Morphological and Functional Effects of Ultrasound on Blood–Brain Barrier Transitory Opening: An In Vitro Study on Rat Brain Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
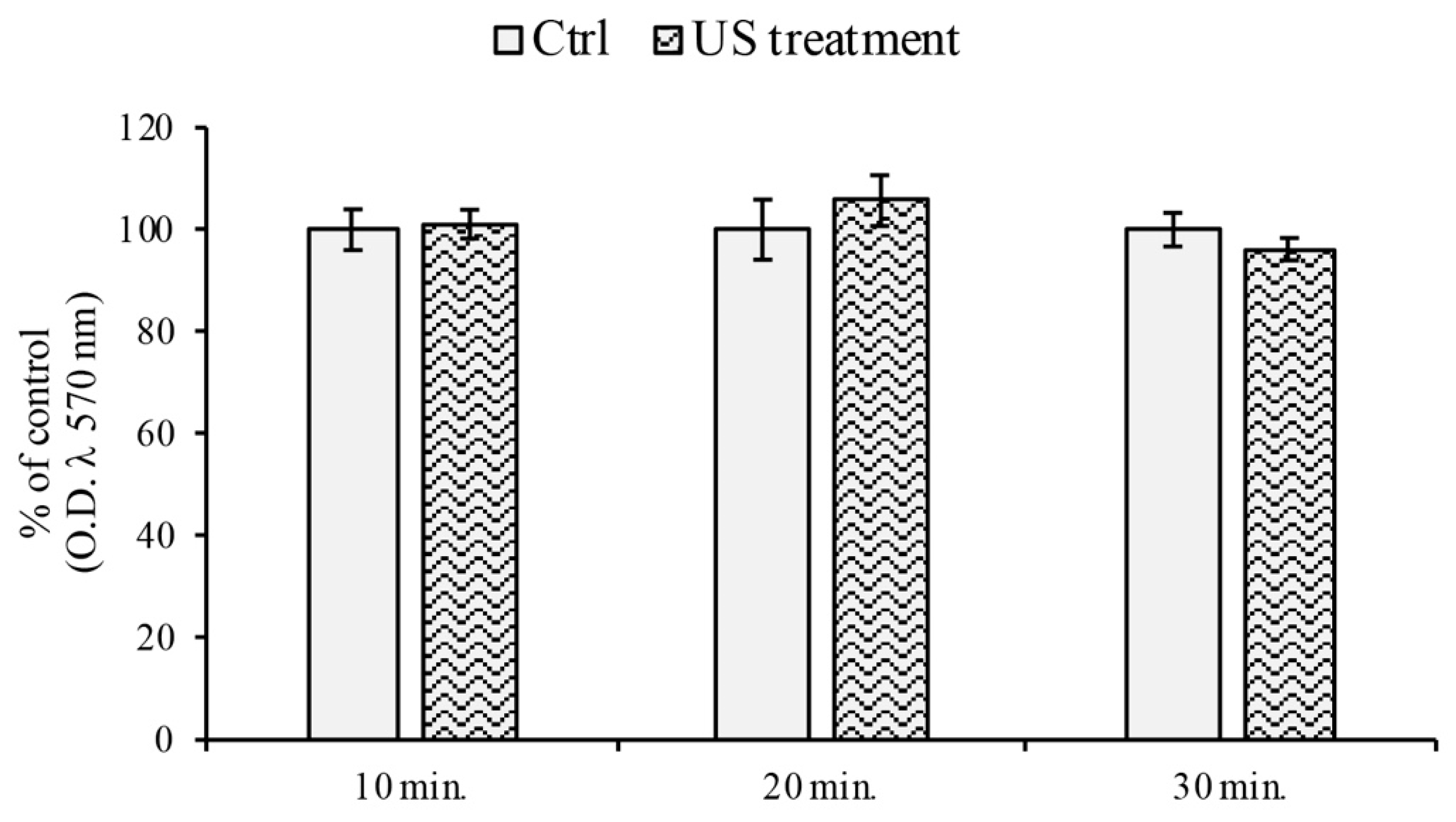
2.2. MTT Assay
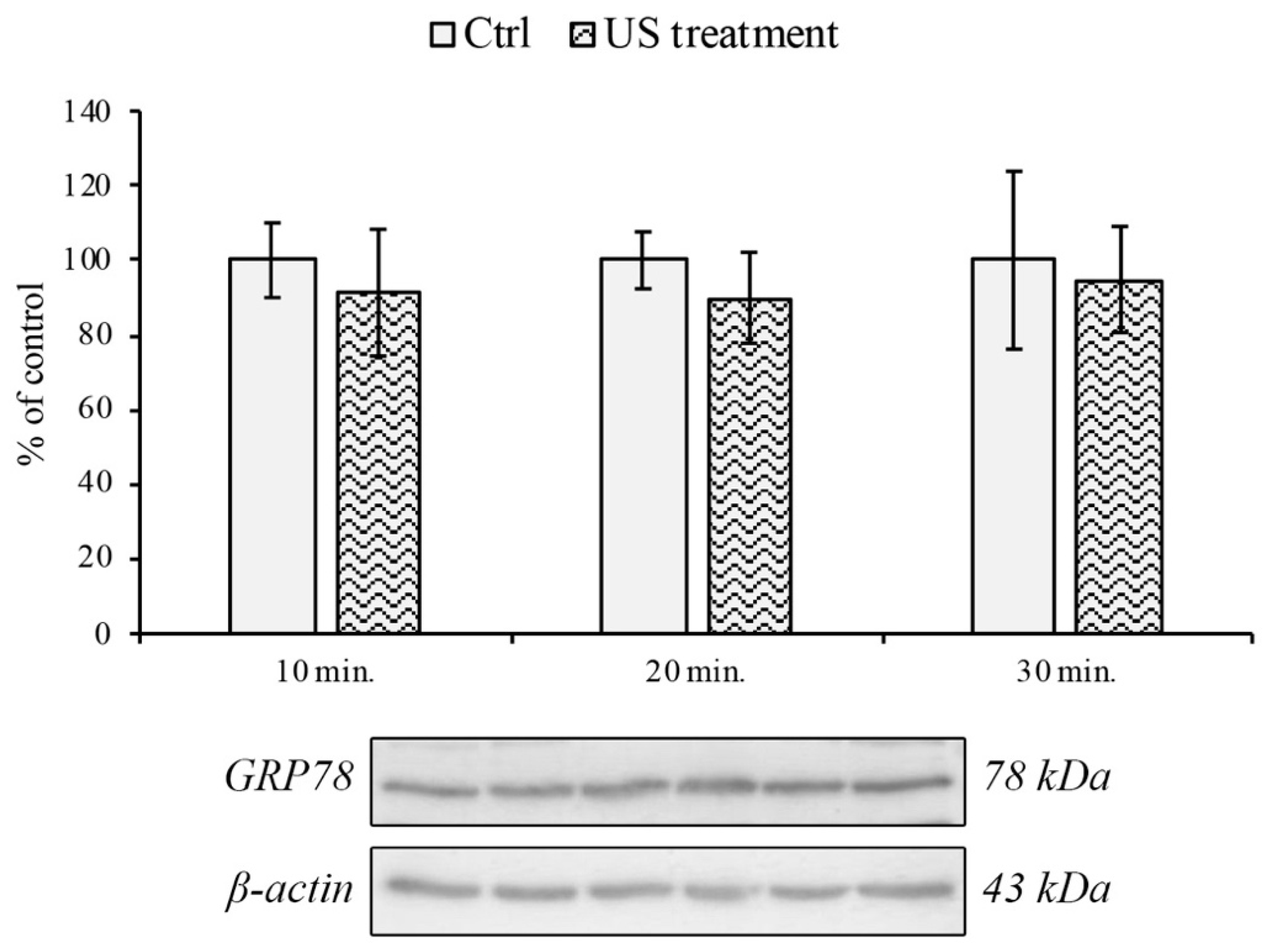
2.3. Western Blotting Analysis
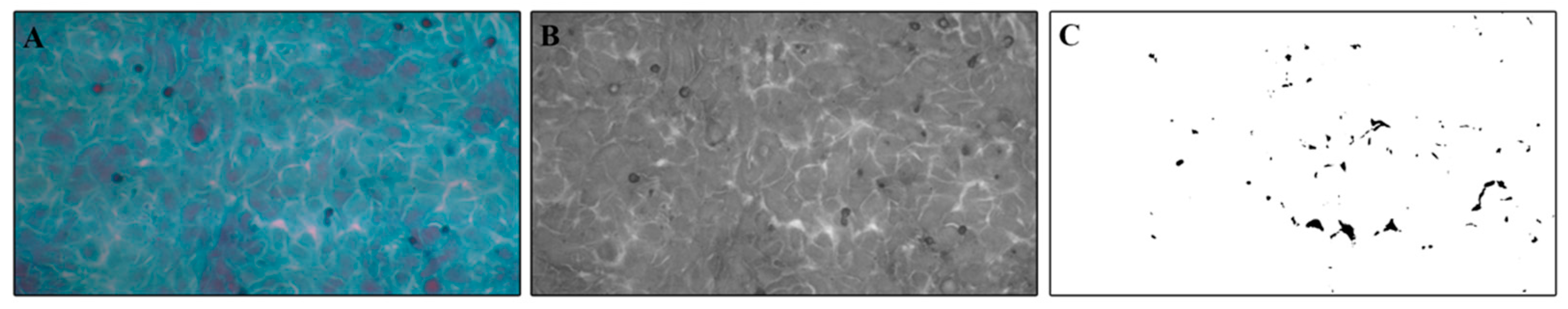
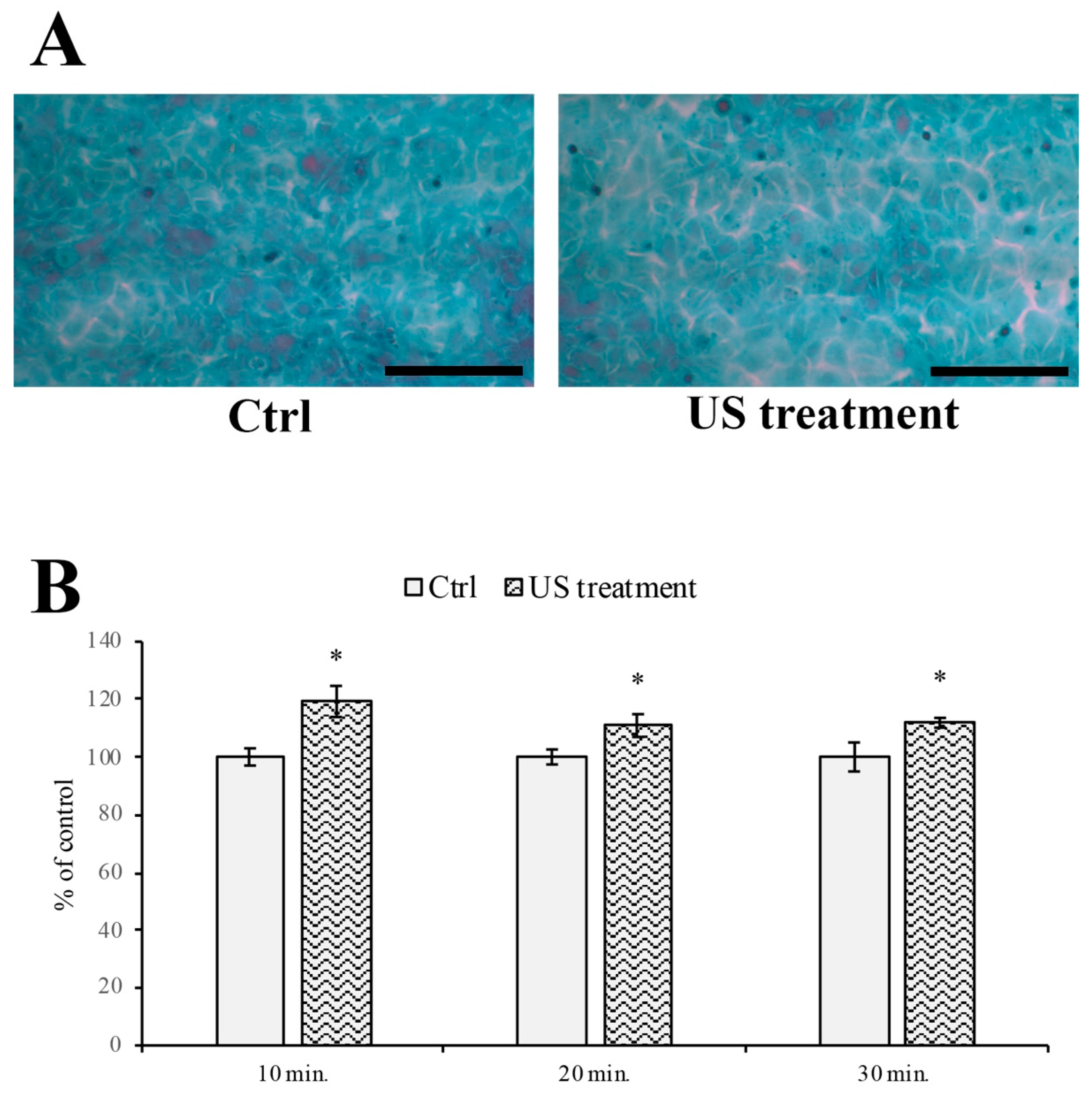
2.4. Papanicolaou Cytological Staining and Intercellular Spaces Evaluation
2.5. Immunofluorescent Labeling
2.6. Statistical Analysis
3. Results
3.1. Cell Viability Assay
3.2. Endoplasmic Reticulum and Oxidative Stress Evaluation
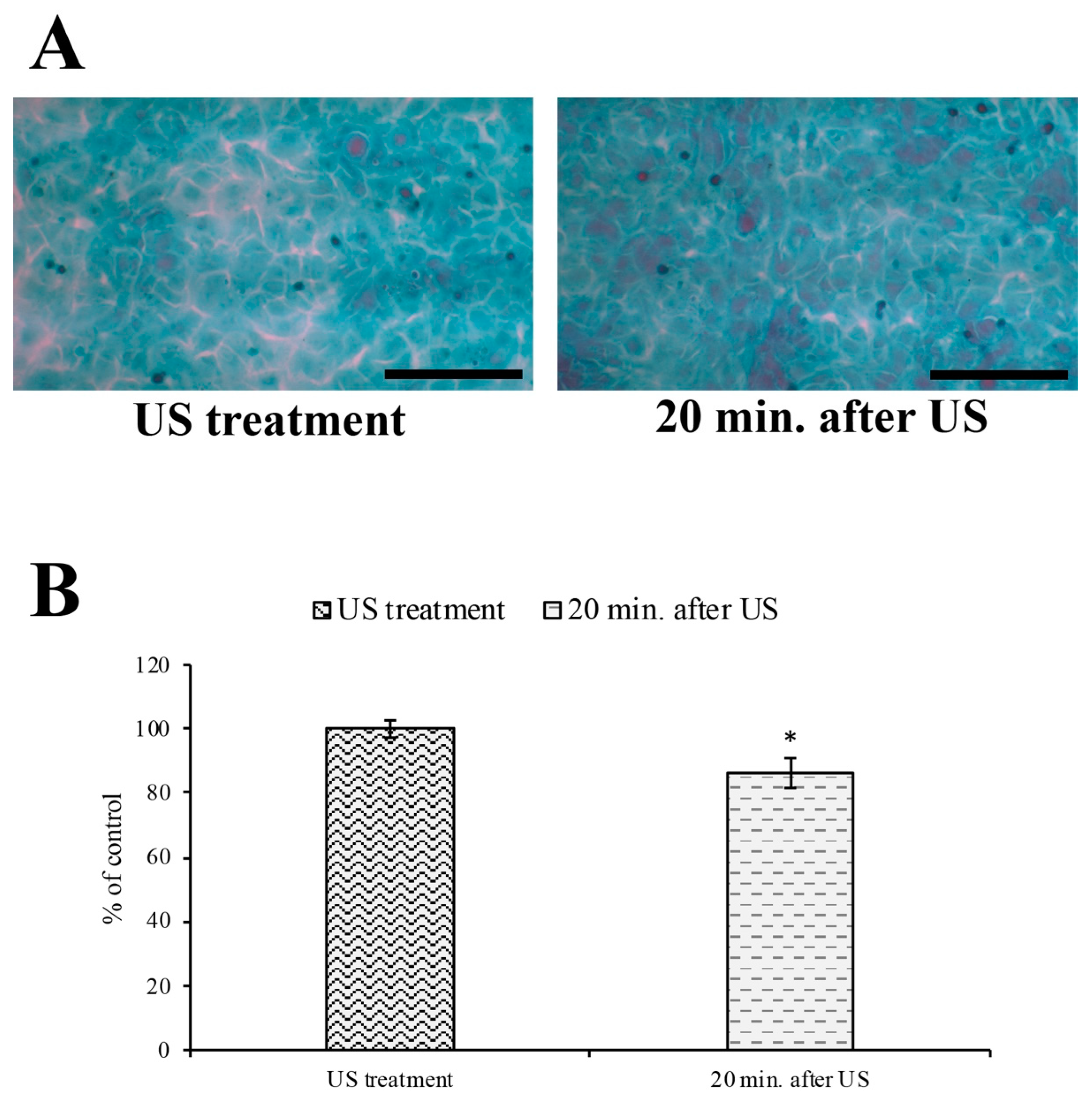
3.3. Evaluation of Intercellular Spaces
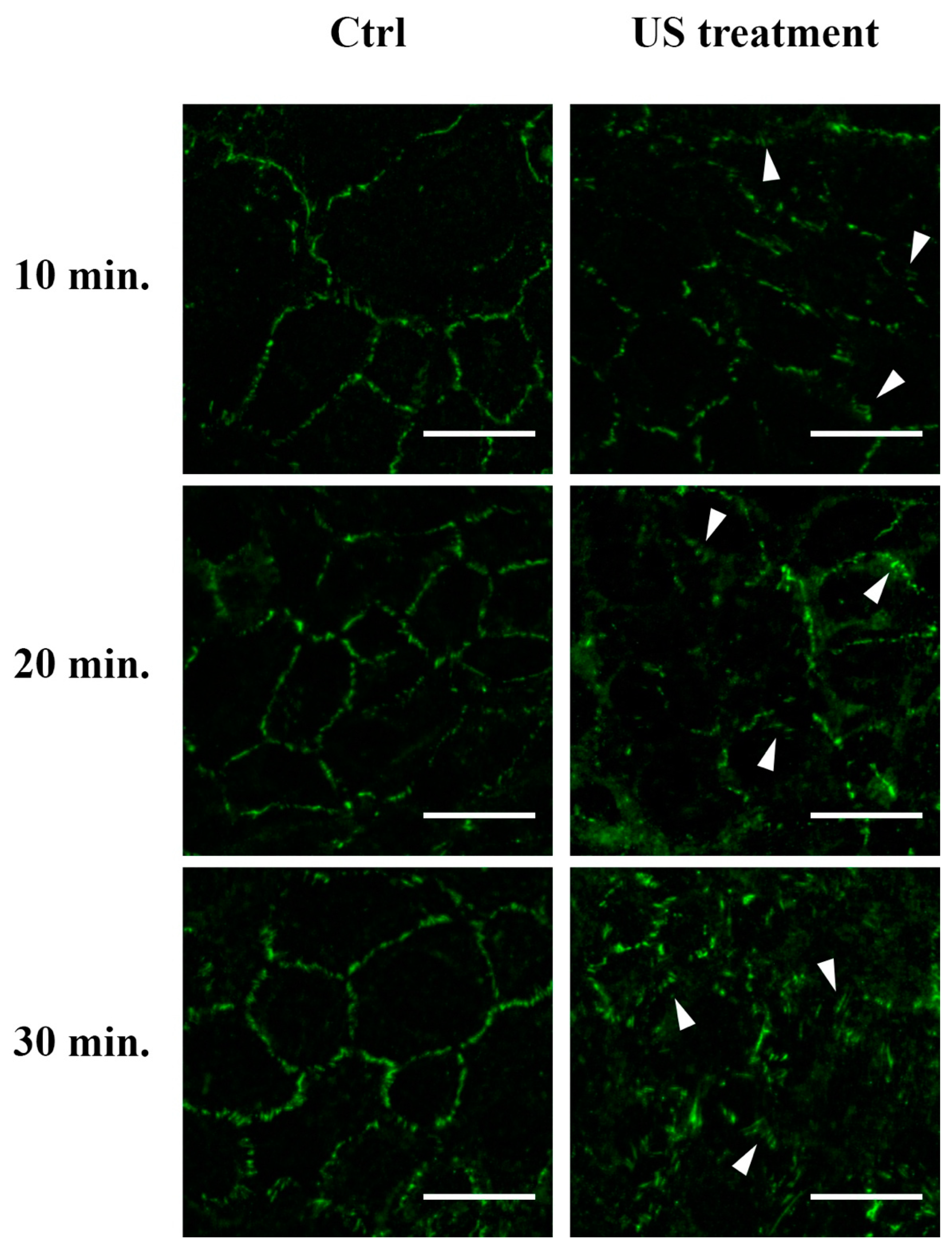
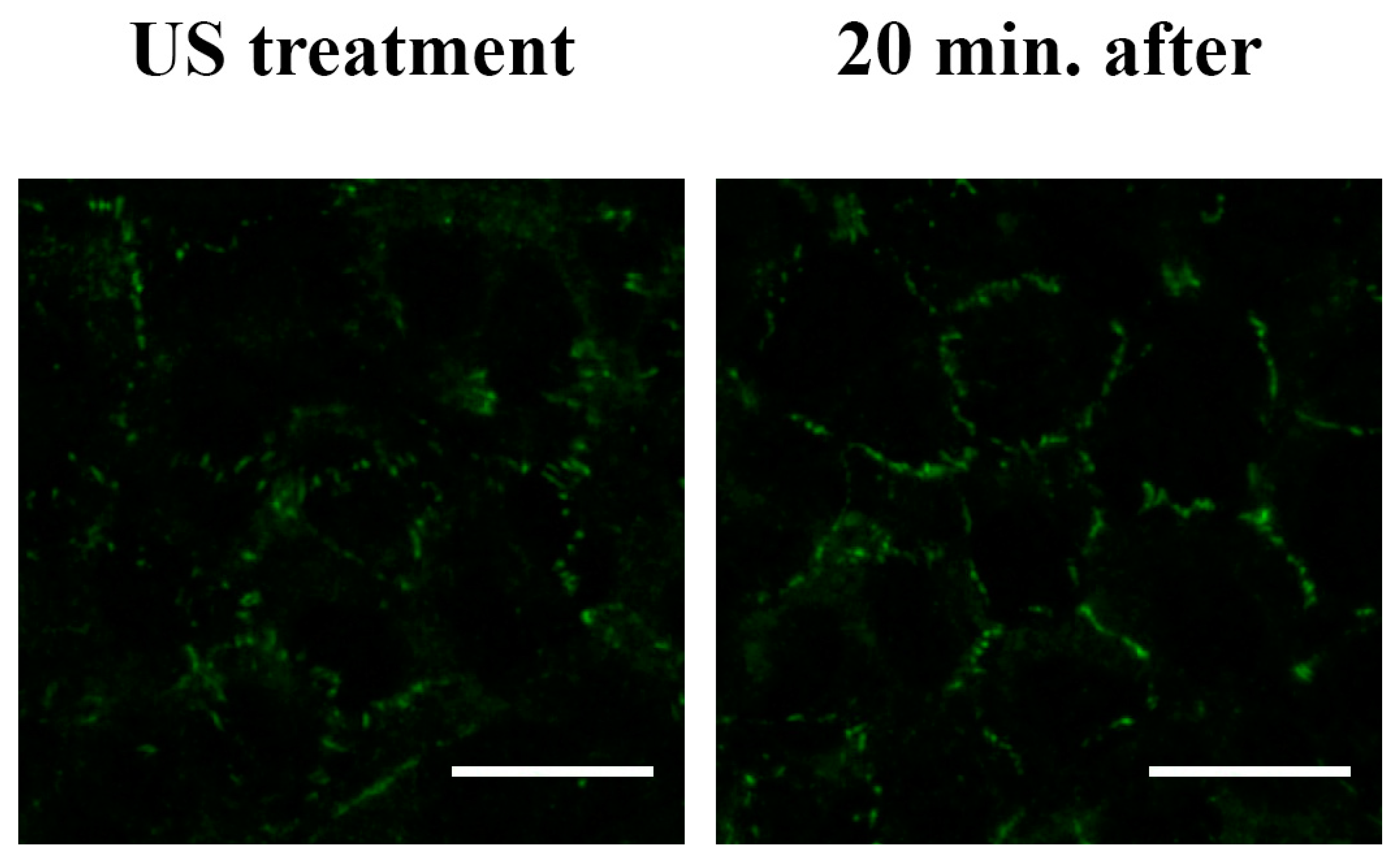
3.4. F-actin and Tight Junction ZO-1 Distribution Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Mayeux, R. Alzheimer Disease: Epidemiology, Diagnostic Criteria, Risk Factors and Biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M.; Tabassum, N. Role of Environmental Toxicants on Neurodegenerative Disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional Proteins of the Blood-Brain Barrier: New Insights into Function and Dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.-G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood–Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Hameroff, S.; Trakas, M.; Duffield, C.; Annabi, E.; Gerace, M.B.; Boyle, P.; Lucas, A.; Amos, Q.; Buadu, A.; Badal, J.J. Transcranial Ultrasound (TUS) Effects on Mental States: A Pilot Study. Brain Stimul. 