Polar Chromosomes—Challenges of a Risky Path
Abstract
:1. Introduction—Chromosome Congression and Alignment
2. Different Routes to Chromosome Biorientation—Curios Case of Polar Chromosomes
3. General Models of Chromosome Alignment
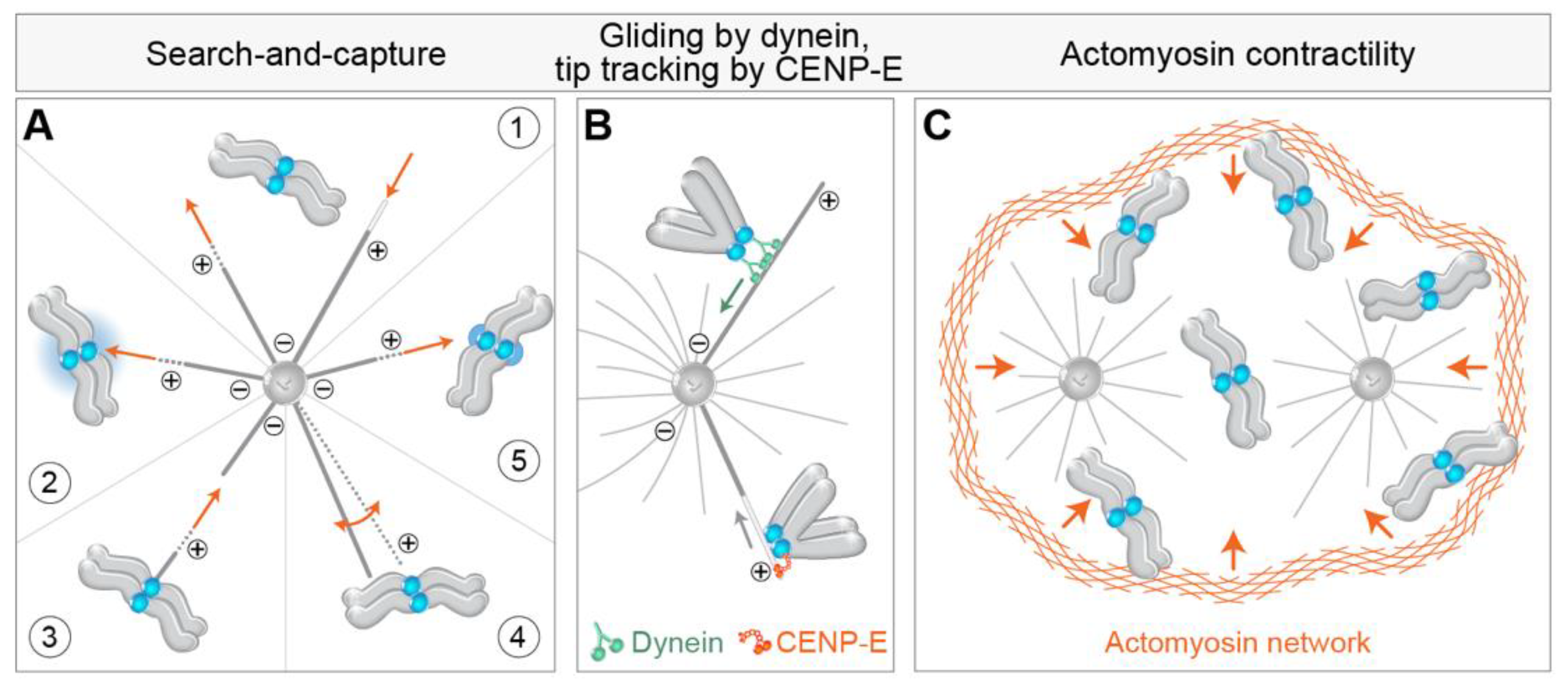
3.1. Search-and-Capture Model
3.2. Oscillating at the Equator—Maintenance of Alignment
4. Biomechanical and Molecular Aspects of Polar Chromosome Congression
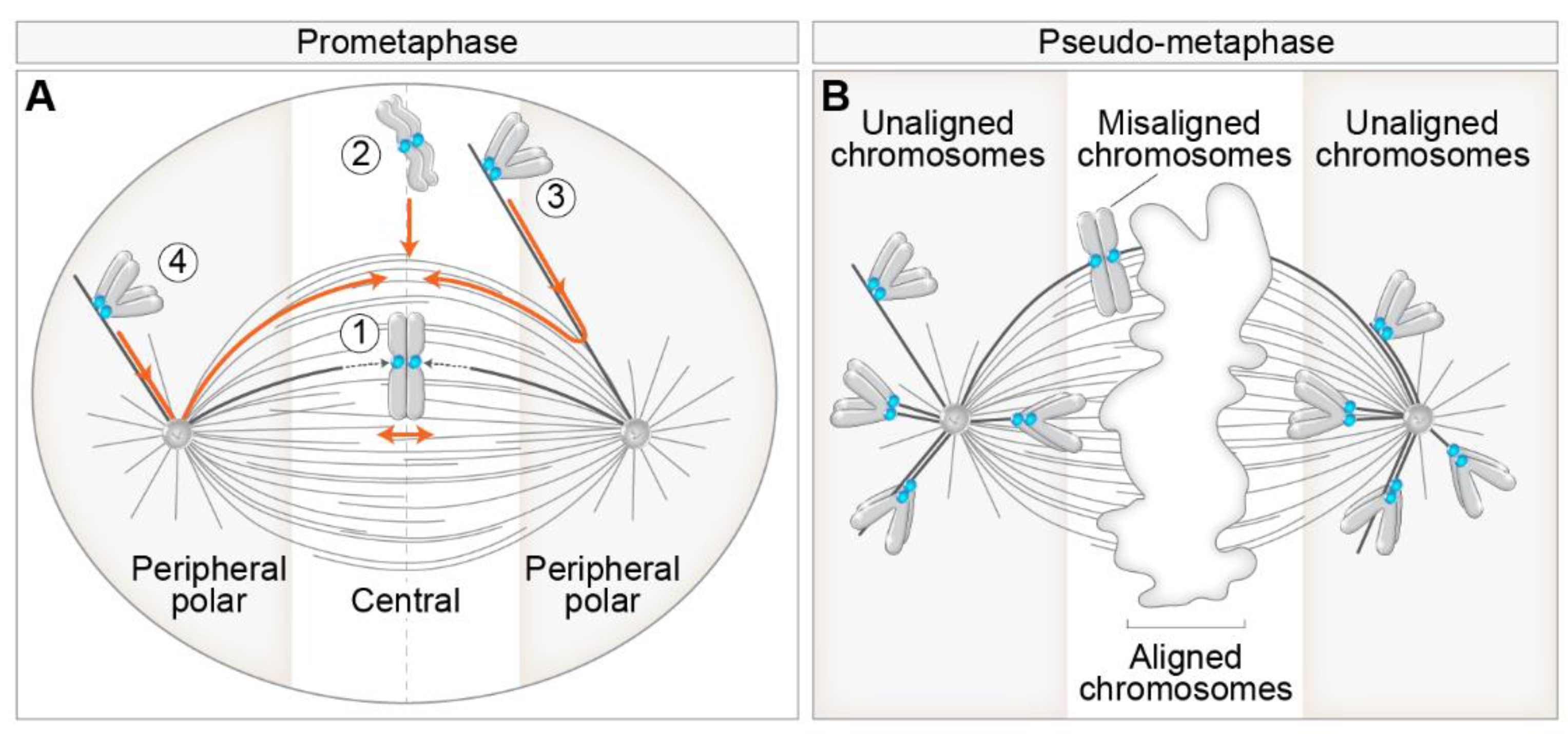
4.1. Getting to the Spindle—Movements towards and across the Polar Region
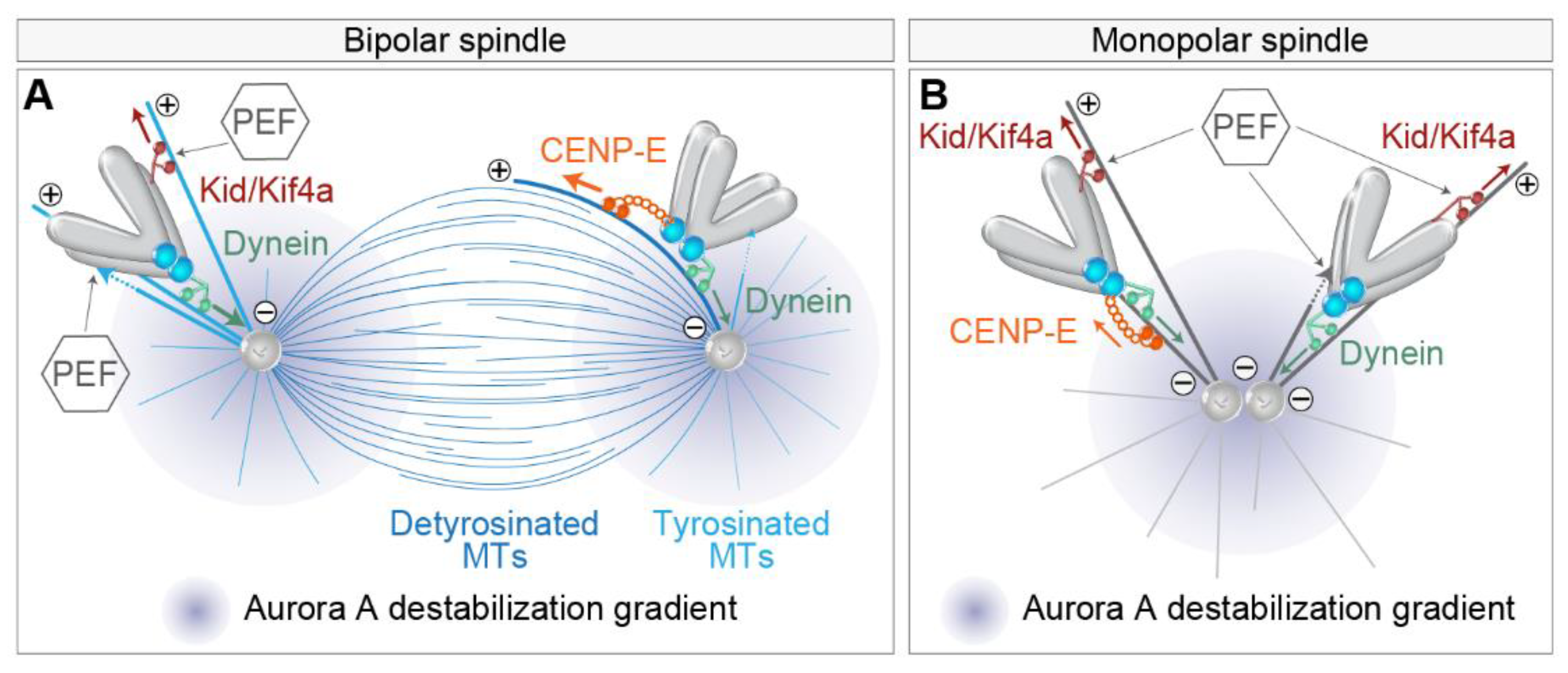
4.2. Getting to the Equator—Congression from the Spindle Pole to the Spindle Midplane
4.3. How Do Polar Chromosomes Set Their Distance to the Spindle Pole?
4.4. Significance of Centrosome Prepositioning during Prophase
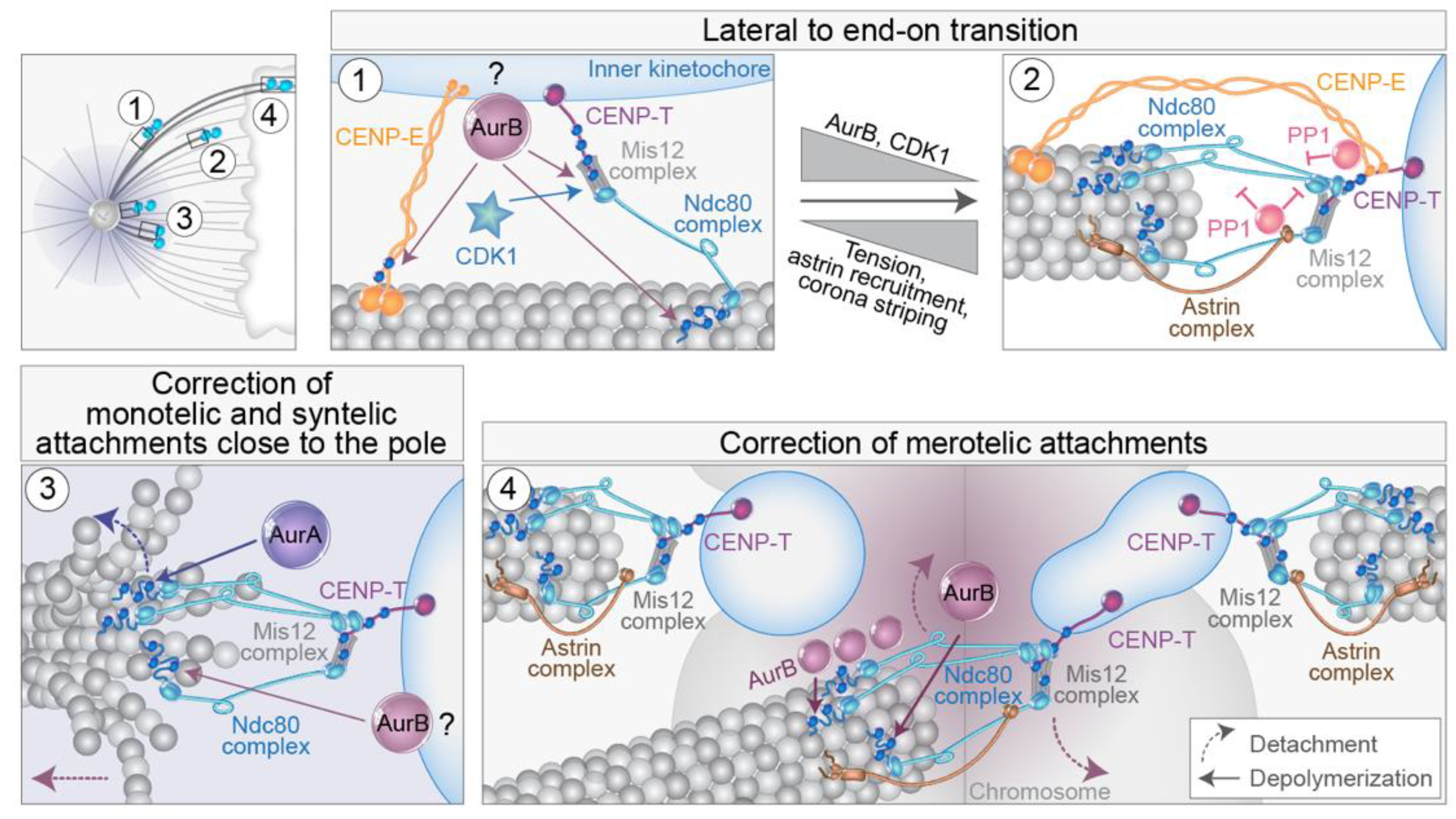
5. Regulation of Lateral to End-on Conversion and Error Correction
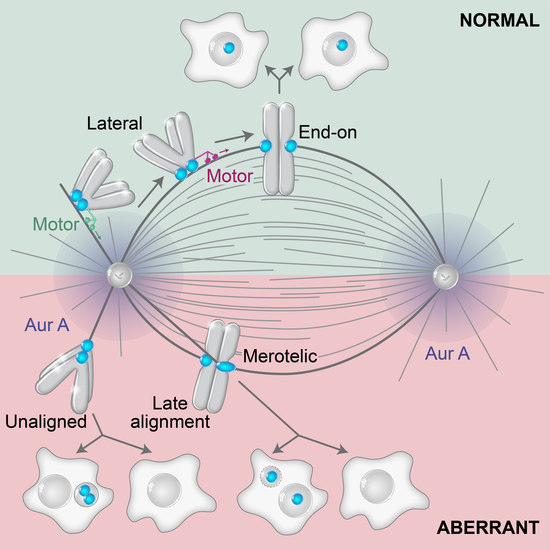
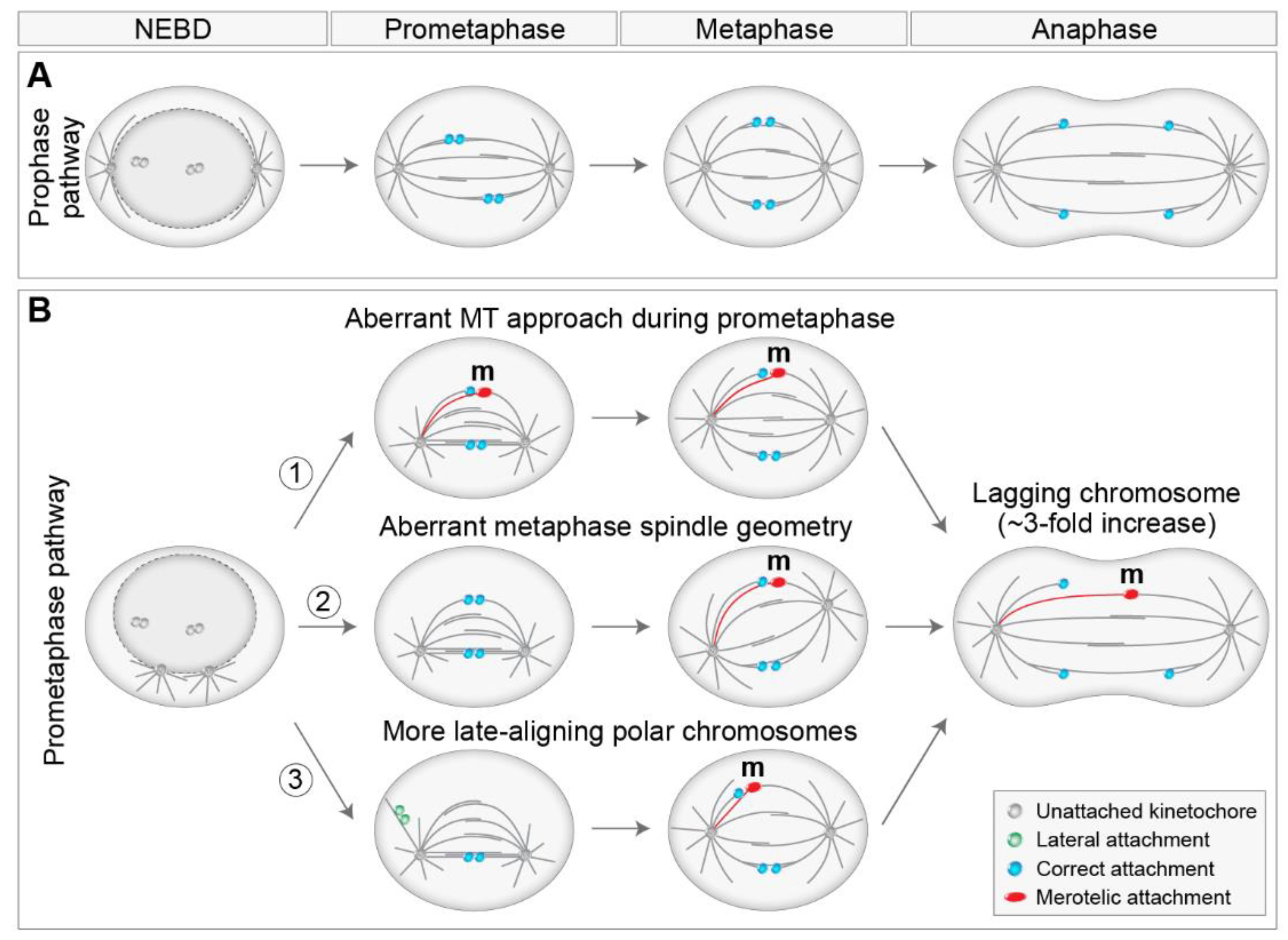
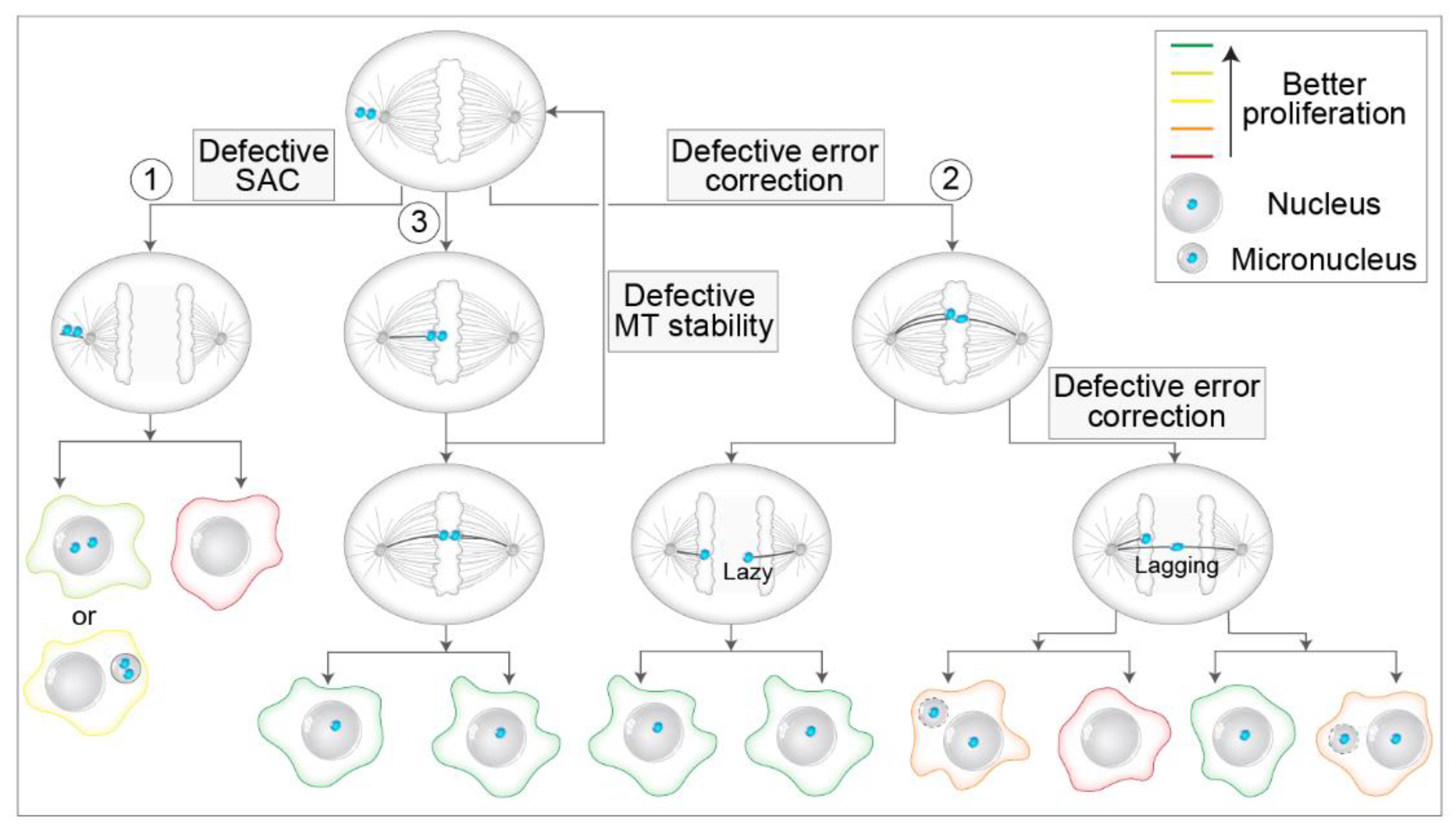
6. Mis-Segregation and Aneuploidy of Polar Chromosomes
6.1. Different Ways to Mis-Segregation through Polar Chromosomes
6.2. Polar Chromosomes in Cancer—Aneuploidy and CIN
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntosh, J.R.; Molodtsov, M.I.; Ataullakhanov, F.I. Biophysics of Mitosis. Q. Rev. Biophys. 2012, 45, 147. [Google Scholar] [CrossRef] [PubMed]
- Prosser, S.L.; Pelletier, L. Mitotic Spindle Assembly in Animal Cells: A Fine Balancing Act. Nat. Rev. Mol. Cell Biol. 2017, 18, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Pavin, N.; Tolić, I.M. Self-Organization and Forces in the Mitotic Spindle. Annu. Rev. Biophys. 2016, 45, 279–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, A.P.; Cheeseman, I.M. Kinetochore Assembly throughout the Cell Cycle. Semin. Cell Dev. Biol. 2021, 117, 62–74. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Maiato, H. Prometaphase. Semin. Cell Dev. Biol. 2021, 117, 52–61. [Google Scholar] [CrossRef]
- Nunes, V.; Ferreira, J.G. From the Cytoskeleton to the Nucleus: An Integrated View on Early Spindle Assembly. Semin. Cell Dev. Biol. 2021, 117, 42–51. [Google Scholar] [CrossRef]
- Maiato, H.; Gomes, A.M.; Sousa, F.; Barisic, M. Mechanisms of Chromosome Congression during Mitosis. Biology 2017, 6, 13. [Google Scholar] [CrossRef]
- Walczak, C.E.; Cai, S.; Khodjakov, A. Mechanisms of Chromosome Behaviour during Mitosis. Nat. Rev. Mol. Cell Biol. 2010, 11, 91–102. [Google Scholar] [CrossRef]
- Vukušić, K.; Tolić, I.M. Anaphase B: Long-Standing Models Meet New Concepts. Semin. Cell Dev. Biol. 2021, 117, 127–139. [Google Scholar] [CrossRef]
- Asbury, C.L. Anaphase A: Disassembling Microtubules Move Chromosomes toward Spindle Poles. Biology 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Lara-Gonzalez, P.; Pines, J.; Desai, A. Spindle Assembly Checkpoint Activation and Silencing at Kinetochores. Semin. Cell Dev. Biol. 2021, 117, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Gregan, J.; Polakova, S.; Zhang, L.; Tolić-Nørrelykke, I.M.; Cimini, D. Merotelic Kinetochore Attachment: Causes and Effects. Trends Cell Biol. 2011, 21, 374–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampson, M.A.; Grishchuk, E.L. Mechanisms to Avoid and Correct Erroneous Kinetochore-Microtubule Attachments. Biology 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Amon, A. Context Is Everything: Aneuploidy in Cancer. Nat. Rev. Genet. 2019, 21, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Storchova, Z. Consequences of Mitotic Failure - The Penalties and the Rewards. Semin. Cell Dev. Biol. 2021, 117, 149–158. [Google Scholar] [CrossRef]
- Gerlich, D.; Beaudouin, J.; Kalbfuss, B.; Daigle, N.; Eils, R.; Ellenberg, J. Global Chromosome Positions Are Transmitted through Mitosis in Mammalian Cells. Cell 2003, 112, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Renda, F.; Khodjakov, A. Role of Spatial Patterns and Kinetochore Architecture in Spindle Morphogenesis. Semin. Cell Dev. Biol. 2021, 117, 75–85. [Google Scholar] [CrossRef]
- Barisic, M.; Aguiar, P.; Geley, S.; Maiato, H. Kinetochore Motors Drive Congression of Peripheral Polar Chromosomes by Overcoming Random Arm-Ejection Forces. Nat. Cell Biol. 2014, 16, 1249–1256. [Google Scholar] [CrossRef]
- McEwen, B.F.; Chan, G.K.T.; Zubrowski, B.; Savoian, M.S.; Sauer, M.T.; Yen, T.J. CENP-E Is Essential for Reliable Bioriented Spindle Attachment, but Chromosome Alignment Can Be Achieved via Redundant Mechanisms in Mammalian Cells. Mol. Biol. Cell 2001, 12, 2776–2789. [Google Scholar] [CrossRef] [Green Version]
- Sikirzhytski, V.; Renda, F.; Tikhonenko, I.; Magidson, V.; McEwen, B.F.; Khodjakov, A. Microtubules Assemble near Most Kinetochores during Early Prometaphase in Human Cells. J. Cell Biol. 2018, 217, 2647–2659. [Google Scholar] [CrossRef] [Green Version]
- Magidson, V.; O’Connell, C.B.; Lončarek, J.; Paul, R.; Mogilner, A.; Khodjakov, A. The Spatial Arrangement of Chromosomes during Prometaphase Facilitates Spindle Assembly. Cell 2011, 146, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magidson, V.; Paul, R.; Yang, N.; Ault, J.G.; O’Connell, C.B.; Tikhonenko, I.; Mcewen, B.F.; Mogilner, A.; Khodjakov, A. Adaptive Changes in the Kinetochore Architecture Facilitate Proper Spindle Assembly. Nat. Cell Biol. 2015, 17, 1134–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renda, F.; Miles, C.; Tikhonenko, I.; Fisher, R.; Carlini, L.; Kapoor, T.M.; Mogilner, A.; Khodjakov, A. Non-Centrosomal Microtubules at Kinetochores Promote Rapid Chromosome Biorientation during Mitosis in Human Cells. Curr. Biol. 2022, 32, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Auckland, P.; McAinsh, A.D. Building an Integrated Model of Chromosome Congression. J. Cell Sci. 2015, 128, 3363–3374. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.A.A.; Bonday, Z.Q.; Putkey, F.R.; Kops, G.J.P.L.; Silk, A.D.; Cleveland, D.W. Centromere-Associated Protein-E Is Essential for the Mammalian Mitotic Checkpoint to Prevent Aneuploidy Due to Single Chromosome Loss. J. Cell Biol. 2003, 162, 551–563. [Google Scholar] [CrossRef]
- Gasic, I.; Nerurkar, P.; Meraldi, P. Centrosome Age Regulates Kinetochore–Microtubule Stability and Biases Chromosome Mis-Segregation. Elife 2015, 4, e07909. [Google Scholar] [CrossRef]
- Jaqaman, K.; King, E.M.; Amaro, A.C.; Winter, J.R.; Dorn, J.F.; Elliott, H.L.; Mchedlishvili, N.; McClelland, S.E.; Porter, I.M.; Posch, M.; et al. Kinetochore Alignment within the Metaphase Plate Is Regulated by Centromere Stiffness and Microtubule Depolymerases. J. Cell Biol. 2010, 188, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Roos, U.P. Light and Electron Microscopy of Rat Kangaroo Cells in Mitosis. III. Patterns of Chromosome Behavior during Prometaphase. Chromosoma 1976, 54, 363–385. [Google Scholar] [CrossRef]
