Lower Metabolic Potential and Impaired Metabolic Flexibility in Human Lymph Node Stromal Cells from Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Tissue Culture
2.2. Seahorse Oxidation Assays
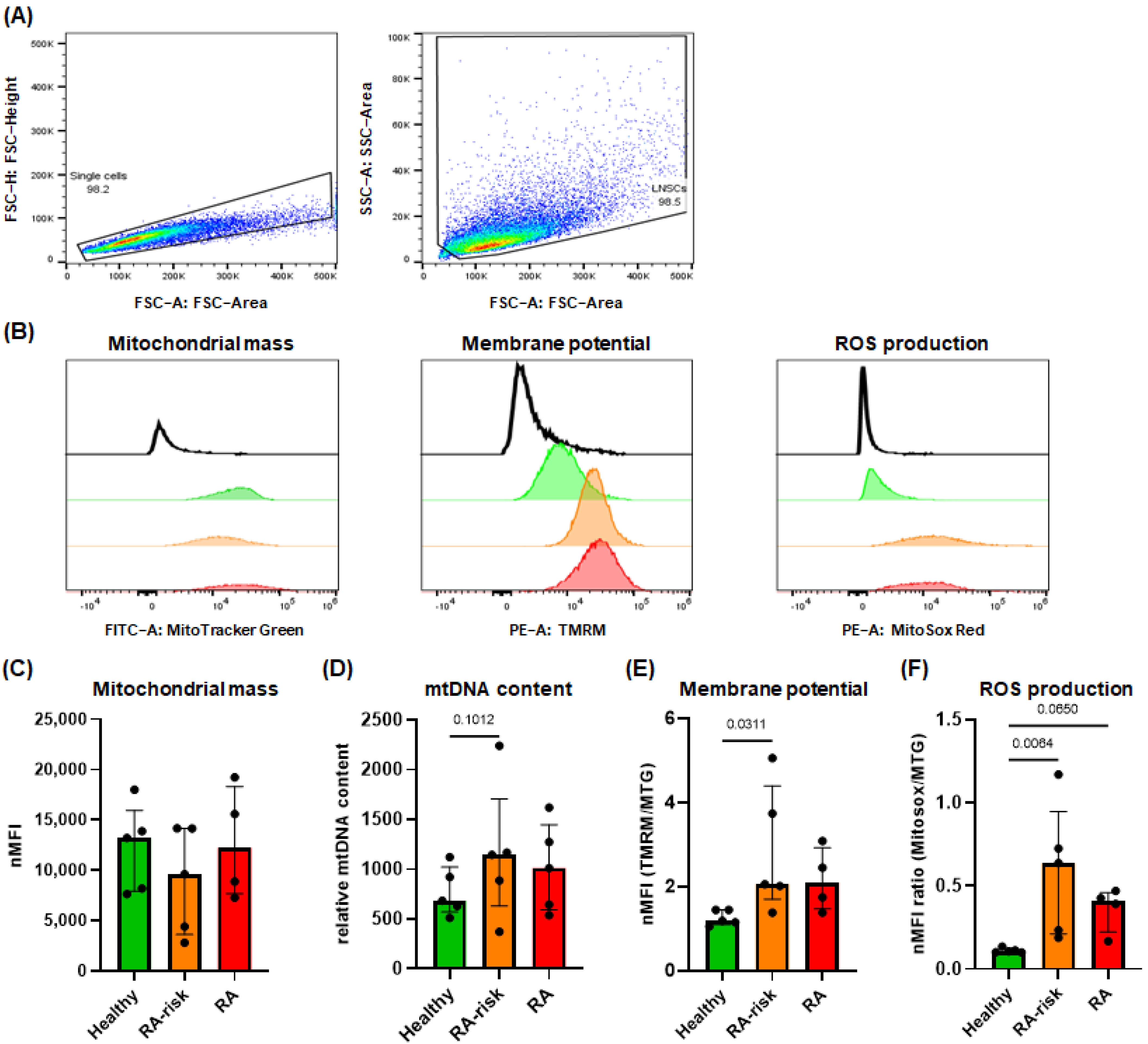
2.3. Flow Cytometry
2.4. Beta-Oxidation
2.5. Quantitative Real-Time PCR
2.6. Mitochondrial DNA Quantitation
2.7. Statistics
3. Results
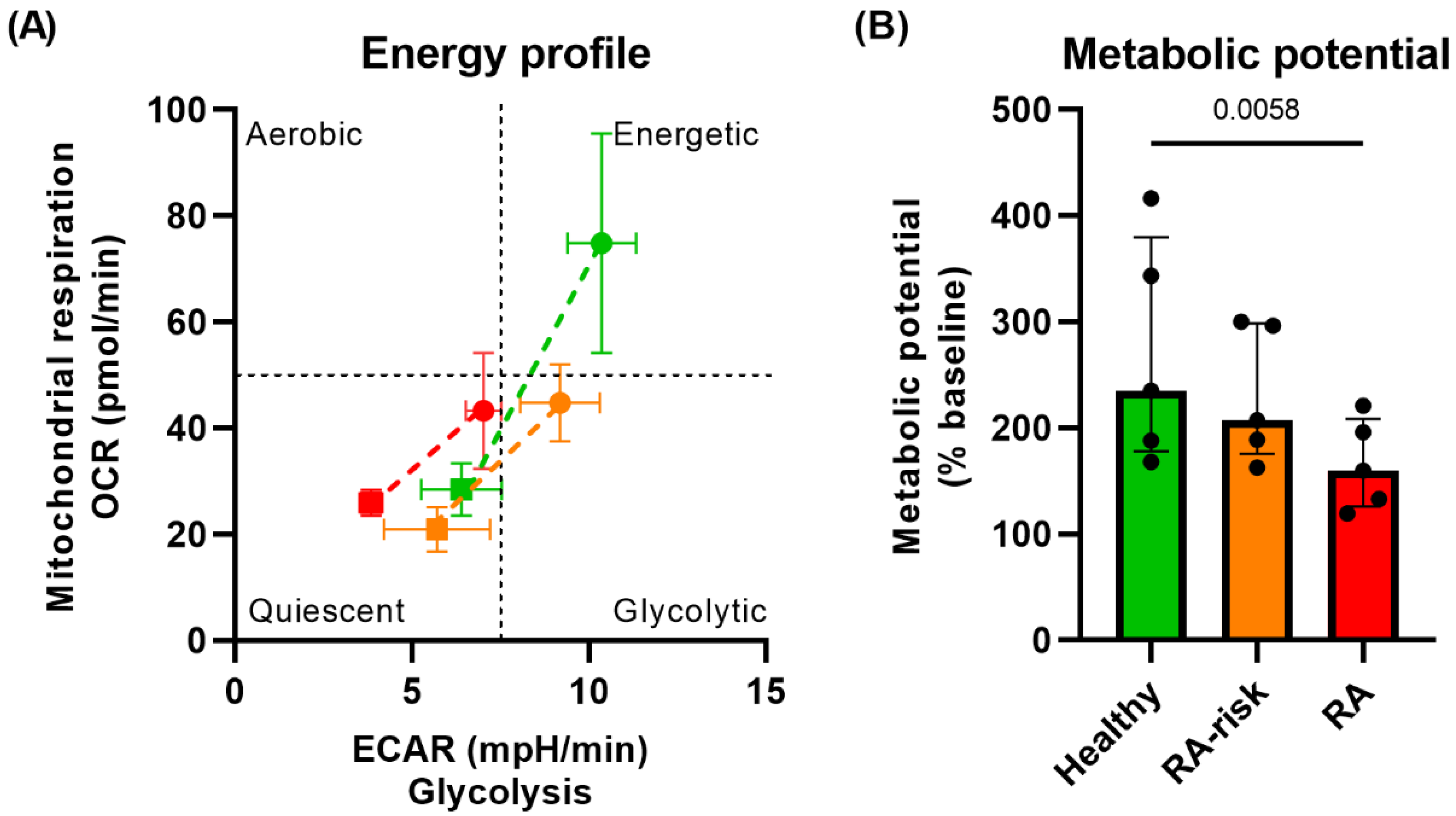
3.1. Lower Mitochondrial Respiration in RA-Risk and RA LNSCs
3.2. Lower Metabolic Potential in RA LNSCs
3.3. Increased Mitochondrial Membrane Potential and ROS Production in RA-Risk LNSCs
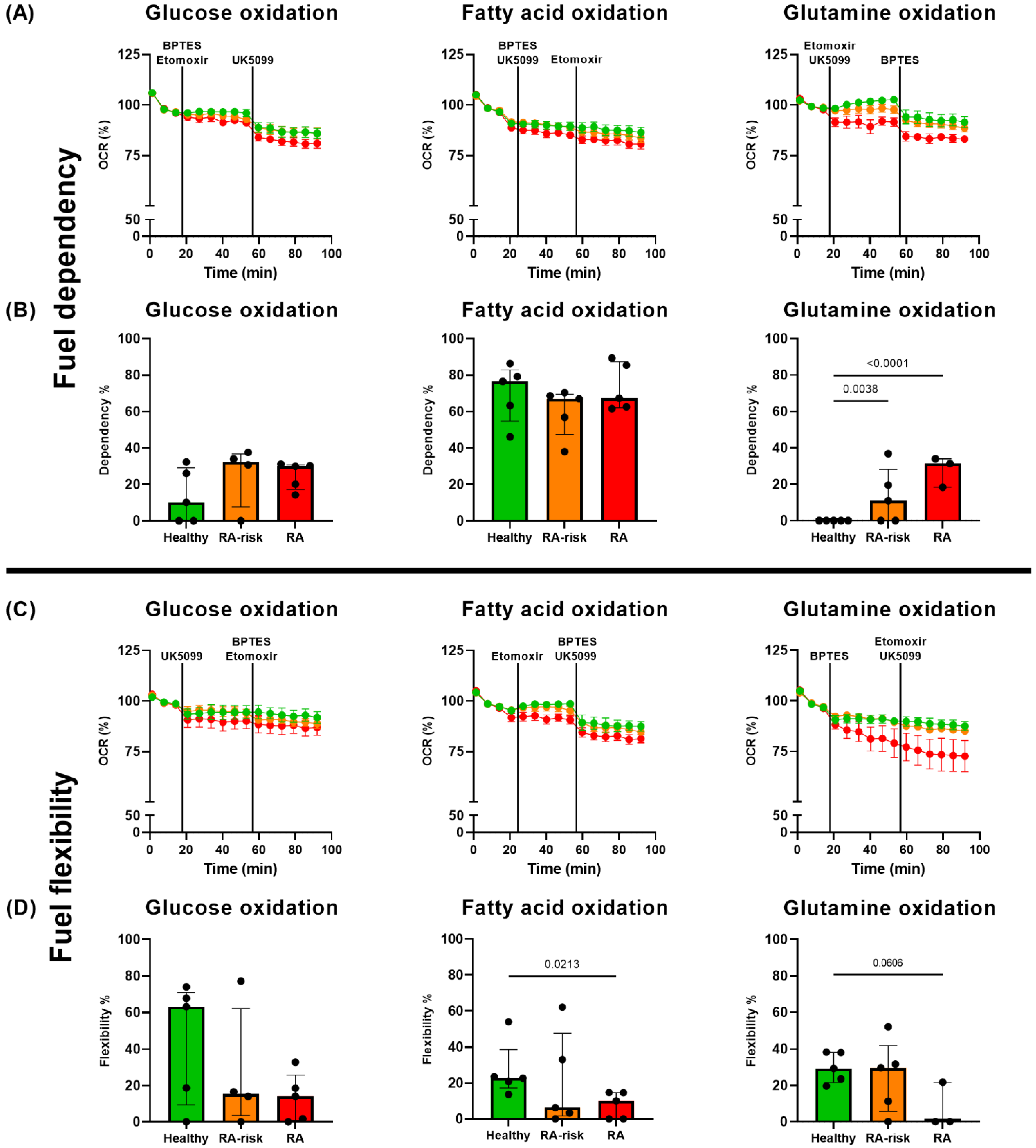
3.4. Fatty Acid β-Oxidation in Cultured LNSCs
3.5. Altered Substrate Use in RA LNSCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- England, B.R.; Thiele, G.M.; Mikuls, T.R. Anticitrullinated protein antibodies: Origin and role in the pathogenesis of rheumatoid arthritis. Curr. Opin. Rheumatol. 2017, 29, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Karlson, E.W.; van Schaardenburg, D.; van der Helm-van Mil, A.H. Strategies to predict rheumatoid arthritis development in at-risk populations. Rheumatology (Oxf.) 2016, 55, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Rantapaa-Dahlqvist, S.; de Jong, B.A.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; van Venrooij, W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 2741–2749. [Google Scholar] [CrossRef]
- de Hair, M.J.; van de Sande, M.G.; Ramwadhdoebe, T.H.; Hansson, M.; Landewe, R.; van der Leij, C.; Maas, M.; Serre, G.; van Schaardenburg, D.; Klareskog, L.; et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: Implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 513–522. [Google Scholar] [CrossRef] [Green Version]
- van de Sande, M.G.; de Hair, M.J.; van der Leij, C.; Klarenbeek, P.L.; Bos, W.H.; Smith, M.D.; Maas, M.; de Vries, N.; van Schaardenburg, D.; Dijkmans, B.A.; et al. Different stages of rheumatoid arthritis: Features of the synovium in the preclinical phase. Ann. Rheum. Dis. 2011, 70, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, R.; Mebius, R.E. Stromal cell-immune cell interactions. Annu. Rev. Immunol. 2011, 29, 23–43. [Google Scholar] [CrossRef]
