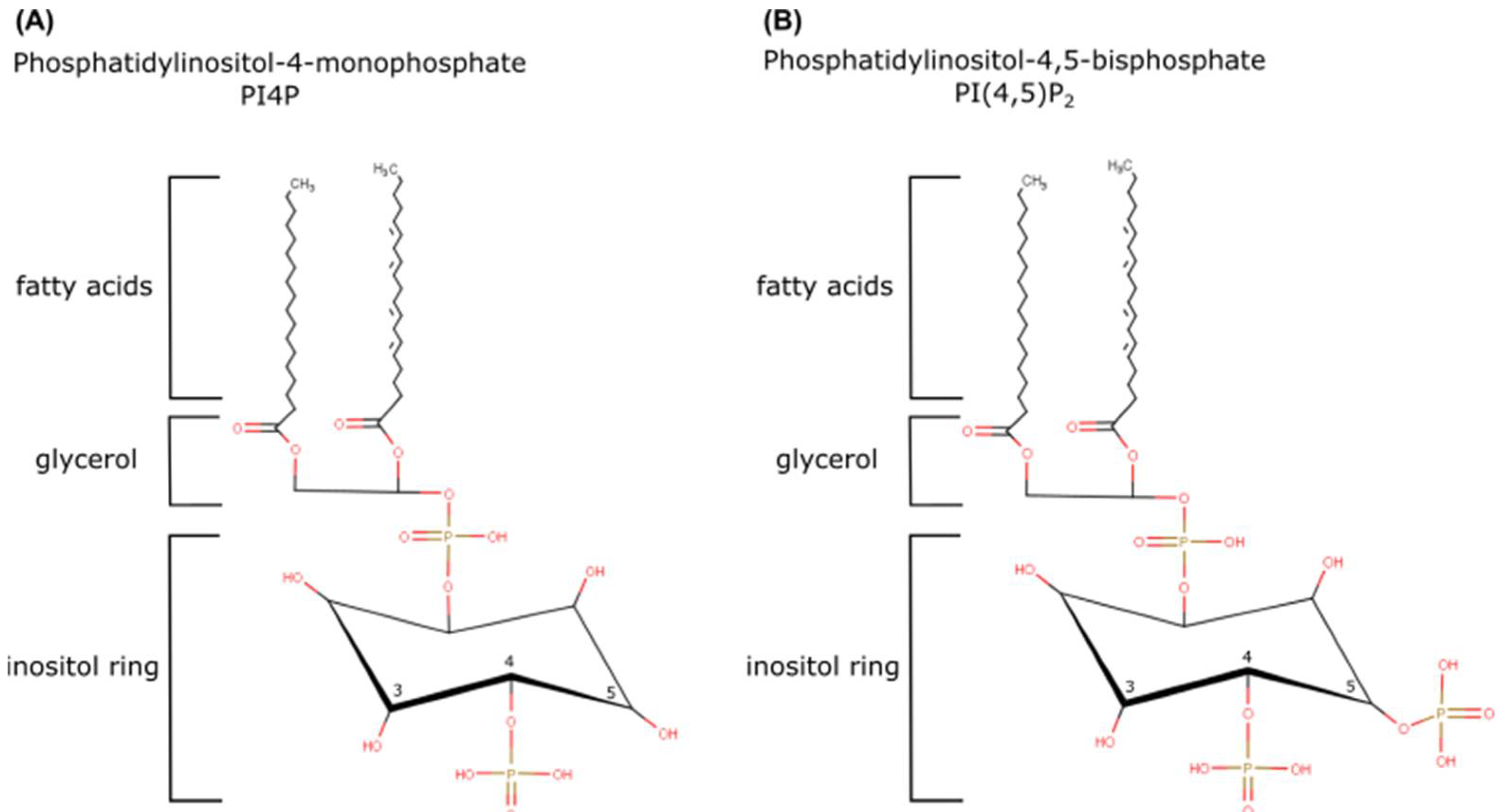
The (re)Birth of PI4P and PI(4,5)P2
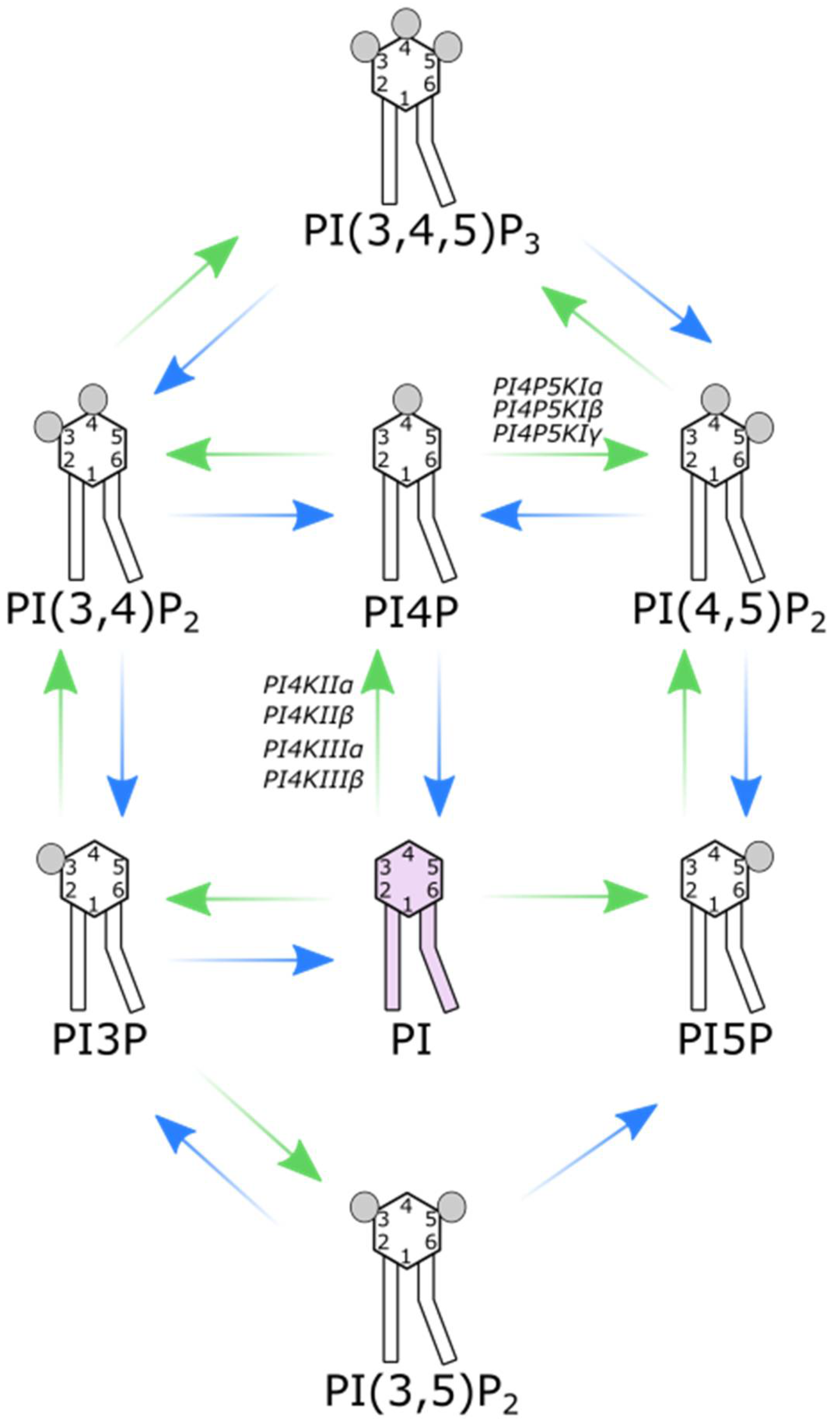
2. PI4Ks
2.1. PI4KIIα
2.2. PI4KIIβ
2.3. PI4Kα
2.4. PI4Kβ
3. PI4P5KIs
3.1. PI4P5KIα
3.2. PI4P5KIβ
3.3. PI4P5KIγ
4. PI4P: A Huge Burden on a Small Lipid
4.1. PI4P at the Golgi Apparatus: Glycosylation and Anterograde Trafficking
4.2. PI4P at the Plasma Membrane
4.3. PI4P at Membrane-Contact Sites
5. PI(4,5)P2: A Dual Role
5.1. PI(4,5)P2 in Clathrin-Mediated Endocytosis
5.2. PI(4,5)P2 as a Signaling Molecule
6. Tools for Detection of Intracellular Phosphoinositides
6.1. Antibodies
6.2. Genetically Encoded Probes
6.3. Mass Spectrometry
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, G.M. The Cell: A Molecular Approach, 8th ed.; Oxford University Press: New York, NY, USA, 2019; p. 783. [Google Scholar]
- Dowham, W. Advances in Lipobiology; Gross, R.W., Ed.; Jai Press Inc.: London, UK, 1997; Volume 2, p. 355. [Google Scholar]
- Sun, Y.; Thapa, N.; Hedman, A.C.; Anderson, R.A. Phosphatidylinositol 4,5-bisphosphate: Targeted production and signaling. Bioessays 2013, 35, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K. Review of PIP2 in Cellular Signaling, Functions and Diseases. Int. J. Mol. Sci. 2020, 21, 8342. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Cockcroft, S. Phosphatidylinositol(4,5)bisphosphate: Diverse functions at the plasma membrane. Essays Biochem. 2020, 64, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, S.; Kurisu, S.; Takenawa, T. Dynamic shaping of cellular membranes by phospholipids and membrane-deforming proteins. Physiol. Rev. 2014, 94, 1219–1248. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, M.-W.; Wu, D.-C.; Huang, Y.-B.; Huang, H.-T.; Chen, C.-L. The role of phosphoinositide-regulated actin reorganization in chemotaxis and cell migration. Br. J. Pharmacol. 2014, 171, 5541–5554. [Google Scholar] [CrossRef]
- Bout, I.V.D.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef]
- Izard, T.; Brown, D.T. Mechanisms and Functions of Vinculin Interactions with Phospholipids at Cell Adhesion Sites. J. Biol. Chem. 2016, 291, 2548–2555. [Google Scholar] [CrossRef]
- Hammond, G.R.; Burke, J.E. Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr. Opin. Cell Biol. 2020, 63, 57–67. [Google Scholar] [CrossRef]
- Dickson, E.J.; Hille, B. Understanding phosphoinositides: Rare, dynamic, and essential membrane phospholipids. Biochem. J. 2019, 476, 1–23. [Google Scholar] [CrossRef]
- Pemberton, J.G.; Kim, Y.J.; Balla, T. Integrated regulation of the phosphatidylinositol cycle and phosphoinositide-driven lipid transport at ER-PM contact sites. Traffic 2019, 21, 200–219. [Google Scholar] [CrossRef]
- Castano, E.; Yildirim, S.; Fáberová, V.; Krausová, A.; Uličná, L.; Paprčková, D.; Sztacho, M.; Hozák, P. Nuclear Phosphoinositides—Versatile Regulators of Genome Functions. Cells 2019, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Olivença, D.V.; Uliyakina, I.; Fonseca, L.L.; Amaral, M.D.; Voit, E.O.; Pinto, F.R. A Mathematical Model of the Phosphoinositide Pathway. Sci. Rep. 2018, 8, 3904. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Triscott, J.; Emerling, B.M.; Hammond, G.R.V. Beyond PI3Ks: Targeting phosphoinositide kinases in disease. Nat. Rev. Drug Discov. 2022, 22, 357–386. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P.; Joseph, A.; Krishnan, H.; Singh, P.; Saha, S. Phosphoinositides: Regulators of Nervous System Function in Health and Disease. Front. Mol. Neurosci. 2019, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Ernest James Phillips, T.; Maguire, E. Phosphoinositides: Roles in the Development of Microglial-Mediated Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 652593. [Google Scholar] [CrossRef]
- Ijuin, T. Phosphoinositide phosphatases in cancer cell dynamics—Beyond PI3K and PTEN. Semin. Cancer Biol. 2019, 59, 50–65. [Google Scholar] [CrossRef]
- Balla, T. Phosphoinositides: Tiny Lipids with Giant Impact on Cell Regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef]
- Beziau, A.; Brand, D.; Piver, E. The Role of Phosphatidylinositol Phosphate Kinases during Viral Infection. Viruses 2020, 12, 1124. [Google Scholar] [CrossRef]
- O’donnell, V.B.; Rossjohn, J.; Wakelam, M.J. Phospholipid signaling in innate immune cells. J. Clin. Investig. 2018, 128, 2670–2679. [Google Scholar] [CrossRef]
- Okkenhaug, K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 2013, 31, 675–704. [Google Scholar] [CrossRef]
- Zewe, J.P.; Miller, A.M.; Sangappa, S.; Wills, R.C.; Goulden, B.D.; Hammond, G.R. Probing the subcellular distribution of phosphatidylinositol reveals a surprising lack at the plasma membrane. J. Cell Biol. 2020, 219, e201906127. [Google Scholar] [CrossRef]