- Mitchison, T.; Kirschner, M. Dynamic Instability of Microtubule Growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Heald, R.; Khodjakov, A. Thirty Years of Search and Capture: The Complex Simplicity of Mitotic Spindle Assembly. J. Cell Biol. 2015, 211, 1103–1111. [Google Scholar] [CrossRef]
- Kirschner, M.; Mitchison, T. Beyond Self-Assembly: From Microtubules to Morphogenesis. Cell 1986, 45, 329–342. [Google Scholar] [CrossRef]
- Paul, R.; Wollman, R.; Silkworth, W.T.; Nardi, I.K.; Cimini, D.; Mogilner, A. Computer Simulations Predict That Chromosome Movements and Rotations Accelerate Mitotic Spindle Assembly without Compromising Accuracy. Proc. Natl. Acad. Sci. USA 2009, 106, 15708–15713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollman, R.; Cytrynbaum, E.N.; Jones, J.T.; Meyer, T.; Scholey, J.M.; Mogilner, A. Efficient Chromosome Capture Requires a Bias in the “search-and-Capture” Process during Mitotic-Spindle Assembly. Curr. Biol. 2005, 15, 828–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, O.M.; LeBerre, M.; Dimitracopoulos, A.; Bonazzi, D.; Zlotek-Zlotkiewicz, E.; Picone, R.; Duke, T.; Piel, M.; Baum, B. Mitotic Rounding Alters Cell Geometry to Ensure Efficient Bipolar Spindle Formation. Dev. Cell 2013, 25, 270–283. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, C.B.; Lončarek, J.; Kaláb, P.; Khodjakov, A. Relative Contributions of Chromatin and Kinetochores to Mitotic Spindle Assembly. J. Cell Biol. 2009, 187, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Kalinina, I.; Nandi, A.; Delivani, P.; Chacón, M.R.; Klemm, A.H.; Ramunno-Johnson, D.; Krull, A.; Lindner, B.; Pavin, N.; Tolić-Nørrelykke, I.M. Pivoting of Microtubules around the Spindle Pole Accelerates Kinetochore Capture. Nat. Cell Biol. 2013, 15, 82–87. [Google Scholar] [CrossRef]
- Nicklas, R.B. How Cells Get the Right Chromosomes. Science 1997, 275, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Rieder, C.L.; Salmon, E.D. Motile Kinetochores and Polar Ejection Forces Dictate Chromosome Position on the Vertebrate Mitotic Spindle. J. Cell Biol. 1994, 124, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Iemura, K.; Yoshizaki, Y.; Kuniyasu, K.; Tanaka, K. Attenuated Chromosome Oscillation as a Cause of Chromosomal Instability in Cancer Cells. Cancers 2021, 13, 4531. [Google Scholar] [CrossRef]
- Amaro, A.C.; Samora, C.P.; Holtackers, R.; Wang, E.; Kingston, I.J.; Alonso, M.; Lampson, M.; McAinsh, A.D.; Meraldi, P. Molecular Control of Kinetochore-Microtubule Dynamics and Chromosome Oscillations. Nat. Cell Biol. 2010, 12, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Stumpff, J.; von Dassow, G.; Wagenbach, M.; Asbury, C.; Wordeman, L. The Kinesin-8 Motor Kif18A Suppresses Kinetochore Movements to Control Mitotic Chromosome Alignment. Dev. Cell 2008, 14, 252–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cane, S.; Ye, A.A.; Luks-Morgan, S.J.; Maresca, T.J. Elevated Polar Ejection Forces Stabilize Kinetochore-Microtubule Attachments. J. Cell Biol. 2013, 200, 203–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, K.; Cheng, J.; Hunt, A.J. The Distribution of Polar Ejection Forces Determines the Amplitude of Chromosome Directional Instability. Curr. Biol. 2009, 19, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajtez, J.; Solomatina, A.; Novak, M.; Polak, B.; Vukušić, K.; Rüdiger, J.; Cojoc, G.; Milas, A.; Šumanovac Šestak, I.; Risteski, P.; et al. Overlap Microtubules Link Sister K-Fibres and Balance the Forces on Bi-Oriented Kinetochores. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vukušić, K.; Ponjavić, I.; Buđa, R.; Risteski, P.; Tolić, I.M. Microtubule-Sliding Modules Based on Kinesins EG5 and PRC1-Dependent KIF4A Drive Human Spindle Elongation. Dev. Cell 2021, 56, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Risteski, P.; Božan, D.; Jagrić, M.; Bosilj, A.; Pavin, N.; Tolić, I.M. Coordinated Poleward Flux of Sister Kinetochore Fibers Drives Chromosome Alignment. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kapoor, T.M.; Lampson, M.A.; Hergert, P.; Cameron, L.; Cimini, D.; Salmon, E.D.; McEwen, B.F.; Khodjakov, A. Chromosomes Can Congress to the Metaphase Plate before Biorientation. Science 2006, 311, 388–391. [Google Scholar] [CrossRef] [Green Version]
- Risteski, P.; Jagrić, M.; Pavin, N.; Tolić, I.M. Biomechanics of Chromosome Alignment at the Spindle Midplane. Curr. Biol. 2021, 31, R574–R585. [Google Scholar] [CrossRef]
- McNeill, P.A.; Berns, M.W. Chromosome Behavior after Laser Microirradiation of a Single Kinetochore in Mitotic PtK2 Cells. J. Cell Biol. 1981, 88, 543–553. [Google Scholar] [CrossRef]
- Cai, S.; O’Connell, C.B.; Khodjakov, A.; Walczak, C.E. Chromosome Congression in the Absence of Kinetochore Fibres. Nat. Cell Biol. 2009, 11, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Wandke, C.; Barisic, M.; Sigl, R.; Rauch, V.; Wolf, F.; Amaro, A.C.; Tan, C.H.; Pereira, A.J.; Kutay, U.; Maiato, H.; et al. Human Chromokinesins Promote Chromosome Congression and Spindle Microtubule Dynamics during Mitosis. J. Cell Biol. 2012, 198, 847–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putkey, F.R.; Cramer, T.; Morphew, M.K.; Silk, A.D.; Johnson, R.S.; McIntosh, J.R.; Cleveland, D.W. Unstable Kinetochore-Microtubule Capture and Chromosomal Instability Following Deletion of CENP-E. Dev. Cell 2002, 3, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Gudimchuk, N.; Vitre, B.; Kim, Y.; Kiyatkin, A.; Cleveland, D.W.; Ataullakhanov, F.I.; Grishchuk, E.L. Kinetochore Kinesin CENP-E Is a Processive Bi-Directional Tracker of Dynamic Microtubule Tips. Nat. Cell Biol. 2013, 15, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Ye, A.A.; Deretic, J.; Hoel, C.M.; Hinman, A.W.; Cimini, D.; Welburn, J.P.; Maresca, T.J. Aurora A Kinase Contributes to a Pole-Based Error Correction Pathway. Curr. Biol. 2015, 25, 1842–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, A.L.; Lan, W.; Stukenberg, P.T. Aurora B Is Enriched at Merotelic Attachment Sites, Where It Regulates MCAK. Curr. Biol. 2006, 16, 1705–1710. [Google Scholar] [CrossRef] [Green Version]
- Lampson, M.A.; Renduchitala, K.; Khodjakov, A.; Kapoor, T.M. Correcting Improper Chromosome-Spindle Attachments during Cell Division. Nat. Cell Biol. 2004, 6, 232–237. [Google Scholar] [CrossRef]
- Rieder, C.L.; Alexander, S.P. Kinetochores Are Transported Poleward along a Single Astral Microtubule during Chromosome Attachment to the Spindle in Newt Lung Cells. J. Cell Biol. 1990, 110, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P.; Rieder, C.L. Chromosome Motion during Attachment to the Vertebrate Spindle: Initial Saltatory-like Behavior of Chromosomes and Quantitative Analysis of Force Production by Nascent Kinetochore Fibers. J. Cell Biol. 1991, 113, 805–815. [Google Scholar] [CrossRef]
