Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis
Abstract
1. Introduction
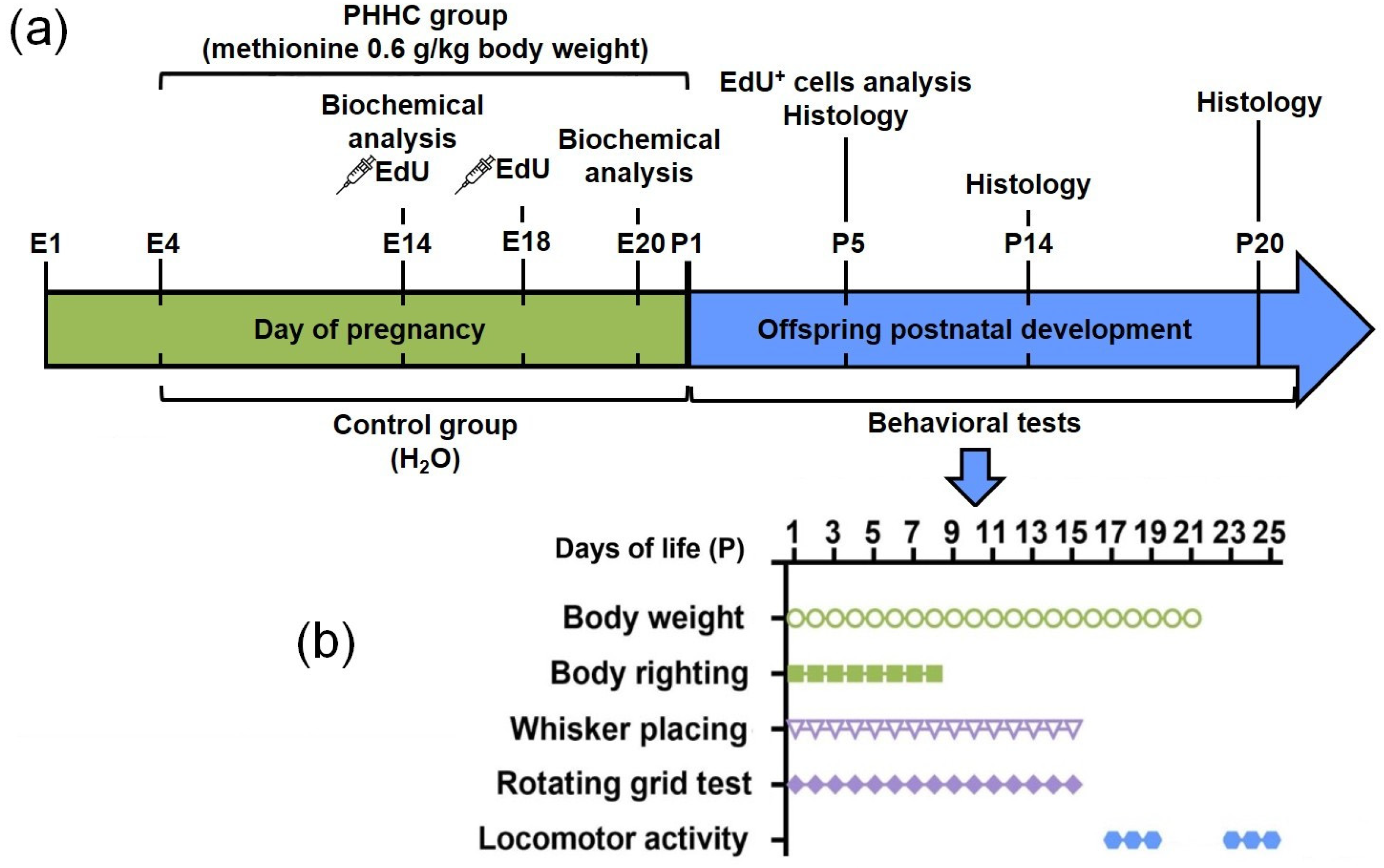
2. Materials and Methods
2.1. Experimental Animals
2.2. Chronic Methionine Treatment
2.3. Offspring Somatic Development
2.4. Animal Behavior
2.4.1. Body Righting
2.4.2. Whisker Placing
2.4.3. Rotating Grid
2.4.4. Locomotor Activity
2.5. Analysis of Migration and Positioning of Cortical Neurons Generated on E14 or E18
2.6. Electron Microscopy
2.7. Quantitative PCR (RT-qPCR)
2.8. Western Blot Analysis
2.9. Gelatin Zymography
2.10. Caspase-3 Activity
2.11. Oxyblot Analysis
2.12. Measurement of Lipid Peroxidation
2.13. Statistical Analysis
3. Results
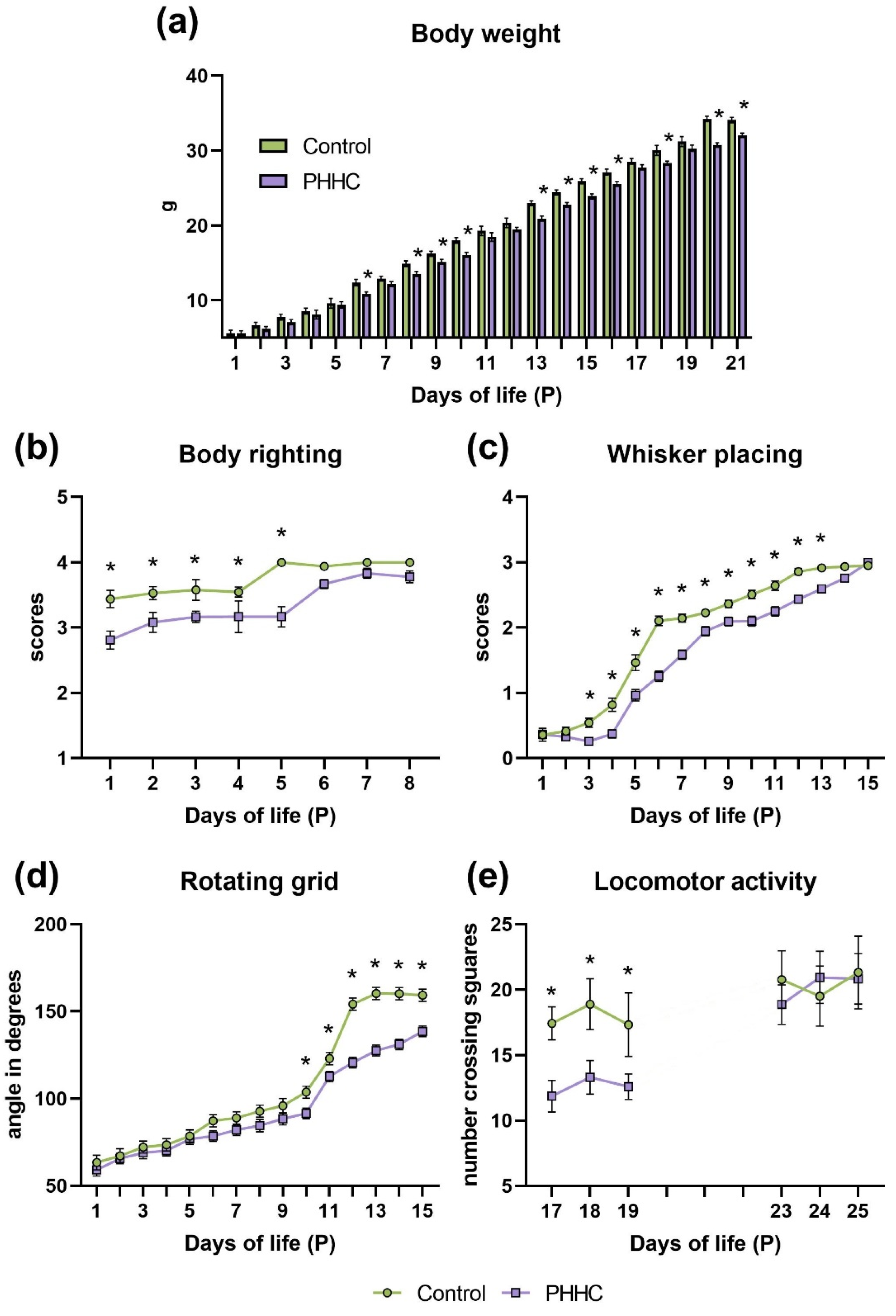
3.1. Body Weight (P1-P21)
3.2. Animal Behavior
3.2.1. Body Righting (P1-P8)
3.2.2. Whisker Placing (P1-P15)
3.2.3. Rotating Grid (P1-P15)
3.2.4. Locomotor Activity (P17-P19 and P23-P25)
3.3. Analysis of Migration and Positioning of Cortical Neurons Generated on E14 or E18
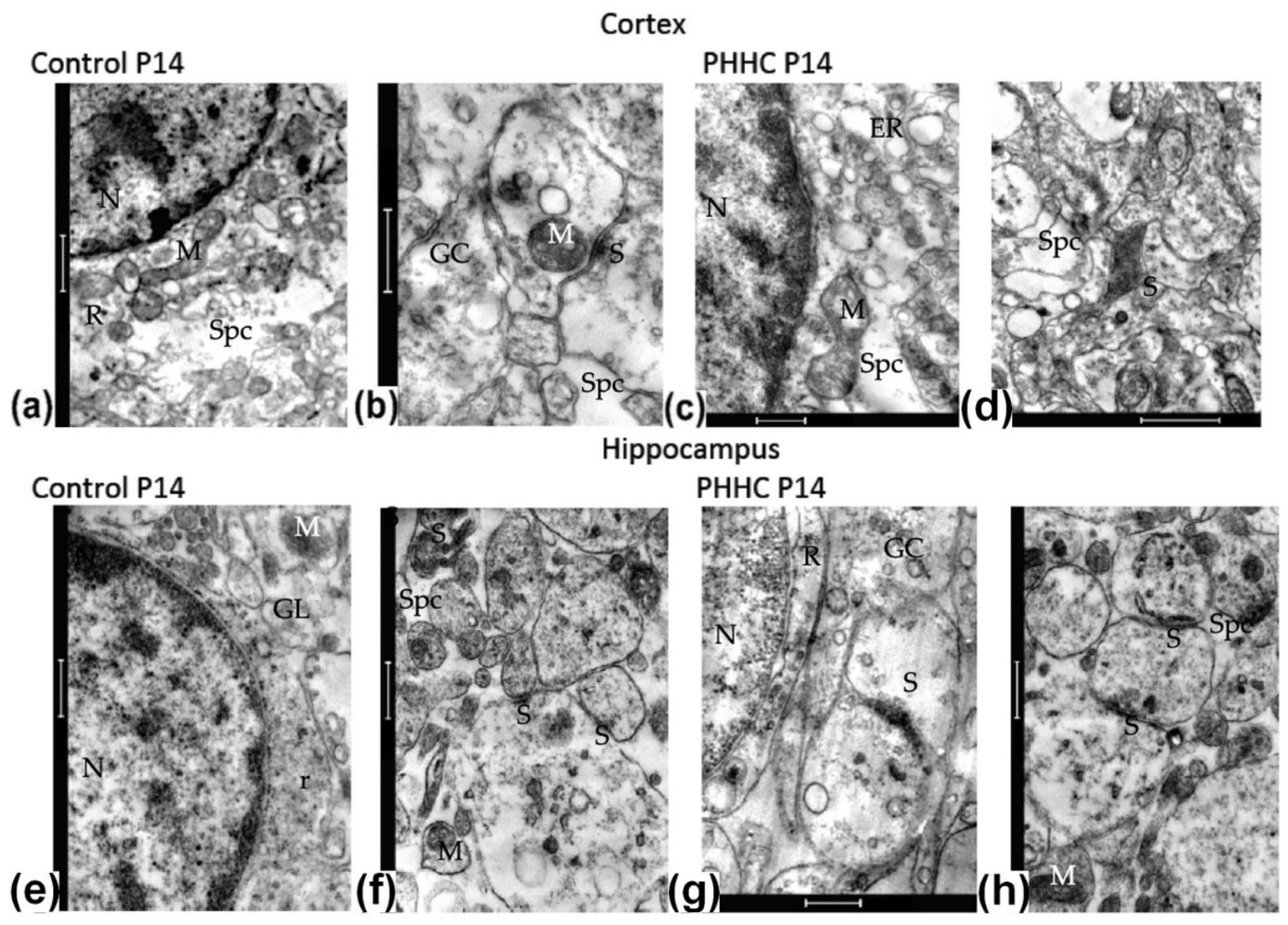
3.4. Electron Microscopy
3.4.1. Neural Tissue Formation and Maturing in P5 Pups
3.4.2. Neural Tissue Formation and Maturing in P14 Pups
3.4.3. Neural Tissue Formation and Maturing in P20 Pups
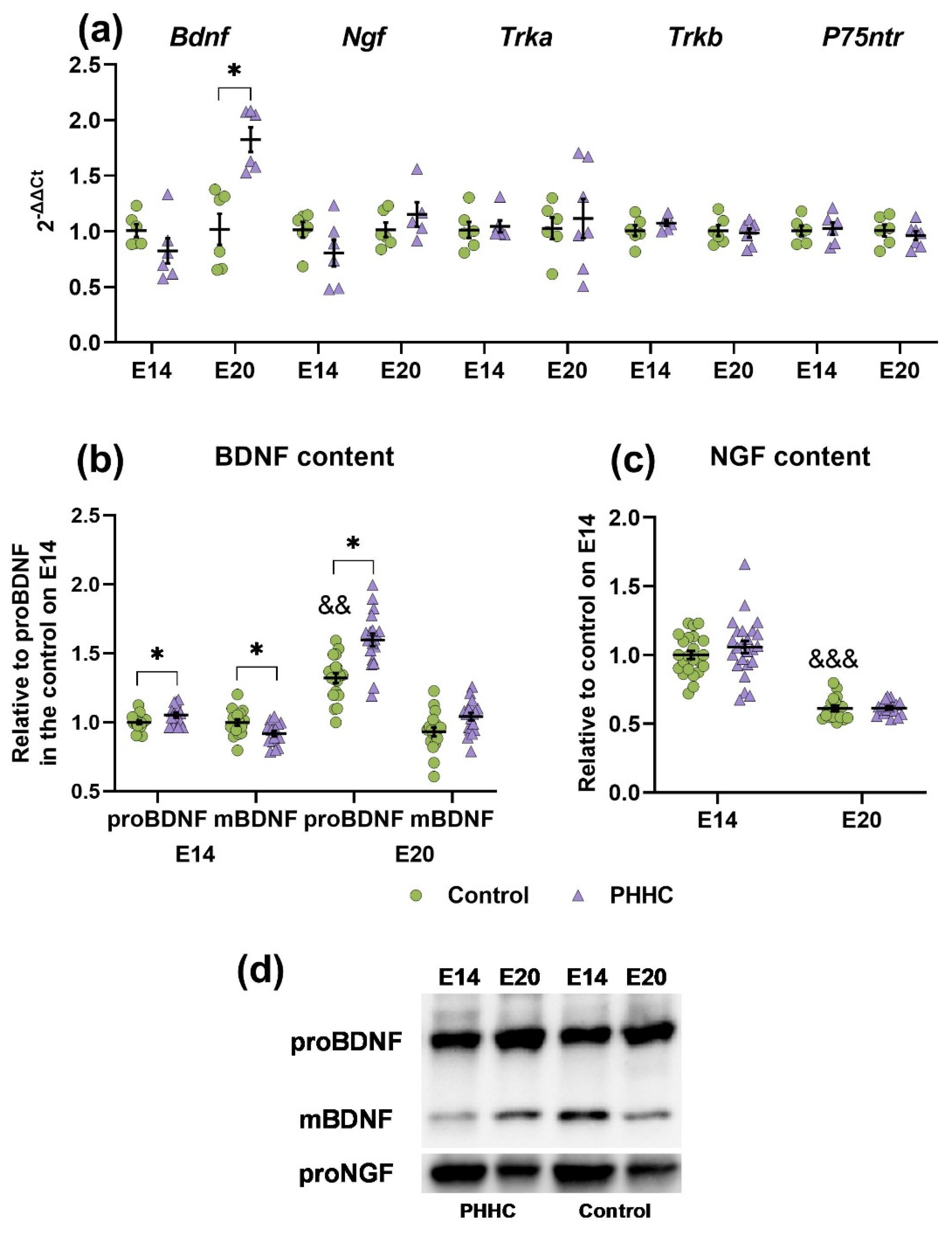
3.5. Analysis of the Neurotrophin System Components in the Rat Fetal Brain
3.6. Analysis of Caspase-3 and Caspase-8 in the Rat Fetal Brain
3.7. Analysis of the Angiogenesis System Components in the Rat Fetal Brain
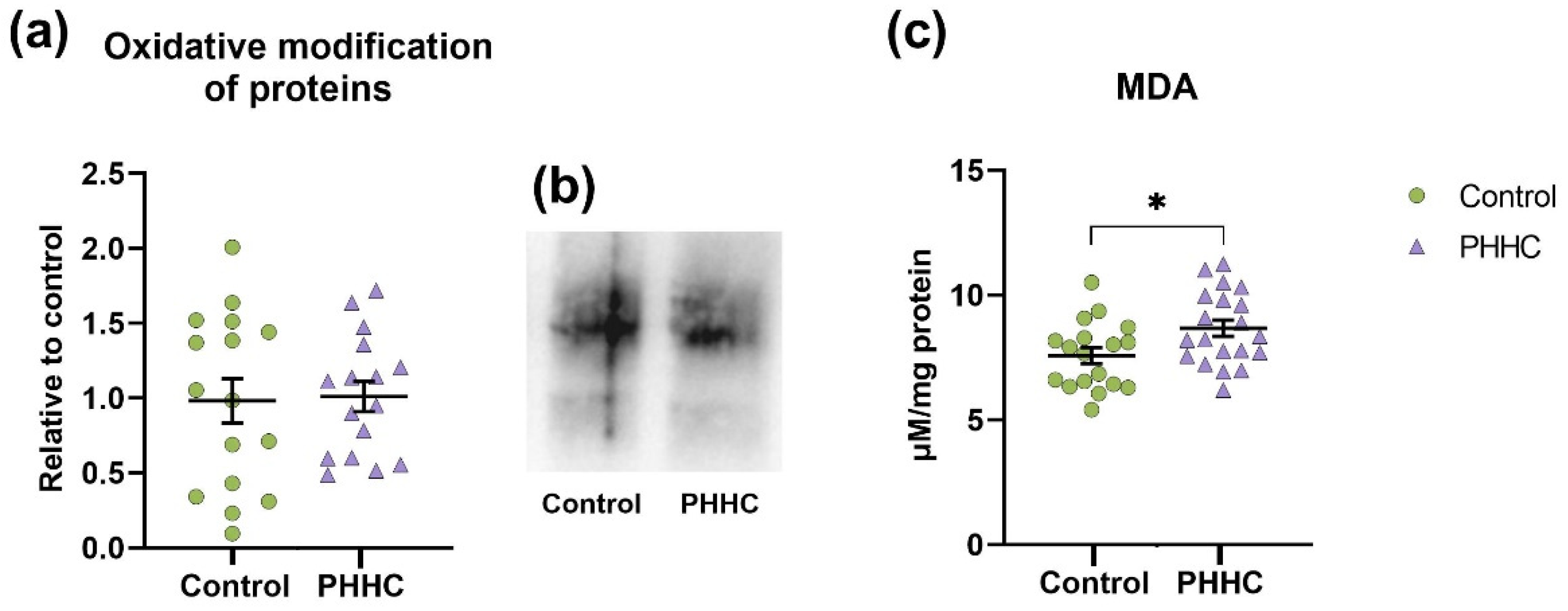
3.8. Oxidative Modifications of Macromolecules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Blaise, S.A.; Nedelec, E.; Schroeder, H.; Alberto, J.M.; Bossenmeyer-Pourie, C.; Gueant, J.L.; Daval, J.L. Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am. J. Pathol. 2007, 170, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Schweinberger, B.M.; Rodrigues, A.F.; Dos Santos, T.M.; Rohden, F.; Barbosa, S.; da Luz Soster, P.R.; Partata, W.A.; Faccioni-Heuser, M.C.; Wyse, A.T.S. Methionine administration in pregnant rats causes memory deficit in the offspring and alters ultrastructure in brain tissue. Neurotox. Res. 2018, 33, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Shcherbitskaia, A.D.; Vasilev, D.S.; Milyutina, Y.P.; Tumanova, N.L.; Zalozniaia, I.V.; Kerkeshko, G.O.; Arutjunyan, A.V. Maternal Hyperhomocysteinemia Induces Neuroinflammation and Neuronal Death in the Rat Offspring Cortex. Neurotox. Res. 2020, 38, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Shcherbitskaia, A.D.; Vasilev, D.S.; Milyutina, Y.P.; Tumanova, N.L.; Mikhel, A.V.; Zalozniaia, I.V.; Arutjunyan, A.V. Prenatal Hyperhomocysteinemia Induces Glial Activation and Alters Neuroinflammatory Marker Expression in Infant Rat Hippocampus. Cells 2021, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, A.A.; Ferreira, A.G.; Wyse, A.T. Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration. Metab. Brain Dis. 2010, 25, 199–206. [Google Scholar] [CrossRef]
- dos Santos, T.M.; Ramires Júnior, O.V.; Alves, V.S.; Coutinho-Silva, R.; Savio, L.E.B.; Wyse, A.T.S. Hyperhomocysteinemia alters cytokine gene expression, cytochrome c oxidase activity and oxidative stress in striatum and cerebellum of rodents. Life Sci. 2021, 277, 119386. [Google Scholar] [CrossRef]
