Changes in Inflammatory Markers in Patients with Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evaluation of Patients
2.3. Blood Samples
2.4. Balloon Pulmonary Angioplasty
2.5. Statistics
3. Results
3.1. Study Groups
3.2. Hemodynamic and Clinical Effects of BPA
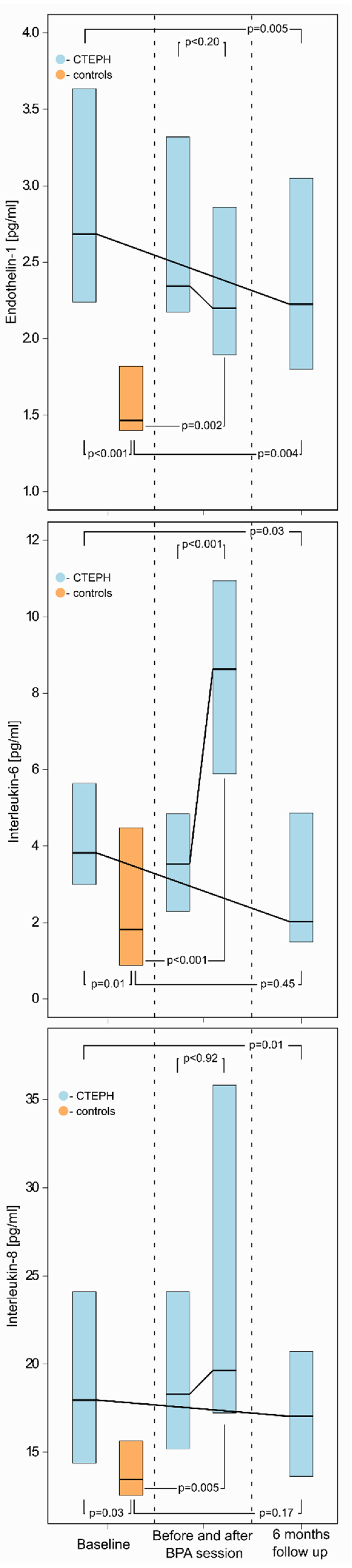
3.3. Baseline Levels of Selected Biomarkers in CTEPH Patients and Controls
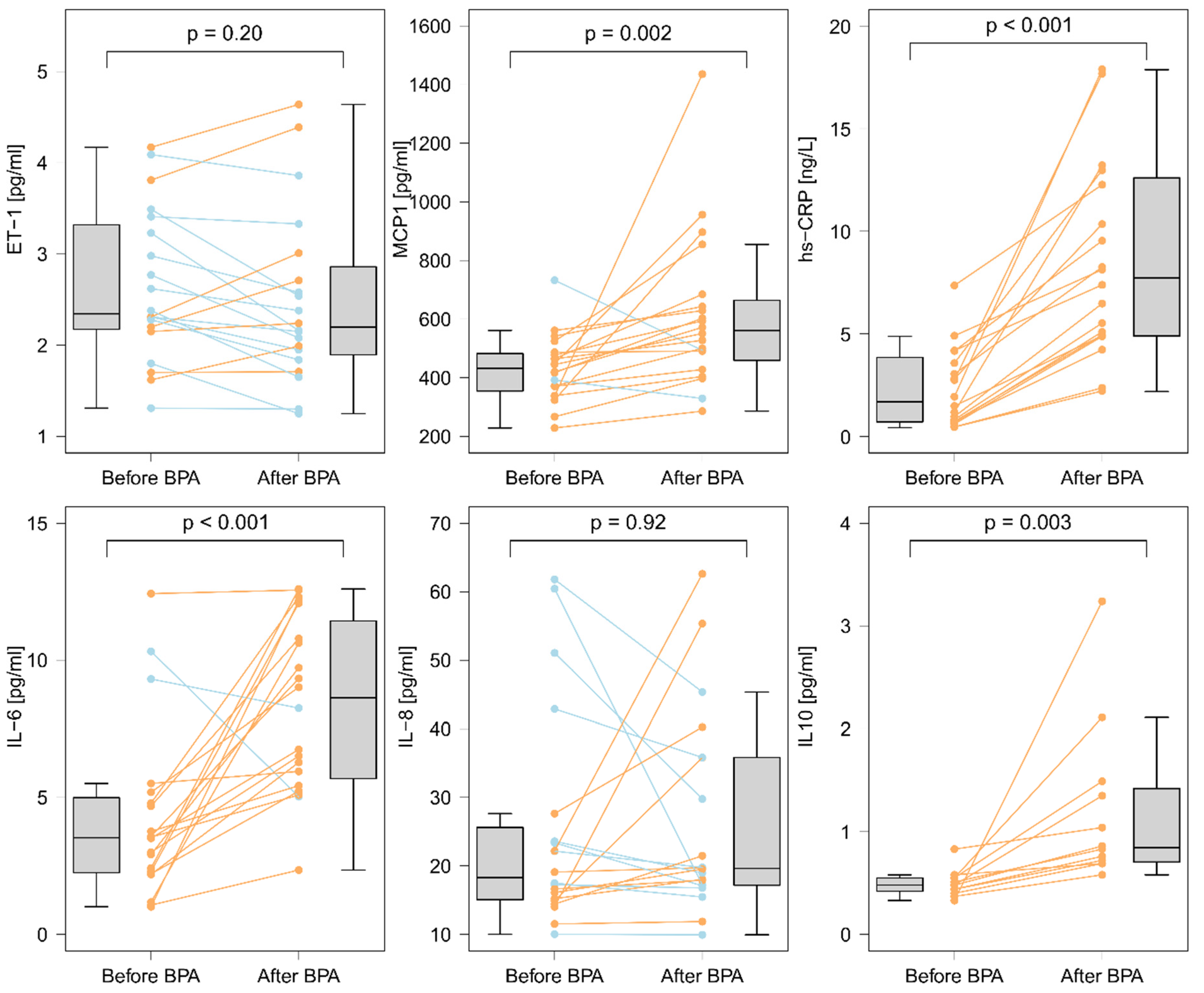
3.4. Changes in Biomarker Levels after a Single BPA Session
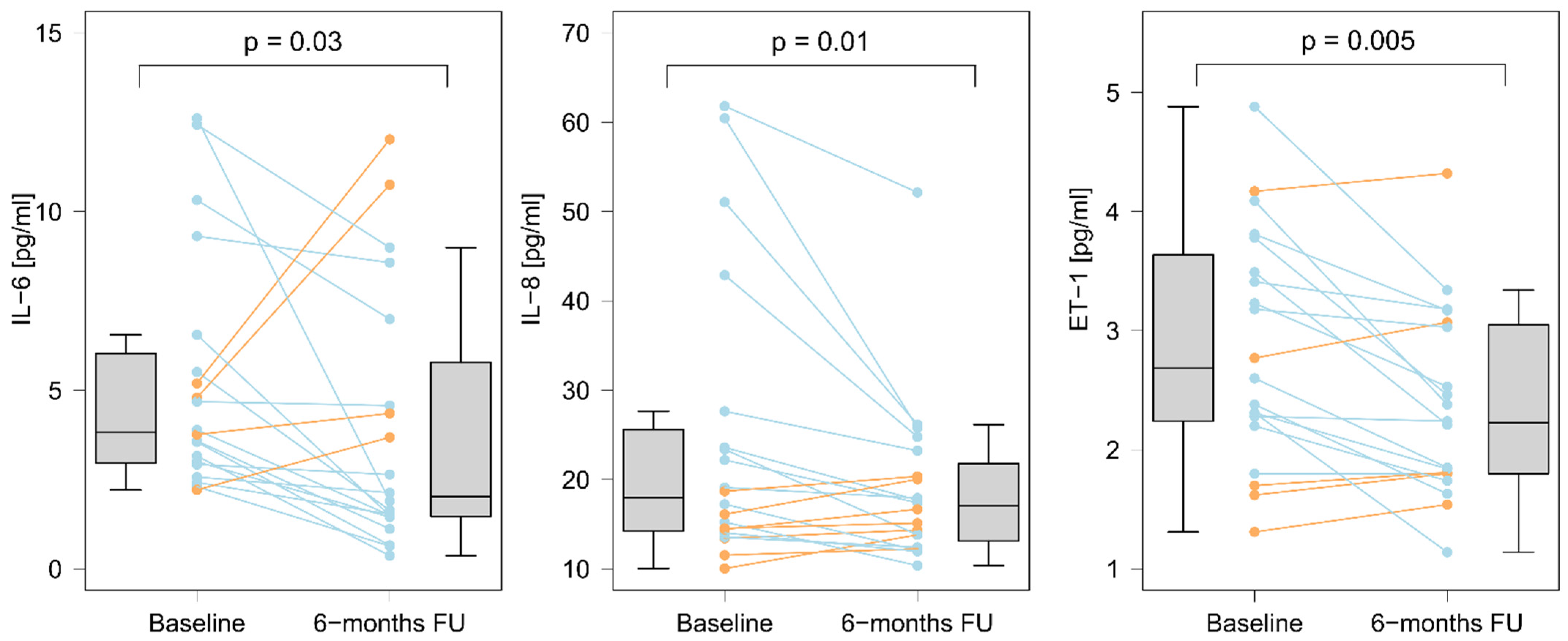
3.5. Changes in Selected Biomarkers Levels after Completion of BPA Treatment
3.6. Associations between Levels of Biomarkers and BPA Treatment Outcomes
3.6.1. The Effect of a Single BPA Session
3.6.2. The Effect of Completed BPA Treatment
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Dzikowska-Diduch, O.; Mroczek, E.; Mularek-Kubzdela, T.; Chrzanowski, Ł.; Skoczylas, I.; Tomaszewski, M.; Peregud-Pogorzelska, M.; Karasek, D.; Lewicka, E.; et al. Characteristics and outcomes of patients with chronic thromboembolic pulmonary hypertension in the era of modern therapeutic approaches: Data from the Polish multicenter registry (BNP-PL). Ther. Adv. Chronic Dis. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Vachiery, J.L.; Gibbs, S. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Kopeć, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Kuśmierczyk, B.; Pruszczyk, P.; Błaszczak, P.; Lewicka, E.; et al. Database of Pulmonary Hypertension in the Polish Population (BNP-PL): Design of the registry. Kardiol. Pol. 2019, 77, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lang, I.M. Current understanding of the pathophysiology of chronic thromboembolic pulmonary hypertension. Thromb. Res. 2018, 164, 136–144. [Google Scholar] [CrossRef]
- Quarck, R.; Wynants, M.; Verbeken, E.; Meyns, B.; Delcroix, M. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2015, 46, 431–443. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K. Crosstalk between endothelial cell and thrombus in chronic thromboembolic pulmonary hypertension: Perspective. Histol. Histopathol. 2012, 28, 185–193. [Google Scholar]
- Zhang, M.; Zhang, Y.-X.; Pang, W.; Zhai, Z.; Wang, C. Circulating biomarkers in chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2019, 9, 2045894019844480. [Google Scholar] [CrossRef]