2013, 6, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Reinz, E.; Fatar, M.; Hennerici, M.G.; Meairs, S. Clearance of Albumin Following Ultrasound-Induced Blood–Brain Barrier Opening Is Mediated by Glial but Not Neuronal Cells. Brain Res. 2011, 1411, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jordão, J.F.; Thévenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.-Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K.; et al. Amyloid-β Plaque Reduction, Endogenous Antibody Delivery and Glial Activation by Brain-Targeted, Transcranial Focused Ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Götz, J. Scanning Ultrasound Removes Amyloid-b and Restores Memory in an Alzheimer’s Disease Mouse Model. Sci. Transl. Med. 2015, 7, 278ra33. [Google Scholar] [CrossRef]
- Bystritsky, A.; Korb, A.S.; Douglas, P.K.; Cohen, M.S.; Melega, W.P.; Mulgaonkar, A.P.; DeSalles, A.; Min, B.-K.; Yoo, S.-S. A Review of Low-Intensity Focused Ultrasound Pulsation. Brain Stimul. 2011, 4, 125–136. [Google Scholar] [CrossRef]
- Gavrilov, L.R.; Tsirulnikov, E.M.; Davies, I.A.I. Application of Focused Ultrasound for the Stimulation of Neural Structures. Ultrasound Med. Biol. 1996, 22, 179–192. [Google Scholar] [CrossRef]
- Foley, J.L.; Little, J.W.; Vaezy, S. Image-Guided High-Intensity Focused Ultrasound for Conduction Block of Peripheral Nerves. Ann. Biomed. Eng. 2006, 35, 109–119. [Google Scholar] [CrossRef]