- Skibbens, R.V.; Skeen, V.P.; Salmon, E.D. Directional Instability of Kinetochore Motility during Chromosome Congression and Segregation in Mitotic Newt Lung Cells: A Push-Pull Mechanism. J. Cell Biol. 1993, 122, 859–875. [Google Scholar] [CrossRef] [Green Version]
- Hayden, J.H.; Bowser, S.S.; Rieder, C.L. Kinetochores Capture Astral Microtubules during Chromosome Attachment to the Mitotic Spindle: Direct Visualization in Live Newt Lung Cells. J. Cell Biol. 1990, 111, 1039–1045. [Google Scholar] [CrossRef]
- Yang, Z.; Tulu, U.S.; Wadsworth, P.; Rieder, C.L. Kinetochore Dynein Is Required for Chromosome Motion and Congression Independent of the Spindle Checkpoint. Curr. Biol. 2007, 17, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barisic, M.; Sohm, B.; Mikolcevic, P.; Wandke, C.; Rauch, V.; Ringer, T.; Hess, M.; Bonn, G.; Geley, S. Spindly/CCDC99 Is Required for Efficient Chromosome Congression and Mitotic Checkpoint Regulation. Mol. Biol. Cell 2010, 21, 1968–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassmann, R.; Holland, A.J.; Varma, D.; Wan, X.; Çivril, F.; Cleveland, D.W.; Oegema, K.; Salmon, E.D.; Desai, A. Removal of Spindly from Microtubule-Attached Kinetochores Controls Spindle Checkpoint Silencing in Human Cells. Genes Dev. 2010, 24, 957–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yu, W.; Liang, Y.; Zhu, X. Kinetochore Dynein Generates a Poleward Pulling Force to Facilitate Congression and Full Chromosome Alignment. Cell Res. 2007, 17, 701–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, G.; Ikeda, M.; Iemura, K.; Amin, M.A.; Kuriyama, S.; Tanaka, M.; Mizuno, N.; Osakada, H.; Haraguchi, T.; Tanaka, K. Lateral Attachment of Kinetochores to Microtubules Is Enriched in Prometaphase Rosette and Facilitates Chromosome Alignment and Bi-Orientation Establishment. Sci. Rep. 2018, 8, 3888. [Google Scholar] [CrossRef] [Green Version]
- Lecland, N.; Lüders, J. The Dynamics of Microtubule Minus Ends in the Human Mitotic Spindle. Nat. Cell Biol. 2014, 16, 770–778. [Google Scholar] [CrossRef]
- Vasquez-Limeta, A.; Loncarek, J. Human Centrosome Organization and Function in Interphase and Mitosis. Semin. Cell Dev. Biol. 2021, 117, 30–41. [Google Scholar] [CrossRef]
- Booth, A.J.R.; Yue, Z.; Eykelenboom, J.K.; Stiff, T.; Luxton, G.W.G.; Hochegger, H.; Tanaka, T.U. Contractile Acto-Myosin Network on Nuclear Envelope Remnants Positions Human Chromosomes for Mitosis. Elife 2019, 8, e46902. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Cramer, L.P.; Baum, B.; McGee, K.M. Myosin II-Dependent Cortical Movement Is Required for Centrosome Separation and Positioning during Mitotic Spindle Assembly. Cell 2004, 117, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Lénárt, P.; Bacher, C.P.; Daigle, N.; Hand, A.R.; Eils, R.; Terasaki, M.; Ellenberg, J. A Contractile Nuclear Actin Network Drives Chromosome Congression in Oocytes. Nature 2005, 436, 812–818. [Google Scholar] [CrossRef]
- Mandeville, E.C.; Rieder, C.L. Keratin Filaments Restrict Organelle Migration into the Forming Spindle of Newt Pneumocytes. Cell Motil. Cytoskelet. 1990, 15, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Wang, S.; Heidinger, J.M.; Shumaker, D.K.; Adam, S.A.; Goldman, R.D.; Zheng, Y. A Mitotic Lamin B Matrix Induced by RanGTP Required for Spindle Assembly. Science 2006, 311, 1887–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Tsai, M.Y.; Wang, S.; Lu, B.; Chen, R.; Yates, J.R.; Zhu, X.; Zheng, Y. Requirement for Nudel and Dynein for Assembly of the Lamin B Spindle Matrix. Nat. Cell Biol. 2009, 11, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaseda, K.; McAinsh, A.D.; Cross, R.A. Dual Pathway Spindle Assembly Increases Both the Speed and the Fidelity of Mitosis. Biol. Open 2012, 1, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, V.; Dantas, M.; Castro, D.; Vitiello, E.; Wang, I.; Carpi, N.; Balland, M.; Piel, M.; Aguiar, P.; Maiato, H.; et al. Centrosome-Nuclear Axis Repositioning Drives the Assembly of a Bipolar Spindle Scaffold to Ensure Mitotic Fidelity. Mol. Biol. Cell 2020, 31, 1675–1690. [Google Scholar] [CrossRef]
- Khodjakov, A.; Cole, R.W.; McEwen, B.F.; Buttle, K.F.; Rieder, C.L. Chromosome Fragments Possessing Only One Kinetochore Can Congress to the Spindle Equator. J. Cell Biol. 1997, 136, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.W.; Sakowicz, R.; Goldstein, L.S.B.; Cleveland, D.W. CENP-E Is a plus End-Directed Kinetochore Motor Required for Metaphase Chromosome Alignment. Cell 1997, 91, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Yen, T.J.; Li, G.; Schaar, B.T.; Szilak, I.; Cleveland, D.W. CENP-E Is a Putative Kinetochore Motor That Accumulates Just before Mitosis. Nature 1992, 359, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.J.; Compton, D.A.; Wise, D.; Zinkowski, R.P.; Brinkley, B.R.; Earnshaw, W.C.; Cleveland, D.W. CENP-E, a Novel Human Centromere-Associated Protein Required for Progression from Metaphase to Anaphase. EMBO J. 1991, 10, 1245–1254. [Google Scholar] [CrossRef]
- Rieder, C.L. The Formation, Structure, and Composition of the Mammalian Kinetochore and Kinetochore Fiber. Int. Rev. Cytol. 1982, 79, 1–58. [Google Scholar] [CrossRef]
- Pfarr, C.M.; Coue, M.; Grissom, P.M.; Hays, T.S.; Porter, M.E.; McIntosh, J.R. Cytoplasmic Dynein Is Localized to Kinetochores during Mitosis. Nature 1990, 345, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Craske, B.; Welburn, J.P.I. Leaving No-One behind: How CENP-E Facilitates Chromosome Alignment. Essays Biochem. 2020, 64, 313. [Google Scholar] [CrossRef] [PubMed]
- Barisic, M.; Silva E Sousa, R.; Tripathy, S.K.; Magiera, M.M.; Zaytsev, A.V.; Pereira, A.L.; Janke, C.; Grishchuk, E.L.; Maiato, H. Microtubule Detyrosination Guides Chromosomes during Mitosis. Science 2015, 348, 799–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barisic, M.; Maiato, H. The Tubulin Code: A Navigation System for Chromosomes during Mitosis. Trends Cell Biol. 2016, 26, 766–775. [Google Scholar] [CrossRef]
- Kaláb, P.; Pralle, A.; Isacoff, E.Y.; Heald, R.; Weis, K. Analysis of a RanGTP-Regulated Gradient in Mitotic Somatic Cells. Nature 2006, 440, 697–701. [Google Scholar] [CrossRef]