- Desale, S.E.; Chinnathambi, S. Phosphoinositides signaling modulates microglial actin remodeling and phagocytosis in Alzheimer’s disease. Cell Commun. Signal. 2021, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Traynor-Kaplan, A.; Kruse, M.; Dickson, E.J.; Dai, G.; Vivas, O.; Yu, H.; Whittington, D.; Hille, B. Fatty-acyl chain profiles of cellular phosphoinositides. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 513–522. [Google Scholar] [CrossRef]
- Nakatsu, F.; Baskin, J.M.; Chung, J.; Tanner, L.B.; Shui, G.; Lee, S.Y.; Pirruccello, M.; Hao, M.; Ingolia, N.T.; Wenk, M.R.; et al. PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 2012, 199, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Posor, Y.; Jang, W.; Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 2022, 23, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.; Mao, Y. The structure of phosphoinositide phosphatases: Insights into substrate specificity and catalysis. Biochim. Biophys. Acta 2015, 1851, 698–710. [Google Scholar] [CrossRef]
- Ramos, A.R.; Ghosh, S.; Erneux, C. The impact of phosphoinositide 5-phosphatases on phosphoinositides in cell function and human disease. J. Lipid Res. 2019, 60, 276–286. [Google Scholar] [CrossRef]
- Minogue, S.; Waugh, M.G.; De Matteis, M.A.; Stephens, D.J.; Berditchevski, F.; Hsuan, J.J. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J. Cell Sci. 2006, 119, 571–581. [Google Scholar] [CrossRef]
- Dornan, G.L.; McPhail, J.A.; Burke, J.E. Type III phosphatidylinositol 4 kinases: Structure, function, regulation, signalling and involvement in disease. Biochem. Soc. Trans. 2016, 44, 260–266. [Google Scholar] [CrossRef]
- Boura, E.; Nencka, R. Phosphatidylinositol 4-kinases: Function, structure, and inhibition. Exp. Cell Res. 2015, 337, 136–145. [Google Scholar] [CrossRef]
- Li, Y.-P.; Mikrani, R.; Hu, Y.-F.; Faran Ashraf Baig, M.M.; Abbas, M.; Akhtar, F.; Xu, M. Research progress of phosphatidylinositol 4-kinase and its inhibitors in inflammatory diseases. Eur. J. Pharmacol. 2021, 907, 174300. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E. Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol. Cell 2018, 71, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Brill, J.A. Cinderella story: PI4P goes from precursor to key signaling molecule. Crit. Rev. Biochem. Mol. Biol. 2013, 49, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Klíma, M.; Baumlova, A.; Chalupska, M.; Hřebabecký, H.; Dejmek, M.; Nencka, R.; Boura, E. The high-resolution crystal structure of phosphatidylinositol 4-kinase IIβ and the crystal structure of phosphatidylinositol 4-kinase IIα containing a nucleoside analogue provide a structural basis for isoform-specific inhibitor design. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.J.; Sun, H.Q.; Yamamoto, M.; Wlodarski, P.; Kunii, K.; Martinez, M.; Barylko, B.; Albanesi, J.P.; Yin, H.L. Type II phosphatidylinositol 4-kinase β is a cytosolic and peripheral membrane protein that is recruited to the plasma membrane and activated by Rac-GTP. J. Biol. Chem. 2002, 277, 46586–46593. [Google Scholar] [CrossRef]
- Waugh, M.G. The Great Escape: How phosphatidylinositol 4-kinases and PI4P promote vesicle exit from the Golgi (and drive cancer). Biochem. J. 2019, 476, 2321–2346. [Google Scholar] [CrossRef]
- De Matteis, M.A.; Wilson, C.; D’Angelo, G. Phosphatidylinositol-4-phosphate: The Golgi and beyond. Bioessays 2013, 35, 612–622. [Google Scholar] [CrossRef]
- Heath, C.M.; Stahl, P.D.; Barbieri, M. Lipid kinases play crucial and multiple roles in membrane trafficking and signaling. Histol. Histopathol. 2003, 18, 989–998. [Google Scholar]
- Craige, B.; Salazar, G.; Faundez, V. Phosphatidylinositol-4-kinase type II alpha contains an ap-3–sorting motif and a kinase domain that are both required for endosome traffic. Mol. Biol. Cell 2008, 19, 1415–1426. [Google Scholar] [CrossRef]
- Pataer, A.; Ozpolat, B.; Shao, R.; Cashman, N.R.; Plotkin, S.S.; Samuel, C.E.; Lin, S.H.; Kabil, N.N.; Wang, J.; Majidi, M.; et al. Therapeutic targeting of the PI4K2A/PKR lysosome network is critical for misfolded protein clearance and survival in cancer cells. Oncogene 2020, 39, 801–813. [Google Scholar] [CrossRef]
- Palamiuc, L.; Ravi, A.; Emerling, B.M. Phosphoinositides in autophagy: Current roles and future insights. FEBS J. 2019, 287, 222–238. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.-Q.; Zhu, X.; Zhang, L.; Albanesi, J.; Levine, B.; Yin, H. GABARAPs regulate PI4P-dependent autophagosome:lysosome fusion. Proc. Natl. Acad. Sci. USA 2015, 112, 7015–7020. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Balla, T. Emerging roles of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate as regulators of multiple steps in autophagy. J. Biochem. 2020, 168, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Finkel, T. A phosphoinositide signalling pathway mediates rapid lysosomal repair. Nature 2022, 609, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Alli-Balogun, G.O.; Gewinner, C.A.; Jacobs, R.; Kriston-Vizi, J.; Waugh, M.; Minogue, S. Phosphatidylinositol 4-kinase IIβ negatively regulates invadopodia formation and suppresses an invasive cellular phenotype. Mol. Biol. Cell 2016, 27, 4033–4042. [Google Scholar] [CrossRef]
