Rescue of Misfolded Organic Cation Transporter 3 Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cell Culture
2.3. Radiotracer Uptake Assays
2.4. Confocal Microscopy
2.5. Immunoblotting (IB)
2.6. Data and Statistical Analysis
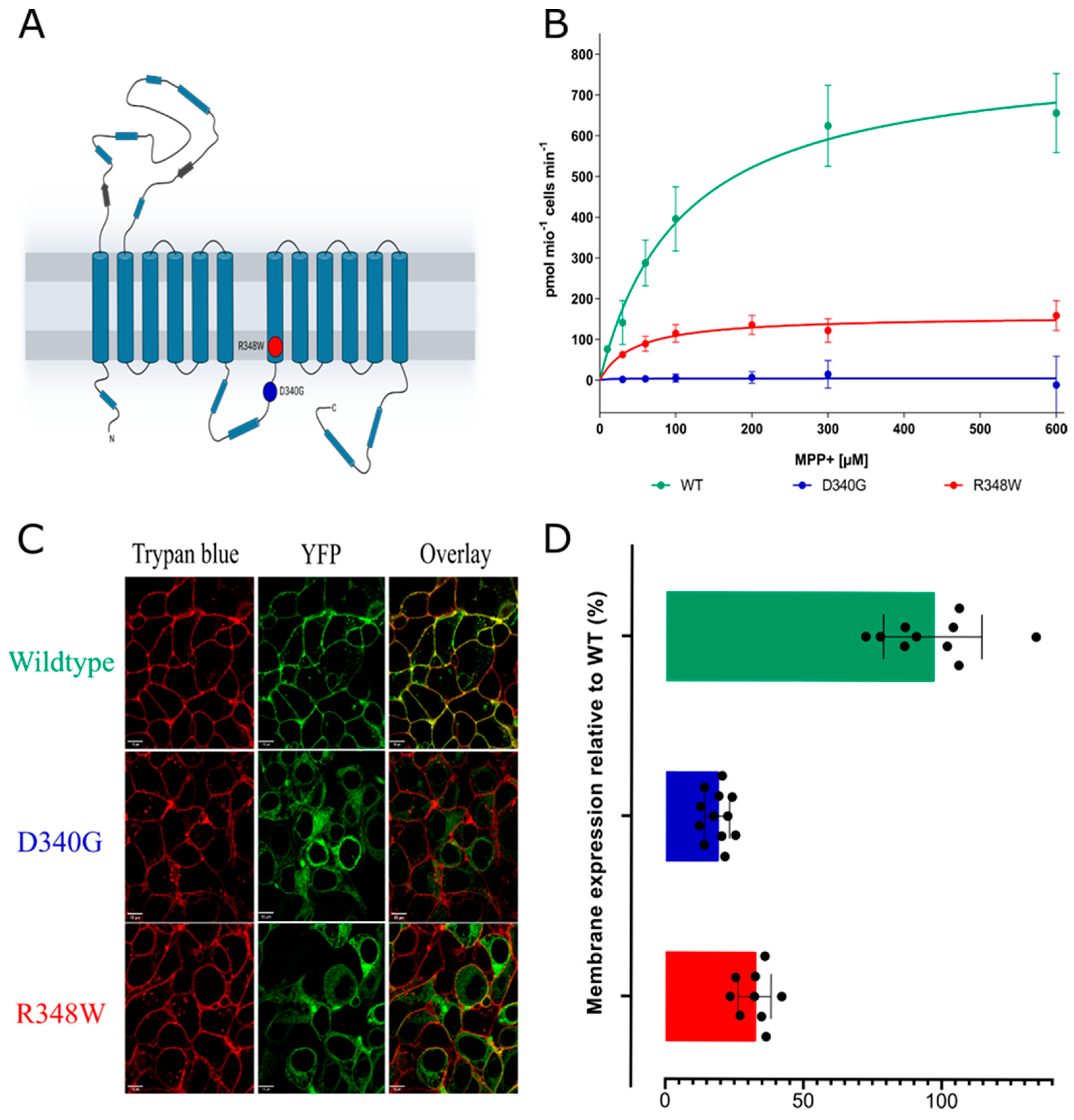
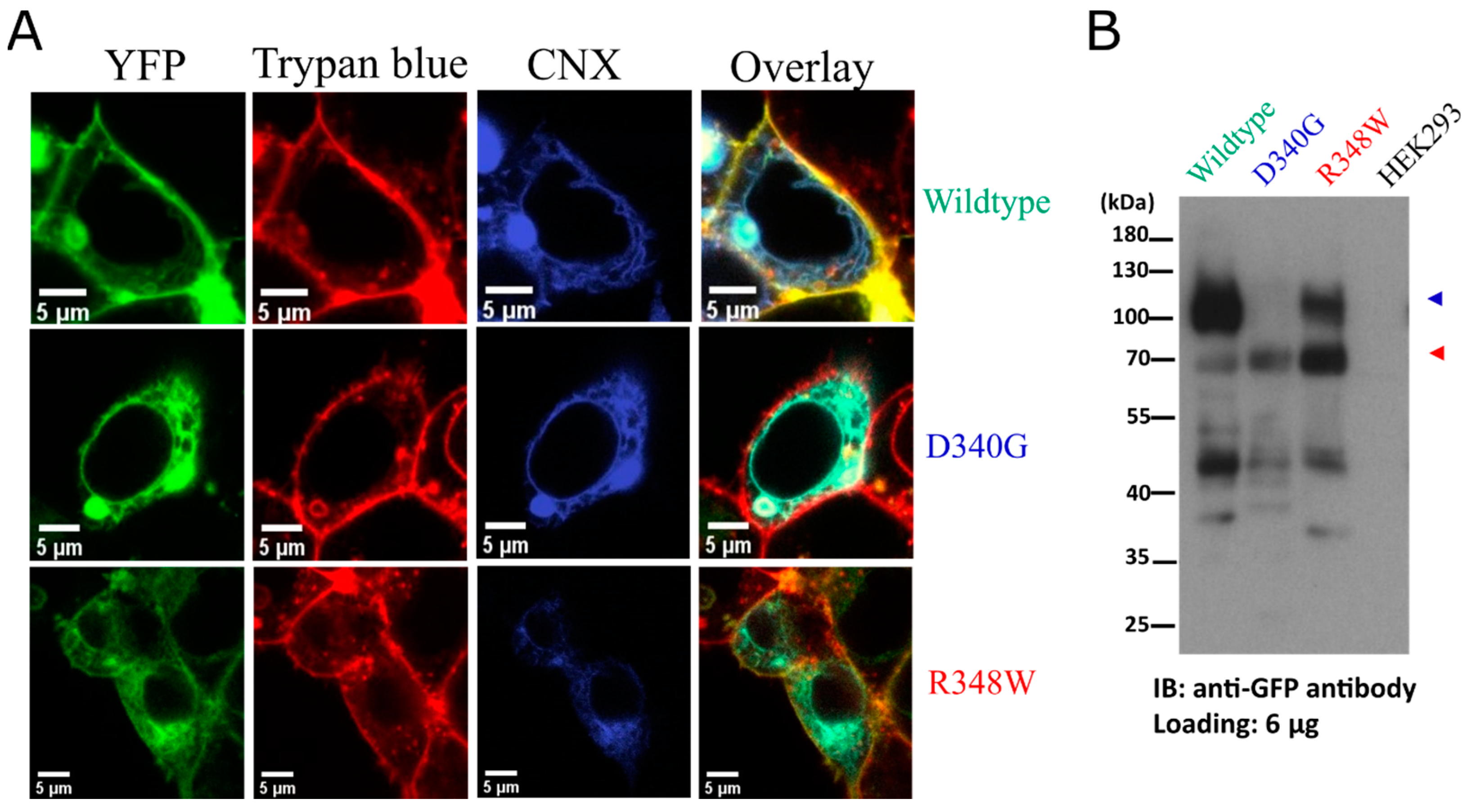
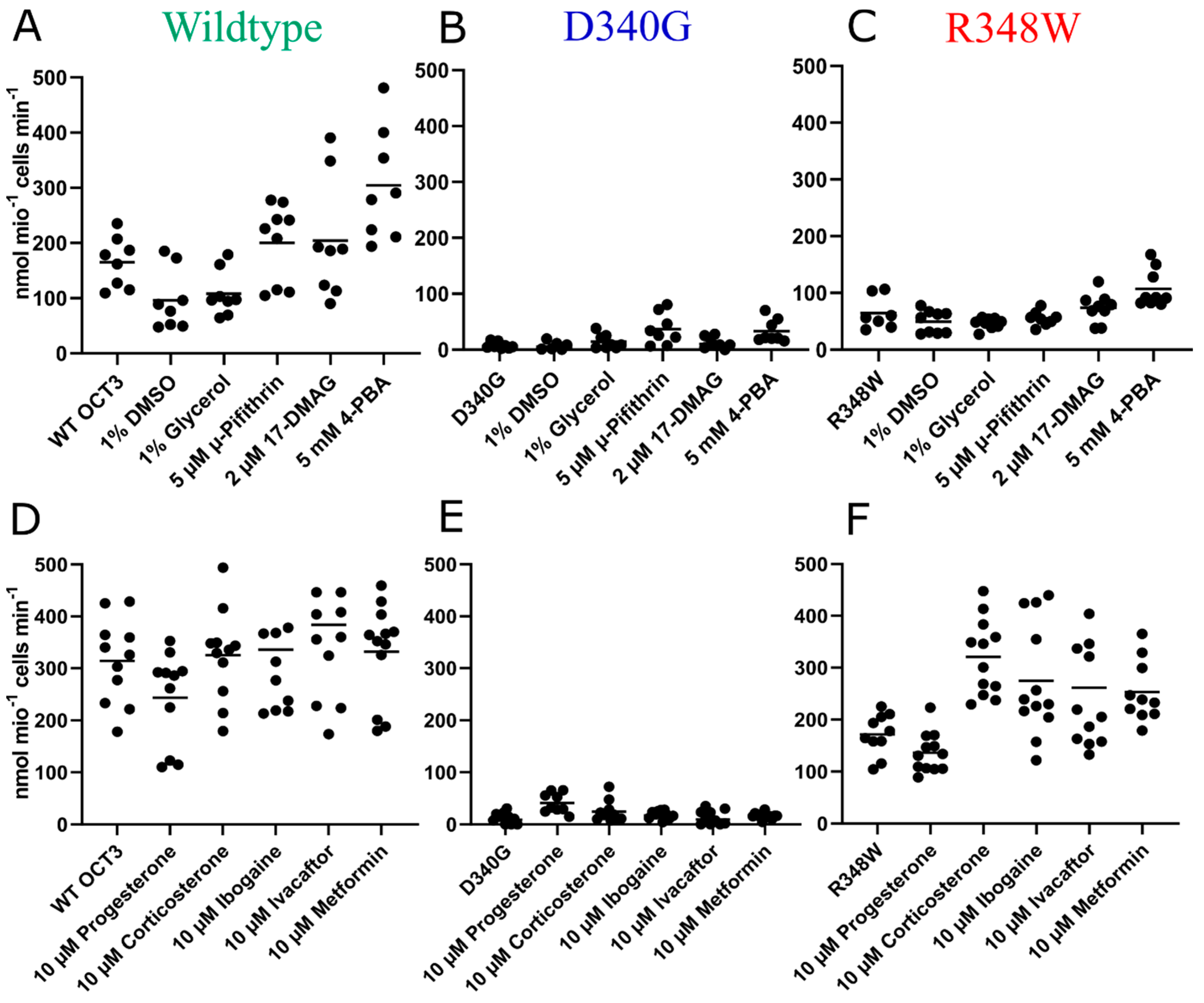
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef] [PubMed]
- Courousse, T.; Gautron, S. Role of organic cation transporters (OCTs) in the brain. Pharmacol. Ther. 2015, 146, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.J. Organic Cation Transporters in Brain Catecholamine Homeostasis. Handb. Exp. Pharmacol. 2021, 266, 187–197. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, S.; Shu, Y. Drug-Drug Interactions at Organic Cation Transporter 1. Front. Pharmacol. 2021, 12, 628705. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.C.; Matsson, P.; Azimi, M.; Zhou, X.; Handin, N.; Yee, S.W.; Artursson, P.; Giacomini, K.M. High Throughput Screening of a Prescription Drug Library for Inhibitors of Organic Cation Transporter 3, OCT3. Pharm. Res. 2022, 39, 1599–1613. [Google Scholar] [CrossRef]
- Saad, A.A.A.; Zhang, F.; Refat, M.; Mohammed, E.A.H.; Zhang, M.; Chen, Y.; Al Hamyari, B.; Alafifi, J.; Wu, X.a. Tamsulosin alters the pharmacokinetics of metformin via inhibition of renal multidrug and toxin extrusion protein 1 and organic cation transporter 2 in rats. J. Pharm. Biomed. Anal. 2022, 212, 114666. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Li, M.; Zhao, M.; Reddy Gopireddy, R.; Teoh, J.P.; Xu, B.; Zhu, C.; Ireton, K.E.; Srinivasan, S.; et al. Intracellular β(1)-Adrenergic Receptors and Organic Cation Transporter 3 Mediate Phospholamban Phosphorylation to Enhance Cardiac Contractility. Circ. Res. 2021, 128, 246–261. [Google Scholar] [CrossRef]
- Song, W.; Luo, Q.; Zhang, Y.; Zhou, L.; Liu, Y.; Ma, Z.; Guo, J.; Huang, Y.; Cheng, L.; Meng, Z.; et al. Organic cation transporter 3 (Oct3) is a distinct catecholamines clearance route in adipocytes mediating the beiging of white adipose tissue. PLoS Biol. 2019, 17, e2006571. [Google Scholar] [CrossRef]