- Sampath, S.C.; Ohi, R.; Leismann, O.; Salic, A.; Pozniakovski, A.; Funabiki, H. The Chromosomal Passenger Complex Is Required for Chromatin-Induced Microtubule Stabilization and Spindle Assembly. Cell 2004, 118, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Polak, B.; Risteski, P.; Lesjak, S.; Tolić, I.M. PRC1-labeled Microtubule Bundles and Kinetochore Pairs Show One-to-one Association in Metaphase. EMBO Rep. 2017, 18, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Vukušić, K.; Buđa, R.; Bosilj, A.; Milas, A.; Pavin, N.; Tolić, I.M. Microtubule Sliding within the Bridging Fiber Pushes Kinetochore Fibers Apart to Segregate Chromosomes. Dev. Cell 2017, 43, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Matković, J.; Ghosh, S.; Ćosić, M.; Barišić, M.; Pavin, N.; Tolić, I.M. Kinetochore- and Chromosome-Driven Transition of Microtubules into Bundles Promotes Spindle Assembly. bioRxiv 2022. [Google Scholar] [CrossRef]
- Khodjakov, A.; Cole, R.W.; Oakley, B.R.; Rieder, C.L. Centrosome-Independent Mitotic Spindle Formation in Vertebrates. Curr. Biol. 2000, 10, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Maiato, H.; Rieder, C.L.; Khodjakov, A. Kinetochore-Driven Formation of Kinetochore Fibers Contributes to Spindle Assembly during Animal Mitosis. J. Cell Biol. 2004, 167, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikirzhytski, V.; Magidson, V.; Steinman, J.B.; He, J.; le Berre, M.; Tikhonenko, I.; Ault, J.G.; McEwen, B.F.; Chen, J.K.; Sui, H.; et al. Direct Kinetochore-Spindle Pole Connections Are Not Required for Chromosome Segregation. J. Cell Biol. 2014, 206, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, A.F.; Roudot, P.; Legant, W.R.; Betzig, E.; Danuser, G.; Gerlich, D.W. Augmin Accumulation on Long-Lived Microtubules Drives Amplification and Kinetochore-Directed Growth. J. Cell Biol. 2019, 218, 2150–2168. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.C.; Soares-De-Oliveira, J.; Drpic, D.; Aguiar, P.; Nio, A.; Pereira, J.; Maiato, H.; Cheeseman, L.P.; Damas, J.; Lewin, H.A.; et al. Augmin-Dependent Microtubule Self-Organization Drives Kinetochore Fiber Maturation in Mammals. Cell Rep. 2022, 39, 110610. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Heuser, J.E.; Waterman, C.M.; Cleveland, D.W. CENP-E Combines a Slow, Processive Motor and a Flexible Coiled Coil to Produce an Essential Motile Kinetochore Tether. J. Cell Biol. 2008, 181, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.L.; Draviam, V.M. Lateral to End-on Conversion of Chromosome-Microtubule Attachment Requires Kinesins Cenp-e and MCAK. Curr. Biol. 2013, 23, 1514–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Anderson, K.L.; Cleveland, D.W. The Microtubule-Dependent Motor Centromere-Associated Protein E (CENP-E) Is an Integral Component of Kinetochore Corona Fibers That Link Centromeres to Spindle Microtubules. J. Cell Biol. 1997, 139, 435–447. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.F.; Heagle, A.B.; Cassels, G.O.; Buttle, K.F.; Rieder, C.L. Kinetochore Fiber Maturation in PtK1 Cells and Its Implications for the Mechanisms of Chromosome Congression and Anaphase Onset. J. Cell Biol. 1997, 137, 1567–1580. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, E.; Morphew, M.; McIntosh, R.J. Electron Tomography Reveals Aspects of Spindle Structure Important for Mechanical Stability at Metaphase. Mol. Biol. Cell 2020, 31, 184–195. [Google Scholar] [CrossRef]
- Kiewisz, R.; Fabig, G.; Conway, W.; Needleman, D.; Müller-Reichert, T. Three-Dimensional Structure of the Kinetochore-Fibers in Human Mitotic Spindles. bioRxiv 2021. [Google Scholar] [CrossRef]
- Chan, G.K.T.; Jablonski, S.A.; Sudakin, V.; Hittle, J.C.; Yen, T.J. Human Bubr1 Is a Mitotic Checkpoint Kinase That Monitors Cenp-E Functions at Kinetochores and Binds the Cyclosome/APC. J. Cell Biol. 1999, 146, 941. [Google Scholar] [CrossRef]
- Barisic, M.; Maiato, H. Dynein Prevents Erroneous Kinetochore-Microtubule Attachments in Mitosis. Cell Cycle 2015, 14, 3356. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.F.; Feijão, T.; Vromans, M.J.M.; Sunkel, C.E.; Lens, S.M.A. Aurora B Kinase Cooperates with CENP-E to Promote Timely Anaphase Onset. Chromosoma 2010, 119, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Chmátal, L.; Yang, K.; Schultz, R.M.; Lampson, M.A. Spatial Regulation of Kinetochore Microtubule Attachments by Destabilization at Spindle Poles in Meiosis I. Curr. Biol. 2015, 25, 1835–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ault, J.G.; Demarco, A.J.; Salmon, E.D.; Rieder, C.L. Studies on the Ejection Properties of Asters: Astral Microtubule Turnover Influences the Oscillatory Behavior and Positioning of Mono-Oriented Chromosomes. J. Cell Sci. 1991, 99, 701–710. [Google Scholar] [CrossRef]
- Brouhard, G.J.; Hunt, A.J. Microtubule Movements on the Arms of Mitotic Chromosomes: Polar Ejection Forces Quantified in Vitro. Proc. Natl. Acad. Sci. USA 2005, 102, 13903–13908. [Google Scholar] [CrossRef] [Green Version]
- Drpic, D.; Pereira, A.J.; Barisic, M.; Maresca, T.J.; Maiato, H. Polar Ejection Forces Promote the Conversion from Lateral to End-on Kinetochore-Microtubule Attachments on Mono-Oriented Chromosomes. Cell Rep. 2015, 13, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Nicklas, R.B.; Ward, S.C. Elements of Error Correction in Mitosis: Microtubule Capture, Release, and Tension. J. Cell Biol. 1994, 126, 1241–1253. [Google Scholar] [CrossRef]
- Levesque, A.A.; Compton, D.A. The Chromokinesin Kid Is Necessary for Chromosome Arm Orientation and Oscillation, but Not Congression, on Mitotic Spindles. J. Cell Biol. 2001, 154, 1135–1146. [Google Scholar] [CrossRef] [Green Version]
- Iemura, K.; Tanaka, K. Chromokinesin Kid and Kinetochore Kinesin CENP-E Differentially Support Chromosome Congression without End-on Attachment to Microtubules. Nat. Commun. 2015, 6, 6447. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, T.M.; Mayer, T.U.; Coughlin, M.L.; Mitchison, T.J. Probing Spindle Assembly Mechanisms with Monastrol, a Small Molecule Inhibitor of the Mitotic Kinesin, Eg5. J. Cell Biol. 2000, 150, 975–988. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.G.; Moree, B.; Hickey, J.M.; Kilmartin, J.V.; Salmon, E.D. HNuf2 Inhibition Blocks Stable Kinetochore-Microtubule Attachment and Induces Mitotic Cell Death in HeLa Cells. J. Cell Biol. 2002, 159, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLuca, J.G.; Gall, W.E.; Ciferri, C.; Cimini, D.; Musacchio, A.; Salmon, E.D. Kinetochore Microtubule Dynamics and Attachment Stability Are Regulated by Hec1. Cell 2006, 127, 969–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapitein, L.C.; Peterman, E.J.G.; Kwok, B.H.; Kim, J.H.; Kapoor, T.M.; Schmidt, C.F. The Bipolar Mitotic Kinesin Eg5 Moves on Both Microtubules That It Crosslinks. Nature 2005, 435, 114–118. [Google Scholar] [CrossRef]
- Cao, J.; Crest, J.; Fasulo, B.; Sullivan, W. Cortical Actin Dynamics Facilitate Early-Stage Centrosome Separation. Curr. Biol. 2010, 20, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.A.; van Heesbeen, R.G.H.P.; Meaders, J.L.; Geers, E.F.; Fernandez-Garcia, B.; Medema, R.H.; Tanenbaum, M.E. Nuclear Envelope-Associated Dynein Drives Prophase Centrosome Separation and Enables Eg5-Independent Bipolar Spindle Formation. EMBO J. 2012, 31, 4179. [Google Scholar] [CrossRef] [Green Version]