- Li, J.; Gao, Z.; Zhao, D.; Zhang, L.; Qiao, X.; Zhao, Y.; Ding, H.; Zhang, P.; Lu, J.; Liu, J.; et al. PI-273, a Substrate-Competitive, Specific Small-Molecule Inhibitor of PI4KIIα, Inhibits the Growth of Breast Cancer Cells. Cancer Res. 2017, 77, 6253–6266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Zhang, J.; Kang, H.; Qin, Z.; Chen, C. PI4KIIα is a novel regulator of tumor growth by its action on angiogenesis and HIF-1α regulation. Oncogene 2010, 29, 2550–2559. [Google Scholar] [CrossRef] [PubMed]
- Banerji, S.; Ngo, M.; Lane, C.F.; Robinson, C.-A.; Minogue, S.; Ridgway, N.D. Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the golgi apparatus requires phosphatidylinositol 4-kinase IIα. Mol. Biol. Cell 2010, 21, 4141–4150. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Gao, Z.; Kang, H.; Rong, G.; Zhang, X.; Chen, C. Dual inhibition of EGFR at protein and activity level via combinatorial blocking of PI4KIIα as anti-tumor strategy. Protein Cell 2014, 5, 457–468. [Google Scholar] [CrossRef]
- Chu, K.M.; Minogue, S.; Hsuan, J.J.; Waugh, M.G. Differential effects of the phosphatidylinositol 4-kinases, PI4KIIα and PI4KIIIβ, on Akt activation and apoptosis. Cell Death Dis. 2010, 1, e106. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of theEGFRin cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Go, G.; Lee, S.H. The Cellular Prion Protein: A Promising Therapeutic Target for Cancer. Int. J. Mol. Sci. 2020, 21, 9208. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Luikart, B.W. Striking a balance: PIP2 and PIP3 signaling in neuronal health and disease. Explor. Neuroprot. Ther. 2021, 1, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.P.; Al-Shawi, R.; Minogue, S.; Waugh, M.G.; Wiedemann, C.; Evangelou, S.; Loesch, A.; Sihra, T.S.; King, R.; Warner, T.T.; et al. Loss of phosphatidylinositol 4-kinase 2α activity causes late onset degeneration of spinal cord axons. Proc. Natl. Acad. Sci. USA 2009, 106, 11535–11539. [Google Scholar] [CrossRef]
- Robinson, J.W.; Leshchyns’ka, I.; Farghaian, H.; Hughes, W.E.; Sytnyk, V.; Neely, G.G.; Cole, A.R. PI4KIIα phosphorylation by GSK3 directs vesicular trafficking to lysosomes. Biochem. J. 2014, 464, 145–156. [Google Scholar] [CrossRef]
- Kang, M.S.; Baek, S.-H.; Chun, Y.S.; Moore, A.Z.; Landman, N.; Berman, D.; Yang, H.O.; Morishima-Kawashima, M.; Osawa, S.; Funamoto, S.; et al. modulation of lipid kinase Pi4kIIα activity and lipid raft association of presenilin 1 underlies γ-secretase inhibition by ginsenoside (20S)-Rg3. J. Biol. Chem. 2013, 288, 20868–20882. [Google Scholar] [CrossRef]
- Dafsari, H.S.; Pemberton, J.G.; Ferrer, E.A.; Yammine, T.; Farra, C.; Mohammadi, M.H.; Ghayoor Karimiani, E.; Hashemi, N.; Souaid, M.; Sabbagh, S.; et al. PI4K2A deficiency causes innate error in intracellular trafficking with developmental and epileptic-dyskinetic encephalopathy. Ann. Clin. Transl. Neurol. 2022, 9, 1345–1358. [Google Scholar] [CrossRef]
- Mohamed, M.; Gardeitchik, T.; Balasubramaniam, S.; Guerrero-Castillo, S.; Dalloyaux, D.; van Kraaij, S.; Venselaar, H.; Hoischen, A.; Urban, Z.; Brandt, U.; et al. Novel defect in phosphatidylinositol 4-kinase type 2-alpha (PI4K2A) at the membrane-enzyme interface is associated with metabolic cutis laxa. J. Inherit. Metab. Dis. 2020, 43, 1382–1391. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhang, P.; Gao, Z.; Zhao, Y.; Qiao, X.; Chen, C. PI4KIIα regulates insulin secretion and glucose homeostasis via a PKD-dependent pathway. Biophys. Rep. 2018, 4, 25–38. [Google Scholar] [CrossRef]
- Jović, M.; Kean, M.J.; Szentpetery, Z.; Polevoy, G.; Gingras, A.-C.; Brill, J.A.; Balla, T. Two phosphatidylinositol 4-kinases control lysosomal delivery of the Gaucher disease enzyme, β-glucocerebrosidase. Mol. Biol. Cell 2012, 23, 1533–1545. [Google Scholar] [CrossRef]
- Salazar, G.; Zlatic, S.; Craige, B.; Peden, A.A.; Pohl, J.; Faundez, V. Hermansky-pudlak syndrome protein complexes associate with phosphatidylinositol 4-kinase type II α in neuronal and non-neuronal cells. J. Biol. Chem. 2009, 284, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, N.; Çelik, E.; Baslar, Z.; Celkan, T.T. A rare cause of thrombocyte dysfunction: Hermansky-Pudlak syndrome. Türk Pediatri Arşivi 2014, 49, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, M.; O’Connor, M.N.; Kriston-Vizi, J.; White, I.J.; Al-Shawi, R.; Simons, J.P.; Mössinger, J.; Haucke, V.; Cutler, D.F. Type II PI4-kinases control Weibel-Palade body biogenesis and von Willebrand factor structure in Human endothelial cells. J. Cell Sci. 2016, 129, 2096–2105. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.; Yu, H.; Zhai, Y.; Gao, Z.; Liu, Y.; Pang, X.; Zhang, L.; Schulten, K.; Sun, F.; et al. Molecular insights into the membrane-associated phosphatidylinositol 4-kinase IIα. Nat. Commun. 2014, 5, 3552. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tan, X.; Liu, X.; Yu, J.; Bota-Rabassedas, N.; Niu, Y.; Luo, J.; Xi, Y.; Zong, C.; Creighton, C.J.; et al. Addiction to Golgi-resident PI4P synthesis in chromosome 1q21.3–amplified lung adenocarcinoma cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023537118. [Google Scholar] [CrossRef]
