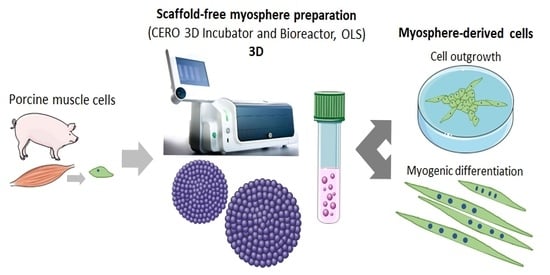
Preparation of Spheroids from Primary Pig Cells in a Mid-Scale Bioreactor Retaining Their Myogenic Potential
Abstract
1. Introduction
2. Materials and Methods
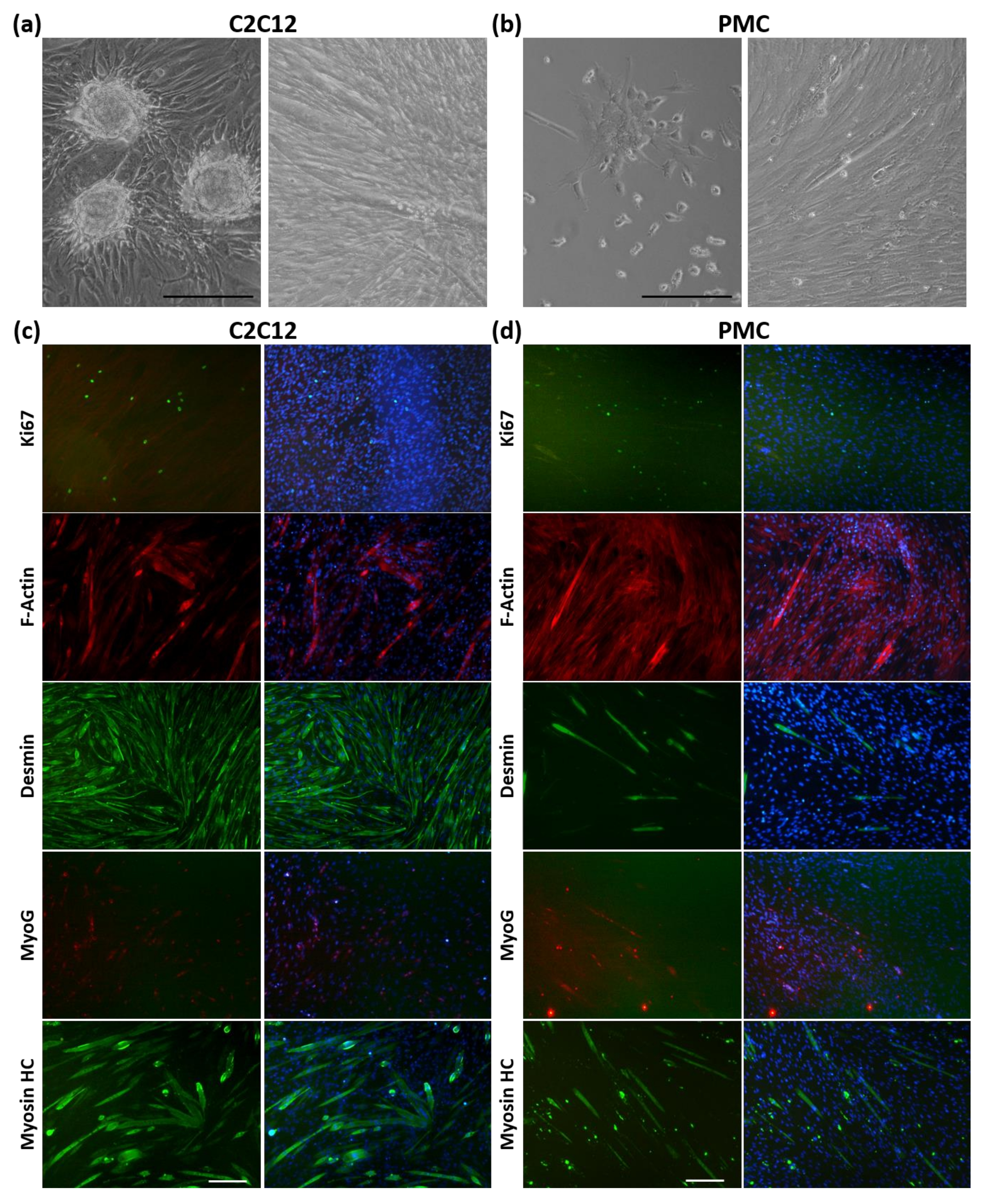
2.1. Cell Culture
2.2. Suspension Culture for Spheroid Formation
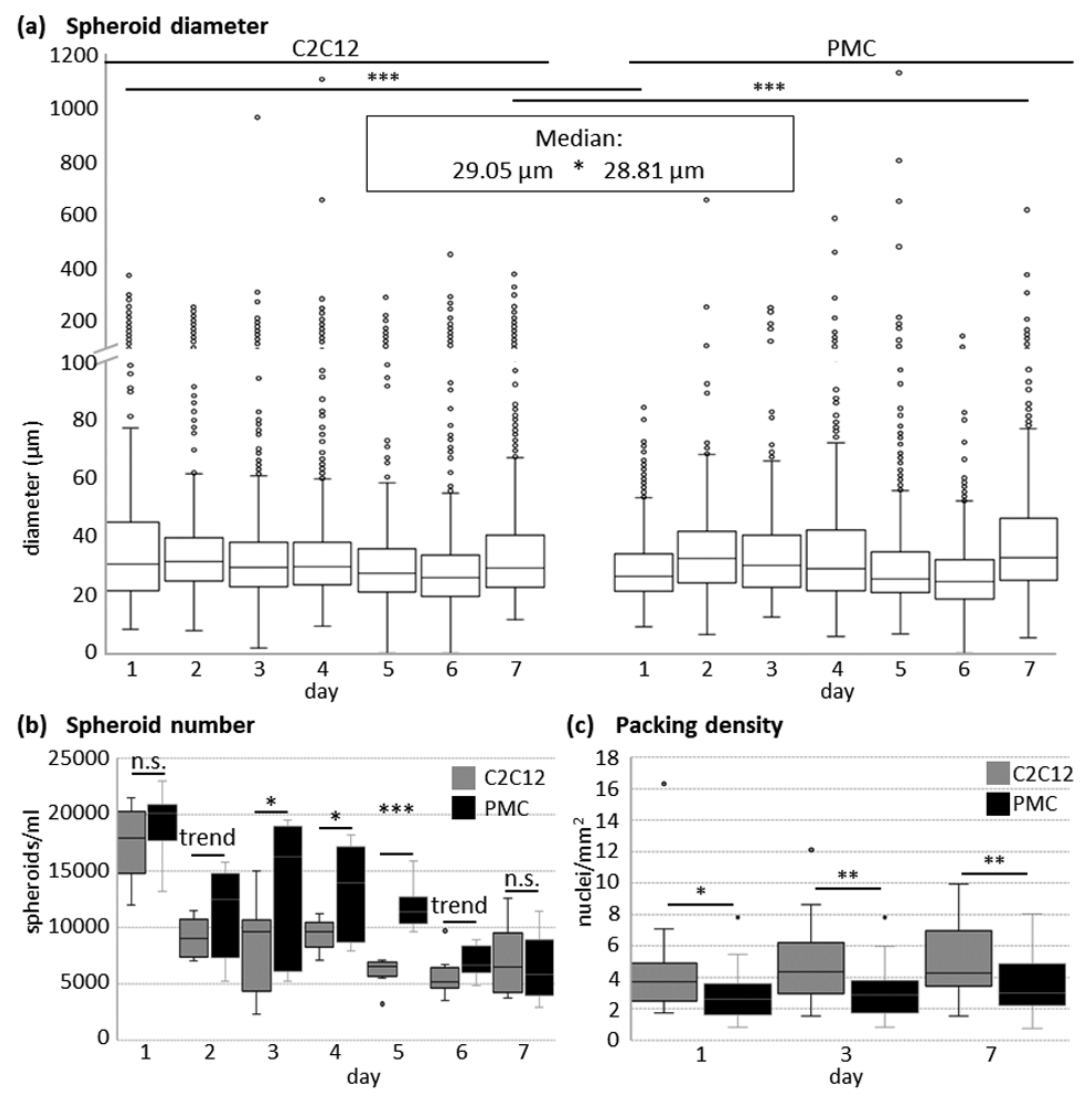
2.3. Quantitative Analyses of Spheroids
2.4. Histological Analysis of Spheroids
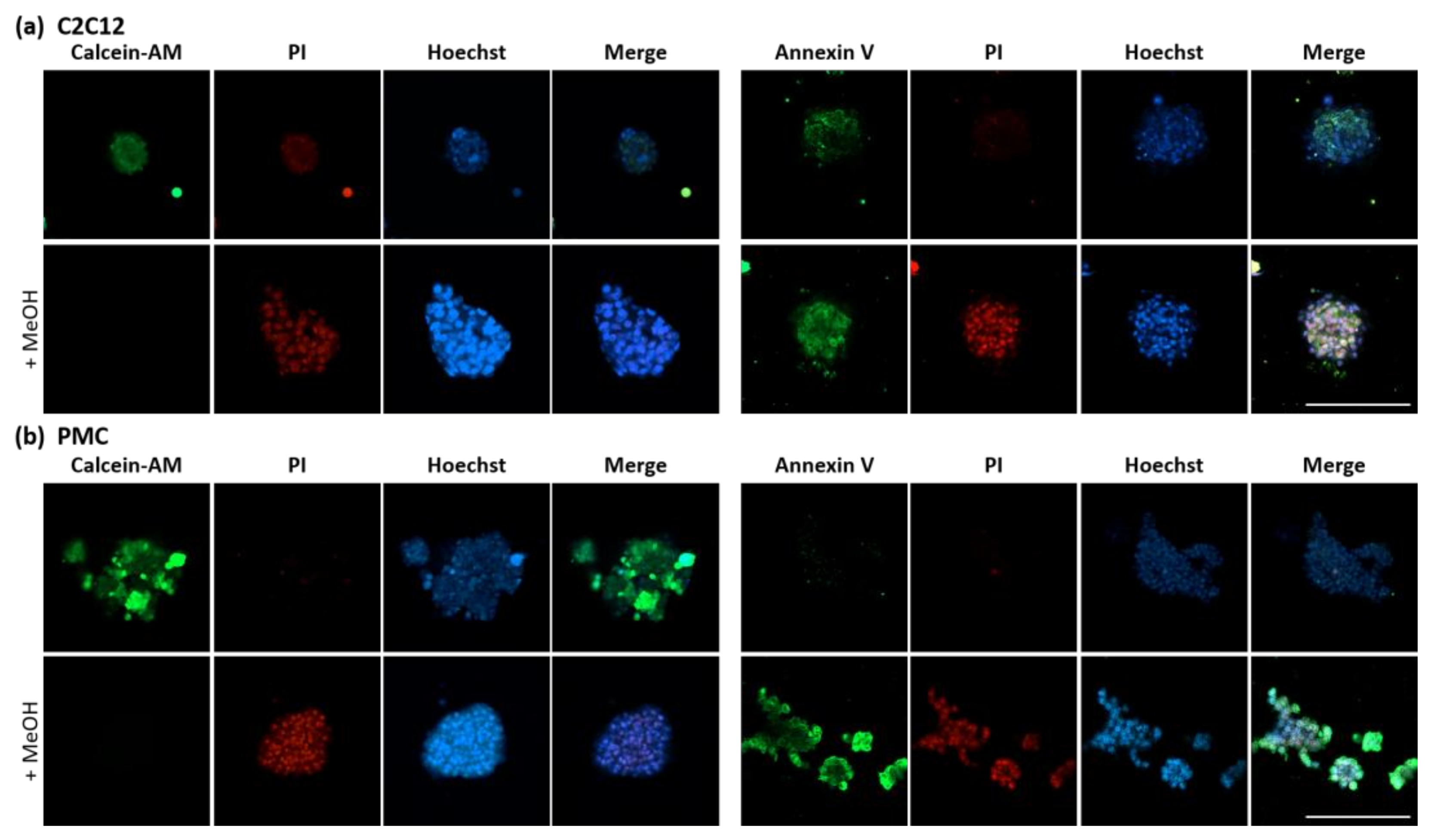
2.5. Analysis of Spheroid Viability
2.6. Myogenic Differentiation of Cells from Spheroids
2.7. Statistical Analyses
3. Results
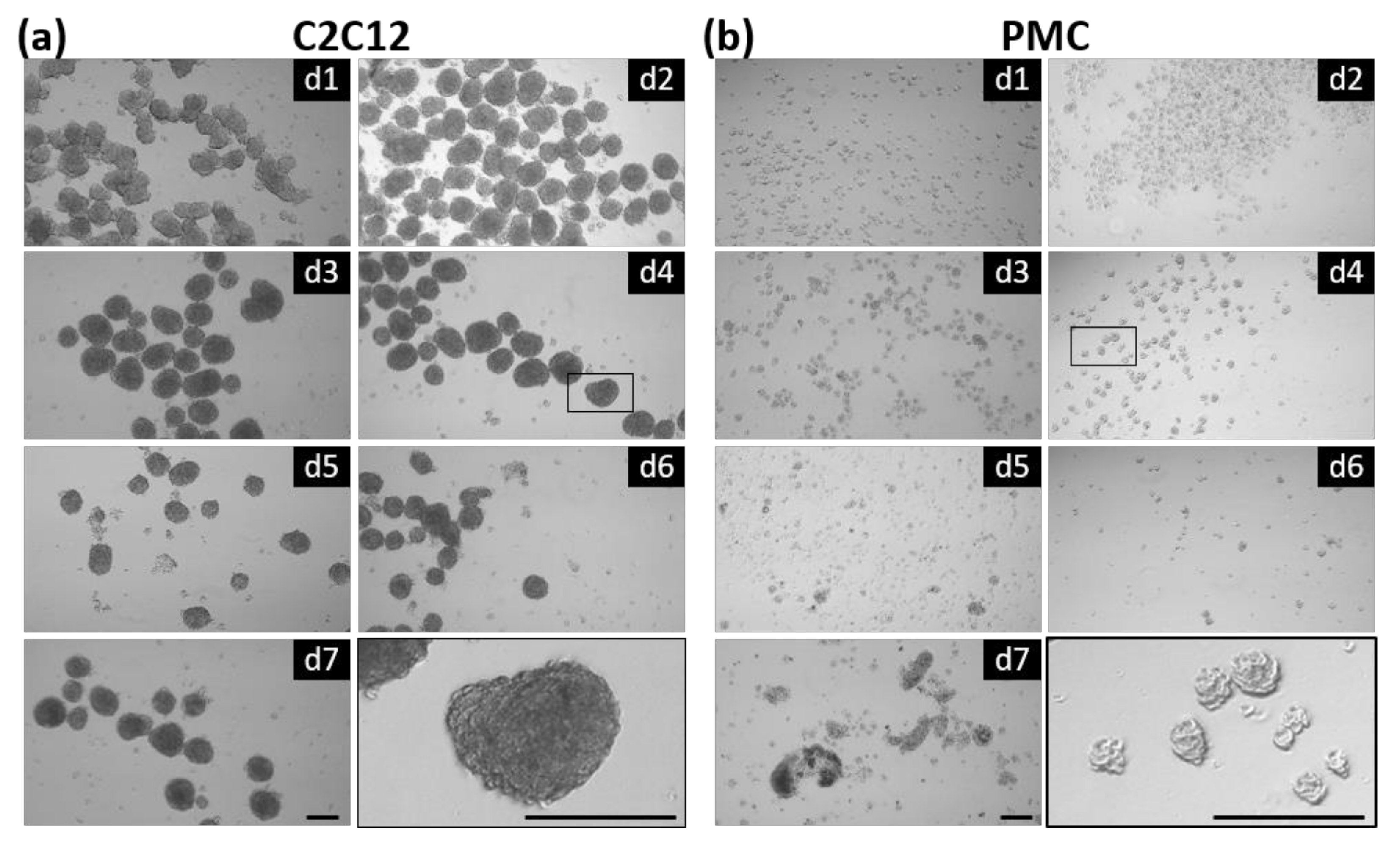
3.1. Formation of Spheroids from Muscle Cells in the CERO Incubator and Bioreactor
3.2. Protein Expression of the Myogenic Markers and Extracellular Matrix in Spheroids
3.3. Viability and Myogenic Potential of Spheroids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, R.G.; Greenman, M.J.; Mall, F.P.; Jackson, C.M. Observations of the living developing nerve fiber. Anat. Rec. 1907, 1, 116–128. [Google Scholar] [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Jiwlawat, S.; Lynch, E.; Glaser, J.; Smit-Oistad, I.; Jeffrey, J.; Van Dyke, J.M.; Suzuki, M. Differentiation and sarcomere formation in skeletal myocytes directly prepared from human induced pluripotent stem cells using a sphere-based culture. Differentiation 2017, 96, 70–81. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.L.; Joshi, R.; Luker, G.D.; Tavana, H. Engineered Breast Cancer Cell Spheroids Reproduce Biologic Properties of Solid Tumors. Adv. Healthcare Mater. 2016, 5, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.S. Neurospheres: Insights into neural stem cell biology. J. Neurosci. Res. 2004, 78, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Jagirdar, R.M.; Papazoglou, E.D.; Pitaraki, E.; Kouliou, O.A.; Rouka, E.; Giannakou, L.; Giannopoulos, S.; Sinis, S.I.; Hatzoglou, C.; Gourgoulianis, K.I.; et al. Cell and extracellular matrix interaction models in benign mesothelial and malignant pleural mesothelioma cells in 2D and 3D in-vitro. Clin. Exp. Pharmacol. Physiol. 2021, 48, 543–552. [Google Scholar] [CrossRef]
- Papazoglou, E.D.; Jagirdar, R.M.; Kouliou, O.A.; Pitaraki, E.; Hatzoglou, C.; Gourgoulianis, K.I.; Zarogiannis, S.G. In Vitro Characterization of Cisplatin and Pemetrexed Effects in Malignant Pleural Mesothelioma 3D Culture Phenotypes. Cancers 2019, 11, 1446. [Google Scholar] [CrossRef]
- Gerogianni, I.; Pitaraki, E.; Jagirdar, R.M.; Kouliou, O.; Giannakou, L.; Giannopoulos, S.; Papazoglou, E.; Hatzoglou, C.; Gourgoulianis, K.I.; Zarogiannis, S.G. 2-Deoxy-glucose Enhances the Effect of Cisplatin and Pemetrexed in Reducing Malignant Pleural Mesothelioma Cell Proliferation But Not Spheroid Growth. Anticancer Res. 2019, 39, 3809–3814. [Google Scholar] [CrossRef]