- de Simone, A.; Nédélec, F.; Gönczy, P. Dynein Transmits Polarized Actomyosin Cortical Flows to Promote Centrosome Separation. Cell Rep. 2016, 14, 2250–2262. [Google Scholar] [CrossRef] [Green Version]
- Toso, A.; Winter, J.R.; Garrod, A.J.; Amaro, A.C.; Meraldi, P.; McAinsh, A.D. Kinetochore-Generated Pushing Forces Separate Centrosomes during Bipolar Spindle Assembly. J. Cell Biol. 2009, 184, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.T.; Wojcik, E.J.; Sanders, M.A.; McGrail, M.; Hays, T.S. Cytoplasmic Dynein Is Required for the Nuclear Attachment and Migration of Centrosomes during Mitosis in Drosophila. J. Cell Biol. 1999, 146, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Silkworth, W.T.; Nardi, I.K.; Paul, R.; Mogilner, A.; Cimini, D. Timing of Centrosome Separation Is Important for Accurate Chromosome Segregation. Mol. Biol. Cell 2012, 23, 401–411. [Google Scholar] [CrossRef]
- Nam, H.J.; Naylor, R.M.; van Deursen, J.M. Centrosome Dynamics as a Source of Chromosomal Instability. Trends Cell Biol. 2015, 25, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.J.; van Deursen, J.M. Cyclin B2 and P53 Control Proper Timing of Centrosome Separation. Nat. Cell Biol. 2014, 16, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Foreman, O.; Wigle, D.A.; Kosari, F.; Vasmatzis, G.; Salisbury, J.L.; van Deursen, J.; Galardy, P.J. USP44 Regulates Centrosome Positioning to Prevent Aneuploidy and Suppress Tumorigenesis. J. Clin. Investig. 2012, 122, 4362–4373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganem, N.J.; Godinho, S.A.; Pellman, D. A Mechanism Linking Extra Centrosomes to Chromosomal Instability. Nature 2009, 460, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lončarek, J.; Kisurina-Evgenieva, O.; Vinogradova, T.; Hergert, P.; la Terra, S.; Kapoor, T.M.; Khodjakov, A. The Centromere Geometry Essential for Keeping Mitosis Error Free Is Controlled by Spindle Forces. Nature 2007, 450, 745–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuniyasu, K.; Iemura, K.; Tanaka, K. Delayed Chromosome Alignment to the Spindle Equator Increases the Rate of Chromosome Missegregation in Cancer Cell Lines. Biomolecules 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; McCollum, D. A Role for Metaphase Spindle Elongation Forces in Correction of Merotelic Kinetochore Attachments. Curr. Biol. 2012, 22, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Tame, M.A.; Raaijmakers, J.A.; Afanasyev, P.; Medema, R.H. Chromosome Misalignments Induce Spindle-Positioning Defects. EMBO Rep. 2016, 17, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.L.; Anzola, J.V.; Davis, R.L.; Yoon, M.; Motamedi, A.; Kroll, A.; Seo, C.P.; Hsia, J.E.; Kim, S.K.; Mitchell, J.W.; et al. Reversible Centriole Depletion with an Inhibitor of Polo-like Kinase 4. Science 2015, 348, 1155. [Google Scholar] [CrossRef] [Green Version]
- Sir, J.H.; Pütz, M.; Daly, O.; Morrison, C.G.; Dunning, M.; Kilmartin, J.V.; Gergely, F. Loss of Centrioles Causes Chromosomal Instability in Vertebrate Somatic Cells. J. Cell Biol. 2013, 203, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, J.; Dumont, S. Spindle Assembly Checkpoint Satisfaction Occurs via End-on but Not Lateral Attachments under Tension. J. Cell Biol. 2017, 216, 1533–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monda, J.K.; Cheeseman, I.M. The Kinetochore-Microtubule Interface at a Glance. J. Cell Sci. 2018, 131, jcs214577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.A.; Wallace, D.A.; Varma, D. The Ndc80 Complex Is Essential for the Initial Kinetochore-Microtubule Capture during Early Mitosis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tauchman, E.C.; Boehm, F.J.; DeLuca, J.G. Stable Kinetochore–Microtubule Attachment Is Sufficient to Silence the Spindle Assembly Checkpoint in Human Cells. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheeseman, I.M.; Chappie, J.S.; Wilson-Kubalek, E.M.; Desai, A. The Conserved KMN Network Constitutes the Core Microtubule-Binding Site of the Kinetochore. Cell 2006, 127, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, T.; Amano, M.; Suzuki, A.; Backer, C.B.; Welburn, J.P.; Dong, Y.; McEwen, B.F.; Shang, W.H.; Suzuki, E.; Okawa, K.; et al. CCAN Makes Multiple Contacts with Centromeric DNA to Provide Distinct Pathways to the Outer Kinetochore. Cell 2008, 135, 1039–1052. [Google Scholar] [CrossRef] [Green Version]
- Hara, M.; Ariyoshi, M.; Okumura, E.I.; Hori, T.; Fukagawa, T. Multiple Phosphorylations Control Recruitment of the KMN Network onto Kinetochores. Nat. Cell Biol. 2018, 20, 1378–1388. [Google Scholar] [CrossRef]
- Nishino, T.; Rago, F.; Hori, T.; Tomii, K.; Cheeseman, I.M.; Fukagawa, T. CENP-T Provides a Structural Platform for Outer Kinetochore Assembly. EMBO J. 2013, 32, 424–436. [Google Scholar] [CrossRef]
- Kalantzaki, M.; Kitamura, E.; Zhang, T.; Mino, A.; Novák, B.; Tanaka, T.U. Kinetochore–Microtubule Error Correction Is Driven by Differentially Regulated Interaction Modes. Nat. Cell Biol. 2015, 17, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, R.L.; Conti, D.; Tamura, N.; Braun, D.; Ramalingam, R.A.; Cieslinski, K.; Ries, J.; Draviam, V.M. Aurora-B Kinase Pathway Controls the Lateral to End-on Conversion of Kinetochore-Microtubule Attachments in Human Cells. Nat. Commun. 2017, 8, 150. [Google Scholar] [CrossRef]
- Nilsson, J. Protein Phosphatases in the Regulation of Mitosis. J. Cell Biol. 2019, 218, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Holland, A.J.; Lan, W.; Cleveland, D.W. Aurora Kinases and Protein Phosphatase 1 Mediate Chromosome Congression through Regulation of CENP-E. Cell 2010, 142, 444–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, S.; Janczyk, P.; Qu, Q.; Brautigam, C.A.; Stukenberg, P.T.; Yu, H.; Gorbsky, G.J. The Human SKA Complex Drives the Metaphase-Anaphase Cell Cycle Transition by Recruiting Protein Phosphatase 1 to Kinetochores. Elife 2016, 5, e12902. [Google Scholar] [CrossRef] [PubMed]
- Kiyomitsu, T.; Obuse, C.; Yanagida, M. Human Blinkin/AF15q14 Is Required for Chromosome Alignment and the Mitotic Checkpoint through Direct Interaction with Bub1 and BubR1. Dev. Cell 2007, 13, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Hanisch, A.; Silljé, H.H.W.; Nigg, E.A. Timely Anaphase Onset Requires a Novel Spindle and Kinetochore Complex Comprising Ska1 and Ska2. EMBO J. 2006, 25, 5504–5515. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Vader, G.; Vromans, M.J.M.; Lampson, M.A.; Lens, S.M.A. Sensing Chromosome Bi-Orientation by Spatial Separation of Aurora B Kinase from Kinetochore Substrates. Science 2009, 323, 1350–1353. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Conti, D.; Shrestha, R.L.; Braun, D.; Draviam, V.M. Counteraction between Astrin-PP1 and Cyclin-B-CDK1 Pathways Protects Chromosome-Microtubule Attachments Independent of Biorientation. Nat. Commun. 2021, 12, 7010. [Google Scholar] [CrossRef]