- Moreira, D.S.; Figueiro, P.W.; Siebert, C.; Prezzi, C.A.; Rohden, F.; Guma, F.C.R.; Manfredini, V.; Wyse, A.T.S. Chronic Mild Hyperhomocysteinemia Alters Inflammatory and Oxidative/Nitrative Status and Causes Protein/DNA Damage, as well as Ultrastructural Changes in Cerebral Cortex: Is Acetylsalicylic Acid Neuroprotective? Neurotox. Res. 2018, 33, 580–592. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar] [CrossRef]
- Navarrete-Muñoz, E.M.; Valera-Gran, D.; Garcia-de-la-Hera, M.; Gonzalez-Palacios, S.; Riaño, I.; Murcia, M.; Lertxundi, A.; Guxens, M.; Tardón, A.; Amiano, P.; et al. High doses of folic acid in the periconceptional period and risk of low weight for gestational age at birth in a population based cohort study. Eur. J. Nutr. 2017, 58, 241–251. [Google Scholar] [CrossRef]
- Moffat, J.J.; Ka, M.; Jung, E.M.; Kim, W.Y. Genes and brain malformations associated with abnormal neuron positioning. Mol. Brain 2015, 8, 72. [Google Scholar] [CrossRef]
- Mayeur, S.; Lukaszewski, M.A.; Breton, C.; Storme, L.; Vieau, D.; Lesage, J. Do neurotrophins regulate the feto-placental development? Med. Hypotheses 2011, 76, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Kodomari, I.; Wada, E.; Nakamura, S.; Wada, K. Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem. Int. 2009, 54, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Kale, A.; Joshi, S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 2020, 83, 102075. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, M.; Shudai, T.; Nitta, A.; Nomoto, H.; Furukawa, Y.; Furukawa, S. Brain-derived neurotrophic factor alters cell migration of particular progenitors in the developing mouse cerebral cortex. Neurosci. Lett. 2002, 317, 21–24. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Sun, Y.; Li, H.-Y.; Lim, Y.; Zhong, J.-H.; Zhou, X.-F. Endogenous proBDNF is a negative regulator of migration of cerebellar granule cells in neonatal mice. Eur. J. Neurosci. 2011, 33, 1376–1384. [Google Scholar] [CrossRef]
- Masoudi, R.; Ioannou, M.S.; Coughlin, M.D.; Pagadala, P.; Neet, K.E.; Clewes, O.; Allen, S.J.; Dawbarn, D.; Fahnestock, M. Biological Activity of Nerve Growth Factor Precursor Is Dependent upon Relative Levels of Its Receptors. J. Biol. Chem. 2009, 284, 18424–18433. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Kenchappa, R.S.; Zampieri, N.; Chao, M.V.; Barker, P.A.; Teng, H.K.; Hempstead, B.L.; Carter, B.D. Ligand-Dependent Cleavage of the P75 Neurotrophin Receptor Is Necessary for NRIF Nuclear Translocation and Apoptosis in Sympathetic Neurons. Neuron 2006, 50, 219–232. [Google Scholar] [CrossRef]
- Fan, Y.-j.; Wu, L.L.-y.; Li, H.-y.; Wang, Y.-J.; Zhou, X.-F. Differential effects of pro-BDNF on sensory neurons after sciatic nerve transection in neonatal rats. Eur. J. Neurosci. 2008, 27, 2380–2390. [Google Scholar] [CrossRef]
- Yano, H.; Torkin, R.; Martin, L.A.; Chao, M.V.; Teng, K.K. Proneurotrophin-3 Is a Neuronal Apoptotic Ligand: Evidence for Retrograde-Directed Cell Killing. J. Neurosci. 2009, 29, 14790–14802. [Google Scholar] [CrossRef]
