Separase Control and Cohesin Cleavage in Oocytes: Should I Stay or Should I Go?
Abstract
:1. Introduction
2. Separase
2.1. Separase Control in Mitosis
2.1.1. Securin
2.1.2. Cyclin B1
2.1.3. Sgo2/Mad2
2.1.4. Limitations
2.1.5. Separase Inactivation at the Exit from Mitosis
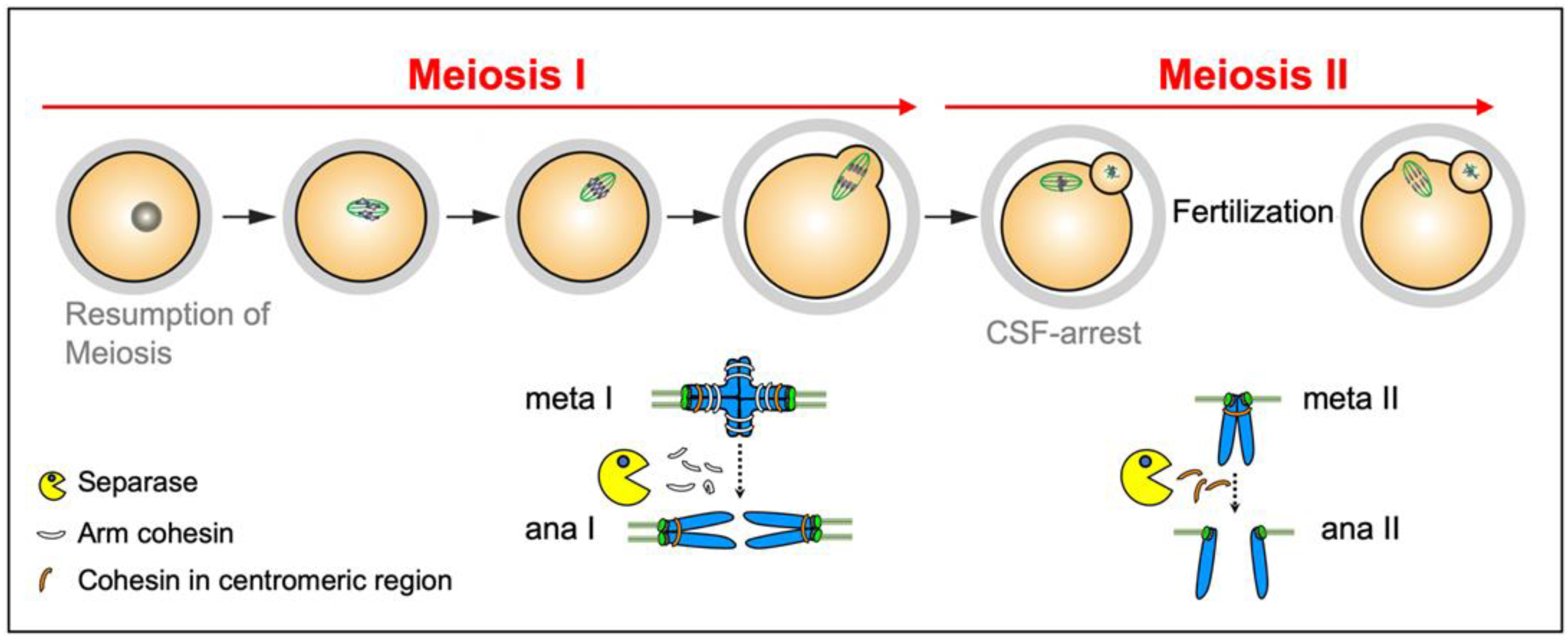
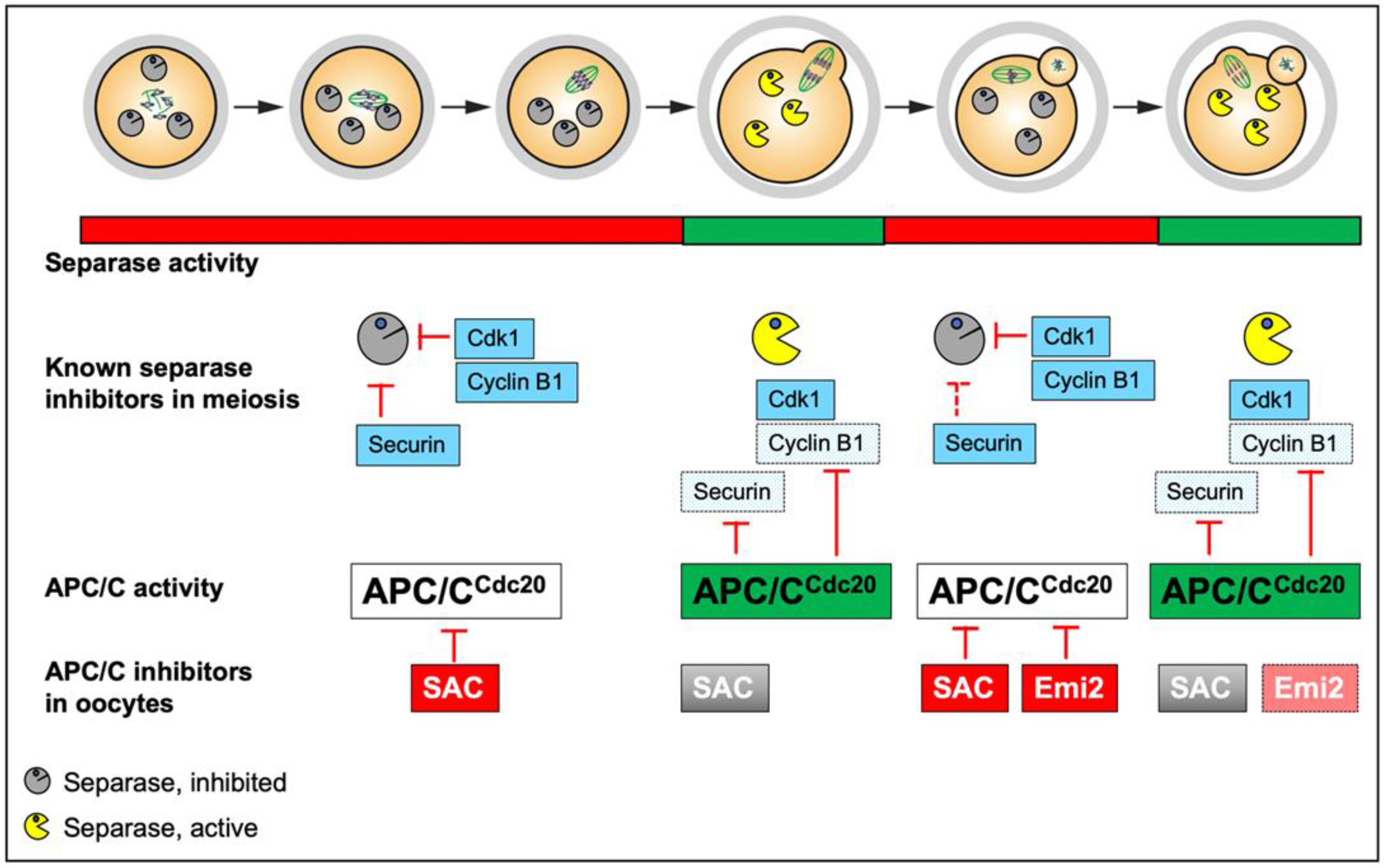
2.2. Separase Control in Meiosis
2.2.1. Securin
2.2.2. Cyclin B1
2.2.3. Pin1 and Sgo2/Mad2
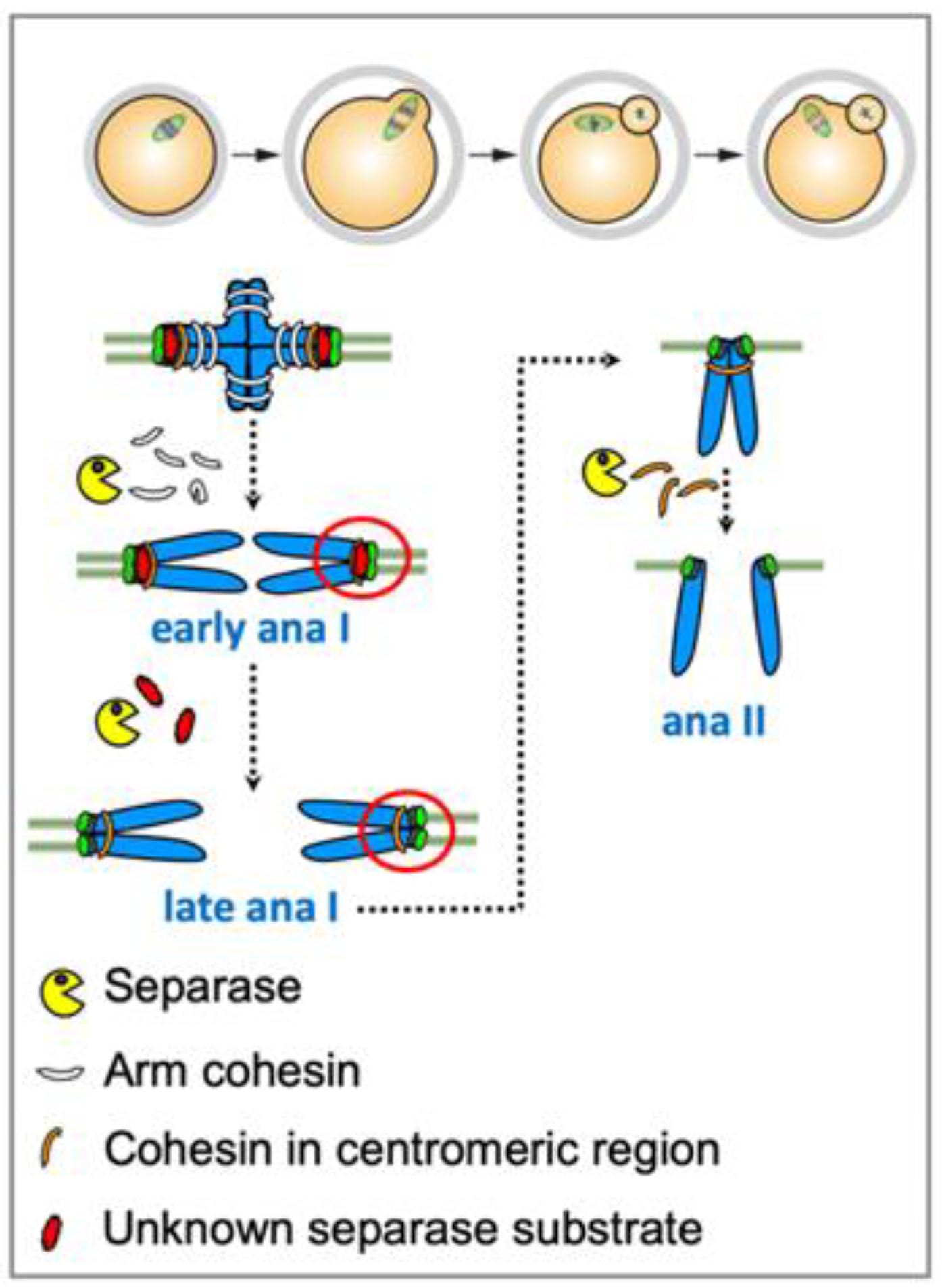
3. The Meiotic Cohesin Subunit Rec8
3.1. Rec8 Phosphorylation for Cleavage in Lower Eukaryotes
3.2. Aurora B/C Kinases Phosphorylate Rec8 in Mammalian Oocytes for Separase-Dependent Cleavage
4. Cohesin Protection and Deprotection
4.1. No Tension-Dependent Removal of Proteins Protecting Centromeric Cohesin
4.2. Is There Cohesin Protection by Sgo2 in Meiosis II?
4.3. Accessibility of Centromeric Rec8
5. Perspectives
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petronczki, M.; Siomos, M.F.; Nasmyth, K. Un menage a quatre: The molecular biology of chromosome segregation in meiosis. Cell 2003, 112, 423–440. [Google Scholar] [CrossRef] [Green Version]
- MacLennan, M.; Crichton, J.H.; Playfoot, C.J.; Adams, I.R. Oocyte development, meiosis and aneuploidy. Semin. Cell Dev. Biol. 2015, 45, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihajlovic, A.I.; FitzHarris, G. Segregating Chromosomes in the Mammalian Oocyte. Curr. Biol. 2018, 28, R895–R907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ur, S.N.; Corbett, K.D. Architecture and Dynamics of Meiotic Chromosomes. Annu. Rev. Genet. 2021, 55, 497–526. [Google Scholar] [CrossRef]
- Grey, C.; de Massy, B. Chromosome Organization in Early Meiotic Prophase. Front. Cell Dev. Biol. 2021, 9, 688878. [Google Scholar] [CrossRef]
- Pyatnitskaya, A.; Borde, V.; De Muyt, A. Crossing and zipping: Molecular duties of the ZMM proteins in meiosis. Chromosoma 2019, 128, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.P.; Orr-Weaver, T.L. Chromosome segregation during meiosis: Building an unambivalent bivalent. Curr. Top. Dev. Biol. 1998, 37, 263–299. [Google Scholar] [CrossRef]
- Lister, L.M.; Kouznetsova, A.; Hyslop, L.A.; Kalleas, D.; Pace, S.L.; Barel, J.C.; Nathan, A.; Floros, V.; Adelfalk, C.; Watanabe, Y.; et al. Age-related meiotic segregation errors in Mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 2010, 20, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Hodges, C.A.; Revenkova, E.; Jessberger, R.; Hassold, T.J.; Hunt, P.A. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat. Genet. 2005, 37, 1351–1355. [Google Scholar] [CrossRef]