- Huang, K.M.; Zavorka Thomas, M.; Magdy, T.; Eisenmann, E.D.; Uddin, M.E.; DiGiacomo, D.F.; Pan, A.; Keiser, M.; Otter, M.; Xia, S.H.; et al. Targeting OCT3 attenuates doxorubicin-induced cardiac injury. Proc. Natl. Acad. Sci. USA 2021, 118, e2020168118. [Google Scholar] [CrossRef]
- Gu, J.; Wang, L.; Li, T.; Tang, S.; Wang, Y.; Zhang, W.; Jiang, X. Role and mechanism of organic cation transporter 3 in oxaliplatin treatment of colon cancer in vitro and in vivo. Oncol. Rep. 2019, 42, 1355–1364. [Google Scholar] [CrossRef]
- Li, Q.; Peng, X.; Yang, H.; Rodriguez, J.-A.; Shu, Y. Contribution of Organic Cation Transporter 3 to Cisplatin Cytotoxicity in Human Cervical Cancer Cells. J. Pharm. Sci. 2012, 101, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Vialou, V.; Balasse, L.; Callebert, J.; Launay, J.M.; Giros, B.; Gautron, S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J. Neurochem. 2008, 106, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.J.; Hurley, M.M.; Chan, J.; Pickel, V.M. Organic cation transporter 3 (OCT3) is localized to intracellular and surface membranes in select glial and neuronal cells within the basolateral amygdaloid complex of both rats and mice. Brain Struct. Funct. 2017, 222, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.J.; Lowry, C.A.; Orchinik, M. Corticosterone-Sensitive Monoamine Transport in the Rat Dorsomedial Hypothalamus: Potential Role for Organic Cation Transporter 3 in Stress-Induced Modulation of Monoaminergic Neurotransmission. J. Neurosci. 2006, 26, 8758. [Google Scholar] [CrossRef]
- Maier, J.; Niello, M.; Rudin, D.; Daws, L.C.; Sitte, H.H. The Interaction of Organic Cation Transporters 1-3 and PMAT with Psychoactive Substances. Handb. Exp. Pharmacol. 2021, 266, 199–214. [Google Scholar] [CrossRef]
- Daws, L.C. Organic Cation Transporters in Psychiatric Disorders. Handb. Exp. Pharmacol. 2021, 266, 215–239. [Google Scholar] [CrossRef]
- Conn, P.M.; Ulloa-Aguirre, A.; Ito, J.; Janovick, J.A. G Protein-Coupled Receptor Trafficking in Health and Disease: Lessons Learned to Prepare for Therapeutic Mutant Rescue in Vivo. Pharmacol. Rev. 2007, 59, 225. [Google Scholar] [CrossRef]
- Freissmuth, M.; Stockner, T.; Sucic, S. SLC6 Transporter Folding Diseases and Pharmacochaperoning. Handb. Exp. Pharmacol. 2018, 245, 249–270. [Google Scholar] [CrossRef]