- Conti, D.; Gul, P.; Islam, A.; Martín-Durán, J.M.; Pickersgill, R.W.; Draviam, V.M. Kinetochores Attached to Microtubule-Ends Are Stabilised by Astrin Bound Pp1 to Ensure Proper Chromosome Segregation. Elife 2019, 8, e49325. [Google Scholar] [CrossRef]
- Sacristan, C.; Ahmad, M.U.D.; Keller, J.; Fermie, J.; Groenewold, V.; Tromer, E.; Fish, A.; Melero, R.; Carazo, J.M.; Klumperman, J.; et al. Dynamic Kinetochore Size Regulation Promotes Microtubule Capture and Chromosome Biorientation in Mitosis. Nat. Cell Biol. 2018, 20, 800–810. [Google Scholar] [CrossRef]
- Rendli, P.M.; Gasic, I.; Meraldi, P.; Nigg, E.A.; Santamaria, A. The Ska complex promotes Aurora B activity to ensure chromosome biorientation. J. Cell Biol. 2016, 215, 77–93. [Google Scholar] [CrossRef]
- Barbosa, J.; Sunkel, C.E.; Conde, C. The Role of Mitotic Kinases and the RZZ Complex in Kinetochore-Microtubule Attachments: Doing the Right Link. Front. Cell Dev. Biol. 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Cimini, D.; Moree, B.; Canman, J.C.; Salmon, E.D. Merotelic Kinetochore Orientation Occurs Frequently during Early Mitosis in Mammalian Tissue Cells and Error Correction Is Achieved by Two Different Mechanisms. J. Cell Sci. 2003, 116, 4213–4225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimini, D.; Wan, X.; Hirel, C.B.; Salmon, E.D. Aurora Kinase Promotes Turnover of Kinetochore Microtubules to Reduce Chromosome Segregation Errors. Curr. Biol. 2006, 16, 1711–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McVey, S.L.; Cosby, J.K.; Nannas, N.J. Aurora B Tension Sensing Mechanisms in the Kinetochore Ensure Accurate Chromosome Segregation. Int. J. Mol. Sci. 2021, 22, 8818. [Google Scholar] [CrossRef] [PubMed]
- Carmena, M.; Ruchaud, S.; Earnshaw, W.C. Making the Auroras Glow: Regulation of Aurora A and B Kinase Function by Interacting Proteins. Curr. Opin. Cell Biol. 2009, 21, 796. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Renda, F.; Zhang, H.; Gokden, A.; Wu, D.Z.; Chenoweth, D.M.; Khodjakov, A.; Lampson, M.A. Tension Promotes Kinetochore-Microtubule Release by Aurora B Kinase. J. Cell Biol. 2021, 220, e202007030. [Google Scholar] [CrossRef]
- Salimian, K.J.; Ballister, E.R.; Smoak, E.M.; Wood, S.; Panchenko, T.; Lampson, M.A.; Black, B.E. Feedback Control in Sensing Chromosome Biorientation by the Aurora B Kinase. Curr. Biol. 2011, 21, 1158–1165. [Google Scholar] [CrossRef] [Green Version]
- Welburn, J.P.I.; Vleugel, M.; Liu, D.; Yates, J.R.; Lampson, M.A.; Fukagawa, T.; Cheeseman, I.M. Aurora B Phosphorylates Spatially Distinct Targets to Differentially Regulate the Kinetochore-Microtubule Interface. Mol. Cell 2010, 38, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Hindriksen, S.; Lens, S.M.A.; Hadders, M.A. The Ins and Outs of Aurora B Inner Centromere Localization. Front. Cell Dev. Biol. 2017, 5, 112. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Sandri, B.J.; Tank, D.; McClellan, M.; Harasymiw, L.A.; Yang, Q.; Parker, L.L.; Gardner, M.K. A Gradient in Metaphase Tension Leads to a Scaled Cellular Response in Mitosis. Dev. Cell 2019, 49, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Dudka, D.; Noatynska, A.; Smith, C.A.; Liaudet, N.; McAinsh, A.D.; Meraldi, P. Complete Microtubule–Kinetochore Occupancy Favours the Segregation of Merotelic Attachments. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Zaytsev, A.V.; Godzi, M.; Ataullakhanov, F.I.; Grishchuk, E.L.; Stukenberg, P.T. The Binding of Borealin to Microtubules Underlies a Tension Independent Kinetochore-Microtubule Error Correction Pathway. Nat. Commun. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hadders, M.A.; Hindriksen, S.; Truong, M.A.; Mhaskar, A.N.; Pepijn Wopken, J.; Vromans, M.J.M.; Lens, S.M.A. Untangling the Contribution of Haspin and Bub1 to Aurora B Function during Mitosis. J. Cell Biol. 2020, 219, e201907087. [Google Scholar] [CrossRef]
- Hengeveld, R.C.C.; Vromans, M.J.M.; Vleugel, M.; Hadders, M.A.; Lens, S.M.A. Inner Centromere Localization of the CPC Maintains Centromere Cohesion and Allows Mitotic Checkpoint Silencing. Nat. Commun. 2017, 8, 15542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broad, A.J.; DeLuca, J.G. The Right Place at the Right Time: Aurora B Kinase Localization to Centromeres and Kinetochores. Essays Biochem. 2020, 64, 299. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Z.; Chen, Q.; Yan, H.; Zhang, M.; Zhou, L.; Xu, J.; Lu, W.; Wang, F. Centromere-Localized Aurora B Kinase Is Required for the Fidelity of Chromosome Segregation. J. Cell Biol. 2020, 219, e201907092. [Google Scholar] [CrossRef]
- Broad, A.J.; DeLuca, K.F.; DeLuca, J.G. Aurora B Kinase Is Recruited to Multiple Discrete Kinetochore and Centromere Regions in Human Cells. J. Cell Biol. 2020, 219, e201905144. [Google Scholar] [CrossRef]
- Yoo, T.Y.; Choi, J.M.; Conway, W.; Yu, C.H.; Pappu, R.V.; Needleman, D.J. Measuring NDC80 Binding Reveals the Molecular Basis of Tension-Dependent Kinetochore-Microtubule Attachments. Elife 2018, 7, e36392. [Google Scholar] [CrossRef]
- Auckland, P.; Clarke, N.I.; Royle, S.J.; McAinsh, A.D. Congressing Kinetochores Progressively Load Ska Complexes to Prevent Force-Dependent Detachment. J. Cell Biol. 2017, 216, 1623–1639. [Google Scholar] [CrossRef] [Green Version]
- Akiyoshi, B.; Sarangapani, K.K.; Powers, A.F.; Nelson, C.R.; Reichow, S.L.; Arellano-Santoyo, H.; Gonen, T.; Ranish, J.A.; Asbury, C.L.; Biggins, S. Tension Directly Stabilizes Reconstituted Kinetochore-Microtubule Attachments. Nature 2010, 468, 576–579. [Google Scholar] [CrossRef] [Green Version]
- DeLuca, K.F.; Meppelink, A.; Broad, A.J.; Mick, J.E.; Peersen, O.B.; Pektas, S.; Lens, S.M.A.; DeLuca, J.G. Aurora A Kinase Phosphorylates Hec1 to Regulate Metaphase Kinetochore-Microtubule Dynamics. J. Cell Biol. 2018, 217, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Iemura, K.; Natsume, T.; Maehara, K.; Kanemaki, M.T.; Tanaka, K. Chromosome Oscillation Promotes Aurora A-Dependent Hec1 Phosphorylation and Mitotic Fidelity. J. Cell Biol. 2021, 220, e202006116. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Bader, J.R.; Tauhata, S.B.F.; Raycroft, M.; Hornick, J.; Pfister, K.K.; Lane, W.S.; Chan, G.K.; Hinchcliffe, E.H.; Vaughan, P.S.; et al. Phosphorylation Regulates Targeting of Cytoplasmic Dynein to Kinetochores during Mitosis. J. Cell Biol. 2008, 183, 819–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, J.R.; Kasuboski, J.M.; Winding, M.; Vaughan, P.S.; Hinchcliffe, E.H.; Vaughan, K.T. Polo-like Kinase1 Is Required for Recruitment of Dynein to Kinetochores during Mitosis. J. Biol. Chem. 2011, 286, 20769–20777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, W.C.; Fava, L.L.; Uldschmid, A.; Schmitz, M.H.A.; Gerlich, D.W.; Nigg, E.A.; Santamaria, A. Mitotic Control of Kinetochore-Associated Dynein and Spindle Orientation by Human Spindly. J. Cell Biol. 2009, 185, 859. [Google Scholar] [CrossRef] [Green Version]