- Bojjireddy, N.; Botyanszki, J.; Hammond, G.; Creech, D.; Peterson, R.; Kemp, D.C.; Snead, M.; Brown, R.; Morrison, A.; Wilson, S.; et al. Pharmacological and genetic targeting of the pi4ka enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 2014, 289, 6120–6132. [Google Scholar] [CrossRef]
- Dornan, G.L.; Dalwadi, U.; Hamelin, D.J.; Hoffmann, R.M.; Yip, C.K.; Burke, J.E. Probing the Architecture, Dynamics, and Inhibition of the PI4KIIIα/TTC7/FAM126 Complex. J. Mol. Biol. 2018, 430, 3129–3142. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, X.; Zheng, G.; Jia, G.; Li, Z.; Ding, X.; Lei, L.; Yuan, L.; Xu, S.; Gao, N. Targeting PI4KA sensitizes refractory leukemia to chemotherapy by modulating the ERK/AMPK/OXPHOS axis. Theranostics 2022, 12, 6972–6988. [Google Scholar] [CrossRef]
- Dorobantu, C.M.; Harak, C.; Klein, R.; van der Linden, L.; Strating, J.R.P.M.; van der Schaar, H.M.; Lohmann, V.; van Kuppeveld, F.J.M. Tyrphostin AG1478 Inhibits Encephalomyocarditis Virus and Hepatitis C Virus by Targeting Phosphatidylinositol 4-Kinase IIIα. Antimicrob. Agents Chemother. 2016, 60, 6402–6406. [Google Scholar] [CrossRef]
- Zhang, Z.; Venditti, R.; Ran, L.; Liu, Z.; Vivot, K.; Schürmann, A.; Bonifacino, J.S.; De Matteis, M.A.; Ricci, R. Distinct changes in endosomal composition promote NLRP3 inflammasome activation. Nat. Immunol. 2023, 24, 30–41. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, C.-H.; Tong, C.; Yang, Y.; Wang, Z.; Lam, S.M.; Wang, D.; Li, R.; Shui, G.; Shi, Y.S.; et al. Activity-dependent PI4P synthesis by PI4KIIIα regulates long-term synaptic potentiation. Cell Rep. 2022, 38, 110452. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Prats, A.; Bjelobaba, I.; Aldworth, Z.; Baba, T.; Abebe, D.; Kim, Y.J.; Stojilkovic, S.S.; Stopfer, M.; Balla, T. Schwann-Cell-Specific Deletion of Phosphatidylinositol 4-Kinase Alpha Causes Aberrant Myelination. Cell Rep. 2018, 23, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, B.; Sbrissa, D.; Pressprich, M.; Kim, S.; Vaishampayan, U.; Cher, M.L.; Chinni, S. Adaptor proteins mediate CXCR4 and PI4KA crosstalk in prostate cancer cells and the significance of PI4KA in bone tumor growth. Res. Sq. 2023, 1–13. [Google Scholar] [CrossRef]
- Rutaganira, F.U.; Fowler, M.L.; McPhail, J.A.; Gelman, M.A.; Nguyen, K.; Xiong, A.; Dornan, G.L.; Tavshanjian, B.; Glenn, J.S.; Shokat, K.M.; et al. Design and Structural Characterization of Potent and Selective Inhibitors of Phosphatidylinositol 4 Kinase IIIβ. J. Med. Chem. 2016, 59, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Arita, M. Phosphatidylinositol-4 kinase III beta and oxysterol-binding protein accumulate unesterified cholesterol on poliovirus-induced membrane structure. Microbiol. Immunol. 2014, 58, 239–256. [Google Scholar] [CrossRef]
- Spickler, C.; Lippens, J.; Laberge, M.-K.; Desmeules, S.; Bellavance, E.; Garneau, M.; Guo, T.; Hucke, O.; Leyssen, P.; Neyts, J.; et al. Phosphatidylinositol 4-kinase III beta is essential for replication of human rhinovirus and its inhibition causes a lethal phenotype in vivo. Antimicrob. Agents Chemother. 2013, 57, 3358–3368. [Google Scholar] [CrossRef]
- Strating, J.R.; van der Linden, L.; Albulescu, L.; Bigay, J.; Arita, M.; Delang, L.; Leyssen, P.; van der Schaar, H.M.; Lanke, K.H.; Thibaut, H.J.; et al. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep. 2015, 10, 600–615. [Google Scholar] [CrossRef]
- van der Schaar, H.M.; Leyssen, P.; Thibaut, H.J.; de Palma, A.; van der Linden, L.; Lanke, K.H.W.; Lacroix, C.; Verbeken, E.; Conrath, K.; MacLeod, A.M.; et al. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIβ. Antimicrob. Agents Chemother. 2013, 57, 4971–4981. [Google Scholar] [CrossRef]
- Phillpotts, R.J.; Wallace, J.; Tyrrell, D.; Tagart, V.B. Therapeutic activity of enviroxime against rhinovirus infection in volunteers. Antimicrob. Agents Chemother. 1983, 23, 671–675. [Google Scholar] [CrossRef]
- Paquet, T.; Le Manach, C.; Cabrera, D.G.; Younis, Y.; Henrich, P.P.; Abraham, T.S.; Lee, M.C.S.; Basak, R.; Ghidelli-Disse, S.; Lafuente-Monasterio, M.J.; et al. Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase. Sci. Transl. Med. 2017, 9, eaad9735. [Google Scholar] [CrossRef]
- Mohammed, R.; Asres, M.S.; Gudina, E.K.; Adissu, W.; Johnstone, H.; Marrast, A.C.; Donini, C.; Duparc, S.; Yilma, D. Efficacy, Safety, Tolerability, and Pharmacokinetics of MMV390048 in Acute Uncomplicated Malaria. Am. J. Trop. Med. Hyg. 2023, 108, 81–84. [Google Scholar] [CrossRef]
