Functional Characterization of Endothelial Cells Differentiated from Porcine Epiblast Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Reagents and Antibodies
2.3. In Vitro Differentiation of Endothelial Cells from Porcine Epiblast Stem Cells
2.4. Magnetic-Activated Cells Soring (MACS)
2.5. Proliferation Assay
2.6. Flow Cytometry Analysis
2.7. Immunocytochemistry
2.8. Quantitative Polymerase Chain Reaction
2.9. Three-Dimensional Spheroid Sprouting Assay
2.10. Capillary-Like Structure Formation Assay
2.11. Dil-Acetylated-LDL Uptake Assay
2.12. Statistical Analysis
3. Results
3.1. Separating Endothelial Cells Differentiated from pEpiSCs
3.2. Proliferation of pEpiSCs-Derived ECs
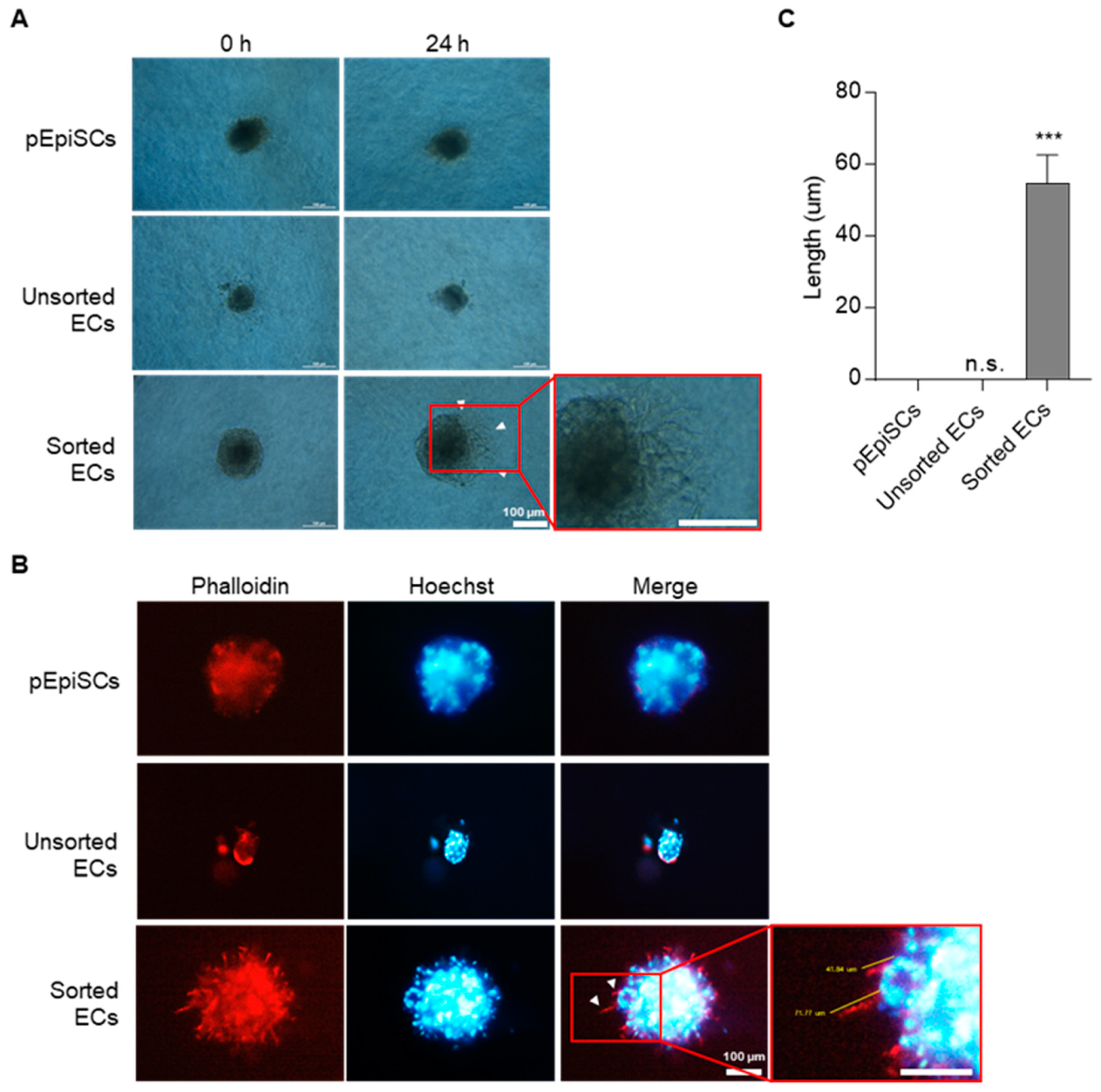
3.3. Angiogenic Function of pEpiSCs-Derived ECs by Three-Dimensional Spheroid Sprouting
3.4. Vessel Organization of pEpiSCs-Derived ECs Using Capillary-like Structure Formation
3.5. Acetylated Low Density Lipoprotein Uptake of pEpiSCs-Derived ECs
4. Discussion
5. Conclusions
Author Contributions
Funding
International Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wei, L.H.; Bauer, P.M.; Wu, G.; del Soldato, P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.D. Endothelial cell function and thrombosis. Bailliere’s Best Pract. Res. Clin. Haematol. 1999, 12, 329–341. [Google Scholar] [CrossRef]
- Wang, W.; Deng, M.; Liu, X.; Ai, W.; Tang, Q.; Hu, J. TLR4 activation induces nontolerant inflammatory response in endothelial cells. Inflammation 2011, 34, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.Y.; Blann, A.D.; Patel, J.; Freestone, B.; Hughes, E.; Lip, G.Y. Endothelial dysfunction and damage in congestive heart failure: Relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation 2004, 110, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Harvey, L.D.; Ayon, R.J.; Babicheva, A.; Bonnet, S.; Chan, S.Y.; Yuan, J.X.; Perez, V.J. Endothelial dysfunction in pulmonary arterial hypertension: An evolving landscape (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045893217752912. [Google Scholar] [CrossRef]
- Colmenero, I.; Santonja, C.; Alonso-Riano, M.; Noguera-Morel, L.; Hernandez-Martin, A.; Andina, D.; Wiesner, T.; Rodriguez-Peralto, J.L.; Requena, L.; Torrelo, A. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: Histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br. J. Dermatol. 2020, 183, 729–737. [Google Scholar] [CrossRef]
- Munzel, D.; Lehle, K.; Haubner, F.; Schmid, C.; Birnbaum, D.E.; Preuner, J.G. Impact of diabetic serum on endothelial cells: An in-vitro-analysis of endothelial dysfunction in diabetes mellitus type 2. Biochem. Biophys. Res. Commun. 2007, 362, 238–244. [Google Scholar] [CrossRef]