- Bocchi, L.; Branca, J.J.V.; Pacini, S.; Cosentino, A.; Morucci, G.; Ruggiero, M. Effect of Ultrasounds on Neurons and Microglia: Cell Viability and Automatic Analysis of Cell Morphology. Biomed. Signal Process. Control 2015, 22, 44–53. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Pacini, S.; Morucci, G.; Bocchi, L.; Cosentino, A.; Boni, E.; Ruggiero, M. Effect of Ultrasound and Selenium on Human Neurons in Vitro. Arch. Ital. De Biol. 2018, 156, 153–163. [Google Scholar] [CrossRef]
- Cosentino, A.; Boni, E.; Pacini, S.; Branca, J.; Morucci, G.; Ruggiero, M.; Bocchi, L. Morphological Analysis of Neurons: Automatic Identification of Elongations. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) IEEE, Milan, Italy, 25–29 August 2015; pp. 8131–8134. [Google Scholar]
- Arif, W.M.; Elsinga, P.H.; Gasca-Salas, C.; Versluis, M.; Martínez-Fernández, R.; Dierckx, R.A.J.O.; Borra, R.J.H.; Luurtsema, G. Focused Ultrasound for Opening Blood-Brain Barrier and Drug Delivery Monitored with Positron Emission Tomography. J. Control. Release 2020, 324, 303–316. [Google Scholar] [CrossRef]
- Librizzi, L.; Uva, L.; Raspagliesi, L.; Gionso, M.; Regondi, M.C.; Durando, G.; DiMeco, F.; de Curtis, M.; Prada, F. Ultrasounds Induce Blood–Brain Barrier Opening across a Sonolucent Polyolefin Plate in an in Vitro Isolated Brain Preparation. Sci. Rep. 2022, 12, 2906. [Google Scholar] [CrossRef] [PubMed]
- Konofagou, E.E.; Tunga, Y.-S.; Choia, J.; Deffieuxa, T.; Baseria, B.; Vlachosa, F. Ultrasound-Induced Blood-Brain Barrier Opening. Curr. Pharm. Biotechnol. 2012, 13, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Lelu, S.; Afadzi, M.; Berg, S.; Aslund, A.K.O.; Torp, S.H.; Sattler, W.; Davies, C.D.L. Primary Porcine Brain Endothelial Cells as In Vitro Model to Study Effects of Ultrasound and Microbubbles on Blood–Brain Barrier Function. IEEE Trans. Ultrason. Ferroelectr. Freq. Contr. 