- Kunkl, M.; Porciello, N.; Mastrogiovanni, M.; Capuano, C.; Lucantoni, F.; Moretti, C.; Persson, J.L.; Galandrini, R.; Buzzetti, R.; Tuosto, L. ISA-2011B, a Phosphatidylinositol 4-Phosphate 5-Kinase α Inhibitor, Impairs CD28-Dependent Costimulatory and Pro-inflammatory Signals in Human T Lymphocytes. Front. Immunol. 2017, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Semenas, J.; Miftakhova, R.; Simoulis, A.; Robinson, B.; Gjörloff Wingren, A.; Mongan, N.P.; Heery, D.M.; Johnson, H.; Abrahamsson, P.-A.; et al. Targeted suppression of AR-V7 using PIP5K1α inhibitor overcomes enzalutamide resistance in prostate cancer cells. Oncotarget 2016, 7, 63065–63081. [Google Scholar] [CrossRef]
- Semenas, J.; Hedblom, A.; Miftakhova, R.R.; Sarwar, M.; Larsson, R.; Shcherbina, L.; Johansson, M.E.; Härkönen, P.; Sterner, O.; Persson, J.L. The role of PI3K/AKT-related PIP5K1α and the discovery of its selective inhibitor for treatment of advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2014, 111, E3689–E3698. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Huang, W.; Ge, X.; Xue, L.; Zhao, W.; Xue, J. Type Iγ phosphatidylinositol phosphate kinase promotes tumor growth by facilitating Warburg effect in colorectal cancer. Ebiomedicine 2019, 44, 375–386. [Google Scholar] [CrossRef]
- Wieffer, M.; Cibrian Uhalte, E.; Posor, Y.; Otten, C.; Branz, K.; Schütz, I.; Mössinger, J.; Schu, P.; Abdelilah-Seyfried, S.; Krauß, M.; et al. PI4K2β/AP-1-Based TGN-Endosomal Sorting Regulates Wnt Signaling. Curr. Biol. 2013, 23, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Takasuga, S.; Sasaki, J.; Kofuji, S.; Eguchi, S.; Yamazaki, M.; Suzuki, A. Mammalian phosphoinositide kinases and phosphatases. Prog. Lipid Res. 2009, 48, 307–343. [Google Scholar] [CrossRef]
- Sobocińska, J.; Roszczenko-Jasinska, P.; Zaręba-Kozioł, M.; Hromada-Judycka, A.; Matveichuk, O.; Traczyk, G.; Łukasiuk, K.; Kwiatkowska, K. Lipopolysaccharide Upregulates Palmitoylated Enzymes of the Phosphatidylinositol Cycle: An Insight from Proteomic Studies. Mol. Cell. Proteom. 2018, 17, 233–254. [Google Scholar] [CrossRef]
- Misehe, M.; Klima, M.; Matoušová, M.; Chalupská, D.; Dejmek, M.; Šála, M.; Mertlíková-Kaiserová, H.; Boura, E.; Nencka, R. Structure-based design and modular synthesis of novel PI4K class II inhibitors bearing a 4-aminoquinazoline scaffold. Bioorg. Med. Chem. Lett. 2022, 76, 129010. [Google Scholar] [CrossRef]
- Clayton, E.L.; Minogue, S.; Waugh, M.G. Mammalian phosphatidylinositol 4-kinases as modulators of membrane trafficking and lipid signaling networks. Prog. Lipid Res. 2013, 52, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Kim, Y.J.; Varnai, P.; Szentpetery, Z.; Knight, Z.; Shokat, K.M.; Balla, T. Maintenance of hormone-sensitive phosphoinositide pools in the plasma membrane requires phosphatidylinositol 4-kinase IIIα. Mol. Biol. Cell 2008, 19, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Jardine, S.; Dhingani, N.; Muise, A.M. TTC7A: Steward of Intestinal Health. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, J.M.; Kim, D.H.; Kim, I.A. Inhibition of PI4K IIIα radiosensitizes in human tumor xenograft and immune-competent syngeneic murine tumor model. Oncotarget 2017, 8, 110392–110405. [Google Scholar] [CrossRef]
- Ziyad, S.; Riordan, J.D.; Cavanaugh, A.M.; Su, T.; Hernandez, G.E.; Hilfenhaus, G.; Morselli, M.; Huynh, K.; Wang, K.; Chen, J.-N.; et al. A Forward Genetic Screen Targeting the Endothelium Reveals a Regulatory Role for the Lipid Kinase Pi4ka in Myelo- and Erythropoiesis. Cell Rep. 2018, 22, 1211–1224. [Google Scholar] [CrossRef]
- Adhikari, H.; Kattan, W.E.; Kumar, S.; Zhou, P.; Hancock, J.F.; Counter, C.M. Oncogenic KRAS is dependent upon an EFR3A-PI4KA signaling axis for potent tumorigenic activity. Nat. Commun. 2021, 12, 5248. [Google Scholar] [CrossRef] [PubMed]
- Ilboudo, A.; Nault, J.-C.; Dubois-Pot-Schneider, H.; Corlu, A.; Zucman-Rossi, J.; Samson, M.; Le Seyec, J. Overexpression of phosphatidylinositol 4-kinase type IIIα is associated with undifferentiated status and poor prognosis of human hepatocellular carcinoma. BMC Cancer 2014, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.T.; Howard, M.F.; Wisniewski, E.; Popitsch, N.; Knight, S.J.; Keays, D.A.; Quaghebeur, G.; Cox, H.; Cox, P.; Balla, T.; et al. Germline recessive mutations in PI4KA are associated with perisylvian polymicrogyria, cerebellar hypoplasia and arthrogryposis. Hum. Mol. Genet. 2015, 24, 3732–3741. [Google Scholar] [CrossRef] [PubMed]
- Verdura, E.; Rodríguez-Palmero, A.; Vélez-Santamaria, V.; Planas-Serra, L.; de la Calle, I.; Raspall-Chaure, M.; Roubertie, A.; Benkirane, M.; Saettini, F.; Pavinato, L.; et al. Biallelic PI4KA variants cause a novel neurodevelopmental syndrome with hypomyelinating leukodystrophy. Brain 2021, 144, 2659–2669. [Google Scholar] [CrossRef]
- Morrow, A.A.; Alipour, M.A.; Bridges, D.; Yao, Z.; Saltiel, A.R.; Lee, J.M. The lipid kinase PI4KIIIβ is highly expressed in breast tumors and activates akt in cooperation with Rab11a. Mol. Cancer Res. 2014, 12, 1492–1508. [Google Scholar] [CrossRef]