- Kasuboski, J.M.; Bader, J.R.; Vaughan, P.S.; Tauhata, S.B.F.; Winding, M.; Morrissey, M.A.; Joyce, M.V.; Boggess, W.; Vos, L.; Chan, G.K.; et al. Zwint-1 Is a Novel Aurora B Substrate Required for the Assembly of a Dynein-Binding Platform on Kinetochores. Mol. Biol. Cell 2011, 22, 3318–3330. [Google Scholar] [CrossRef] [PubMed]
- Taveras, C.; Liu, C.; Mao, Y. A Tension-Independent Mechanism Reduces Aurora B-Mediated Phosphorylation upon Microtubule Capture by CENP-E at the Kinetochore. Cell Cycle 2019, 18, 1349. [Google Scholar] [CrossRef]
- Mammel, A.E.; Huang, H.Z.; Gunn, A.L.; Choo, E.; Hatch, E.M. Chromosome Length and Gene Density Contribute to Micronuclear Membrane Stability. Life Sci. Alliance 2021, 5, e202101210. [Google Scholar] [CrossRef]
- Soto, M.; García-Santisteban, I.; Krenning, L.; Medema, R.H.; Raaijmakers, J.A. Chromosomes Trapped in Micronuclei Are Liable to Segregation Errors. J. Cell Sci. 2018, 131, jcs214742. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.S.; Holland, A.J. The Impact of Mitotic Errors on Cell Proliferation and Tumorigenesis. Genes Dev. 2018, 32, 620–638. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.J.; Resio, B.; Pellman, D. Causes and Consequences of Aneuploidy in Cancer. Nat. Rev. Genet. 2012, 13, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Vera-Rodriguez, M.; Chavez, S.L.; Rubio, C.; Reijo Pera, R.A.; Simon, C. Prediction Model for Aneuploidy in Early Human Embryo Development Revealed by Single-Cell Analysis. Nat. Commun. 2015, 6, 7601. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Diez, C.; Paim, L.M.G.; FitzHarris, G. Cell-Size-Independent Spindle Checkpoint Failure Underlies Chromosome Segregation Error in Mouse Embryos. Curr. Biol. 2019, 29, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yost, S.; de Wolf, B.; Hanks, S.; Zachariou, A.; Marcozzi, C.; Clarke, M.; de Voer, R.M.; Etemad, B.; Uijttewaal, E.; Ramsay, E.; et al. Biallelic TRIP13 Mutations Predispose to Wilms Tumor and Chromosome Missegregation. Nat. Genet. 2017, 49, 1148–1151. [Google Scholar] [CrossRef] [Green Version]
- Collin, P.; Nashchekina, O.; Walker, R.; Pines, J. The Spindle Assembly Checkpoint Works like a Rheostat Rather than a Toggle Switch. Nat. Cell Biol. 2013, 15, 1378–1385. [Google Scholar] [CrossRef] [Green Version]
- Dick, A.E.; Gerlich, D.W. Kinetic Framework of Spindle Assembly Checkpoint Signalling. Nat. Cell Biol. 2013, 15, 1370–1377. [Google Scholar] [CrossRef] [Green Version]
- Rieder, C.L.; Cole, R.W.; Khodjakov, A.; Sluder, G. The Checkpoint Delaying Anaphase in Response to Chromosome Monoorientation Is Mediated by an Inhibitory Signal Produced by Unattached Kinetochores. J. Cell Biol. 1995, 130, 941–948. [Google Scholar] [CrossRef]
- Tovini, L.; McClelland, S.E. Impaired CENP-E Function Renders Large Chromosomes More Vulnerable to Congression Failure. Biomolecules 2019, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Ferrandiz, N.; Downie, L.; Starling, G.P.; Royle, S.J. Endomembranes promote chromosome missegregation by ensheathing misaligned chromosomes. J. Cell Biol. 2022, 221, e202203021. [Google Scholar] [CrossRef]
- Weaver, B.A.A.; Silk, A.D.; Montagna, C.; Verdier-Pinard, P.; Cleveland, D.W. Aneuploidy Acts Both Oncogenically and as a Tumor Suppressor. Cancer Cell 2007, 11, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Daum, J.R.; Potapova, T.A.; Sivakumar, S.; Daniel, J.J.; Flynn, J.N.; Rankin, S.; Gorbsky, G.J. Cohesion Fatigue Induces Chromatid Separation in Cells Delayed at Metaphase. Curr. Biol. 2011, 21, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, D.A.; Rieder, C.L. Mitotic Checkpoint Slippage in Humans Occurs via Cyclin B Destruction in the Presence of an Active Checkpoint. Curr. Biol. 2006, 16, 1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etemad, B.; Kuijt, T.E.F.; Kops, G.J.P.L. Kinetochore–Microtubule Attachment Is Sufficient to Satisfy the Human Spindle Assembly Checkpoint. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sen, O.; Harrison, J.U.; Burroughs, N.J.; McAinsh, A.D. Kinetochore Life Histories Reveal an Aurora-B-Dependent Error Correction Mechanism in Anaphase. Dev. Cell 2021, 56, 3082–3099. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kwon, M.; Mannino, M.; Yang, N.; Renda, F.; Khodjakov, A.; Pellman, D. Nuclear Envelope Assembly Defects Link Mitotic Errors to Chromothripsis. Nature 2018, 561, 551–555. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA Damage in Micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Uetake, Y.; Sluder, G. Prolonged Prometaphase Blocks Daughter Cell Proliferation despite Normal Completion of Mitosis. Curr. Biol. 2010, 20, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Santaguida, S.; Richardson, A.; Iyer, D.R.; M’Saad, O.; Zasadil, L.; Knouse, K.A.; Wong, Y.L.; Rhind, N.; Desai, A.; Amon, A. Chromosome Mis-Segregation Generates Cell-Cycle-Arrested Cells with Complex Karyotypes That Are Eliminated by the Immune System. Dev. Cell 2017, 41, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Chunduri, N.K.; Menges, P.; Zhang, X.; Wieland, A.; Gotsmann, V.L.; Mardin, B.R.; Buccitelli, C.; Korbel, J.O.; Willmund, F.; Kschischo, M.; et al. Systems Approaches Identify the Consequences of Monosomy in Somatic Human Cells. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Hellmuth, S.; Stemmann, O. Separase-triggered apoptosis enforces minimal length of mitosis. Nature 2020, 580, 542–547. [Google Scholar] [CrossRef]
- Papini, D.; Levasseur, M.D.; Higgins, J.M.G. The Aurora B Gradient Sustains Kinetochore Stability in Anaphase. Cell Rep. 2021, 37, 109818. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.; de Sousa, F.; Gomes, A.M.; Afonso, O.; Ferreira, L.T.; Figueiredo, A.C.; Maiato, H. An Anaphase Surveillance Mechanism Prevents Micronuclei Formation from Frequent Chromosome Segregation Errors. Cell Rep. 2021, 37, 109783. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Compton, D.A. Chromosome Missegregation in Human Cells Arises through Specific Types of Kinetochore-Microtubule Attachment Errors. Proc. Natl. Acad. Sci. USA 2011, 108, 17974–17978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, C.L.; Malaby, H.L.H.; Sepaniac, L.A.; Martin, W.; Byers, C.; Czechanski, A.; Messinger, D.; Tang, M.; Ohi, R.; Reinholdt, L.G.; et al. Mitotic Chromosome Alignment Ensures Mitotic Fidelity by Promoting Interchromosomal Compaction during Anaphase. J. Cell Biol. 2019, 218, 1148. [Google Scholar] [CrossRef] [Green Version]