2017, 64, 281–290. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Becatti, M.; Paternostro, F.; Gulisano, M.; Ghelardini, C.; Salvemini, D.; Di Cesare Mannelli, L.; Pacini, A. Oxaliplatin-Induced Blood Brain Barrier Loosening: A New Point of View on Chemotherapy-Induced Neurotoxicity. Oncotarget 2018, 9, 23426–23438. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Mello, T.; Becatti, M.; Pazzagli, L.; Colzi, I.; Gonnelli, C.; Carrino, D.; Paternostro, F.; et al. Effects of Cadmium on ZO-1 Tight Junction Integrity of the Blood Brain Barrier. Int. J. Mol. Sci. 2019, 20, 6010. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.; Branca, J.J.V.; Becatti, M.; Paternostro, F.; Morucci, G.; Gulisano, M.; Di Cesare Mannelli, L.; Pacini, A. Alcohol-Induced Blood-Brain Barrier Impairment: An In Vitro Study. Int. J. Environ. Res. Public Health 2021, 18, 2683. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J.; Hirschberg, H. Site-Specific Opening of the Blood-Brain Barrier. J. Biophotonics 2010, 3, 356–367. [Google Scholar] [CrossRef]
- Lee, A.S. The ER Chaperone and Signaling Regulator GRP78/BiP as a Monitor of Endoplasmic Reticulum Stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssière, J.-L. Mitochondrial Reactive Oxygen Species in Cell Death Signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Series Introduction: Neurodegeneration: What Is It and Where Are We? J. Clin. Invest. 2003, 111, 3–10. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Cascione, M.; De Matteis, V.; Leporatti, S.; Rinaldi, R. The New Frontiers in Neurodegenerative Diseases Treatment: Liposomal-Based Strategies. Front. Bioeng. Biotechnol. 2020, 8, 566767. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, Z.; Talaei, S.; Aghamiri, S.; Goradel, N.H.; Jafarpour, A.; Negahdari, B. Overcoming the Blood–Brain Barrier in Neurodegenerative Disorders and Brain Tumours. IET Nanobiotechnol. 2020, 14, 441–448. [Google Scholar] [CrossRef]
- Poovaiah, N.; Davoudi, Z.; Peng, H.; Schlichtmann, B.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Treatment of Neurodegenerative Disorders through the Blood–Brain Barrier Using Nanocarriers. Nanoscale 2018, 10, 16962–16983. [Google Scholar] [CrossRef] [PubMed]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of Ultrasound in Medicine. Acta Inform. Med. 2011, 19, 168. [Google Scholar] [CrossRef]
- Blackmore, J.; Shrivastava, S.; Sallet, J.; Butler, C.R.; Cleveland, R.O. Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound Med. Biol. 2019, 45, 1509–1536. [Google Scholar] [CrossRef] [PubMed]
- Clennell, B.; Steward, T.G.J.; Elley, M.; Shin, E.; Weston, M.; Drinkwater, B.W.; Whitcomb, D.J. Transient Ultrasound Stimulation Has Lasting Effects on Neuronal Excitability. Brain Stimul. 2021, 14, 217–225. [Google Scholar] [CrossRef]
- Miller, M.W.; Ziskin, M.C. Biological Consequences of Hyperthermia. Ultrasound Med. Biol. 1989, 15, 707–722. [Google Scholar] [CrossRef]