- Marston, A.L.; Wassmann, K. Multiple Duties for Spindle Assembly Checkpoint Kinases in Meiosis. Front. Cell Dev. Biol. 2017, 5, 109. [Google Scholar] [CrossRef]
- Nikalayevich, E.; El Jailani, S.; Dupre, A.; Cladiere, D.; Gryaznova, Y.; Fosse, C.; Buffin, E.; Touati, S.A.; Wassmann, K. Aurora B/C-dependent phosphorylation promotes Rec8 cleavage in mammalian oocytes. Curr. Biol. 2022, 32, 2281–2290.e4. [Google Scholar] [CrossRef]
- Rattani, A.; Wolna, M.; Ploquin, M.; Helmhart, W.; Morrone, S.; Mayer, B.; Godwin, J.; Xu, W.; Stemmann, O.; Pendas, A.; et al. Sgol2 provides a regulatory platform that coordinates essential cell cycle processes during meiosis I in oocytes. eLife 2013, 2, e01133. [Google Scholar] [CrossRef] [Green Version]
- Llano, E.; Gomez, R.; Gutierrez-Caballero, C.; Herran, Y.; Sanchez-Martin, M.; Vazquez-Quinones, L.; Hernandez, T.; de Alava, E.; Cuadrado, A.; Barbero, J.L.; et al. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008, 22, 2400–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailhes, J.B.; Hilliard, C.; Fuseler, J.W.; London, S.N. Okadaic acid, an inhibitor of protein phosphatase 1 and 2A, induces premature separation of sister chromatids during meiosis I and aneuploidy in mouse oocytes in vitro. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2003, 11, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Chambon, J.P.; Touati, A.S.; Berneau, S.; Hebras, C.; Groeme, R.; Cladière, D.; Dumollard, R.; McDougall, A.; Wassmann, K. The PP2A inhibitor I2PP2A is essential for sister chromatid segregation in meiosis II. Curr. Biol. 2013, 23, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamenz, J.; Hauf, S. Time To Split Up: Dynamics of Chromosome Separation. Trends Cell Biol. 2017, 27, 42–54. [Google Scholar] [CrossRef]
- Rosen, L.E.; Klebba, J.E.; Asfaha, J.B.; Ghent, C.M.; Campbell, M.G.; Cheng, Y.; Morgan, D.O. Cohesin cleavage by separase is enhanced by a substrate motif distinct from the cleavage site. Nat. Commun. 2019, 10, 5189. [Google Scholar] [CrossRef] [Green Version]
- Waizenegger, I.C.; Hauf, S.; Meinke, A.; Peters, J.M. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 2000, 103, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, T.; Sykora, M.M.; Veld, P.J.H.I.; Mechtler, K.; Peters, J.M. Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc. Natl. Acad. Sci. USA 2013, 110, 13404–13409. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, A.; Wutz, G.; Huet, S.; Jaritz, M.; Wuensche, A.; Schirghuber, E.; Davidson, I.F.; Tang, W.; Cisneros, D.A.; Bhaskara, V.; et al. Wapl is an essential regulator of chromatin structure and chromosome segregation. Nature 2013, 501, 564–568. [Google Scholar] [CrossRef]