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. JoVE 2011, 51, 2720. Available online: http://www.jove.com/details.php?id=2720. [CrossRef]
- Ravichandran, A.; Liu, Y.; Teoh, S.H. Review: Bioreactor design towards generation of relevant engineered tissues: Focus on clinical translation. J. Tissue Eng. Regen. Med. 2018, 12, e7–e22. [Google Scholar] [CrossRef]
- Justice, B.A.; Badr, N.A.; Felder, R.A. 3D cell culture opens new dimensions in cell-based assays. Drug Discov. Today 2009, 14, 102–107. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- De Waele, J.; Reekmans, K.; Daans, J.; Goossens, H.; Berneman, Z.; Ponsaerts, P. 3D culture of murine neural stem cells on decellularized mouse brain sections. Biomaterials 2015, 41, 122–131. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dessauge, F.; Schleder, C.; Perruchot, M.H.; Rouger, K. 3D in vitro models of skeletal muscle: Myopshere, myobundle and bioprinted muscle construct. Vet. Res. 2021, 52, 72. [Google Scholar] [CrossRef]
- Rando, T.A.; Blau, H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994, 125, 1275–1287. [Google Scholar] [CrossRef]
- Arsic, N.; Mamaeva, D.; Lamb, N.J.; Fernandez, A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp. Cell Res. 2008, 314, 1266–1280. [Google Scholar] [CrossRef]