- Reesink, H.J.; Meijer, R.C.; Lutter, R.; Boomsma, F.; Jansen, H.M.; Kloek, J.J.; Bresser, P. Hemodynamic and Clinical Correlates of Endothelin-1 in Chronic Thromboembolic Pulmonary Hypertension. Circ. J. 2006, 70, 1058–1063. [Google Scholar] [CrossRef][Green Version]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined Balloon Pulmonary Angioplasty for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef]
- Magoń, W.; Stępniewski, J.; Waligóra, M.; Jonas, K.; Podolec, P.; Kopeć, G. Pulmonary Artery Elastic Properties After Balloon Pulmonary Angioplasty in Patients With Inoperable Chronic Thromboembolic Pulmonary Hypertension. Can. J. Cardiol. 2019, 35, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Darocha, S.; Roik, M.; Kopeć, G.; Araszkiewicz, A.; Furdal, M.; Lewandowski, M.; Jacheć, W.; Grabka, M.; Banaszkiewicz, M.; Pietrasik, A.; et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: A multicentre registry. EuroIntervention 2021. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Stępniewski, J.; Magoń, W.; Waligóra, M.; Podolec, P. Prolonged catheter balloon inflation for the treatment of hemoptysis complicating balloon pulmonary angioplasty. Pol. Arch. Intern. Med. 2017, 127, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Łabyk, A.; Wretowski, D.; Potępa, M.; Pruszczyk, P.; Roik, M. Refined balloon pulmonary angioplasty as a first line therapy of complex thromboembolic lesions in patients with chronic thromboembolic pulmonary hypertension. Pol. Arch. Intern. Med. 2020, 130, 805–806. [Google Scholar] [CrossRef]
- Piłka, M.; Darocha, S.; Banaszkiewicz, M.; Florczyk, M.; Wieteska, M.; Dobosiewicz, A.; Mańczak, M.; Mańczak, R.; Pietrasik, A.; Pietura, R.; et al. The evolution of electrocardiographic signs of right ventricular overload after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Pol. Arch. Intern. Med. 2019, 129, 451–459. [Google Scholar] [CrossRef]
- Quarck, R.; Nawrot, T.; Meyns, B.; Delcroix, M. C-Reactive Protein: A New Predictor of Adverse Outcome in Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2009, 53, 1211–1218. [Google Scholar] [CrossRef]
- Van Kan, C.; van der Plas, M.N.; Reesink, H.J.; van Steenwijk, R.P.; Kloek, J.J.; Tepaske, R.; Bonta, P.I.; Bresser, P. Hemodynamic and ventilatory responses during exercise in chronic thromboembolic disease. J. Thorac. Cardiovasc. Surg. 2016, 152, 763–771. [Google Scholar] [CrossRef]