- Schmidt, D.E.; Manca, M.; Hoefer, I.E. Circulating endothelial cells in coronary artery disease and acute coronary syndrome. Trends Cardiovasc. Med. 2015, 25, 578–587. [Google Scholar] [CrossRef]
- Thapa, N.; Hong, H.Y.; Sangeetha, P.; Kim, I.S.; Yoo, J.; Rhee, K.; Oh, G.T.; Kwon, I.C.; Lee, B.H. Identification of a peptide ligand recognizing dysfunctional endothelial cells for targeting atherosclerosis. J. Control. Release 2008, 131, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Topper, J.N.; Nagel, T.; Anderson, K.R.; Garcia-Cardena, G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. N. Y. Acad. Sci. 2000, 902, 230–239; discussion 239–240. [Google Scholar] [CrossRef] [PubMed]
- Goya, K.; Otsuki, M.; Xu, X.; Kasayama, S. Effects of the prostaglandin I2 analogue, beraprost sodium, on vascular cell adhesion molecule-1 expression in human vascular endothelial cells and circulating vascular cell adhesion molecule-1 level in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2003, 52, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Kim, S.; von Recum, H. Endothelial stem cells and precursors for tissue engineering: Cell source, differentiation, selection, and application. Tissue Eng. Part B Rev. 2008, 14, 133–147. [Google Scholar] [CrossRef]
- Reed, D.M.; Foldes, G.; Harding, S.E.; Mitchell, J.A. Stem cell-derived endothelial cells for cardiovascular disease: A therapeutic perspective. Br. J. Clin. Pharmacol. 2013, 75, 897–906. [Google Scholar] [CrossRef]
- Wu, J.; Belmonte, J.C.I. Dynamic pluripotent stem cell states and their applications. Cell. Stem. Cell 2015, 17, 509–525. [Google Scholar] [CrossRef]
- Ying, Q.L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.; Smith, A. The ground state of embryonic stem cell self-renewal. Nature 2008, 453, 519–523. [Google Scholar] [CrossRef]
- Brons, I.G.; Smithers, L.E.; Trotter, M.W.; Rugg-Gunn, P.; Sun, B.; Chuva de Sousa Lopes, S.M.; Howlett, S.K.; Clarkson, A.; Ahrlund-Richter, L.; Pedersen, R.A.; et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007, 448, 191–195. [Google Scholar] [CrossRef]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Kalishman, J.; Golos, T.G.; Durning, M.; Harris, C.P.; Becker, R.A.; Hearn, J.P. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. USA 1995, 92, 7844–7848. [Google Scholar] [CrossRef]
- Aoi, T.; Yae, K.; Nakagawa, M.; Ichisaka, T.; Okita, K.; Takahashi, K.; Chiba, T.; Yamanaka, S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 2008, 321, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Markoulaki, S.; Schorderet, P.; Carey, B.W.; Beard, C.; Wernig, M.; Creyghton, M.P.; Steine, E.J.; Cassady, J.P.; Foreman, R.; et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 2008, 133, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Dvorak, P.; Hampl, A. Basic fibroblast growth factor and its receptors in human embryonic stem cells. Folia Histochem. Cytobiol. 2005, 43, 203–208. [Google Scholar]