- Nishiyama, T.; Ladurner, R.; Schmitz, J.; Kreidl, E.; Schleiffer, A.; Bhaskara, V.; Bando, M.; Shirahige, K.; Hyman, A.A.; Mechtler, K.; et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 2010, 143, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, R.; Gillespie, P.J.; Hirano, T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr. Biol. 2006, 16, 2406–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shintomi, K.; Hirano, T. Releasing cohesin from chromosome arms in early mitosis: Opposing actions of Wapl-Pds5 and Sgo1. Genes Dev. 2009, 23, 2224–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, N.R.; Wassmann, K.; Anger, M.; Schuh, M.; Wirth, K.G.; Xu, H.; Helmhart, W.; Kudo, H.; McKay, M.; Maro, B.; et al. Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 2006, 126, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, S.; Gomez, H.L.; Pendas, A.M.; Stemmann, O. Securin-independent regulation of separase by checkpoint-induced shugoshin-MAD2. Nature 2020, 580, 536–541. [Google Scholar] [CrossRef]
- Waizenegger, I.; Gimenez-Abian, J.F.; Wernic, D.; Peters, J.M. Regulation of human separase by securin binding and autocleavage. Curr. Biol. 2002, 12, 1368–1378. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Raia, P.; Ghent, C.M.; Raisch, T.; Sadian, Y.; Cavadini, S.; Sabale, P.M.; Barford, D.; Raunser, S.; Morgan, D.O.; et al. Structural basis of human separase regulation by securin and CDK1-cyclin B1. Nature 2021, 596, 138–142. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, X.; Yu, H. Structural basis of cohesin cleavage by separase. Nature 2016, 532, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, S.; Pohlmann, C.; Brown, A.; Bottger, F.; Sprinzl, M.; Stemmann, O. Positive and negative regulation of vertebrate separase by Cdk1-cyclin B1 may explain why securin is dispensable. J. Biol. Chem. 2015, 290, 8002–8010. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, C.; Zhang, S.; Barford, D. Visualizing the complex functions and mechanisms of the anaphase promoting complex/cyclosome (APC/C). Open Biol. 2017, 7, 170204. [Google Scholar] [CrossRef]
- Etemad, B.; Kops, G.J. Attachment issues: Kinetochore transformations and spindle checkpoint silencing. Curr. Opin Cell Biol. 2016, 39, 101–108. [Google Scholar] [CrossRef]