- Cremasco, V.; Woodruff, M.C.; Onder, L.; Cupovic, J.; Nieves-Bonilla, J.M.; Schildberg, F.A.; Chang, J.; Cremasco, F.; Harvey, C.J.; Wucherpfennig, K.; et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat. Immunol. 2014, 15, 973–981. [Google Scholar] [CrossRef]
- Cohen, J.N.; Guidi, C.J.; Tewalt, E.F.; Qiao, H.; Rouhani, S.J.; Ruddell, A.; Farr, A.G.; Tung, K.S.; Engelhard, V.H. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 2010, 207, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Grasso, C.; Pierie, C.; Mebius, R.E.; van Baarsen, L.G.M. Lymph node stromal cells: Subsets and functions in health and disease. Trends Immunol. 2021, 42, 920–936. [Google Scholar] [CrossRef]
- Luther, S.A.; Tang, H.L.; Hyman, P.L.; Farr, A.G.; Cyster, J.G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 12694–12699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, S.N.; Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 2009, 9, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.D.; Turley, S.J. Fibroblastic reticular cells: Organization and regulation of the T lymphocyte life cycle. J. Immunol. 2015, 194, 1389–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, L.; Creusot, R.J.; Pager, C.T.; Sarnow, P.; Fathman, C.G. Reduced DEAF1 function during type 1 diabetes inhibits translation in lymph node stromal cells by suppressing Eif4g3. J. Mol. Cell Biol. 2013, 5, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadafi, R.; Gago de Graça, C.; Keuning, E.D.; Koning, J.J.; de Kivit, S.; Konijn, T.; Henri, S.; Borst, J.; Reijmers, R.M.; van Baarsen, L.G.M.; et al. Lymph Node Stromal Cells Generate Antigen-Specific Regulatory T Cells and Control Autoreactive T and B Cell Responses. Cell Rep. 2020, 30, 4110–4123.e4114. [Google Scholar] [CrossRef]