- Kabeche, L.; Compton, D.A. Cyclin A Regulates Kinetochore Microtubules to Promote Faithful Chromosome Segregation. Nature 2013, 502, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Bakhoum, S.F.; Genovese, G.; Compton, D.A. Deviant Kinetochore Microtubule Dynamics Underlie Chromosomal Instability. Curr. Biol. 2009, 19, 1937–1942. [Google Scholar] [CrossRef] [Green Version]
- Bakhoum, S.F.; Thompson, S.L.; Manning, A.L.; Compton, D.A. Genome Stability Is Ensured by Temporal Control of Kinetochore-Microtubule Dynamics. Nat. Cell Biol. 2009, 11, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Knouse, K.A.; Lopez, K.E.; Bachofner, M.; Amon, A. Chromosome Segregation Fidelity in Epithelia Requires Tissue Architecture. Cell 2018, 175, 200. [Google Scholar] [CrossRef] [Green Version]
- Worrall, J.T.; Tamura, N.; Mazzagatti, A.; Shaikh, N.; van Lingen, T.; Bakker, B.; Spierings, D.C.J.; Vladimirou, E.; Foijer, F.; McClelland, S.E. Non-Random Mis-Segregation of Human Chromosomes. Cell Rep. 2018, 23, 3366. [Google Scholar] [CrossRef]
- Drpic, D.; Almeida, A.C.; Aguiar, P.; Renda, F.; Damas, J.; Lewin, H.A.; Larkin, D.M.; Khodjakov, A.; Maiato, H. Chromosome Segregation Is Biased by Kinetochore Size. Curr. Biol. 2018, 28, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- Dumont, M.; Gamba, R.; Gestraud, P.; Klaasen, S.; Worrall, J.T.; de Vries, S.G.; Boudreau, V.; Salinas-Luypaert, C.; Maddox, P.S.; Lens, S.M.; et al. Human Chromosome-Specific Aneuploidy Is Influenced by DNA-Dependent Centromeric Features. EMBO J. 2020, 39, e102924. [Google Scholar] [CrossRef] [PubMed]
- Fachinetti, D.; Han, J.S.; McMahon, M.A.; Ly, P.; Abdullah, A.; Wong, A.J.; Cleveland, D.W. DNA Sequence-Specific Binding of CENP-B Enhances the Fidelity of Human Centromere Function. Dev. Cell 2015, 33, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torosantucci, L.; de Santis Puzzonia, M.; Cenciarelli, C.; Rens, W.; Degrassi, F. Aneuploidy in Mitosis of PtK1 Cells Is Generated by Random Loss and Nondisjunction of Individual Chromosomes. J. Cell Sci. 2009, 122, 3455–3461. [Google Scholar] [CrossRef] [Green Version]
- Silk, A.D.; Zasadil, L.M.; Holland, A.J.; Vitre, B.; Cleveland, D.W.; Weaver, B.A. Chromosome Missegregation Rate Predicts Whether Aneuploidy Will Promote or Suppress Tumors. Proc. Natl. Acad. Sci. USA 2013, 110, E4134–E4141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-Ruiz, M.; Muzzopappa, M.; Milán, M. Tumor Suppressor Roles of CENP-E and Nsl1 in Drosophila Epithelial Tissues. Cell Cycle 2014, 13, 1450–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakala, M.; Aggarwal, M.; Sniffen, C.; Zasadil, L.; Carroll, A.; Ma, D.; Su, X.A.; Wangsa, D.; Meyer, A.; Sieben, C.J.; et al. Clonal Selection of Stable Aneuploidies in Progenitor Cells Drives High-Prevalence Tumorigenesis. Genes Dev. 2021, 35, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Kung, P.P.; Martinez, R.; Zhu, Z.; Zager, M.; Blasina, A.; Rymer, I.; Hallin, J.; Xu, M.; Carroll, C.; Chionis, J.; et al. Chemogenetic Evaluation of the Mitotic Kinesin CENP-E Reveals a Critical Role in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2014, 13, 2104–2115. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ling, K.; Wu, X.; Cao, J.; Liu, B.; Li, S.; Si, Q.; Cai, Y.; Yan, C.; Zhang, Y.; et al. Reduced Expression of Cenp-e in Human Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 156–158. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, M.; Nishida, N.; Iwatsuki, M.; Ishimaru, S.; Mimori, K.; Tanaka, F.; Nakagawa, T.; Sato, T.; Sugihara, K.; Hoon, D.S.B.; et al. Kinesin 18A Expression: Clinical Relevance to Colorectal Cancer Progression. Int. J. Cancer 2011, 129, 2543–2552. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, C.; Chen, H.; Li, L.; Guo, L.; Jiang, W.; Lu, S.H. Kif18A Is Involved in Human Breast Carcinogenesis. Carcinogenesis 2010, 31, 1676–1684. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, M.; Lee, J.H.; Sengupta, K.; Ried, T.; Rane, S.; Misteli, T. Tumor Formation via Loss of a Molecular Motor Protein. Curr. Biol. 2006, 16, 1559–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, N.; Shaikh, N.; Muliaditan, D.; Soliman, T.N.; McGuinness, J.R.; Maniati, E.; Moralli, D.; Durin, M.-A.; Green, C.M.; Balkwill, F.R.; et al. Specific Mechanisms of Chromosomal Instability Indicate Therapeutic Sensitivities in High-Grade Serous Ovarian Carcinoma. Cancer Res. 2020, 80, 4946–4959. [Google Scholar] [CrossRef] [PubMed]
- Ertych, N.; Stolz, A.; Stenzinger, A.; Weichert, W.; Kaulfuß, S.; Burfeind, P.; Aigner, A.; Wordeman, L.; Bastians, H. Increased Microtubule Assembly Rates Influence Chromosomal Instability in Colorectal Cancer Cells. Nat. Cell Biol. 2014, 16, 779–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlén, A.; Martin, C.; Miron, S.; Julien, M.; Theillet, F.X.; Ropars, V.; Sessa, G.; Beaurepere, R.; Boucherit, V.; Duchambon, P.; et al. Proper chromosome alignment depends on BRCA2 phosphorylation by PLK1. Nat. Commun. 2020, 11, 1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowald, K.; Mantovan, M.; Passos, J.; Buccitelli, C.; Mardin, B.R.; Korbel, J.O.; Jechlinger, M.; Sotillo, R. Negative Selection and Chromosome Instability Induced by Mad2 Overexpression Delay Breast Cancer but Facilitate Oncogene-Independent Outgrowth. Cell Rep. 2016, 15, 2679–2691. [Google Scholar] [CrossRef] [Green Version]
- Salgueiro, L.; Buccitelli, C.; Rowald, K.; Somogyi, K.; Kandala, S.; Korbel, J.O.; Sotillo, R. Acquisition of chromosome instability is a mechanism to evade oncogene addiction. EMBO Mol. Med. 2020, 12, e10941. [Google Scholar] [CrossRef]
- Hintzen, D.C.; Soto, M.; Schubert, M.; Bakker, B.; Spierings, D.C.J.; Szuhai, K.; Lansdorp, P.M.; Foijer, F.; Medema, R.H.; Raaijmakers, J.A. Monosomies, trisomies and segmental aneuploidies differentially affect chromosomal stability. bioRxiv 2021. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukušić, K.; Tolić, I.M. Polar Chromosomes—Challenges of a Risky Path. Cells 2022, 11, 1531. https://doi.org/10.3390/cells11091531
Vukušić K, Tolić IM. Polar Chromosomes—Challenges of a Risky Path. Cells. 2022; 11(9):1531. https://doi.org/10.3390/cells11091531
Chicago/Turabian StyleVukušić, Kruno, and Iva M. Tolić. 2022. "Polar Chromosomes—Challenges of a Risky Path" Cells 11, no. 9: 1531. https://doi.org/10.3390/cells11091531
APA StyleVukušić, K., & Tolić, I. M. (2022). Polar Chromosomes—Challenges of a Risky Path. Cells, 11(9), 1531. https://doi.org/10.3390/cells11091531