- Stanton, M.; Ettarh, R.; Arango, D.; Tonra, M.; Brennan, P. Diagnostic Ultrasound Induces Change within Numbers of Cryptal Mitotic and Apoptotic Cells in Small Intestine. Life Sci. 2001, 68, 1471–1475. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Wang, X.; Guo, L.; Su, X.; Liu, Q. Membrane Damage Effect of Continuous Wave Ultrasound on K562 Human Leukemia Cells. J. Ultrasound Med. 2012, 31, 1977–1986. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Schlicher, R.K.; Hicks, H.K.; Prausnitz, M.R. Saving Cells from Ultrasound-Induced Apoptosis: Quantification of Cell Death and Uptake Following Sonication and Effects of Targeted Calcium Chelation. Ultrasound Med. Biol. 2010, 36, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; Couraud, P.-O. Rat Brain Endothelial Cell Lines for the Study of Blood–Brain Barrier Permeability and Transport Functions. Cell. Mol. Neurobiol. 2005, 25, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Levario-Diaz, V.; Bhaskar, P.; Carmen Galan, M.; Barnes, A.C. Effect of Acoustic Standing Waves on Cellular Viability and Metabolic Activity. Sci. Rep. 2020, 10, 8493. [Google Scholar] [CrossRef] [PubMed]
- Cucullo, L. The Role of Shear Stress in Blood-Brain Barrier Endothelial Physiology. BMC Neurosci. 2011, 15, 40. [Google Scholar] [CrossRef]
- Qiu, W.; Kass, D.A.; Hu, Q.; Ziegelstein, R.C. Determinants of Shear Stress-Stimulated Endothelial Nitric Oxide Production Assessed in Real-Time by 4,5-Diaminofluorescein Fluorescence. Biochem. Biophys. Res. Commun. 2001, 286, 328–335. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Kong, X.; Gao, X.; Gu, Y.; Zhang, J. Oscillatory Shear Stress Induces Oxidative Stress via TLR4 Activation in Endothelial Cells. Mediat. Inflamm. 2019, 2019, 7162976. [Google Scholar] [CrossRef]