- Chouthai, N.S.; Sampers, J.; Desai, N.; Smith, G.M. Changes in Neurotrophin Levels in Umbilical Cord Blood From Infants With Different Gestational Ages and Clinical Conditions. Pediatr. Res. 2003, 53, 965–969. [Google Scholar] [CrossRef]
- Garcés, M.F.; Sanchez, E.; Torres-Sierra, A.L.; Ruíz-Parra, A.I.; Angel-Müller, E.; Alzate, J.P.; Sánchez, Á.Y.; Gomez, M.A.; Romero, X.C.; Castañeda, Z.E.; et al. Brain-derived neurotrophic factor is expressed in rat and human placenta and its serum levels are similarly regulated throughout pregnancy in both species. Clin. Endocrinol. 2014, 81, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Kawamura, N.; Fukuda, J.; Kumagai, J.; Hsueh, A.J.W.; Tanaka, T. Regulation of preimplantation embryo development by brain-derived neurotrophic factor. Dev. Biol. 2007, 311, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Turlejski, K.; Djavadian, R.L. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol. Exp. 2010, 70, 454–467. [Google Scholar]
- Moscatelli, I.; Pierantozzi, E.; Camaioni, A.; Siracusa, G.; Campagnolo, L. p75 neurotrophin receptor is involved in proliferation of undifferentiated mouse embryonic stem cells. Exp. Cell Res. 2009, 315, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Kerjan, G.; Dolan, J.; Haumaitre, C.; Schneider-Maunoury, S.; Fujisawa, H.; Mitchell, K.J.; Chédotal, A. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat. Neurosci. 2005, 8, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sima, J.; Jin, M.; Wang, K.Y.; Xue, X.J.; Zheng, W.; Ding, Y.Q.; Yuan, X.B. Semaphorin-3A guides radial migration of cortical neurons during development. Nat. Neurosci. 2008, 11, 36–44. [Google Scholar] [CrossRef]
- Marín, O.; Rubenstein, J.L.R. Cell Migration in the Forebrain. Annu. Rev. Neurosci. 2003, 26, 441–483. [Google Scholar] [CrossRef]
- Gil, V.; del Río, J. Functions of Plexins/Neuropilins and Their Ligands during Hippocampal Development and Neurodegeneration. Cells 2019, 8, 206. [Google Scholar] [CrossRef]
- Mata, A.; Gil, V.; Pérez-Clausell, J.; Dasilva, M.; González-Calixto, M.C.; Soriano, E.; García-Verdugo, J.M.; Sanchez-Vives, M.V.; del Río, J.A. New functions of Semaphorin 3E and its receptor PlexinD1 during developing and adult hippocampal formation. Sci. Rep. 2018, 8, 1381. [Google Scholar] [CrossRef]
- D’Amelio, M.; Sheng, M.; Cecconi, F. Caspase-3 in the central nervous system: Beyond apoptosis. Trends Neurosci. 2012, 35, 700–709. [Google Scholar] [CrossRef]
- Koz, S.T.; Gouwy, N.T.; Demir, N.; Nedzvetsky, V.S.; Etem, E.; Baydas, G. Effects of maternal hyperhomocysteinemia induced by methionine intake on oxidative stress and apoptosis in pup rat brain. Int. J. Dev. Neurosci. 2010, 28, 325–329. [Google Scholar] [CrossRef]
- Arutjunyan, A.V.; Milyutina, Y.P.; Shcherbitskaia, A.D.; Kerkeshko, G.O.; Zalozniaia, I.V.; Mikhel, A.V. Neurotrophins of the fetal brain and placenta in prenatal hyperhomocysteinemia. Biochemistry 2020, 85, 248–259. [Google Scholar] [CrossRef]