- Kasture, A.; Stockner, T.; Freissmuth, M.; Sucic, S. An unfolding story: Small molecules remedy misfolded monoamine transporters. Int. J. Biochem. Cell Biol. 2017, 92, 1–5. [Google Scholar] [CrossRef]
- Bhat, S.; El-Kasaby, A.; Freissmuth, M.; Sucic, S. Functional and Biochemical Consequences of Disease Variants in Neurotransmitter Transporters: A Special Emphasis on Folding and Trafficking Deficits. Pharmacol. Ther. 2021, 222, 107785. [Google Scholar] [CrossRef]
- El-Kasaby, A.; Koban, F.; Sitte, H.H.; Freissmuth, M.; Sucic, S. A cytosolic relay of heat shock proteins HSP70-1A and HSP90β monitors the folding trajectory of the serotonin transporter. J. Biol. Chem. 2014, 289, 28987–29000. [Google Scholar] [CrossRef] [PubMed]
- El-Kasaby, A.; Just, H.; Malle, E.; Stolt-Bergner, P.C.; Sitte, H.H.; Freissmuth, M.; Kudlacek, O. Mutations in the carboxyl-terminal SEC24 binding motif of the serotonin transporter impair folding of the transporter. J. Biol. Chem. 2010, 285, 39201–39210. [Google Scholar] [CrossRef] [PubMed]
- Ponleitner, M.; Szöllősi, D.; El-Kasaby, A.; Koban, F.; Freissmuth, M.; Stockner, T. Thermal Unfolding of the Human Serotonin Transporter: Differential Effect by Stabilizing and Destabilizing Mutations and Cholesterol on Thermodynamic and Kinetic Stability. Mol. Pharmacol. 2022, 101, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Koban, F.; El-Kasaby, A.; Häusler, C.; Stockner, T.; Simbrunner, B.M.; Sitte, H.H.; Freissmuth, M.; Sucic, S. A salt bridge linking the first intracellular loop with the C terminus facilitates the folding of the serotonin transporter. J. Biol. Chem. 2015, 290, 13263–13278. [Google Scholar] [CrossRef] [PubMed]
- El-Kasaby, A.; Kasture, A.; Koban, F.; Hotka, M.; Asjad, H.M.M.; Kubista, H.; Freissmuth, M.; Sucic, S. Rescue by 4-phenylbutyrate of several misfolded creatine transporter-1 variants linked to the creatine transporter deficiency syndrome. Neuropharmacology 2019, 161, 107572. [Google Scholar] [CrossRef]
- Bhat, S.; Guthrie, D.A.; Kasture, A.; El-Kasaby, A.; Cao, J.; Bonifazi, A.; Ku, T.; Giancola, J.B.; Hummel, T.; Freissmuth, M.; et al. Tropane-Based Ibogaine Analog Rescues Folding-Deficient Serotonin and Dopamine Transporters. ACS Pharmacol. Transl. Sci. 2021, 4, 503–516. [Google Scholar] [CrossRef]