- Romagnoli, C.; Iantomasi, T.; Brandi, M.L. Available In Vitro Models for Human Satellite Cells from Skeletal Muscle. Int. J. Mol. Sci. 2021, 22, 13221. [Google Scholar] [CrossRef]
- Westerman, K.A.; Penvose, A.; Yang, Z.; Allen, P.D.; Vacanti, C.A. Adult muscle ’stem’ cells can be sustained in culture as free-floating myospheres. Exp. Cell Res. 2010, 316, 1966–1976. [Google Scholar] [CrossRef]
- Aguanno, S.; Petrelli, C.; Di Siena, S.; De Angelis, L.; Pellegrini, M.; Naro, F. A Three-Dimensional Culture Model of Reversibly Quiescent Myogenic Cells. Stem Cells Int. 2019, 2019, 7548160. [Google Scholar] [CrossRef]
- Marquette, M.L.; Byerly, D.; Sognier, M. A novel in vitro three-dimensional skeletal muscle model. In Vitro Cell Dev. Biol. Anim. 2007, 43, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Zhang, H.; Zhou, T.; Duan, Y.; Shou, H.; Yu, S.; Gao, C. Spheroids of Endothelial Cells and Vascular Smooth Muscle Cells Promote Cell Migration in Hyaluronic Acid and Fibrinogen Composite Hydrogels. Research 2020, 2020, 8970480. [Google Scholar] [CrossRef] [PubMed]
- Sarig, R.; Baruchi, Z.; Fuchs, O.; Nudel, U.; Yaffe, D. Regeneration and transdifferentiation potential of muscle-derived stem cells propagated as myospheres. Stem Cells 2006, 24, 1769–1778. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Chen, C.; Stoelzel, K.; Kaufmann, A.M.; Albers, A.E. Human skeletal muscle-derived stem cells retain stem cell properties after expansion in myosphere culture. Exp. Cell Res. 2011, 317, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Naldaiz-Gastesi, N.; Goicoechea, M.; Aragon, I.M.; Perez-Lopez, V.; Fuertes-Alvarez, S.; Herrera-Imbroda, B.; Lopez de Munain, A.; De Luna-Diaz, R.; Baptista, P.M.; Fernandez, M.A.; et al. Isolation and characterization of myogenic precursor cells from human cremaster muscle. Sci. Rep. 2019, 9, 3454. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, T.; Meyer, M.G.; Krakora, D.; Suzuki, M. Isolation and in vitro propagation of human skeletal muscle progenitor cells from fetal muscle. Cell Biol. Int. 2013, 37, 191–196. [Google Scholar] [CrossRef][Green Version]
- Rouger, K.; Larcher, T.; Dubreil, L.; Deschamps, J.Y.; Le Guiner, C.; Jouvion, G.; Delorme, B.; Lieubeau, B.; Carlus, M.; Fornasari, B.; et al. Systemic delivery of allogenic muscle stem cells induces long-term muscle repair and clinical efficacy in duchenne muscular dystrophy dogs. Am. J. Pathol. 2011, 179, 2501–2518. [Google Scholar] [CrossRef]
- Verbruggen, S.; Luining, D.; Van Essen, A.; Post, M.J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 2018, 70, 503–512. [Google Scholar] [CrossRef]