- Yip, L.; Su, L.; Sheng, D.; Chang, P.; Atkinson, M.; Czesak, M.; Albert, P.R.; Collier, A.R.; Turley, S.J.; Fathman, C.G.; et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat. Immunol. 2009, 10, 1026–1033. [Google Scholar] [CrossRef] [Green Version]
- Karouzakis, E.; Hähnlein, J.; Grasso, C.; Semmelink, J.F.; Tak, P.P.; Gerlag, D.M.; Gay, S.; Ospelt, C.; van Baarsen, L.G.M. Molecular Characterization of Human Lymph Node Stromal Cells During the Earliest Phases of Rheumatoid Arthritis. Front. Immunol. 2019, 10, 1863. [Google Scholar] [CrossRef] [Green Version]
- Hähnlein, J.S.; Nadafi, R.; de Jong, T.; Ramwadhdoebe, T.H.; Semmelink, J.F.; Maijer, K.I.; Zijlstra, I.A.; Maas, M.; Gerlag, D.M.; Geijtenbeek, T.B.H.; et al. Impaired lymph node stromal cell function during the earliest phases of rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 35. [Google Scholar] [CrossRef] [Green Version]
- Hähnlein, J.S.; Ramwadhdoebe, T.H.; Semmelink, J.F.; Choi, I.Y.; Berger, F.H.; Maas, M.; Gerlag, D.M.; Tak, P.P.; Geijtenbeek, T.B.H.; van Baarsen, L.G.M. Distinctive expression of T cell guiding molecules in human autoimmune lymph node stromal cells upon TLR3 triggering. Sci. Rep. 2018, 8, 1736. [Google Scholar] [CrossRef] [Green Version]
- Everts, B.; Amiel, E.; Huang, S.C.-C.; Smith, A.M.; Chang, C.-H.; Lam, W.Y.; Redmann, V.; Freitas, T.C.; Blagih, J.; van der Windt, G.J.W.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar] [CrossRef]
- Degauque, N.; Brosseau, C.; Brouard, S. Regulation of the Immune Response by the Inflammatory Metabolic Microenvironment in the Context of Allotransplantation. Front. Immunol. 2018, 9, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, W.J.; Ahl, P.J.; Connolly, J.E. Metabolism Is Central to Tolerogenic Dendritic Cell Function. Mediat. Inflamm. 2016, 2016, 2636701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patente, T.A.; Pelgrom, L.R.; Everts, B. Dendritic cells are what they eat: How their metabolism shapes T helper cell polarization. Curr. Opin. Immunol. 