- Ng, J.; Barral, S.; De La Fuente Barrigon, C.; Lignani, G.; Erdem, F.A.; Wallings, R.; Privolizzi, R.; Rossignoli, G.; Alrashidi, H.; Heasman, S.; et al. Gene therapy restores dopamine transporter expression and ameliorates pathology in iPSC and mouse models of infantile parkinsonism. Sci. Transl. Med. 2021, 13, 1564. [Google Scholar] [CrossRef]
- Asjad, H.M.M.; Kasture, A.; El-Kasaby, A.; Sackel, M.; Hummel, T.; Freissmuth, M.; Sucic, S. Pharmacochaperoning in a Drosophila model system rescues human dopamine transporter variants associated with infantile/juvenile parkinsonism. J. Biol. Chem. 2017, 292, 19250–19265. [Google Scholar] [CrossRef]
- Sutton, C.; Williams, E.Q.; Homsi, H.; Beerepoot, P.; Nazari, R.; Han, D.; Ramsey, A.J.; Mash, D.C.; Olson, D.E.; Blough, B.; et al. Structure-Activity Relationships of Dopamine Transporter Pharmacological Chaperones. Front. Cell Neurosci. 2022, 16, 832536. [Google Scholar] [CrossRef]
- Beerepoot, P.; Lam, V.M.; Salahpour, A. Pharmacological Chaperones of the Dopamine Transporter Rescue Dopamine Transporter Deficiency Syndrome Mutations in Heterologous Cells. J. Biol. Chem. 2016, 291, 22053–22062. [Google Scholar] [CrossRef]
- Fischer, F.P.; Kasture, A.S.; Hummel, T.; Sucic, S. Molecular and Clinical Repercussions of GABA Transporter 1 Variants Gone Amiss: Links to Epilepsy and Developmental Spectrum Disorders. Front. Mol. Biosci. 2022, 9, 834498. [Google Scholar] [CrossRef] [PubMed]
- Kasture, A.; El-Kasaby, A.; Szöllősi, D.; Asjad, H.M.M.; Grimm, A.; Stockner, T.; Hummel, T.; Freissmuth, M.; Sucic, S. Functional Rescue of a Misfolded Drosophila melanogaster Dopamine Transporter Mutant Associated with a Sleepless Phenotype by Pharmacological Chaperones. J. Biol. Chem. 2016, 291, 20876–20890. [Google Scholar] [CrossRef] [PubMed]
- de la Rocha-Muñoz, A.; Melgarejo, E.; Aragón, C.; López-Corcuera, B. Rescue of two trafficking-defective variants of the neuronal glycine transporter GlyT2 associated to hyperekplexia. Neuropharmacology 2021, 189, 108543. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Newman, A.H.; Freissmuth, M. How to rescue misfolded SERT, DAT and NET: Targeting conformational intermediates with atypical inhibitors and partial releasers. Biochem. Soc. Trans. 2019, 47, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.B.; Yasothan, U.; Kirkpatrick, P. Ivacaftor. Nat. Rev. Drug Discov. 2012, 11, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Janovick, J.A.; Spicer, T.P.; Bannister, T.D.; Smith, E.; Ganapathy, V.; Scampavia, L. Chemical validation and optimization of pharmacoperones targeting vasopressin type 2 receptor mutant. Biochem. J. 2018, 475, 2941–2953. [Google Scholar] [CrossRef]