- Grosjean, F.; De Amici, M.; Klersy, C.; Marchi, G.; Sciortino, A.; Spaltini, F.; Pin, M.; Grazioli, V.; Celentano, A.; Vanini, B.; et al. High preoperative plasma endothelin-1 levels are associated with increased acute kidney injury risk after pulmonary endarterectomy. J. Nephrol. 2018, 31, 881–888. [Google Scholar] [CrossRef]
- Kurzyna, M.; Araszkiewicz, A.; Błaszczak, P.; Grabka, M.; Hawranek, M.; Kopeć, G.; Mroczek, E.; Zembala, M.; Torbicki, A.; Ochała, A. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society’s Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Kardiol. Pol. 2015, 73, 63–68. [Google Scholar] [CrossRef]
- Emile, F.; Mohamed, L.; Pamela, M.; Delphine, B.; Sylvie, L.; Yu, T. Pulmonary angioplasty without anticoagulants may reduce the rate of hemoptysis and make the procedure safer. J. Am. Coll. Cardiol. 2020, 75 (Suppl. S1), 2100. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Bębnowska, D.; Hrynkiewicz, R.; Dworzyński, J.; Niedźwiedzka-Rystwej, P.; Kopeć, G.; Grywalska, E. Role of the Immune System Elements in Pulmonary Arterial Hypertension. J. Clin. Med. 2021, 10, 3757. [Google Scholar] [CrossRef]
- von Haehling, S.; von Bardeleben, R.S.; Kramm, T.; Thiermann, Y.; Niethammer, M.; Doehner, W.; Anker, S.D.; Munzel, T.; Mayer, E.; Genth-Zotz, S. Inflammation in right ventricular dysfunction due to thromboembolic pulmonary hypertension. Int. J. Cardiol. 2010, 144, 206–211. [Google Scholar] [CrossRef]
- Yang, M.; Deng, C.; Wu, D.; Zhong, Z.; Lv, X.; Huang, Z.; Lian, N.; Liu, K.; Zhang, Q. The role of mononuclear cell tissue factor and inflammatory cytokines in patients with chronic thromboembolic pulmonary hypertension. J. Thromb. Thrombolysis 2016, 42, 38–45. [Google Scholar] [CrossRef]
- Koudstaal, T.; van Uden, D.; van Hulst, J.A.C.; Heukels, P.; Bergen, I.M.; Geenen, L.W.; Baggen, V.J.M.; Bosch, A.E.V.D.; Toorn, L.M.V.D.; Chandoesing, P.P.; et al. Plasma markers in pulmonary hypertension subgroups correlate with patient survival. Respir. Res. 2021, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Soon, E.; Holmes, A.M.; Barker, L.; Treacy, C.; Suntharalingham, J.; Toshner, M.; Nicklin, P.; Walker, C.; Budd, D.; Jenkins, D.; et al. S97 Inflammatory cytokines are elevated in patients with operable chronic thromboembolic pulmonary hypertension and predict outcome post-endarterectomy. Thorax 2010, 65 (Suppl. S4), A45. [Google Scholar] [CrossRef]
