Association of CSF GAP-43 and APOE ε4 with Cognition in Mild Cognitive Impairment and Alzheimer’s Disease
Abstract
1. Background
2. Methods
2.1. ADNI Database Description
2.2. Participants and Classification Criteria
2.3. CSF Measurements
2.4. Neuroimaging Methods and Cognitive Assessments
2.5. Statistical Methods
3. Results
3.1. CSF GAP-43 Levels in Different Diagnostic Groups
3.2. Characteristics of Included Participants
3.3. Correlations of CSF GAP-43 Levels with CSF Core AD Biomarkers
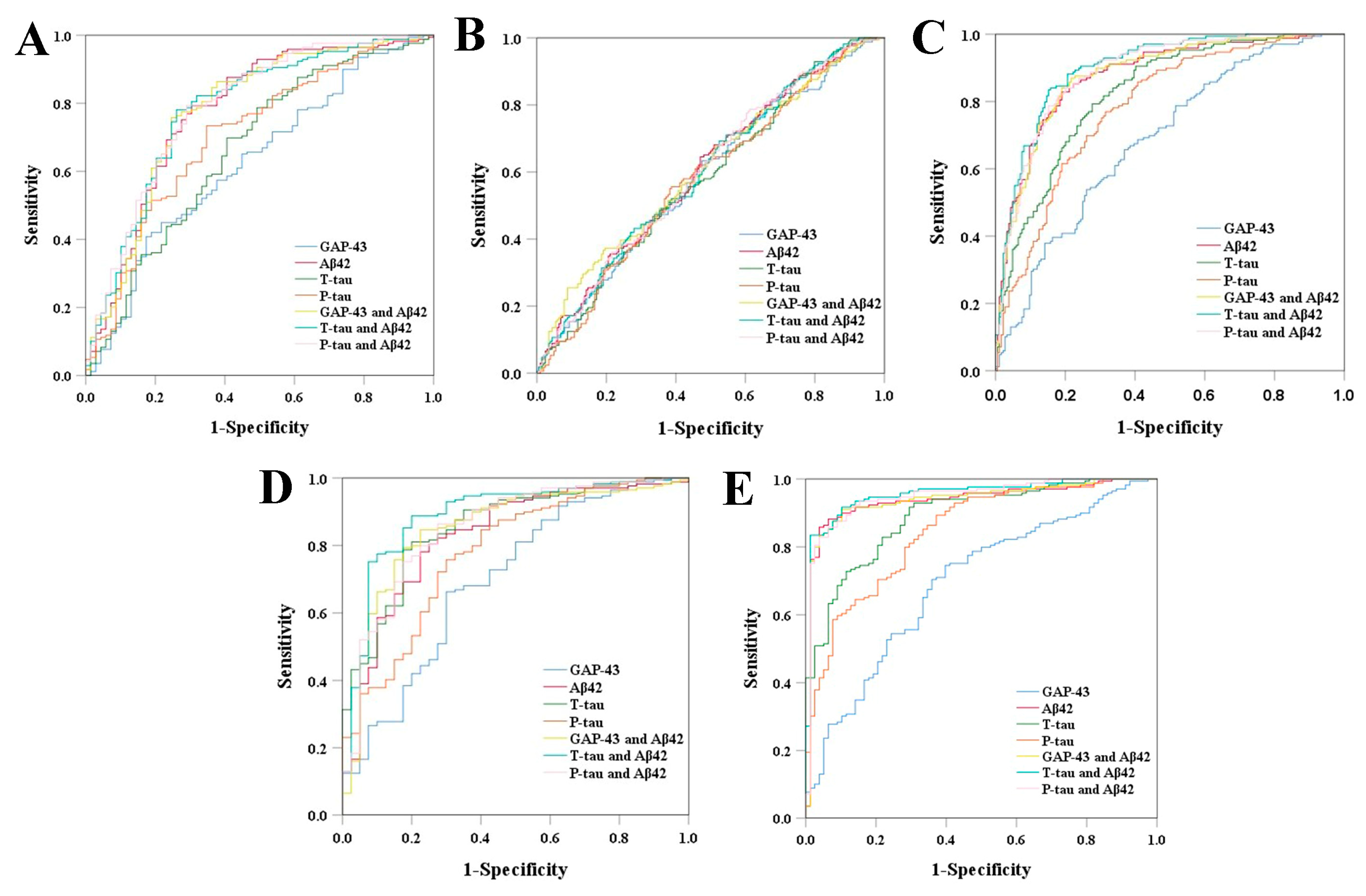
3.4. Diagnostic Ability of CSF GAP-43 and CSF Core AD Biomarkers
3.5. Cross-Sectional Correlations of CSF GAP-43 with MMSE, Hippocampus Volumes, and Ventricular Volumes
3.6. Longitudinal Associations between Baseline CSF GAP-43 and MMSE Progression
3.7. Ability of CSF GAP-43 to Predict Future Cognitive Impairment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Counts, S.E.; Alldred, M.J.; Che, S.; Ginsberg, S.D.; Mufson, E.J. Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology 2014, 79, 172–179. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Scheff, S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 1990, 27, 457–464. [Google Scholar] [CrossRef]
- Denny, J.B. Molecular mechanisms, biological actions, and neuropharmacology of the growth-associated protein GAP-43. Curr. Neuropharmacol. 2006, 4, 293–304. [Google Scholar] [CrossRef]
- Neve, R.L.; Finch, E.A.; Bird, E.D.; Benowitz, L.I. Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3638–3642. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Ng, S.C.; Hsu, D.W. Aberrant GAP-43 gene expression in Alzheimer’s disease. Am. J. Pathol. 1995, 147, 934–946. [Google Scholar]
- Rekart, J.L.; Quinn, B.; Mesulam, M.M.; Routtenberg, A. Subfield-specific increase in brain growth protein in postmortem hippocampus of Alzheimer’s patients. Neuroscience 2004, 126, 579–584. [Google Scholar] [CrossRef]