- Hellmuth, S.; Bottger, F.; Pan, C.; Mann, M.; Stemmann, O. PP2A delays APC/C-dependent degradation of separase-associated but not free securin. EMBO J. 2014, 33, 1134–1147. [Google Scholar] [CrossRef]
- Shindo, N.; Kumada, K.; Hirota, T. Separase sensor reveals dual roles for separase coordinating cohesin cleavage and cdk1 inhibition. Dev. Cell 2012, 23, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Jallepalli, P.V.; Waizenegger, I.C.; Bunz, F.; Langer, S.; Speicher, M.R.; Peters, J.M.; Kinzler, K.W.; Vogelstein, B.; Lengauer, C. Securin is required for chromosomal stability in human cells. Cell 2001, 105, 445–457. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Huang, X.; Zhang, P. Securin is not required for cellular viability, but is required for normal growth of mouse embryonic fibroblasts. Curr. Biol. 2001, 11, 1197–1201. [Google Scholar] [CrossRef] [Green Version]
- Pfleghaar, K.; Heubes, S.; Cox, J.; Stemmann, O.; Speicher, M.R. Securin is not required for chromosomal stability in human cells. PLoS Biol. 2005, 3, e416. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, R.; Melmed, S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol. Endocrinol. 2001, 15, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Taylor, S.S. Cyclin-B1-mediated inhibition of excess separase is required for timely chromosome disjunction. J. Cell Sci. 2006, 119, 3325–3336. [Google Scholar] [CrossRef] [Green Version]
- Gorr, I.H.; Boos, D.; Stemmann, O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 2005, 19, 135–141. [Google Scholar] [CrossRef]
- Stemmann, O.; Zou, H.; Gerber, S.A.; Gygi, S.P.; Kirschner, M.W. Dual inhibition of sister chromatid separation at metaphase. Cell 2001, 107, 715–726. [Google Scholar] [CrossRef]
- Huang, X.; Hatcher, R.; York, J.P.; Zhang, P. Securin and separase phosphorylation act redundantly to maintain sister chromatid cohesion in mammalian cells. Mol. Biol. Cell 2005, 16, 4725–4732. [Google Scholar] [CrossRef] [Green Version]
- Afonso, O.; Castellani, C.M.; Cheeseman, L.P.; Ferreira, J.G.; Orr, B.; Ferreira, L.T.; Chambers, J.J.; Morais-de-Sa, E.; Maresca, T.J.; Maiato, H. Spatiotemporal control of mitotic exit during anaphase by an aurora B-Cdk1 crosstalk. eLife 2019, 8, e47646. [Google Scholar] [CrossRef]
- Collin, P.; Nashchekina, O.; Walker, R.; Pines, J. The spindle assembly checkpoint works like a rheostat rather than a toggle switch. Nat. Cell Biol. 2013, 15, 1378–1385. [Google Scholar] [CrossRef] [Green Version]