- Ataam, J.A.; Amsallem, M.; Guihaire, J.; Haddad, F.; Lamrani, L.; Stephan, F.; Jaïs, X.; Humbert, M.; Mercier, O.; Fadel, E. Preoperative C-reactive protein predicts early postoperative outcomes after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2021, 161, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-Term Outcome of Patients With Chronic Thromboembolic Pulmonary Hypertension:Results From an International Prospective Registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef]
- Langer, F.; Schramm, R.; Bauer, M.; Tscholl, D.; Kunihara, T.; Schafers, H.-J. Cytokine Response to Pulmonary Thromboendarterectomy. Chest 2004, 126, 135–141. [Google Scholar] [CrossRef]
- Shimokawahara, H.; Ogawa, A.; Mizoguchi, H.; Yagi, H.; Ikemiyagi, H.; Matsubara, H. Vessel Stretching Is a Cause of Lumen Enlargement Immediately After Balloon Pulmonary Angioplasty: Intravascular Ultrasound Analysis in Patients With Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2018, 11, e006010. [Google Scholar] [CrossRef]
- Magoń, W.; Stępniewski, J.; Waligóra, M.; Jonas, K.; Przybylski, R.; Sikorska, M.; Podolec, P.; Kopeć, G. Virtual Histology to Evaluate Mechanisms of Pulmonary Artery Lumen Enlargement in Response to Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2020, 9, 1655. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Murbraech, K.; Ragnarsson, A.; Gude, E.; Andersen, R.; Fiane, A.E.; Andreassen, J.; Aakhus, S.; Andreassen, A.K. Echocardiographic evidence of right ventricular functional improvement after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. J. Heart Lung Transplant. 2016, 35, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Smolders, V.; Lodder, K.; Rodríguez, C.; Tura-Ceide, O.; Barberà, J.; Jukema, J.; Quax, P.; Goumans, M.; Kurakula, K. The Inflammatory Profile of CTEPH-Derived Endothelial Cells Is a Possible Driver of Disease Progression. Cells 2021, 10, 737. [Google Scholar] [CrossRef]
- Thenappan, T.; Ormiston, M.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef] [PubMed]
- Stam, K.; Van Duin, R.W.B.; Uitterdijk, A.; Krabbendam-Peters, I.; Sorop, O.; Danser, A.H.J.; Duncker, D.J.; Merkus, D. Pulmonary microvascular remodeling in chronic thrombo-embolic pulmonary hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L951–L964. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Keller, T.; Wolter, J.S.; Ajnwojner, R.; Peters, K.; Haas, M.A.; Roller, F.C.; Breithecker, A.; Rieth, A.J.; et al. Dynamics of high-sensitivity cardiac troponin T during therapy with balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. PLoS ONE 2018, 13, e0204683. [Google Scholar] [CrossRef] [PubMed]