- Aguiari, P.; Leo, S.; Zavan, B.; Vindigni, V.; Rimessi, A.; Bianchi, K.; Franzin, C.; Cortivo, R.; Rossato, M.; Vettor, R.; et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1226–1231. [Google Scholar] [CrossRef]
- Wobus, A.M.; Rohwedel, J.; Maltsev, V.; Hescheler, J. In vitro differentiation of embryonic stem cells into cardiomyocytes or skeletal muscle cells is specifically modulated by retinoic acid. Rouxs Arch. Dev. Biol. 1994, 204, 36–45. [Google Scholar] [CrossRef]
- Gabriel, J.; Brennan, D.; Elisseeff, J.H.; Beachley, V. Microarray Embedding/Sectioning for Parallel Analysis of 3D Cell Spheroids. Sci. Rep. 2019, 9, 16287. [Google Scholar] [CrossRef] [PubMed]
- Elanzew, A.; Sommer, A.; Pusch-Klein, A.; Brustle, O.; Haupt, S. A reproducible and versatile system for the dynamic expansion of human pluripotent stem cells in suspension. Biotechnol. J. 2015, 10, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Meier, A.; Dehne, A.; Salhotra, A.; Tran, T.A.; Neumann, S.; Schmidt, K.; Meiser, I.; Neubauer, J.C.; Zimmermann, H.; et al. A complete workflow for the differentiation and the dissociation of hiPSC-derived cardiospheres. Stem Cell. Res. 2018, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- OLS OMNI Life Science GmbH & Co. KG. 3D Cell Culture Bioreactor CERO 3D. Available online: https://www.ols-bio.de/products/3d-cell-culture-bioreactor-cero (accessed on 18 March 2022).