- Remnestål, J.; Just, D.; Mitsios, N.; Fredolini, C.; Mulder, J.; Schwenk, J.M.; Uhlén, M.; Kultima, K.; Ingelsson, M.; Kilander, L.; et al. CSF profiling of the human brain enriched proteome reveals associations of neuromodulin and neurogranin to Alzheimer’s disease. Proteom. Clin. Appl. 2016, 10, 1242–1253. [Google Scholar] [CrossRef]
- Andersson, A.; Remnestål, J.; Nellgård, B.; Vunk, H.; Kotol, D.; Edfors, F.; Uhlén, M.; Schwenk, J.M.; Ilag, L.L.; Zetterberg, H.; et al. Development of parallel reaction monitoring assays for cerebrospinal fluid proteins associated with Alzheimer’s disease. Clin. Chim. Acta 2019, 494, 79–93. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Dumanis, S.B.; DiBattista, A.M.; Miessau, M.; Moussa, C.E.; Rebeck, G.W. APOE genotype affects the pre-synaptic compartment of glutamatergic nerve terminals. J. Neurochem. 2013, 124, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Petersen, R.C.; Donohue, M.C.; Gamst, A.; Raman, R.; Thomas, R.G.; Walter, S.; Trojanowski, J.Q.; Shaw, L.M.; Beckett, L.A.; et al. Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: Progress and plans. Alzheimers Dement. 2010, 6, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.C.; Fisher, R.H.; Lewis, A.J.; Zorzitto, M.L.; Snow, W.G.; Reid, D.W.; Nieuwstraten, P. The NINCDS-ADRDA Work Group criteria for the clinical diagnosis of probable Alzheimer’s disease: A clinicopathologic study of 57 cases. Neurology 1988, 38, 359–364. [Google Scholar] [CrossRef]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef]
- Sandelius, Å.; Portelius, E.; Källén, Å.; Zetterberg, H.; Rot, U.; Olsson, B.; Toledo, J.B.; Shaw, L.M.; Lee, V.M.Y.; Irwin, D.J.; et al. Elevated CSF GAP-43 is Alzheimer’s disease specific and associated with tau and amyloid pathology. Alzheimers Dement. 2019, 15, 55–64. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bernstein, M.A.; Fox, N.C.; Thompson, P.; Alexander, G.; Harvey, D.; Borowski, B.; Britson, P.J.; Whitwell, J.L.; Ward, C.; et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 2008, 27, 685–691. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Brinkmalm, A.; Brinkmalm, G.; Honer, W.G.; Frölich, L.; Hausner, L.; Minthon, L.; Hansson, O.; Wallin, A.; Zetterberg, H.; Blennow, K.; et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Kvartsberg, H.; Duits, F.H.; Ingelsson, M.; Andreasen, N.; Öhrfelt, A.; Andersson, K.; Brinkmalm, G.; Lannfelt, L.; Minthon, L.; Hansson, O.; et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Ossenkoppele, R.; Kvartsberg, H.; Brinkmalm, A.; Mattsson-Carlgren, N.; Stomrud, E.; Smith, R.; Zetterberg, H.; Blennow, K.; et al. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 2021, 144, 310–324. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef]
- Roselli, F.; Tirard, M.; Lu, J.; Hutzler, P.; Lamberti, P.; Livrea, P.; Morabito, M.; Almeida, O.F. Soluble beta-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J. Neurosci. 2005, 25, 11061–11070. [Google Scholar] [CrossRef]
- Shemer, I.; Holmgren, C.; Min, R.; Fülöp, L.; Zilberter, M.; Sousa, K.M.; Farkas, T.; Härtig, W.; Penke, B.; Burnashev, N.; et al. Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur. J. Neurosci. 2006, 23, 2035–2047. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
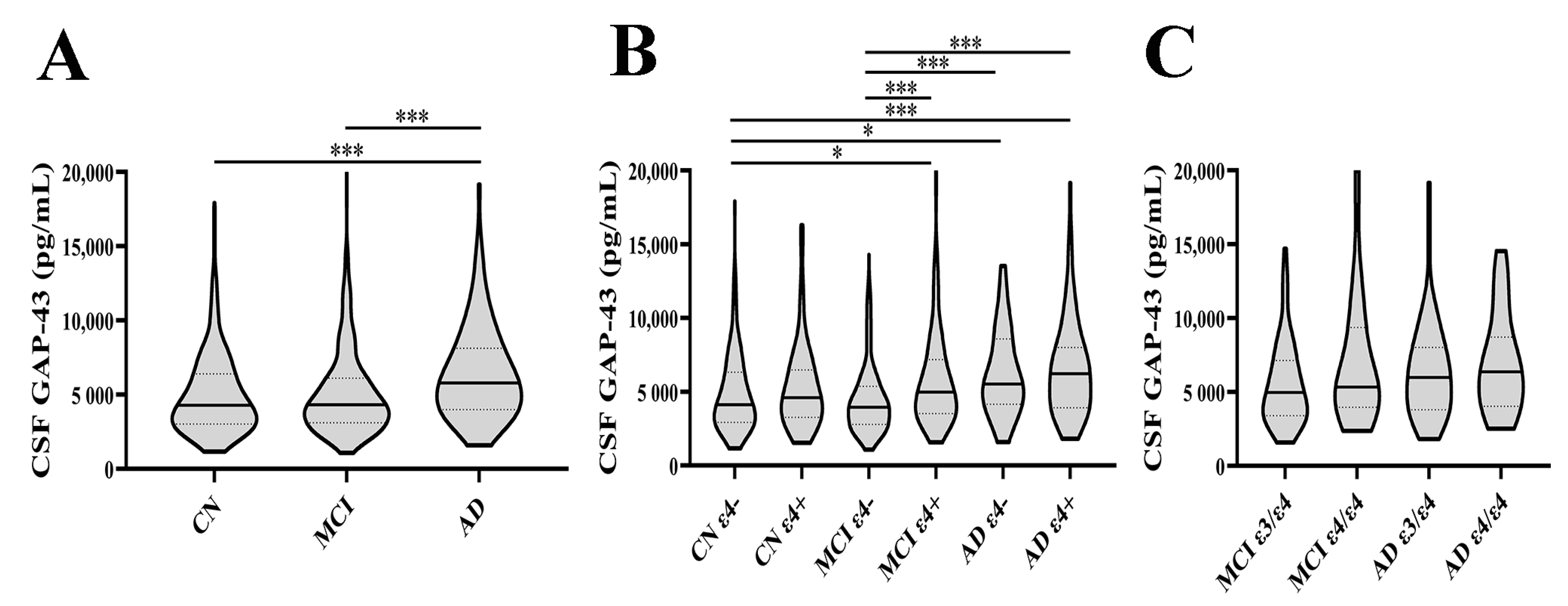


| Baseline Characteristics | CN ε4– (n = 169) | CN ε4+ (n = 69) | MCI ε4– (n = 204) | MCI ε4+ (n = 184) | AD ε4– (n = 40) | AD ε4+ (n = 78) | p Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 73 ± 9 d | 71 ± 9 e | 72 ± 11 e | 71 ± 11 a,e,f | 77 ± 13 b,c,d | 74.5 ± 10 d | <0.001 |
| Female, N (%) | 86 (50.9%) | 44 (63.8%) e | 93 (45.6%) | 81 (44.0%) | 12 (30.0%) b | 35 (44.9%) | 0.013 |
| Education (years) | 16 ± 3 | 17 ± 2 | 16 ± 4 | 16 ± 4 | 16 ± 4 | 16 ± 4 | 0.131 |
| MMSE | 29 ± 2 c,d,e,f | 29 ± 1 c,d,e,f | 29 ± 1 a,b,e,f | 28 ± 3 a,b,e,f | 23 ± 3 a,b,c,d | 24 ± 3 a,b,c,d | <0.001 |
| CSF Aβ42 (pg/mL) | 215 ± 63 b,d,e,f | 159 ± 74.5 a,c,f | 205 ± 82 b,d,e,f | 140.5 ± 51.5 a,c,f | 148 ± 59.5 a,c,f | 124.5 ± 29.3 a,b,c,d,e | <0.001 |
| CSF T-tau (pg/mL) | 54.2 ± 31.5 d,e,f | 63.8 ± 41.2 d,e,f | 56.7 ± 41.5 d,e,f | 89.75 ± 75 a,b,c,f | 106 ± 71.5 a,b,c | 120 ± 75.2 a,b,c,d | <0.001 |