- Wolf, F.; Wandke, C.; Isenberg, N.; Geley, S. Dose-dependent effects of stable cyclin B1 on progression through mitosis in human cells. EMBO J. 2006, 25, 2802–2813. [Google Scholar] [CrossRef]
- Hellmuth, S.; Rata, S.; Brown, A.; Heidmann, S.; Novak, B.; Stemmann, O. Human chromosome segregation involves multi-layered regulation of separase by the peptidyl-prolyl-isomerase Pin1. Mol. Cell 2015, 58, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Vader, G. Pch2(TRIP13): Controlling cell division through regulation of HORMA domains. Chromosoma 2015, 124, 333–339. [Google Scholar] [CrossRef]
- Huang, X.; Andreu-Vieyra, C.V.; Wang, M.; Cooney, A.J.; Matzuk, M.M.; Zhang, P. Preimplantation mouse embryos depend on inhibitory phosphorylation of separase to prevent chromosome missegregation. Mol. Cell. Biol. 2009, 29, 1498–1505. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Andreu-Vieyra, C.V.; York, J.P.; Hatcher, R.; Lu, T.; Matzuk, M.M.; Zhang, P. Inhibitory phosphorylation of separase is essential for genome stability and viability of murine embryonic germ cells. PLoS Biol. 2008, 6, e15. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Gao, X.; Hu, B.; Du, Y.; Zhou, J.; Tian, X.; Huang, X. Separase phosphosite mutation leads to genome instability and primordial germ cell depletion during oogenesis. PLoS ONE 2011, 6, e18763. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yu, H.; Zou, H. Nuclear exclusion of separase prevents cohesin cleavage in interphase cells. Cell Cycle 2006, 5, 2537–2542. [Google Scholar] [CrossRef]
- Holt, J.E.; Lane, S.I.; Jones, K.T. The control of meiotic maturation in mammalian oocytes. Curr. Top. Dev. Biol. 2013, 102, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kornbluth, S. Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era. J. Cell Sci. 2008, 121, 3509–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, A.; Rauh, N.R.; Nigg, E.A.; Mayer, T.U. Cytostatic factor: An activity that puts the cell cycle on hold. J. Cell Sci. 2006, 119, 1213–1218. [Google Scholar] [CrossRef] [Green Version]
- Nikalayevich, E.; Bouftas, N.; Wassmann, K. Detection of Separase Activity Using a Cleavage Sensor in Live Mouse Oocytes. Methods Mol. Biol. 2018, 1818, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.; Levasseur, M.; Homer, H.; Yallop, K.; Murdoch, A.; McDougall, A. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat. Cell Biol. 2003, 5, 1023–1025. [Google Scholar] [CrossRef]