- Yang, N.; Ma, P.; Lang, J.; Zhang, Y.; Deng, J.; Ju, X.; Zhang, G.; Jiang, C. Phosphatidylinositol 4-kinase IIIβ is required for severe acute respiratory syndrome coronavirus spike-mediated cell entry. J. Biol. Chem. 2012, 287, 8457–8467. [Google Scholar] [CrossRef]
- Kremer, L.; Hennes, M.S.E.; Brause, A.; Ursu, A.; Robke, L.; Matsubayashi, H.T.; Nihongaki, Y.; Flegel, M.S.J.; Mejdrová, I.; Eickhoff, J.; et al. Discovery of the Hedgehog Pathway Inhibitor Pipinib that Targets PI4KIIIß. Angew. Chem. Int. Ed. Engl. 2019, 58, 16617–16628. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ghosh, C.; Xing, Y.; Sun, Y. Phosphatidylinositol 4,5-bisphosphate in the Control of Membrane Trafficking. Int. J. Biol. Sci. 2020, 16, 2761–2774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Suzuki, A.; Lian, L.; Min, S.H.; Wang, Z.; Litvinov, R.I.; Stalker, T.J.; Yago, T.; Klopocki, A.G.; et al. Platelets lacking PIP5KIγ have normal integrin activation but impaired cytoskeletal-membrane integrity and adhesion. Blood 2013, 121, 2743–2752. [Google Scholar] [CrossRef]
- Doughman, R.L.; Firestone, A.; Anderson, R.A. Phosphatidylinositol phosphate kinases put PI4,5P 2 in its place. J. Membr. Biol. 2003, 194, 77–89. [Google Scholar] [CrossRef]
- East, M.P.; Laitinen, T.; Asquith, C.R.M. PIP5K1A: A potential target for cancers with KRAS or TP53 mutations. Nat. Rev. Drug Discov. 2020, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Lian, L.; Tang, T.; Stalker, T.J.; Sasaki, T.; Kanaho, Y.; Brass, L.F.; Choi, J.K.; Hartwig, J.H.; et al. Loss of PIP5KIβ demonstrates that PIP5KI isoform-specific PIP2 synthesis is required for IP3 formation. Proc. Natl. Acad. Sci. USA 2008, 105, 14064–14069. [Google Scholar] [CrossRef]
- Sun, Y.; Turbin, D.; Ling, K.; Thapa, N.; Leung, S.; Huntsman, D.G.; Anderson, R. Type I gamma phosphatidylinositol phosphate kinase modulates invasion and proliferation and its expression correlates with poor prognosis in breast cancer. Breast Cancer Res. 2010, 12, R6. [Google Scholar] [CrossRef]
- Schill, N.J.; Hedman, A.C.; Choi, S.; Anderson, R.A. Isoform 5 of PIPKIgamma regulates the endosomal trafficking and degradation of E-cadherin. J. Cell Sci. 2014, 127, 2189–2203. [Google Scholar] [CrossRef]
- Choi, S.; Thapa, N.; Tan, X.; Hedman, A.C.; Anderson, R.A. PIP kinases define PI4,5P2 signaling specificity by association with effectors. Biochim. Biophys. Acta 2015, 1851, 711–723. [Google Scholar] [CrossRef]
- Di Paolo, G.; Moskowitz, H.S.; Gipson, K.; Wenk, M.R.; Voronov, S.; Obayashi, M.; Flavell, R.; Fitzsimonds, R.M.; Ryan, T.A.; De Camilli, P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 2004, 431, 415–422. [Google Scholar] [CrossRef]
- Wang, Y.; Lian, L.; Golden, J.A.; Morrisey, E.E.; Abrams, C.S. PIP5KIγ is required for cardiovascular and neuronal development. Proc. Natl. Acad. Sci. USA 2007, 104, 11748–11753. [Google Scholar] [CrossRef] [PubMed]
- McCrea, H.J.; De Camilli, P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology 2009, 24, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Narkis, G.; Ofir, R.; Landau, D.; Manor, E.; Volokita, M.; Hershkowitz, R.; Elbedour, K.; Birk, O.S. Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKIγ of the phophatidylinsitol pathway. Am. J. Hum. Genet. 2007, 81, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Litvinov, R.I.; Chen, X.; Bach, T.L.; Lian, L.; Petrich, B.G.; Monkley, S.J.; Kanaho, Y.; Critchley, D.R.; Sasaki, T.; et al. Loss of PIP5KIγ, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J. Clin. Investig. 2008, 118, 812–819. [Google Scholar] [CrossRef]
- Chung, J.; Torta, F.; Masai, K.; Lucast, L.; Czapla, H.; Tanner, L.B.; Narayanaswamy, P.; Wenk, M.R.; Nakatsu, F.; De Camilli, P. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER–plasma membrane contacts. Science 2015, 349, 428–432. [Google Scholar] [CrossRef]
- Hammond, G.R.; Machner, M.P.; Balla, T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 2014, 205, 113–126. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, J.; Sun, H.Q.; Martinez, M.; Sun, Y.X.; Macia, E.; Kirchhausen, T.; Albanesi, J.P.; Roth, M.G.; Yin, H.L. Phosphatidylinositol 4 Phosphate Regulates Targeting of Clathrin Adaptor AP-1 Complexes to the Golgi. Cell 2003, 114, 299–310. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.-Q.; Macia, E.; Kirchhausen, T.; Watson, H.; Bonifacino, J.S.; Yin, H.L. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-golgi network and regulates their recognition of the ubiquitin sorting signal. Mol. Biol. Cell 2007, 18, 2646–2655. [Google Scholar] [CrossRef]
- Godi, A.; DI Campli, A.; Konstantakopoulos, A.; Di Tullio, G.; Alessi, D.; Kular, G.S.; Daniele, T.; Marra, P.; Lucocq, J.; De Matteis, M.A. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 2004, 6, 393–404. [Google Scholar] [CrossRef]