| CSF P-tau (pg/mL) | 27.5 ± 19.8 b,d,e,f | 37.5 ± 24.3 a,c,f | 28.6 ± 21.9 b,d,e,f | 42.25 ± 30.1 a,c | 41.25 ± 32.6 a,c | 54.7 ± 32.6 a,b,c | <0.001 |
| CN ε4− | CN ε4+ | MCI ε4− | MCI ε4+ | AD ε4− | AD ε4+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | rs | p | rs | p | rs | p | |
| Aβ42 | 0.078 | 0.312 | 0.056 | 0.649 | −0.094 | 0.180 | −0.306 | <0.001 | 0.379 | 0.016 | −0.078 | 0.498 |
| T-tau | 0.711 | <0.001 | 0.709 | <0.001 | 0.751 | <0.001 | 0.742 | <0.001 | 0.453 | 0.003 | 0.696 | <0.001 |
| P-tau | 0.579 | <0.001 | 0.576 | <0.001 | 0.581 | <0.001 | 0.611 | <0.001 | 0.227 | 0.158 | 0.509 | <0.001 |
| GAP-43 | Aβ42 | T-tau | P-tau | GAP-43 and Aβ42 | T-tau and Aβ42 | P-tau and Aβ42 | |
|---|---|---|---|---|---|---|---|
| CN ε4+ | 0.627 (0.548−0.705) (p = 0.002) | 0.776 (0.706−0.847) (p < 0.001) | 0.664 (0.585−0.743) (p < 0.001) | 0.708 (0.634−0.782) (p < 0.001) | 0.778 (0.708−0.849) (p < 0.001) | 0.782 (0.714−0.850) (p < 0.001) | 0.789 (0.722−0.856) (p < 0.001) |
| MCI ε4- | 0.586 (0.528−0.644) (p = 0.004) | 0.603 (0.546−0.660) (p = 0.001) | 0.582 (0.524−0.639) (p = 0.007) | 0.582 (0.524−0.639) (p = 0.007) | 0.608 (0.551−0.666) (p < 0.001) | 0.602 (0.545−0.659) (p = 0.001) | 0.603 (0.546−0.660) (p = 0.001) |
| MCI ε4+ | 0.686 (0.631−0.741) (p < 0.001) | 0.879 (0.843−0.915) (p < 0.001) | 0.825 (0.783−0.868) (p < 0.001) | 0.785 (0.738−0.832) (p < 0.001) | 0.880 (0.844−0.916) (p < 0.001) | 0.901 (0.870−0.933) (p < 0.001) | 0.888 (0.854−0.922) (p < 0.001) |
| AD ε4- | 0.700 (0.606−0.795) (p < 0.001) | 0.828 (0.754−0.902) (p < 0.001) | 0.855 (0.792−0.918) (p < 0.001) | 0.780 (0.700−0.861) (p < 0.001) | 0.850 (0.779−0.920) (p < 0.001) | 0.890 (0.830−0.951) (p < 0.001) | 0.851 (0.781−0.921) (p < 0.001) |
| AD ε4+ | 0.695 (0.625−0.765) (p < 0.001) | 0.937 (0.903−0.972) (p < 0.001) | 0.889 (0.848−0.931) (p < 0.001) | 0.847 (0.796−0.898) (p < 0.001) | 0.939 (0.905−0.973) (p < 0.001) | 0.956 (0.929−0.982) (p < 0.001) | 0.948 (0.917−0.979) (p < 0.001) |