- Vasilev, D.S.; Dubrovskaya, N.M.; Tumanova, N.L.; Zhuravin, I.A. Prenatal hypoxia in different periods of embryogenesis differentially affects cell migration, neuronal plasticity, and rat behavior in postnatal ontogenesis. Front. Neurosci. 2016, 10, 126. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier/Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Vasilev, D.S.; Tumanova, N.L.; Kim, K.K.; Lavrentyeva, V.V.; Lukomskaya, N.Y.; Zhuravin, I.A.; Magazanik, L.G.; Zaitsev, A.V. Transient morphological alterations in the hippocampus after pentylenetetrazole-induced seizures in rats. Neurochem. Res. 2018, 43, 1671–1682. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Langnaese, K.; John, R.; Schweizer, H.; Ebmeyer, U.; Keilhoff, G. Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol. Biol. 2008, 9, 53. [Google Scholar] [CrossRef]
- Schwarz, A.P.; Malygina, D.A.; Kovalenko, A.A.; Trofimov, A.N.; Zaitsev, A.V. Multiplex qPCR assay for assessment of reference gene expression stability in rat tissues/samples. Mol. Cell. Probes 2020, 53, 101611. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Chen, J.; Dong, W. Chronic antidepressant administration alleviates frontal and hippocampal BDNF deficits in CUMS rat. Brain Res. 2010, 1366, 141–148. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Lu, X.; Jiang, J. Effects of inhibiting the PI3K/Akt/mTOR signaling pathway on the pain of sciatic endometriosis in a rat model. Can. J. Physiol. Pharmacol. 2019, 97, 963–970. [Google Scholar] [CrossRef]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sport. 2017, 27, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Grandone, E.; Colaizzo, D.; Vecchione, G.; Scianname, N.; Notarangelo, A.; Croce, A.I.; Margaglione, M. Homocysteine levels in amniotic fluid. Relationship with birth-weight. Thromb. Haemost. 2006, 95, 625–628. [Google Scholar] [PubMed]
- Makhro, A.V.; Mashkina, A.P.; Solenaya, O.A.; Trunova, O.A.; Kozina, L.S.; Arutyunian, A.V.; Bulygina, E.R. Prenatal hyperhomocysteinemia as a model of oxidative stress of the brain. Bull. Exp. Biol. Med. 2008, 146, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, M.; Blom, H.J.; den Heijer, M. Maternal homocysteine and small-for-gestational-age offspring: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Prada, J.A.; Tsang, R.C. Biological mechanisms of environmentally induced causes of IUGR. Eur. J. Clin. Nutr. 1998, 52 (Suppl. 1), S21–S27; discussion S27–S28. [Google Scholar]
- Arakawa, H.; Erzurumlu, R.S. Role of whiskers in sensorimotor development of C57BL/6 mice. Behav. Brain Res. 2015, 287, 146–155. [Google Scholar] [CrossRef]
- Schallert, T. Behavioral tests for preclinical intervention assessment. NeuroRX 2006, 3, 497–504. [Google Scholar] [CrossRef]
- Beloozerova, I.N.; Sirota, M.G.; Swadlow, H.A.; Orlovsky, G.N.; Popova, L.B.; Deliagina, T.G. Activity of Different Classes of Neurons of the Motor Cortex during Postural Corrections. J. Neurosci. 2003, 23, 7844–7853. [Google Scholar] [CrossRef][Green Version]
- Wise, S.P.; Donoghue, J.P. Motor Cortex of Rodents. In Sensory-Motor Areas and Aspects of Cortical Connectivity; Cerebral Cortex; Plenum Publishing Corp.: New York, NY, USA, 1986; pp. 243–270. [Google Scholar]