- Hammond, G.R.V.; Schiavo, G.; Irvine, R.F. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P2. Biochem. J. 2009, 422, 23–35. [Google Scholar] [CrossRef]
- Cheong, F.Y.; Sharma, V.; Blagoveshchenskaya, A.; Oorschot, V.M.J.; Brankatschk, B.; Klumperman, J.; Freeze, H.H.; Mayinger, P. Spatial Regulation of Golgi Phosphatidylinositol-4-Phosphate is Required for Enzyme Localization and Glycosylation Fidelity. Traffic 2010, 11, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Szentpetery, Z.; Várnai, P.; Balla, T. Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 8225–8230. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.R.V.; Fischer, M.J.; Anderson, K.E.; Holdich, J.; Koteci, A.; Balla, T.; Irvine, R.F. PI4P and PI(4,5)P2 Are Essential But Independent Lipid Determinants of Membrane Identity. Science 2012, 337, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.-C.; Hille, B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005, 15, 370–378. [Google Scholar] [CrossRef]
- Mesmin, B.; Bigay, J.; von Filseck, J.M.; Lacas-Gervais, S.; Drin, G.; Antonny, B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 2013, 155, 830–843. [Google Scholar] [CrossRef]
- Prinz, W.A. Bridging the gap: Membrane contact sites in signaling, metabolism, and organelle dynamics. J. Cell Biol. 2014, 205, 759–769. [Google Scholar] [CrossRef]
- Dickson, E.J.; Jensen, J.B.; Vivas, O.; Kruse, M.; Traynor-Kaplan, A.E.; Hille, B. Dynamic formation of ER–PM junctions presents a lipid phosphatase to regulate phosphoinositides. J. Cell Biol. 2016, 213, 33–48. [Google Scholar] [CrossRef]
- Watt, S.A.; Kular, G.; Fleming, I.N.; Downes, P.; Lucocq, J.M. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ. Biochem. J. 2002, 363, 657–666. [Google Scholar] [CrossRef]
- Cremona, O.; Di Paolo, G.; Wenk, M.R.; Lüthi, A.; Kim, W.T.; Takei, K.; Daniell, L.; Nemoto, Y.; Shears, S.B.; Flavell, R.; et al. Essential Role of Phosphoinositide Metabolism in Synaptic Vesicle Recycling. Cell 1999, 99, 179–188. [Google Scholar] [CrossRef]
- Sobol, M.; Yildirim, S.; Philimonenko, V.V.; Marášek, P.; Castaño, E.; Hozák, P. UBF complexes with phosphatidylinositol 4,5-bisphosphate in nucleolar organizer regions regardless of ongoing RNA polymerase I activity. Nucleus 2013, 4, 478–486. [Google Scholar] [CrossRef]
- Haucke, V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem. Soc. Trans. 2005, 33, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Padrón, D.; Wang, Y.J.; Yamamoto, M.; Yin, H.; Roth, M.G. Phosphatidylinositol phosphate 5-kinase Iβ recruits AP-2 to the plasma membrane and regulates rates of constitutive endocytosis. J. Cell Biol. 2003, 162, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Vicinanza, M.; Di Campli, A.; Polishchuk, E.; Santoro, M.; Di Tullio, G.; Godi, A.; Levtchenko, E.; De Leo, M.G.; Polishchuk, R.; Sandoval, L.; et al. OCRL controls trafficking through early endosomes via PtdIns4,5P2-dependent regulation of endosomal actin. EMBO J. 2011, 30, 4970–4985. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.F. Role of PI(4,5)P2 in vesicle exocytosis and membrane fusion. Subcell Biochem 2012, 59, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Gamper, N.; Shapiro, M.S. Target-specific PIP2 signalling: How might it work? J. Physiol. 2007, 582, 967–975. [Google Scholar] [CrossRef]
- Suh, B.-C.; Hille, B. PIP2 is a necessary cofactor for ion channel function: How and why? Annu. Rev. Biophys. 2008, 37, 175–195. [Google Scholar] [CrossRef]
- Idevall-Hagren, O.; De Camilli, P. Detection and manipulation of phosphoinositides. Biochim. Biophys. Acta 2015, 1851, 736–745. [Google Scholar] [CrossRef]
- Kalasova, I.; Fáberová, V.; Kalendová, A.; Yildirim, S.; Uličná, L.; Venit, T.; Hozák, P. Tools for visualization of phosphoinositides in the cell nucleus. Histochem. Cell Biol. 2016, 145, 485–496. [Google Scholar] [CrossRef]
- Bura, A.; Jurak Begonja, A. Imaging of Intracellular and Plasma Membrane Pools of PI(4,5)P2 and PI4P in Human Platelets. Life 2021, 11, 1331. [Google Scholar] [CrossRef]
- Wills, R.C.; Goulden, B.D.; Hammond, G.R.V. Genetically encoded lipid biosensors. Mol. Biol. Cell 2018, 29, 1526–1532. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Y.; Xu, P.; Xu, T.; Lou, X. Nanoscale Landscape of Phosphoinositides Revealed by Specific Pleckstrin Homology (PH) Domains Using Single-molecule Superresolution Imaging in the Plasma Membrane. J. Biol. Chem. 2015, 290, 26978–26993. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Levine, T. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J. Biol. Chem. 2004, 279, 44683–44689. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.P.; Munro, S. The pleckstrin homology domain of oxysterol-binding protein recognises a determinant specific to Golgi membranes. Curr. Biol. 1998, 8, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, T.P.; Ahn, S.; Meyer, T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr. Biol. 1998, 8, 343–346. [Google Scholar] [CrossRef]