- Said, G.; Grippon, S.; Kirkpatrick, P. Tafamidis. Nat. Rev. Drug Discov. 2012, 11, 185–186. [Google Scholar] [CrossRef]
- Khanppnavar, B.; Maier, J.; Herborg, F.; Gradisch, R.; Lazzarin, E.; Luethi, D.; Yang, J.-W.; Qi, C.; Holy, M.; Jäntsch, K.; et al. Structural basis of organic cation transporter-3 inhibition. Nat. Commun. 2022, 13, 6714. [Google Scholar] [CrossRef]
- Angenoorth, T.J.F.; Stankovic, S.; Niello, M.; Holy, M.; Brandt, S.D.; Sitte, H.H.; Maier, J. Interaction Profiles of Central Nervous System Active Drugs at Human Organic Cation Transporters 1–3 and Human Plasma Membrane Monoamine Transporter. Int. J. Mol. Sci. 2021, 22, 12995. [Google Scholar] [CrossRef]
- Korkhov, V.M.; Milan-Lobo, L.; Zuber, B.; Farhan, H.; Schmid, J.A.; Freissmuth, M.; Sitte, H.H. Peptide-based interactions with calnexin target misassembled membrane proteins into endoplasmic reticulum-derived multilamellar bodies. J. Mol. Biol. 2008, 378, 337–352. [Google Scholar] [CrossRef]
- Sucic, S.; Kasture, A.; Mazhar Asjad, H.M.; Kern, C.; El-Kasaby, A.; Freissmuth, M. When transporters fail to be transported: How to rescue folding-deficient SLC6 transporters. J. Neurol. Neuromed. 2016, 1, 34–40. [Google Scholar] [CrossRef]
- Mofo Mato, E.P.; Guewo-Fokeng, M.; Essop, M.F.; Owira, P.M.O. Genetic polymorphisms of organic cation transporter 1 (OCT1) and responses to metformin therapy in individuals with type 2 diabetes: A systematic review. Medicine 2018, 97, e11349. [Google Scholar] [CrossRef] [PubMed]
- Leabman, M.K.; Huang, C.C.; Stryke, D.; Johns, S.J.; Kawamoto, M.; Ferrin, T.E.; DeYoung, J.; Taylor, T.R.; De La Cruz, M.; Herskowitz, I.; et al. PharmGKB Update: I. Genetic Variants of the Organic Cation Transporter 2 (OCT2, SLC22A2). Pharmacol. Rev. 2003, 55, 399. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kekuda, R.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Chen, J.; Conway, S.J.; Ganapathy, V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J. Biol. Chem. 1998, 273, 32776–32786. [Google Scholar] [CrossRef] [PubMed]