| CN ε4- | CN ε4+ | MCI ε4- | MCI ε4+ | AD ε4- | AD ε4+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Cross-sectional (MLR) | ||||||||||||
| MMSE | 0.069 (−0.082, 0.221) | 0.368 | 0.176 (−0.052, 0.403) | 0.128 | −0.148 (−0.280, −0.015) | 0.029 | −0.179 (−0.321, −0.037) | 0.014 | −0.281 (−0.617, 0.056) | 0.099 | 0.179 (−0.041, 0.399) | 0.109 |
| Bilateral hippocampal volume | −0.117 (−0.227, −0.008) | 0.036 | 0.033 (−0.199, 0.265) | 0.775 | −0.036 (−0.150, 0.078) | 0.535 | −0.026 (−0.146, 0.093) | 0.663 | −0.276 (−0.546, −0.006) | 0.045 | −0.027 (−0.207, 0.152) | 0.762 |
| Ventricular volume | −0.287 (−0.426, −0.147) | <0.001 | −0.163 (−0.393, 0.067) | 0.161 | −0.139 (−0.263, −0.016) | 0.027 | −0.279 (−0.401, −0.158) | <0.001 | −0.283 (−0.597, 0.032) | 0.076 | −0.228 (−0.430, −0.027) | 0.027 |
| Longitudinal (GEE) | ||||||||||||
| MMSE progression | - | - | - | - | −0.197 (−0.372, −0.022) | 0.027 | −0.151 (−0.289, −0.013) | 0.032 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Guo, X.; Zhu, F.; Zhang, Q.; Yang, Y.; for the Alzheimer’s Disease Neuroimaging Initiative. Association of CSF GAP-43 and APOE ε4 with Cognition in Mild Cognitive Impairment and Alzheimer’s Disease. Cells 2023, 12, 13. https://doi.org/10.3390/cells12010013
Zhu Y, Guo X, Zhu F, Zhang Q, Yang Y, for the Alzheimer’s Disease Neuroimaging Initiative. Association of CSF GAP-43 and APOE ε4 with Cognition in Mild Cognitive Impairment and Alzheimer’s Disease. Cells. 2023; 12(1):13. https://doi.org/10.3390/cells12010013
Chicago/Turabian StyleZhu, Yueli, Xiaoming Guo, Feng Zhu, Qin Zhang, Yunmei Yang, and for the Alzheimer’s Disease Neuroimaging Initiative. 2023. "Association of CSF GAP-43 and APOE ε4 with Cognition in Mild Cognitive Impairment and Alzheimer’s Disease" Cells 12, no. 1: 13. https://doi.org/10.3390/cells12010013
APA StyleZhu, Y., Guo, X., Zhu, F., Zhang, Q., Yang, Y., & for the Alzheimer’s Disease Neuroimaging Initiative. (2023). Association of CSF GAP-43 and APOE ε4 with Cognition in Mild Cognitive Impairment and Alzheimer’s Disease. Cells, 12(1), 13. https://doi.org/10.3390/cells12010013