- Terret, M.E.; Wassmann, K.; Waizenegger, I.; Maro, B.; Peters, J.M.; Verlhac, M.H. The Meiosis I-to-Meiosis II Transition in Mouse Oocytes Requires Separase Activity. Curr. Biol. 2003, 13, 1797–1802. [Google Scholar] [CrossRef] [Green Version]
- Ledan, E.; Polanski, Z.; Terret, M.E.; Maro, B. Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev. Biol. 2001, 232, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Karasu, M.E.; Bouftas, N.; Keeney, S.; Wassmann, K. Cyclin B3 promotes anaphase I onset in oocyte meiosis. J. Cell Biol. 2019, 218, 1265–1281. [Google Scholar] [CrossRef]
- Levasseur, M.D.; Thomas, C.; Davies, O.R.; Higgins, J.M.G.; Madgwick, S. Aneuploidy in Oocytes Is Prevented by Sustained CDK1 Activity through Degron Masking in Cyclin B1. Dev. Cell 2019, 48, 672–684.e5. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, K.; Niault, T.; Maro, B. Metaphase I Arrest upon Activation of the Mad2-Dependent Spindle Checkpoint in Mouse Oocytes. Curr. Biol. 2003, 13, 1596–1608. [Google Scholar] [CrossRef]
- Homer, H.A.; McDougall, A.; Levasseur, M.; Murdoch, A.P.; Herbert, M. Mad2 is required for inhibiting securin and cyclin B degradation following spindle depolymerisation in meiosis I mouse oocytes. Reproduction 2005, 130, 829–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homer, H.A.; McDougall, A.; Levasseur, M.; Yallop, K.; Murdoch, A.P.; Herbert, M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005, 19, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hached, K.; Xie, S.Z.; Buffin, E.; Cladiere, D.; Rachez, C.; Sacras, M.; Sorger, P.K.; Wassmann, K. Mps1 at kinetochores is essential for female mouse meiosis I. Development 2011, 138, 2261–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabti, I.; Reis, A.; Levasseur, M.; Stemmann, O.; Jones, K.T. Securin and not CDK1/cyclin B1 regulates sister chromatid disjunction during meiosis II in mouse eggs. Dev. Biol. 2008, 321, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, T.; Schultz, R.M.; Lampson, M.A. Age-dependent susceptibility of chromosome cohesion to premature separase activation in mouse oocytes. Biol. Reprod. 2011, 85, 1279–1283. [Google Scholar] [CrossRef]
- Thomas, C.; Wetherall, B.; Levasseur, M.D.; Harris, R.J.; Kerridge, S.T.; Higgins, J.M.G.; Davies, O.R.; Madgwick, S. A prometaphase mechanism of securin destruction is essential for meiotic progression in mouse oocytes. Nat. Commun. 2021, 12, 4322. [Google Scholar] [CrossRef]
