Thymoquinone-Mediated Modulation of Toll-like Receptors and Pluripotency Factors in Gingival Mesenchymal Stem/Progenitor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. G-MSCs’ Isolation and Culture
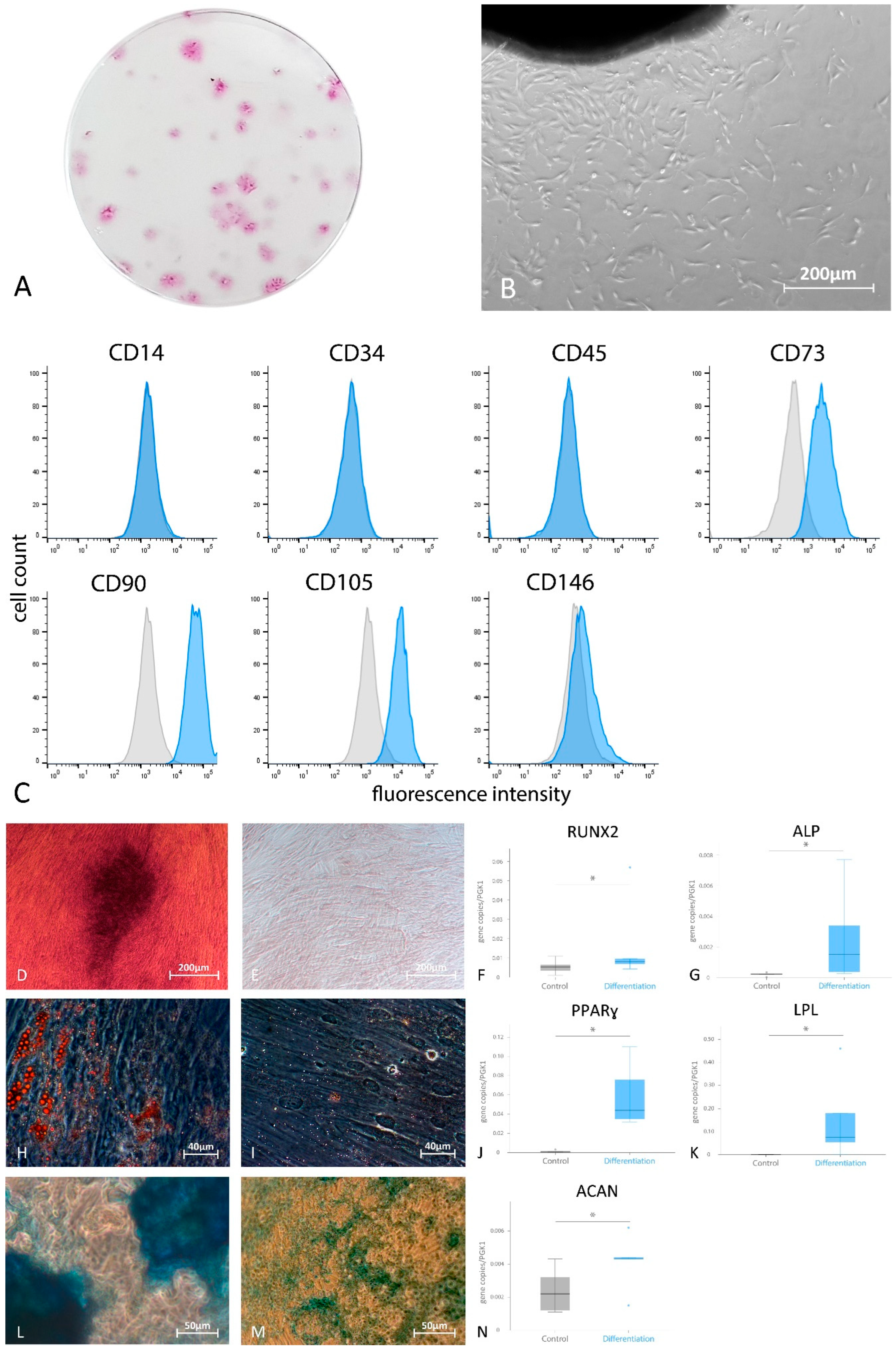
2.2. Colony-Forming Units (CFUs)
2.3. Flowcytometric Analysis of Surface MSC Markers
2.4. Multilineage Differentiation of G-MSCs