- Ioffe, M.E. What is the specificity of the motor cortex in motor learning? In Motor Control, Today and Tomorrow; Academic Press: Sofia, Bulgaria, 1999; pp. 123–137. [Google Scholar]
- Whishaw, I.Q.; Pellis, S.M.; Gorny, B.; Kolb, B.; Tetzlaff, W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 1993, 56, 59–76. [Google Scholar] [CrossRef]
- Wills, T.J.; Cacucci, F.; Burgess, N.; O’Keefe, J. Development of the Hippocampal Cognitive Map in Preweanling Rats. Science 2010, 328, 1573–1576. [Google Scholar] [CrossRef]
- Taube, J.S.; Muller, R.U.; Ranck, J.B. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 1990, 10, 420–435. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ars, C.L.; Nijs, I.M.; Marroun, H.E.; Muetzel, R.; Schmidt, M.; Steenweg-de Graaff, J.; van der Lugt, A.; Jaddoe, V.W.; Hofman, A.; Steegers, E.A.; et al. Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes, cognitive development and psychological functioning: The Generation R Study. Br. J. Nutr. 2016, 122, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Iturbe, S.; Vázquez-Roque, R.A.; De la Cruz-López, F.; Flores, G.; Garcés-Ramírez, L. Dendritic and behavioral changes in rats neonatally treated with homocysteine; A proposal as an animal model to study the attention deficit hyperactivity disorder. J. Chem. Neuroanat. 2022, 119, 102057. [Google Scholar] [CrossRef]
- Rakic, P. Specification of cerebral cortical areas. Science 1988, 241, 170–176. [Google Scholar] [CrossRef]
- Zechel, J.L.; Gamboa, J.L.; Peterson, A.G.; Puchowicz, M.A.; Selman, W.R.; Lust, W.D. Neuronal migration is transiently delayed by prenatal exposure to intermittent hypoxia. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2005, 74, 287–299. [Google Scholar] [CrossRef]
- Stevens, H.E.; Su, T.; Yanagawa, Y.; Vaccarino, F.M. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology 2013, 38, 509–521. [Google Scholar] [CrossRef]
- Golan, M.H.; Mane, R.; Molczadzki, G.; Zuckerman, M.; Kaplan-Louson, V.; Huleihel, M.; Perez-Polo, J.R. Impaired migration signaling in the hippocampus following prenatal hypoxia. Neuropharmacology 2009, 57, 511–522. [Google Scholar] [CrossRef]
- Ang, E.S.B.C.; Gluncic, V.; Duque, A.; Schafer, M.E.; Rakic, P. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12903–12910. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Jarskog, L.F.; Vadlamudi, S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J. Neuroimmunol. 2003, 138, 49–55. [Google Scholar] [CrossRef]
- Marx, C.E.; Vance, B.J.; Jarskog, L.F.; Chescheir, N.C.; Gilmore, J.H. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 levels in human amniotic fluid. Am. J. Obstet. Gynecol. 1999, 181, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Jiao, F.; Yan, X.; Ou, H. Maternal Chronic Folate Supplementation Ameliorates Behavior Disorders Induced by Prenatal High-Fat Diet Through Methylation Alteration of BDNF and Grin2b in Offspring Hippocampus. Mol. Nutr. Food Res. 2017, 61, 1700461. [Google Scholar] [CrossRef] [PubMed]