- Smith, A.G.; Heath, J.K.; Donaldson, D.D.; Wong, G.G.; Moreau, J.; Stahl, M.; Rogers, D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 1988, 336, 688–690. [Google Scholar] [CrossRef]
- Tesar, P.J.; Chenoweth, J.G.; Brook, F.A.; Davies, T.J.; Evans, E.P.; Mack, D.L.; Gardner, R.L.; McKay, R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007, 448, 196–199. [Google Scholar] [CrossRef]
- Xu, R.H.; Peck, R.M.; Li, D.S.; Feng, X.; Ludwig, T.; Thomson, J.A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Method. 2005, 2, 185–190. [Google Scholar] [CrossRef]
- Ying, Q.L.; Nichols, J.; Chambers, I.; Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003, 115, 281–292. [Google Scholar] [CrossRef]
- Daheron, L.; Opitz, S.L.; Zaehres, H.; Lensch, M.W.; Andrews, P.W.; Itskovitz-Eldor, J.; Daley, G.Q. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 2004, 22, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Vallier, L.; Alexander, M.; Pedersen, R.A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005, 118, 4495–4509. [Google Scholar] [CrossRef] [PubMed]
- Alberio, R.; Croxall, N.; Allegrucci, C. Pig epiblast stem cells depend on activin/nodal signaling for pluripotency and self-renewal. Stem Cells Dev. 2010, 19, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.K.; Cho, Y.S.; Kim, I.S.; Jeon, S.B.; Moon, D.K.; Hwangbo, C.; Choi, J.W.; Kim, T.S.; Lee, J.H. A Rho-Associated Coiled-Coil Containing Kinase Inhibitor, Y-27632, Improves Viability of Dissociated Single Cells, Efficiency of Colony Formation, and Cryopreservation in Porcine Pluripotent Stem Cells. Cell. Reprogr. 2019, 21, 37–50. [Google Scholar] [CrossRef]
- Levenberg, S.; Golub, J.S.; Amit, M.; Itskovitz-Eldor, J.; Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 4391–4396. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999, 77, 527–543. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Jeon, S.B.; Seo, B.G.; Baek, S.K.; Lee, H.G.; Shin, J.H.; Lee, I.W.; Kim, H.J.; Moon, S.Y.; Shin, K.C.; Choi, J.W.; et al. Endothelial Cells Differentiated from Porcine Epiblast Stem Cells. Cell. Reprogr. 2021, 23, 89–98. [Google Scholar] [CrossRef]
- Dubois, N.C.; Craft, A.M.; Sharma, P.; Elliott, D.A.; Stanley, E.G.; Elefanty, A.G.; Gramolini, A.; Keller, G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 2011, 29, 1011–1018. [Google Scholar] [CrossRef]