- Marinko, J.T.; Huang, H.; Penn, W.D.; Capra, J.A.; Schlebach, J.P.; Sanders, C.R. Folding and Misfolding of Human Membrane Proteins in Health and Disease: From Single Molecules to Cellular Proteostasis. Chem. Rev. 2019, 119, 5537–5606. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.S.; Ayaub, E.A.; Zhou, W.; Yum, V.; Dickhout, J.G.; Ask, K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Rubenstein, R.C.; Zeitlin, P.L. Sodium 4-phenylbutyrate downregulates Hsc70: Implications for intracellular trafficking of DeltaF508-CFTR. Am. J. Physiol. Cell Physiol. 2000, 278, C259–C267. [Google Scholar] [CrossRef]
- Suaud, L.; Miller, K.; Panichelli, A.E.; Randell, R.L.; Marando, C.M.; Rubenstein, R.C. 4-Phenylbutyrate stimulates Hsp70 expression through the Elp2 component of elongator and STAT-3 in cystic fibrosis epithelial cells. J. Biol. Chem. 2011, 286, 45083–45092. [Google Scholar] [CrossRef]
- Wultsch, T.; Grimberg, G.; Schmitt, A.; Painsipp, E.; Wetzstein, H.; Breitenkamp, A.F.; Grundemann, D.; Schomig, E.; Lesch, K.P.; Gerlach, M.; et al. Decreased anxiety in mice lacking the organic cation transporter 3. J. Neural. Transm. 2009, 116, 689–697. [Google Scholar] [CrossRef]
- Gupta, S.; Wulf, G.; Henjakovic, M.; Koepsell, H.; Burckhardt, G.; Hagos, Y. Human Organic Cation Transporter 1 Is Expressed in Lymphoma Cells and Increases Susceptibility to Irinotecan and Paclitaxel. J. Pharmacol. Exp. Ther. 2012, 341, 16. [Google Scholar] [CrossRef] [PubMed]
- Naka, A.; Takeda, R.; Shintani, M.; Ogane, N.; Kameda, Y.; Aoyama, T.; Yoshikawa, T.; Kamoshida, S. Organic cation transporter 2 for predicting cisplatin-based neoadjuvant chemotherapy response in gastric cancer. Am. J. Cancer Res. 2015, 5, 2285–2293. [Google Scholar] [PubMed]
- Tashiro, A.; Tatsumi, S.; Takeda, R.; Naka, A.; Matsuoka, H.; Hashimoto, Y.; Hatta, K.; Maeda, K.; Kamoshida, S. High expression of organic anion transporter 2 and organic cation transporter 2 is an independent predictor of good outcomes in patients with metastatic colorectal cancer treated with FOLFOX-based chemotherapy. Am. J. Cancer Res. 2014, 4, 528–536. [Google Scholar] [PubMed]