| Variable | Before BPA Treatment | Six Months after Completion of BPA Treatment | p |
|---|---|---|---|
| Age, years | 67 (65–75) | - | - |
| Male sex, n, % | 6 (30%) | - | - |
| Time from onset of symptoms to CTEPH diagnosis, months | 10 (5–22) | - | - |
| WHO functional lass: I/II/III/IV, n, % | 0/0/20/0 0%/0%/100%/0% | 2/12/6/0 10%/60%/30%/0% | <0.001 |
| 6-minute walking distance *, m | 330 (260–380) | 393 (340–450) | 0.01 |
| NT-proBNP, pg/mL | 1726 (521–2678) | 236 (144–722) | 0.002 |
| mPAP *, mmHg | 39 (37–50) | 29 (25–31) | <0.001 |
| RAP, mmHg | 4 (3–7) | 4 (3–6) | 0.26 |
| CI *, L/min/m2 | 2.31 (1.95–2.62) | 2.49 (2.32–3.00) | 0.12 |
| PVR *, WU | 8.9 (6.3–11.1) | 3.9 (3.5–5.7) | <0.001 |
| CTEPH (n = 20) | Controls (n = 10) | p | |
|---|---|---|---|
| hsCRP, mg/L | 2.16 (0.75–4.48) | 2.15 (0.88–2.77) | 0.79 |
| IL-6, pg/mL | 3.82 (2.96–6.03) | 1.81 (0.88–4.48) | 0.01 |
| IL-8, pg/mL | 17.96 (14.23–25.62) | 13.45 (12.46–16.02) | 0.03 |
| IL-10, pg/mL | 0.49 (0.39–0.58) | 0.51 (0.48–0.69) | 0.98 |
| MCP-1, pg/ml | 419 (338–506) | 416 (273–505) | 0.84 |
| ET-1, pg/mL | 2.68 (2.24–3.64) | 1.47 (1.4–1.82) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magoń, W.; Stępniewski, J.; Waligóra, M.; Jonas, K.; Przybylski, R.; Podolec, P.; Kopeć, G. Changes in Inflammatory Markers in Patients with Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty. Cells 2022, 11, 1491. https://doi.org/10.3390/cells11091491
Magoń W, Stępniewski J, Waligóra M, Jonas K, Przybylski R, Podolec P, Kopeć G. Changes in Inflammatory Markers in Patients with Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty. Cells. 2022; 11(9):1491. https://doi.org/10.3390/cells11091491
Chicago/Turabian StyleMagoń, Wojciech, Jakub Stępniewski, Marcin Waligóra, Kamil Jonas, Roman Przybylski, Piotr Podolec, and Grzegorz Kopeć. 2022. "Changes in Inflammatory Markers in Patients with Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty" Cells 11, no. 9: 1491. https://doi.org/10.3390/cells11091491
APA StyleMagoń, W., Stępniewski, J., Waligóra, M., Jonas, K., Przybylski, R., Podolec, P., & Kopeć, G. (2022). Changes in Inflammatory Markers in Patients with Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty. Cells, 11(9), 1491. https://doi.org/10.3390/cells11091491