- Hattori, F.; Chen, H.; Yamashita, H.; Tohyama, S.; Satoh, Y.S.; Yuasa, S.; Li, W.; Yamakawa, H.; Tanaka, T.; Onitsuka, T.; et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat. Method. 2010, 7, 61–66. [Google Scholar] [CrossRef]
- Rust, W.; Balakrishnan, T.; Zweigerdt, R. Cardiomyocyte enrichment from human embryonic stem cell cultures by selection of ALCAM surface expression. Regen. Med. 2009, 4, 225–237. [Google Scholar] [CrossRef]
- Xu, C.; Police, S.; Rao, N.; Carpenter, M.K. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ. Res. 2002, 91, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Zahra, F.T.; Choleva, E.; Sajib, M.S.; Papadimitriou, E.; Mikelis, C.M. In Vitro Spheroid Sprouting Assay of Angiogenesis. Methods Mol. Biol. 2019, 1952, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.H.; Denis, M.; Krimbou, L.; Marcil, M.; Genest, J. Cellular cholesterol homeostasis in vascular endothelial cells. Can. J. Cardiol. 2006, 22, 35B–40B. [Google Scholar] [CrossRef]
- Nowak-Imialek, M.; Kues, W.A.; Petersen, B.; Lucas-Hahn, A.; Herrmann, D.; Haridoss, S.; Oropeza, M.; Lemme, E.; Scholer, H.R.; Carnwath, J.W.; et al. Oct4-enhanced green fluorescent protein transgenic pigs: A new large animal model for reprogramming studies. Stem Cells Develop. 2011, 20, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Zhang, H.; Tang, W.W.C.; Irie, N.; Withey, S.; Klisch, D.; Sybirna, A.; Dietmann, S.; Contreras, D.A.; Webb, R.; et al. Principles of early human development and germ cell program from conserved model systems. Nature 2017, 546, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Gu, M. Efficient Differentiation of Human Pluripotent Stem Cells to Endothelial Cells. Curr. Protoc. Hum. Genet. 2018, 98, e64. [Google Scholar] [CrossRef]
- Guan, X.M.; Cheng, M.; Li, H.; Cui, X.D.; Li, X.; Wang, Y.L.; Sun, J.L.; Zhang, X.Y. Biological properties of bone marrow-derived early and late endothelial progenitor cells in different culture media. Mol. Med. Rep. 2013, 8, 1722–1728. [Google Scholar] [CrossRef][Green Version]
- Abid, M.R.; Guo, S.; Minami, T.; Spokes, K.C.; Ueki, K.; Skurk, C.; Walsh, K.; Aird, W.C. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler. Throm. Vasc. Biol. 2004, 24, 294–300. [Google Scholar] [CrossRef]
- Gerber, H.-P.; McMurtrey, A.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway: Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343. [Google Scholar] [CrossRef] [PubMed]