- Tatsumi, S.; Matsuoka, H.; Hashimoto, Y.; Hatta, K.; Maeda, K.; Kamoshida, S. Organic cation transporter 2 and tumor budding as independent prognostic factors in metastatic colorectal cancer patients treated with oxaliplatin-based chemotherapy. Int. J. Clin. Exp. Pathol. 2013, 7, 204–212. [Google Scholar]
- Yokoo, S.; Masuda, S.; Yonezawa, A.; Terada, T.; Katsura, T.; Inui, K.-I. Significance of Organic Cation Transporter 3 (SLC22A3) Expression for the Cytotoxic Effect of Oxaliplatin in Colorectal Cancer. Drug Metab. Dispos. 2008, 36, 2299. [Google Scholar] [CrossRef]
- Kerb, R.; Brinkmann, U.; Chatskaia, N.; Gorbunov, D.; Gorboulev, V.; Mornhinweg, E.; Keil, A.; Eichelbaum, M.; Koepsell, H. Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics 2002, 12, 591–595. [Google Scholar] [CrossRef]
- Goswami, S.; Gong, L.; Giacomini, K.; Altman, R.B.; Klein, T.E. PharmGKB summary: Very important pharmacogene information for SLC22A1. Pharm. Genom. 2014, 24, 324–328. [Google Scholar] [CrossRef]
- Shu, Y.; Leabman, M.K.; Feng, B.; Mangravite, L.M.; Huang, C.C.; Stryke, D.; Kawamoto, M.; Johns, S.J.; DeYoung, J.; Carlson, E.; et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc. Natl. Acad. Sci. USA 2003, 100, 5902–5907. [Google Scholar] [CrossRef]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef]
- Sundelin, E.; Gormsen, L.C.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Christensen, M.; Brøsen, K.; Frøkiaer, J.; Jessen, N. Genetic Polymorphisms in Organic Cation Transporter 1 Attenuates Hepatic Metformin Exposure in Humans. Clin. Pharmacol. Ther. 2017, 102, 841–848. [Google Scholar] [CrossRef]
- Filipski, K.K.; Mathijssen, R.H.; Mikkelsen, T.S.; Schinkel, A.H.; Sparreboom, A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009, 86, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhang, D.; Lu, W.; Zheng, T.; Wan, L.; Li, Q.; Bao, Y.; Liu, F.; Jia, W. Polymorphism of Organic Cation Transporter 2 Improves Glucose-Lowering Effect of Metformin via Influencing Its Pharmacokinetics in Chinese Type 2 Diabetic Patients. Mol. Diagn. Ther. 2015, 19, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Nezu, J.-I.; Tamai, I.; Oku, A.; Ohashi, R.; Yabuuchi, H.; Hashimoto, N.; Nikaido, H.; Sai, Y.; Koizumi, A.; Shoji, Y.; et al. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat. Genet. 1999, 21, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.W.; Giacomini, K.M. Emerging Roles of the Human Solute Carrier 22 Family. Drug Metab. Dispos. 2021, 50, 1193–1210. [Google Scholar] [CrossRef] [PubMed]