- Parrish, R.R.; Buckingham, S.C.; Mascia, K.L.; Johnson, J.J.; Matyjasik, M.M.; Lockhart, R.M.; Lubin, F.D. Methionine increases BDNF DNA methylation and improves memory in epilepsy. Ann. Clin. Transl. Neurol. 2015, 2, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Tognoli, C.; Rossi, F.; Di Cola, F.; Baj, G.; Tongiorgi, E.; Terova, G.; Saroglia, M.; Bernardini, G.; Gornati, R. Acute stress alters transcript expression pattern and reduces processing of proBDNF to mature BDNF in Dicentrarchus labrax. BMC Neurosci. 2010, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.M.; O’Neill, H.C.; Stitzel, J.A. Developmental nicotine exposure elicits multigenerational disequilibria in proBDNF proteolysis and glucocorticoid signaling in the frontal cortices, striata, and hippocampi of adolescent mice. Biochem. Pharmacol. 2019, 168, 438–451. [Google Scholar] [CrossRef]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of Cell Survival by Secreted Proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef]
- Wang, M.; Xie, Y.; Qin, D. Proteolytic cleavage of proBDNF to mBDNF in neuropsychiatric and neurodegenerative diseases. Brain Res. Bull. 2021, 166, 172–184. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, W.; Zhang, J.; Li, Q.; Ou, H.; Wang, Z.; Li, S.; Huang, X.; Zhao, C. Schizophrenia-associated rs4702 G allele-specific downregulation of FURIN expression by miR-338-3p reduces BDNF production. Schizophr. Res. 2018, 199, 176–180. [Google Scholar] [CrossRef]
- Gerenu, G.; Martisova, E.; Ferrero, H.; Carracedo, M.; Rantamäki, T.; Ramirez, M.J.; Gil-Bea, F.J. Modulation of BDNF cleavage by plasminogen-activator inhibitor-1 contributes to Alzheimer’s neuropathology and cognitive deficits. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 991–1001. [Google Scholar] [CrossRef]
- Abdallah, M.W.; Michel, T.M. Matrix metalloproteinases in autism spectrum disorders. J. Mol. Psychiatry 2013, 1, 16. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Huang, C.-C.; Hsu, K.-S. Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. J. Physiol. 2012, 590, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Bescond, A.; Augier, T.; Chareyre, C.; Garçon, D.; Hornebeck, W.; Charpiot, P. Influence of Homocysteine on Matrix Metalloproteinase-2: Activation and Activity. Biochem. Biophys. Res. Commun. 1999, 263, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Battaglia-Hsu, S.-f.; Akchiche, N.; Noel, N.; Alberto, J.-M.; Jeannesson, E.; Orozco-Barrios, C.E.; Martinez-Fong, D.; Daval, J.-L.; Guéant, J.-L. Vitamin B12 deficiency reduces proliferation and promotes differentiation of neuroblastoma cells and up-regulates PP2A, proNGF, and TACE. Proc. Natl. Acad. Sci. USA 2009, 106, 21930–21935. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Chao, M.V. Shaping neurons: Long and short range effects of mature and proBDNF signalling upon neuronal structure. Neuropharmacology 2014, 76, 603–609. [Google Scholar] [CrossRef]
- Yang, J.; Harte-Hargrove, L.C.; Siao, C.-J.; Marinic, T.; Clarke, R.; Ma, Q.; Jing, D.; LaFrancois, J.J.; Bath, K.G.; Mark, W.; et al. proBDNF Negatively Regulates Neuronal Remodeling, Synaptic Transmission, and Synaptic Plasticity in Hippocampus. Cell Rep. 2014, 7, 796–806. [Google Scholar] [CrossRef]
- McCarthy, D.M.; Zhang, X.; Darnell, S.B.; Sangrey, G.R.; Yanagawa, Y.; Sadri-Vakili, G.; Bhide, P.G. Cocaine Alters BDNF Expression and Neuronal Migration in the Embryonic Mouse Forebrain. J. Neurosci. 2011, 31, 13400–13411. [Google Scholar] [CrossRef]