- Olmer, R.; Engels, L.; Usman, A.; Menke, S.; Malik, M.N.H.; Pessler, F.; Göhring, G.; Bornhorst, D.; Bolten, S.; Abdelilah-Seyfried, S. Differentiation of human pluripotent stem cells into functional endothelial cells in scalable suspension culture. Stem Cell Rep. 2018, 10, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Albelda, S.M. Endothelial and epithelial cell adhesion molecules. Am. J. Respir. Cell Mol. Biol. 1991, 4, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Heng, B.C.; Xu, J.; Zhu, S.; Yuan, C.; Lo, E.C.; Zhang, C. Decellularized extracellular matrix of human umbilical vein endothelial cells promotes endothelial differentiation of stem cells from exfoliated deciduous teeth. J. Biomed. Mater. Res. Part A 2017, 105, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.M.; Costa, E.B.; Orellana, M.D.; Picanco-Castro, V.; Covas, D.T. OP9 Stromal Cells Proteins Involved in Hematoendothelial Differentiation from Human Embryonic Stem Cells. Cell. Reprogr. 2015, 17, 338–346. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequence (5′→3′) | References | |
|---|---|---|---|
| Forward | Reverse | ||
| 18S | TCG GAA CTG AGG CCA TGA TT | GAA TTT CAC CTC TAG CGG CG | NR_046241.1 |
| OCT-3/4 | GGA TAT ACC CAG GCC GAT GT | GTC GTT TGG CTG AAC ACC TT | NM_001113060.1 |
| NANOG | CCC GAA GCA TCC ATT TCC AG | GAT GAC ATC TGC AAG GAG GC | DQ_447201.1 |
| SOX2 | CAT GTC CCA GCA CTA CCA GA | GAG AGA GGC AGT GTA CCG TT | NM_001123197.1 |
| Name | Media | Supplements & Growth Factors |
|---|---|---|
| EGM-2 | EBM-2 | EGM-2 SingleQuot Kit |
| EGM-2-EV | EBM-2 | VEGF excluded EGM-2 SingleQuot Kit |
| Differentiation Media | EBM-2 | 50 ng/mL VEGF-165, VEGF excluded EGM-2 SingleQuot Kit |
| M199 | M199 | 20% PBS, 100 µg/mL heparin, 30 µg/mL ECGS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-H.; Seo, B.-G.; Lee, I.-W.; Kim, H.-J.; Seo, E.-C.; Lee, K.-M.; Jeon, S.-B.; Baek, S.-K.; Kim, T.-S.; Lee, J.-H.; et al. Functional Characterization of Endothelial Cells Differentiated from Porcine Epiblast Stem Cells. Cells 2022, 11, 1524. https://doi.org/10.3390/cells11091524
Shin J-H, Seo B-G, Lee I-W, Kim H-J, Seo E-C, Lee K-M, Jeon S-B, Baek S-K, Kim T-S, Lee J-H, et al. Functional Characterization of Endothelial Cells Differentiated from Porcine Epiblast Stem Cells. Cells. 2022; 11(9):1524. https://doi.org/10.3390/cells11091524
Chicago/Turabian StyleShin, Joon-Hong, Bo-Gyeong Seo, In-Won Lee, Hyo-Jin Kim, Eun-Chan Seo, Kwang-Min Lee, Soo-Been Jeon, Sang-Ki Baek, Tae-Suk Kim, Jeong-Hyung Lee, and et al. 2022. "Functional Characterization of Endothelial Cells Differentiated from Porcine Epiblast Stem Cells" Cells 11, no. 9: 1524. https://doi.org/10.3390/cells11091524
APA StyleShin, J.-H., Seo, B.-G., Lee, I.-W., Kim, H.-J., Seo, E.-C., Lee, K.-M., Jeon, S.-B., Baek, S.-K., Kim, T.-S., Lee, J.-H., Choi, J.-W., Hwangbo, C., & Lee, J.-H. (2022). Functional Characterization of Endothelial Cells Differentiated from Porcine Epiblast Stem Cells. Cells, 11(9), 1524. https://doi.org/10.3390/cells11091524