2019, 58, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gerlag, D.M.; Raza, K.; van Baarsen, L.G.; Brouwer, E.; Buckley, C.D.; Burmester, G.R.; Gabay, C.; Catrina, A.I.; Cope, A.P.; Cornelis, F.; et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: Report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann. Rheum. Dis 2012, 71, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- de Hair, M.J.H.; Zijlstra, I.A.J.; Boumans, M.J.H.; van de Sande, M.G.H.; Maas, M.; Gerlag, D.M.; Tak, P.P. Hunting for the pathogenesis of rheumatoid arthritis: Core-needle biopsy of inguinal lymph nodes as a new research tool. Ann. Rheum. Dis. 2012, 71, 1911–1912. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- de Jong, T.A.; Semmelink, J.F.; Denis, S.W.; van de Sande, M.G.H.; Houtkooper, R.H.L.; van Baarsen, L.G.M. Altered lipid metabolism in synovial fibroblasts of individuals at risk of developing rheumatoid arthritis. J. Autoimmun. 2023, 134, 102974. [Google Scholar] [CrossRef]
- Diekman, E.F.; Ferdinandusse, S.; van der Pol, L.; Waterham, H.R.; Ruiter, J.P.; Ijlst, L.; Wanders, R.J.; Houten, S.M.; Wijburg, F.A.; Blank, A.C.; et al. Fatty acid oxidation flux predicts the clinical severity of VLCAD deficiency. Genet. Med. 2015, 17, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.A.; Fisher-Wellman, K.H.; Neufer, P.D. From OCR and ECAR to energy: Perspectives on the design and interpretation of bioenergetics studies. J. Biol. Chem. 2021, 297, 101140. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pond, C.M.; Mattacks, C.A. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J. Lipid Res. 1996, 36, 2219–2231. [Google Scholar] [CrossRef]
- Gaber, T.; Strehl, C.; Buttgereit, F. Metabolic regulation of inflammation. Nat. Rev. Rheumatol. 2017, 13, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shen, Y.; Hohensinner, P.; Ju, J.; Wen, Z.; Goodman, S.B.; Zhang, H.; Goronzy, J.J.; Weyand, C.M. Deficient Activity of the Nuclease MRE11A Induces T Cell Aging and Promotes Arthritogenic Effector Functions in Patients with Rheumatoid Arthritis. Immunity 2016, 45, 903–916. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Wu, B.; Goodman, S.B.; Berry, G.J.; Goronzy, J.J.; Weyand, C.M. Metabolic Control of Autoimmunity and Tissue Inflammation in Rheumatoid Arthritis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Fearon, U.; Hanlon, M.M.; Wade, S.M.; Fletcher, J.M. Altered metabolic pathways regulate synovial inflammation in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 197, 170–180. [Google Scholar] [CrossRef]



| Healthy n = 5 | RA-Risk Individuals n = 5 | RA Patients n = 5 | |
|---|---|---|---|
| Sex (female, n) (%) | 3 (60) | 4 (80) | 4 (80) |
| Age (years) | 47 (33–58) | 48 (36–50) | 42 (29–44) |
| IgM-RF positive (n) (%) | 0 (0) | 0 (0) | 4 (80) |
| ACPA positive (n) (%) | 0 (0) | 5 (100) | 5 (100) |
| ESR (mm/h) | ND | 5 (2–8.5) | 26 (6.5–40.5) |
| CRP | 0.5 (0.3–3.9) | 1.6 (0.8–3.6) | 3 (1.7–26.5) |
| Treatment (n) (%) | |||
| NSAIDs | - | - | 2 (40) |
| DMARD | - | - | 2 (40) |
| SYBR Green | |||
|---|---|---|---|
| Gene | mRNA Transcript ID | Forward Sequence | Reverse Sequence |
| POLR2G | NM_002696.3 | GAGGTCGTGGATGCTGTTGT | TCTCTGAAGGGATGGAATGTCG |
| RPLP0 | NM_001002.4 | GCAGCATCTACAACCCTGAAGT | GCAGACAGACACTGGCAACAT |
| ACACB | XM_011538265.2 | GCCCCGAGAACCTCAAGAAA | AGAAGCCGCTGTCCATGAAG |
| ACADM | NM_001286044.1 | GGAGTTCACCGAACAGCAGA | AGGGGGACTGGATATTCACCA |
| ACADVL | NM_001033859.2 | ATGCACATCCTCAACAATGG | GAATTTTCTCCCCAAACTGG |
| CPT1A | NM_001876.4 | GCTGACTCGGTACTCTCTGA | GGGTACACGCCAGTGATGAT |
| GFPT1 | NM_001244710.2 | AGTTGGCACAAGGCGAGGTA | TGCTGTCCACACGAGAGAGA |
| GLS | NM_014905.5 | CAGTAGATGGACAGAGGCATTCT | TTAGTCCACTCGGCTCTTTTCC |
| GLUD1 | NM_005271.5 | AACTACCACTTGCTCATGTCTG | ATTGTGTATGCCAAGCCAGAGT |
| PHGDH | NM_006623.4 | AATCTGCGGAAAGTGCTCATC | GGTGGCAGAGCGAACAATAA |
| GLUL | NM_001033044.4 | GGGAGGAGAATGGTCTGAAGTACA | CGATTGGCTACACCAGCAGA |