- Makita, T.; Sucov, H.M.; Gariepy, C.E.; Yanagisawa, M.; Ginty, D.D. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature 2008, 452, 759–763. [Google Scholar] [CrossRef]
- Bellon, A.; Luchino, J.; Haigh, K.; Rougon, G.; Haigh, J.; Chauvet, S.; Mann, F. VEGFR2 (KDR/Flk1) Signaling Mediates Axon Growth in Response to Semaphorin 3E in the Developing Brain. Neuron 2010, 66, 205–219. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Choi, J.-S.; Cha, J.-H.; Choi, J.-Y.; Lee, M.-Y. Expression of vascular endothelial growth factor receptors Flt-1 and Flk-1 in embryonic rat forebrain. Neurosci. Lett. 2007, 425, 131–135. [Google Scholar] [CrossRef]
- Le Bras, B.; Barallobre, M.-J.; Homman-Ludiye, J.; Ny, A.; Wyns, S.; Tammela, T.; Haiko, P.; Karkkainen, M.J.; Yuan, L.; Muriel, M.-P.; et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat. Neurosci. 2006, 9, 340–348. [Google Scholar] [CrossRef]
- Yang, S.-Z.; Zhang, L.-M.; Huang, Y.-L.; Sun, F.-Y. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat. Rec. 2003, 274A, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Raab, S.; Plate, K.H. Different networks, common growth factors: Shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007, 113, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Haigh, J.J.; Morelli, P.I.; Gerhardt, H.; Haigh, K.; Tsien, J.; Damert, A.; Miquerol, L.; Muhlner, U.; Klein, R.; Ferrara, N.; et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 2003, 262, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Raab, S.; Beck, H.; Gaumann, A.; Yüce, A.; Gerber, H.-P.; Plate, K.; Hammes, H.-P.; Ferrara, N.; Breier, G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 2004, 91, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Haigh, K.; Haigh, J.J.; Vasudevan, A. Endothelial VEGF Sculpts Cortical Cytoarchitecture. J. Neurosci. 2013, 33, 14809–14815. [Google Scholar] [CrossRef][Green Version]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X.; Todorov, P. A Novel Review of Homocysteine and Pregnancy Complications. BioMed Res. Int. 2021, 2021, 6652231. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Gaidin, S.G.; Vedunova, M.V.; Babaev, A.A.; Turovsky, E.A. BDNF Overexpression Enhances the Preconditioning Effect of Brief Episodes of Hypoxia, Promoting Survival of GABAergic Neurons. Neurosci. Bull. 2020, 36, 733–760. [Google Scholar] [CrossRef]
- Khurana, P.; Ashraf, Q.M.; Mishra, O.P.; Delivoria-Papadopoulos, M. Effect of hypoxia on caspase-3, -8, and -9 activity and expression in the cerebral cortex of newborn piglets. Neurochem. Res. 2002, 27, 931–938. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Perna, A.F.; Ingrosso, D.; Lombardi, C.; Acanfora, F.; Satta, E.; Cesare, C.M.; Violetti, E.; Romano, M.M.; De Santo, N.G. Possible mechanisms of homocysteine toxicity. Kidney Int. 2003, 63, S137–S140. [Google Scholar] [CrossRef]