- Rwei, P.; Alex Gong, C.-S.; Luo, L.-J.; Lin, M.-B.; Lai, J.-Y.; Liu, H.-L. In Vitro Investigation of Ultrasound-Induced Oxidative Stress on Human Lens Epithelial Cells. Biochem. Biophys. Res. Commun. 2017, 482, 954–960. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, X.; Jiang, P.; Wan, J.M.F.; Qin, P.; Yu, A.C.H. Induction of Endoplasmic Reticulum Stress by Sonoporation: Linkage to Mitochondria-Mediated Apoptosis Initiation. Ultrasound Med. Biol. 2013, 39, 2382–2392. [Google Scholar] [CrossRef]
- Ranaei, E.; Abbasi, N.; Moayeri, A.; Kafashi, M.; Maleki, M.; Kaffashian, M.R. The Synergistic Effects of Ultrasound Waves and Ethanol Exposure on Intracellular Calcium and Reactive Oxygen Species Rates of Hippocampal Cells During the Embryonic Course in Rats. Turk. J. Neurol. 2022, 28. Ahead of Print. [Google Scholar] [CrossRef]
- Draganovic, D.; Lucic, N.; Jojic, D.; Milicevic, S. Correlation of Oxidative Stress Markers with Ultrasound and Cardiotocography Parameters with Hypertension Induced Pregnancy. Acta Inform. Med. 2017, 25, 19. [Google Scholar] [CrossRef]
- Chen, X.; Leow, R.S.; Hu, Y.; Wan, J.M.F.; Yu, A.C.H. Single-Site Sonoporation Disrupts Actin Cytoskeleton Organization. J. R. Soc. Interface 2014, 11, 20140071. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Van Itallie, C.M.; Anderson, J.M. Zonula Occludens-1 and -2 Regulate Apical Cell Structure and the Zonula Adherens Cytoskeleton in Polarized Epithelia. MBoC 2012, 23, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, L.S.; Fanning, A.S. Regulation of Epithelial Permeability by the Actin Cytoskeleton. Cytoskeleton 2011, 68, 653–660. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branca, J.J.V.; Boninsegna, M.; Morucci, G.; Carrino, D.; Nicoletti, C.; Paternostro, F.; Gulisano, M.; Bocchi, L.; Pacini, A. Morphological and Functional Effects of Ultrasound on Blood–Brain Barrier Transitory Opening: An In Vitro Study on Rat Brain Endothelial Cells. Cells 2023, 12, 192. https://doi.org/10.3390/cells12010192
Branca JJV, Boninsegna M, Morucci G, Carrino D, Nicoletti C, Paternostro F, Gulisano M, Bocchi L, Pacini A. Morphological and Functional Effects of Ultrasound on Blood–Brain Barrier Transitory Opening: An In Vitro Study on Rat Brain Endothelial Cells. Cells. 2023; 12(1):192. https://doi.org/10.3390/cells12010192
Chicago/Turabian StyleBranca, Jacopo Junio Valerio, Matteo Boninsegna, Gabriele Morucci, Donatello Carrino, Claudio Nicoletti, Ferdinando Paternostro, Massimo Gulisano, Leonardo Bocchi, and Alessandra Pacini. 2023. "Morphological and Functional Effects of Ultrasound on Blood–Brain Barrier Transitory Opening: An In Vitro Study on Rat Brain Endothelial Cells" Cells 12, no. 1: 192. https://doi.org/10.3390/cells12010192
APA StyleBranca, J. J. V., Boninsegna, M., Morucci, G., Carrino, D., Nicoletti, C., Paternostro, F., Gulisano, M., Bocchi, L., & Pacini, A. (2023). Morphological and Functional Effects of Ultrasound on Blood–Brain Barrier Transitory Opening: An In Vitro Study on Rat Brain Endothelial Cells. Cells, 12(1), 192. https://doi.org/10.3390/cells12010192