- Touati, S.A.; Cladiere, D.; Lister, L.M.; Leontiou, I.; Chambon, J.P.; Rattani, A.; Bottger, F.; Stemmann, O.; Nasmyth, K.; Herbert, M.; et al. Cyclin A2 Is Required for Sister Chromatid Segregation, But Not Separase Control, in Mouse Oocyte Meiosis. Cell Rep. 2012, 2, 1077–1087. [Google Scholar] [CrossRef]
- Lee, J.; Kitajima, T.S.; Tanno, Y.; Yoshida, K.; Morita, T.; Miyano, T.; Miyake, M.; Watanabe, Y. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol. 2008, 10, 42–52. [Google Scholar] [CrossRef]
- Ishiguro, T.; Tanaka, K.; Sakuno, T.; Watanabe, Y. Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat. Cell Biol. 2010, 12, 500–506. [Google Scholar] [CrossRef]
- Katis, V.L.; Lipp, J.J.; Imre, R.; Bogdanova, A.; Okaz, E.; Habermann, B.; Mechtler, K.; Nasmyth, K.; Zachariae, W. Rec8 phosphorylation by casein kinase 1 and Cdc7-Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev. Cell 2010, 18, 397–409. [Google Scholar] [CrossRef]
- Rumpf, C.; Cipak, L.; Dudas, A.; Benko, Z.; Pozgajova, M.; Riedel, C.G.; Ammerer, G.; Mechtler, K.; Gregan, J. Casein kinase 1 is required for efficient removal of Rec8 during meiosis I. Cell Cycle 2010, 9, 2657–2662. [Google Scholar] [CrossRef] [Green Version]
- Rogers, E.; Bishop, J.D.; Waddle, J.A.; Schumacher, J.M.; Lin, R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J. Cell Biol. 2002, 157, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ferrandiz, N.; Barroso, C.; Telecan, O.; Shao, N.; Kim, H.M.; Testori, S.; Faull, P.; Cutillas, P.; Snijders, A.P.; Colaiacovo, M.P.; et al. Spatiotemporal regulation of Aurora B recruitment ensures release of cohesion during C. elegans oocyte meiosis. Nat. Commun. 2018, 9, 834. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.L.; Schindler, K. Specialize and Divide (Twice): Functions of Three Aurora Kinase Homologs in Mammalian Oocyte Meiotic Maturation. Trends Genet. 2017, 33, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Arguello-Miranda, O.; Zagoriy, I.; Mengoli, V.; Rojas, J.; Jonak, K.; Oz, T.; Graf, P.; Zachariae, W. Casein Kinase 1 Coordinates Cohesin Cleavage, Gametogenesis, and Exit from M Phase in Meiosis II. Dev. Cell 2017, 40, 37–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonak, K.; Zagoriy, I.; Oz, T.; Graf, P.; Rojas, J.; Mengoli, V.; Zachariae, W. APC/C-Cdc20 mediates deprotection of centromeric cohesin at meiosis II in yeast. Cell Cycle 2017, 16, 1145–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Yakoubi, W.; Wassmann, K. Meiotic Divisions: No Place for Gender Equality. Adv. Exp. Med. Biol. 2017, 1002, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.; Valdeolmillos, A.; Parra, M.T.; Viera, A.; Carreiro, C.; Roncal, F.; Rufas, J.S.; Barbero, J.L.; Suja, J.A. Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep. 2007, 8, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Gryaznova, Y.; Keating, L.; Touati, S.A.; Cladiere, D.; El Yakoubi, W.; Buffin, E.; Wassmann, K. Kinetochore individualization in meiosis I is required for centromeric cohesin removal in meiosis II. EMBO J. 2021, 40, e106797. [Google Scholar] [CrossRef]
- Mengoli, V.; Jonak, K.; Lyzak, O.; Lamb, M.; Lister, L.M.; Lodge, C.; Rojas, J.; Zagoriy, I.; Herbert, M.; Zachariae, W. Deprotection of centromeric cohesin at meiosis II requires APC/C activity but not kinetochore tension. EMBO J. 2021, 40, e106812. [Google Scholar] [CrossRef]
- El Yakoubi, W.; Buffin, E.; Cladiere, D.; Gryaznova, Y.; Berenguer, I.; Touati, S.A.; Gomez, R.; Suja, J.A.; van Deursen, J.M.; Wassmann, K. Mps1 kinase-dependent Sgo2 centromere localisation mediates cohesin protection in mouse oocyte meiosis I. Nat. Commun. 2017, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Touati, S.A.; Buffin, E.; Cladiere, D.; Hached, K.; Rachez, C.; van Deursen, J.M.; Wassmann, K. Mouse oocytes depend on BubR1 for proper chromosome segregation but not for prophase I arrest. Nat. Commun. 2015, 6, 6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Ishiguro, K.; Nambu, A.; Akiyoshi, B.; Yokobayashi, S.; Kagami, A.; Ishiguro, T.; Pendas, A.M.; Takeda, N.; Sakakibara, Y.; et al. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature 2015, 517, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, N.K.; Ma, J.; Lampson, M.A.; Cheeseman, I.M. Separase cleaves the kinetochore protein Meikin at the meiosis I/II transition. Dev. Cell 2021, 56, 2192–2206.e8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassmann, K. Separase Control and Cohesin Cleavage in Oocytes: Should I Stay or Should I Go? Cells 2022, 11, 3399. https://doi.org/10.3390/cells11213399
Wassmann K. Separase Control and Cohesin Cleavage in Oocytes: Should I Stay or Should I Go? Cells. 2022; 11(21):3399. https://doi.org/10.3390/cells11213399
Chicago/Turabian StyleWassmann, Katja. 2022. "Separase Control and Cohesin Cleavage in Oocytes: Should I Stay or Should I Go?" Cells 11, no. 21: 3399. https://doi.org/10.3390/cells11213399
APA StyleWassmann, K. (2022). Separase Control and Cohesin Cleavage in Oocytes: Should I Stay or Should I Go? Cells, 11(21), 3399. https://doi.org/10.3390/cells11213399