- Boldyrev, A.A. Molecular mechanisms of homocysteine toxicity. Biochemistry 2009, 74, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Pustygina, A.V.; Milyutina, Y.P.; Zaloznyaya, I.V.; Arutyunyan, A.V. Indices of oxidative stress in the brain of newborn rats subjected to prenatal hyperhomocysteinemia. Neurochem. J. 2015, 9, 60–65. [Google Scholar] [CrossRef]






| Gene Symbol RefSeq Accession Number | Nucleotide Sequences (Forward, Reverse, TaqMan-Probe) | Reference |
|---|---|---|
| Ywhaz NM_013011 | GATGAAGCCATTGCTGAACTTG GTCTCCTTGGGTATCCGATGTC ROX-TGAAGAGTCGTACAAAGACAGCACGC-BHQ2 | [38] (primers) [39] (probe) |
| Pgk1 NM_053291 | ATGCAAAGACTGGCCAAGCTAC AGCCACAGCCTCAGCATATTTC HEX-TGCTGGCTGGATGGGCTTGGA-BHQ2 | [38] (primers) [39] (probe) |
| Bdnf NM_001270638.1 | CAGGTCGATTAGGTGGCTTCA CATAAACCACCGACATGGAGC | [40] |
| Ngf NM_001277055.1 | TGCATAGCGTAATGTCCATGTTG CTGTGTCAAGGGAATGCTGAA | [41] |
| Trka (Ntrk1) NM_021589.1 | GACCCCATCCCTGTCTCCTT CCACAGAGACCCCAAAAGGT | This work |
| Trkb (Ntrk2) NM_012731.3 | GACAGTCCTCTGTGGCCAGG TGGCTCTCCCTGGACTCTTT ROX-CCCCAGCCCTGAGGTGCGCA-BHQ2 | This work |
| P75ntr (Ngfr) NM_012610.2 | AGAGGGCACATACTCAGACG TCGACCAGGGATCTCTTCGC FAM-TGCACGCCCTGGGCTGATGCTGAA-BHQ1 | This work |
| Vegfa NM_031836.3 | AGGGTCAAAAACGAAAGCGC CGCGAGTCTGTGTTTTTGCA ROX-CCTGGAGCGTTCACTGTGAGCCT-BHQ2 | This work |
| Vegfb NM_053549.1 | TGGTACCTCTGAGCATGGAA GAGGATCTGCATTCGGACTT | This work |
| Kdr NM_013062.2 | TTCCCGTCCTCAAAGCATCA TGTACGCTGTGCAGGTGTAT FAM-CCCTTCCTGGGACTGTGGCAAAGA-BHQ1 | This work |
| Tests | Day Effect | Group Effect | Interaction Effect |
|---|---|---|---|
| Body weight | F 1;1260 = 96.55, p ≤ 0.001 | F 20;1260 = 1132.92, p ≤ 0.001 | F 20;1260 = 2.48, p ≤ 0.001 |
| Body righting response | F 1;486 = 63.64, p ≤ 0.001 | F 7;486 = 16.434, p ≤ 0.001 | F 7;486 = 2.171, p = 0.036 |
| Whisker placing | F 1;1180 = 137.766, p ≤ 0.001 | F 14;1180 = 297.642, p ≤ 0.001 | F 14;1180 = 5.62, p ≤ 0.001 |
| Rotating grid test | F 1;1179 = 97.582, p ≤ 0.001 | F 14;1179 = 171.151, p ≤ 0.001 | F 14;1179 = 5.637, p ≤ 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilev, D.S.; Shcherbitskaia, A.D.; Tumanova, N.L.; Mikhel, A.V.; Milyutina, Y.P.; Kovalenko, A.A.; Dubrovskaya, N.M.; Inozemtseva, D.B.; Zalozniaia, I.V.; Arutjunyan, A.V. Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis. Cells 2023, 12, 189. https://doi.org/10.3390/cells12010189
Vasilev DS, Shcherbitskaia AD, Tumanova NL, Mikhel AV, Milyutina YP, Kovalenko AA, Dubrovskaya NM, Inozemtseva DB, Zalozniaia IV, Arutjunyan AV. Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis. Cells. 2023; 12(1):189. https://doi.org/10.3390/cells12010189
Chicago/Turabian StyleVasilev, Dmitrii S., Anastasiia D. Shcherbitskaia, Natalia L. Tumanova, Anastasiia V. Mikhel, Yulia P. Milyutina, Anna A. Kovalenko, Nadezhda M. Dubrovskaya, Daria B. Inozemtseva, Irina V. Zalozniaia, and Alexander V. Arutjunyan. 2023. "Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis" Cells 12, no. 1: 189. https://doi.org/10.3390/cells12010189
APA StyleVasilev, D. S., Shcherbitskaia, A. D., Tumanova, N. L., Mikhel, A. V., Milyutina, Y. P., Kovalenko, A. A., Dubrovskaya, N. M., Inozemtseva, D. B., Zalozniaia, I. V., & Arutjunyan, A. V. (2023). Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis. Cells, 12(1), 189. https://doi.org/10.3390/cells12010189