- Miersch, C.; Stange, K.; Rontgen, M. Separation of functionally divergent muscle precursor cell populations from porcine juvenile muscles by discontinuous Percoll density gradient centrifugation. BMC Cell Biol. 2018, 19, 2. [Google Scholar] [CrossRef]
- ATCC. C2C12 (CRL-1772). Available online: https://www.atcc.org/products/crl-1772 (accessed on 22 March 2022).
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Stange, K.; Miersch, C.; Sponder, G.; Roentgen, M. Low birth weight influences the postnatal abundance and characteristics of satellite cell subpopulations in pigs. Sci. Rep. 2020, 10, 6149. [Google Scholar] [CrossRef]
- Murphy, K.C.; Hung, B.P.; Browne-Bourne, S.; Zhou, D.; Yeung, J.; Genetos, D.C.; Leach, J.K. Measurement of oxygen tension within mesenchymal stem cell spheroids. J. R. Soc. Interface 2017, 14, 20160851. [Google Scholar] [CrossRef]
- Shimomura, K.; Ando, W.; Fujie, H.; Hart, D.A.; Yoshikawa, H.; Nakamura, N. Scaffold-free tissue engineering for injured joint surface restoration. J. Exp. Orthop. 2018, 5, 2. [Google Scholar] [CrossRef]
- Vodicka, P.; Smetana, K., Jr.; Dvorankova, B.; Emerick, T.; Xu, Y.Z.; Ourednik, J.; Ourednik, V.; Motlik, J. The miniature pig as an animal model in biomedical research. Ann. N. Y. Acad. Sci. 2005, 1049, 161–171. [Google Scholar] [CrossRef]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabria, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, K.; Pietrzak, P.; Godlewski, M.M.; Piwowarski, J.; Kilianczyk, R.; Guilloteau, P.; Zabielski, R. Intrauterine growth retarded piglet as a model for humans--studies on the perinatal development of the gut structure and function. Reprod. Biol. 2014, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- OLS OMNI Life Science GmbH & Co. KG. Application Note: Generation of HepaRG Spheroids in the CERO; OLS OMNI Life Science GmbH & Co. KG: Bremen, Germany, 2019. [Google Scholar]
- Lei, Y.; Schaffer, D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, E5039–E5048. [Google Scholar] [CrossRef]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Chiu, C.P.; Webster, C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 1983, 32, 1171–1180. [Google Scholar] [CrossRef]
- Mau, M.; Oksbjerg, N.; Rehfeldt, C. Establishment and conditions for growth and differentiation of a myoblast cell line derived from the semimembranosus muscle of newborn piglets. In Vitro Cell Dev. Biol. Anim. 2008, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Miersch, C.; Stange, K.; Hering, S.; Kolisek, M.; Viergutz, T.; Rontgen, M. Molecular and functional heterogeneity of early postnatal porcine satellite cell populations is associated with bioenergetic profile. Sci. Rep. 2017, 7, 45052. [Google Scholar] [CrossRef]
- Furuichi, Y.; Kawabata, Y.; Aoki, M.; Mita, Y.; Fujii, N.L.; Manabe, Y. Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Front. Cell Dev. Biol. 2021, 9, 640399. [Google Scholar] [CrossRef]
- Nakai, N.; Kitai, S.; Iida, N.; Inoue, S.; Nakata, K.; Murakami, T.; Higashida, K. Induction of Autophagy and Changes in Cellular Metabolism in Glucose Starved C2C12 Myotubes. J. Nutr. Sci. Vitaminol. 2020, 66, 41–47. [Google Scholar] [CrossRef]
- Montarras, D.; L’Honore, A.; Buckingham, M. Lying low but ready for action: The quiescent muscle satellite cell. FEBS J. 2013, 280, 4036–4050. [Google Scholar] [CrossRef]