- Koleske, M.L.; McInnes, G.; Brown, J.E.H.; Thomas, N.; Hutchinson, K.; Chin, M.Y.; Koehl, A.; Arkin, M.R.; Schlessinger, A.; Gallagher, R.C.; et al. Functional genomics of OCTN2 variants informs protein-specific variant effect predictor for Carnitine Transporter Deficiency. Proc. Natl. Acad. Sci. USA 2022, 119, e2210247119. [Google Scholar] [CrossRef] [PubMed]
- Magoulas, P.L.; El-Hattab, A.W. Systemic primary carnitine deficiency: An overview of clinical manifestations, diagnosis, and management. Orphanet J. Rare Dis. 2012, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]

| Transporter Variant | Untreated | 4-PBA Pre-Treated | ||
|---|---|---|---|---|
| Km (µM) | Vmax (pmol/106 cells/min) | Km (µM) | Vmax (pmol/106 cells/min) | |
| WT | 127.9 (100.7–162.7) | 798.6 (733.8–873.3) | 110.8 (84.79–136.8) | 1196 (1096–1296) |
| D340G | 249.6 (0.00–1405) | 21.54 (−24.58–67.65) | 574.0 (327.1–820.9) | 134.4 (100.3–168.6) |
| R348W | 46.68 (15.0–112.3) | 90.39 (71.41–116.1) | 99.06 (66.01–132.1) | 206.8 (182.9–230.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angenoorth, T.J.F.; Maier, J.; Stankovic, S.; Bhat, S.; Sucic, S.; Freissmuth, M.; Sitte, H.H.; Yang, J.-W. Rescue of Misfolded Organic Cation Transporter 3 Variants. Cells 2023, 12, 39. https://doi.org/10.3390/cells12010039
Angenoorth TJF, Maier J, Stankovic S, Bhat S, Sucic S, Freissmuth M, Sitte HH, Yang J-W. Rescue of Misfolded Organic Cation Transporter 3 Variants. Cells. 2023; 12(1):39. https://doi.org/10.3390/cells12010039
Chicago/Turabian StyleAngenoorth, Thomas J. F., Julian Maier, Stevan Stankovic, Shreyas Bhat, Sonja Sucic, Michael Freissmuth, Harald H. Sitte, and Jae-Won Yang. 2023. "Rescue of Misfolded Organic Cation Transporter 3 Variants" Cells 12, no. 1: 39. https://doi.org/10.3390/cells12010039
APA StyleAngenoorth, T. J. F., Maier, J., Stankovic, S., Bhat, S., Sucic, S., Freissmuth, M., Sitte, H. H., & Yang, J.-W. (2023). Rescue of Misfolded Organic Cation Transporter 3 Variants. Cells, 12(1), 39. https://doi.org/10.3390/cells12010039