| GOT2 | NM_002080.4 | ATGGGCTTATATGGTGAGCGT | CGCAAATCTGGGGTGTTCAG |
| HK1 | NM_001358263.1 | AGAGGACTGGACCGTCTGAA | GCATGATTCTGGAGAAGTGTGG |
| HMGCR | NM_000859.3 | TTTGGGTATTGCTGGCCTTT | TCCCTTACTTCATCCTGTGAGT |
| LDHA | NM_005566.4 | CAGGTGGTTGAGAGTGCTTATG | TGTCCCAAAATGCAAGGAACAC |
| PFKM | NM_000289.6 | TGGAGATGCCCAAGGTATGAAT | CACTTCCAATCACCGTGCCT |
| PKM | NM_002654.6 | TGGAATGAATGTGGCTCGTCT | GATGGGGTCAGAAGCAAAGC |
| Taqman assays | |||
| Gene | Assay ID | ||
| POLR2G | Hs00275738_m1 | ||
| RPLP0 | Hs00420895_gH | ||
| HK2 | Hs00606086_m1 | ||
| SLC2A1 | Hs00892681_m1 | ||
| PYGM | Hs00989942_m1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jong, T.A.; Semmelink, J.F.; Denis, S.W.; Bolt, J.W.; Maas, M.; van de Sande, M.G.H.; Houtkooper, R.H.L.; van Baarsen, L.G.M. Lower Metabolic Potential and Impaired Metabolic Flexibility in Human Lymph Node Stromal Cells from Patients with Rheumatoid Arthritis. Cells 2023, 12, 1. https://doi.org/10.3390/cells12010001
de Jong TA, Semmelink JF, Denis SW, Bolt JW, Maas M, van de Sande MGH, Houtkooper RHL, van Baarsen LGM. Lower Metabolic Potential and Impaired Metabolic Flexibility in Human Lymph Node Stromal Cells from Patients with Rheumatoid Arthritis. Cells. 2023; 12(1):1. https://doi.org/10.3390/cells12010001
Chicago/Turabian Stylede Jong, Tineke A., Johanna F. Semmelink, Simone W. Denis, Janne W. Bolt, Mario Maas, Marleen G. H. van de Sande, Riekelt H. L. Houtkooper, and Lisa G. M. van Baarsen. 2023. "Lower Metabolic Potential and Impaired Metabolic Flexibility in Human Lymph Node Stromal Cells from Patients with Rheumatoid Arthritis" Cells 12, no. 1: 1. https://doi.org/10.3390/cells12010001
APA Stylede Jong, T. A., Semmelink, J. F., Denis, S. W., Bolt, J. W., Maas, M., van de Sande, M. G. H., Houtkooper, R. H. L., & van Baarsen, L. G. M. (2023). Lower Metabolic Potential and Impaired Metabolic Flexibility in Human Lymph Node Stromal Cells from Patients with Rheumatoid Arthritis. Cells, 12(1), 1. https://doi.org/10.3390/cells12010001