- Ochocki, J.D.; Simon, M.C. Nutrient-sensing pathways and metabolic regulation in stem cells. J. Cell Biol. 2013, 203, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.G.; Garcia-Aznar, J.M. Extracellular matrix density regulates the formation of tumour spheroids through cell migration. PLoS Comput. Biol. 2021, 17, e1008764. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix-What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef]
- Stephenson, M.; Grayson, W. Recent advances in bioreactors for cell-based therapies. F1000 Res. 2018, 7, 517. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Teoh, S.H.; Chong, W.S.; Foo, T.T.; Chng, Y.C.; Choolani, M.; Chan, J. A biaxial rotating bioreactor for the culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials 2009, 30, 2694–2704. [Google Scholar] [CrossRef]
- Mesires, N.T.; Doumit, M.E. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am. J. Physiol. Cell Physiol. 2002, 282, C899–C906. [Google Scholar] [CrossRef]
- Tanaka, K.; Sato, K.; Yoshida, T.; Fukuda, T.; Hanamura, K.; Kojima, N.; Shirao, T.; Yanagawa, T.; Watanabe, H. Evidence for cell density affecting C2C12 myogenesis: Possible regulation of myogenesis by cell-cell communication. Muscle Nerve 2011, 44, 968–977. [Google Scholar] [CrossRef] [PubMed]

| Myosphere Culture Method | Biomaterial | Species | Cell Type | Cultivation Duration | Reference(s) |
|---|---|---|---|---|---|
| Static suspension culture (culture flask/plate) | None | Mouse | C2C12 myoblast cell line | >20 days | [30] |
| 8 days | [31] | ||||
| Primary MDC | Several months | [27,29,33] | |||
| Human | Embryonic stem cell line D3 | 7 days | [40] | ||
| Primary MDC | Up to 5 months | [34] | |||
| 1–2 weeks | [35,36] | ||||
| Dog | Primary MDC | 14 days | [37] | ||
| Hydrogel (hyaluronic acid/fibrinogen) | Human | Vascular smooth muscle cell line (SMCs) | 14 days | [32] | |
| Hanging drop | None | Mouse | C2C12 myoblast cell line | 1 week | [41] |
| Mouse | Primary MDC | 1 week | [41] | ||
| Human | Embryonic stem cell line D3 | 7 days | [40] | ||
| Stirrer flask | Microcarrier (polystyrene or dextran) | Bovine | Primary MDC | 8 days | [38] |
| Rotary cell culture system | None | Mouse | C2C12 myoblast cell line | 8 days | [31] |
| Bioreactor | Cylindric sponge (hyaluron) | Rat | Primary MDC | Up to 25 days | [39] |
| C2C12 | PMCs | |||
|---|---|---|---|---|
| Inoculation Phase | Culture Phase | Inoculation Phase | Culture Phase | |
| Rotation period | 2 s | 1 s | 1 s | 1 s |
| Rotation speed | 80 rpm | 80 rpm | 80 rpm | 80 rpm |
| Rotation pause | 0 s | 0 s | 0 s | 2 s |
| Protocol duration | 12 h | 7 d | 6 h | 7 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stange, K.; Keric, A.; Friese, A.; Röntgen, M. Preparation of Spheroids from Primary Pig Cells in a Mid-Scale Bioreactor Retaining Their Myogenic Potential. Cells 2022, 11, 1453. https://doi.org/10.3390/cells11091453
Stange K, Keric A, Friese A, Röntgen M. Preparation of Spheroids from Primary Pig Cells in a Mid-Scale Bioreactor Retaining Their Myogenic Potential. Cells. 2022; 11(9):1453. https://doi.org/10.3390/cells11091453
Chicago/Turabian StyleStange, Katja, Amir Keric, Andreas Friese, and Monika Röntgen. 2022. "Preparation of Spheroids from Primary Pig Cells in a Mid-Scale Bioreactor Retaining Their Myogenic Potential" Cells 11, no. 9: 1453. https://doi.org/10.3390/cells11091453
APA StyleStange, K., Keric, A., Friese, A., & Röntgen, M. (2022). Preparation of Spheroids from Primary Pig Cells in a Mid-Scale Bioreactor Retaining Their Myogenic Potential. Cells, 11(9), 1453. https://doi.org/10.3390/cells11091453