- Santagata, S.; Boggon, T.J.; Baird, C.L.; Gomez, C.A.; Zhao, J.; Shan, W.S.; Myszka, D.G.; Shapiro, L. G-Protein Signaling through Tubby Proteins. Science 2001, 292, 2041–2050. [Google Scholar] [CrossRef]
- Leitner, M.G.; Thallmair, V.; Wilke, B.U.; Neubert, V.; Kronimus, Y.; Halaszovich, C.R.; Oliver, D. The N-terminal homology (ENTH) domain of Epsin 1 is a sensitive reporter of physiological PI(4,5)P2 dynamics. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1864, 433–442. [Google Scholar] [CrossRef]
- Ford, M.G.J.; Pearse, B.M.F.; Higgins, M.K.; Vallis, Y.; Owen, D.J.; Gibson, A.; Hopkins, C.R.; Evans, F.R.; McMahon, H.T. Simultaneous Binding of PtdIns(4,5)P2 and Clathrin by AP180 in the Nucleation of Clathrin Lattices on Membranes. Science 2001, 291, 1051–1055. [Google Scholar] [CrossRef]
- Hllet, G. Imaging phosphoinositide dynamics using GFP-tagged protein domain. Biol. Cell 2005, 97, 501–518. [Google Scholar] [CrossRef]
- Putney, J.W.; Tomita, T. Phospholipase C signaling and calcium influx. Adv. Biol. Regul. 2012, 52, 152–164. [Google Scholar] [CrossRef]
- Kim, Y.; Shanta, S.R.; Zhou, L.-H.; Kim, K.P. Mass spectrometry based cellular phosphoinositides profiling and phospholipid analysis: A brief review. Exp. Mol. Med. 2010, 42, 1–11. [Google Scholar] [CrossRef]
- Wakelam, M.J.; Clark, J. Methods for analyzing phosphoinositides using mass spectrometry. Biochim. Biophys. Acta 2011, 1811, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Kielkowska, A.; Niewczas, I.; Anderson, K.E.; Durrant, T.N.; Clark, J.; Stephens, L.R.; Hawkins, P.T. A new approach to measuring phosphoinositides in cells by mass spectrometry. Adv. Biol. Regul. 2014, 54, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.Y.F.; Coman, C.; Westhoff, P.; Manke, M.; Sickmann, A.; Borst, O.; Gawaz, M.; Watson, S.P.; Heemskerk, J.W.M.; Ahrends, R. Targeted Phosphoinositides Analysis Using High-Performance Ion Chromatography-Coupled Selected Reaction Monitoring Mass Spectrometry. J. Proteome Res. 2021, 20, 3114–3123. [Google Scholar] [CrossRef] [PubMed]

| Target Enzyme | Inhibitor | Target IC50 (nM) | Off-Targets, IC50 (nM) | State of Clinical Development | Research Area | References |
|---|---|---|---|---|---|---|
| PI4KIIα | PI-273 | 470 [48] | no off-targets, highly selective | in vitro | cancer | [48,67] |
| in vivo | [48] | |||||
| PI4KIIIα | GSK-A1 | 3.1 [15] | >310 [15] | in vitro | basic | [68,69] |
| cancer | [70] | |||||
| viral infection | [71] | |||||
| inflammation | [72] | |||||
| neuronal plasticity | [73] | |||||
| myelination | [74] | |||||
| GSK-F1 | 16 [15] | PI4KIIIβ, PI3Ks >1600 [15] | in vitro | cancer | [75] | |
| in vivo | basic | [68] | ||||
| PI4KIIIβ | IN-9 | 7 [76] | PI4KIIIα, PI3Ks >150 [76] | in vitro | cancer | [67] |
| inflammation | [72] | |||||
| IN-10 | 3.6 [15] | PI4KIIIα, PI3Ks >720 [15,33] | in vitro | inflammation | [72] | |
| T-00127-HEV1 | 60 [32] | PI4KIIIα, PIK3CD 10 000 [32] | in vitro | viral infection | [77,78,79] | |
| in vivo | [78] | |||||
| BF738735 | 5.7 [32] | PI4KIIIα 1700 [33] | in vitro | viral infection | [79,80] | |
| Enviroxime | 120 [32] | PI4KIIIα 1400 [32] | discontinued in phase II clinical trials | viral infection | [15,81] | |
| Plasmodium falciparum PI4KIIIβ | MMV390048 | 28 [82] | no off-targets, highly selective | terminated phase II clinical trials | parasitic infection | [15,83] |
| PI4P5KIα | ISA-2011B | n.d. [15] | p110α n.d. [15] | in vitro | inflammation | [84] |
| cancer | [85,86] | |||||
| in vivo | cancer | [85,86] | ||||
| PI4P5KIγ | UNC3230 | 51 (Kd) [15] | PI5P4Kγ 4 (Kd) [15] | in vitro | cancer | [87] |
| PI | Protein Domain | Intracellular Localization |
|---|---|---|
| PI4P | OSH2-PH [144] | PM |
| OSBP-PH [145] | Golgi | |
| FAPP1-PH [121] | Golgi | |
| P4M SidM [118] | Golgi, PM, LE/Lys | |
| PI(4,5)P2 | PLCδ1-PH [146] | PM |
| Tubby-PX [147] | PM | |
| Epsin1-ENTH/AP180-ANTH [148,149] | PM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bura, A.; Čabrijan, S.; Đurić, I.; Bruketa, T.; Jurak Begonja, A. A Plethora of Functions Condensed into Tiny Phospholipids: The Story of PI4P and PI(4,5)P2. Cells 2023, 12, 1411. https://doi.org/10.3390/cells12101411
Bura A, Čabrijan S, Đurić I, Bruketa T, Jurak Begonja A. A Plethora of Functions Condensed into Tiny Phospholipids: The Story of PI4P and PI(4,5)P2. Cells. 2023; 12(10):1411. https://doi.org/10.3390/cells12101411
Chicago/Turabian StyleBura, Ana, Sara Čabrijan, Iris Đurić, Tea Bruketa, and Antonija Jurak Begonja. 2023. "A Plethora of Functions Condensed into Tiny Phospholipids: The Story of PI4P and PI(4,5)P2" Cells 12, no. 10: 1411. https://doi.org/10.3390/cells12101411
APA StyleBura, A., Čabrijan, S., Đurić, I., Bruketa, T., & Jurak Begonja, A. (2023). A Plethora of Functions Condensed into Tiny Phospholipids: The Story of PI4P and PI(4,5)P2. Cells, 12(10), 1411. https://doi.org/10.3390/cells12101411