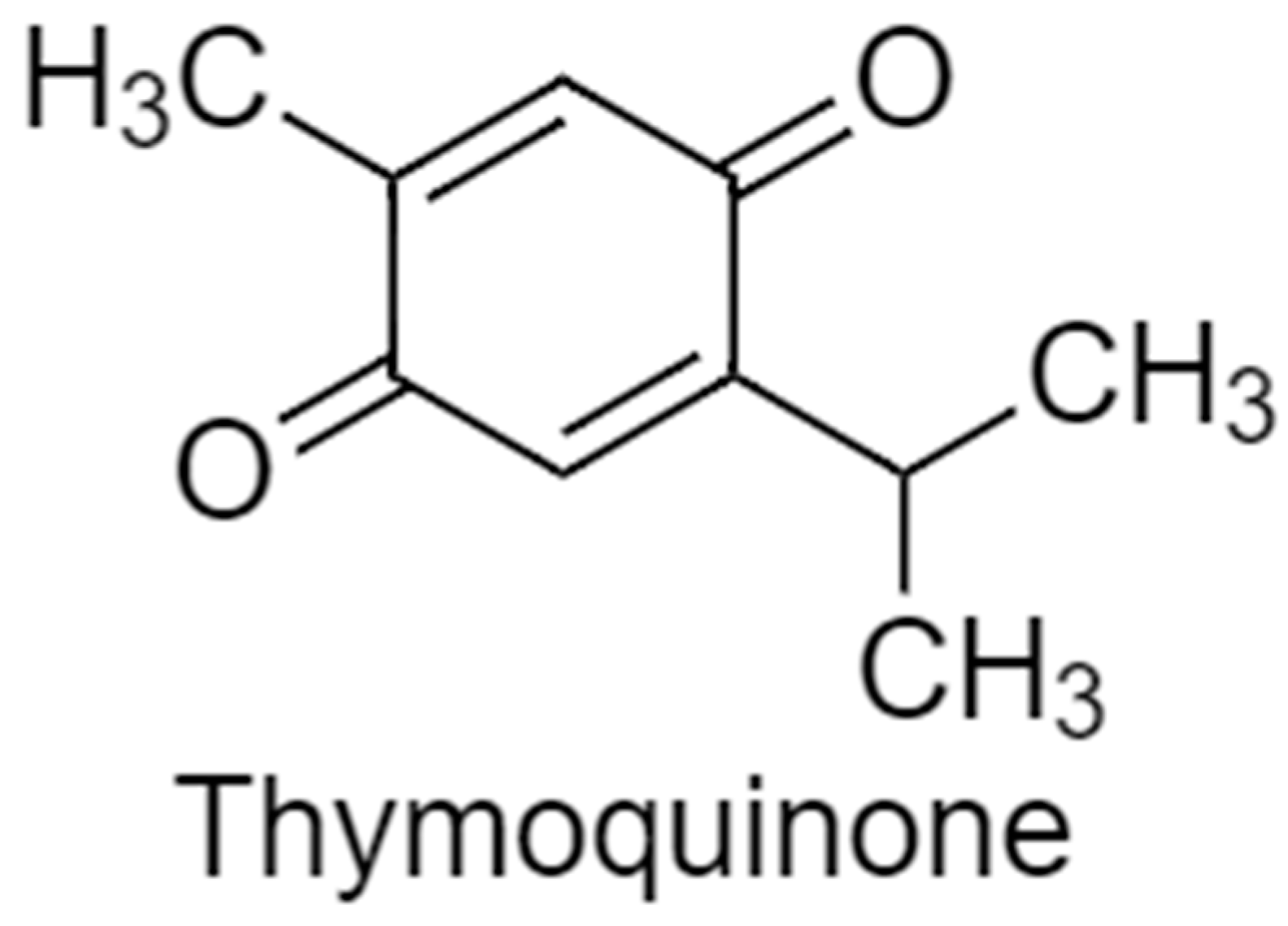
2.5. Thymoquinone Stimulation
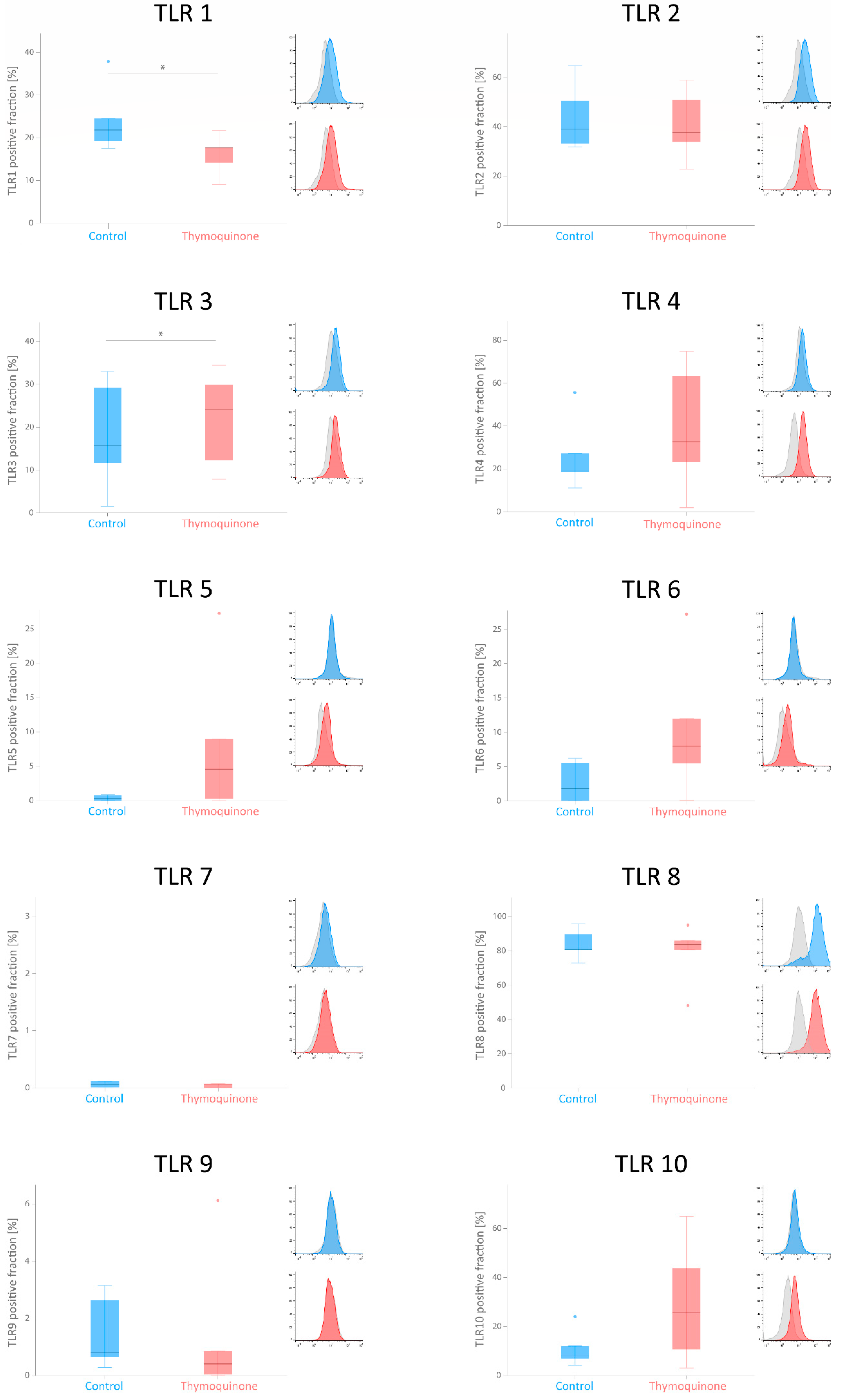
2.6. Flowcytometric Analysis of TLRs in G-MSCs after TQ Stimulation
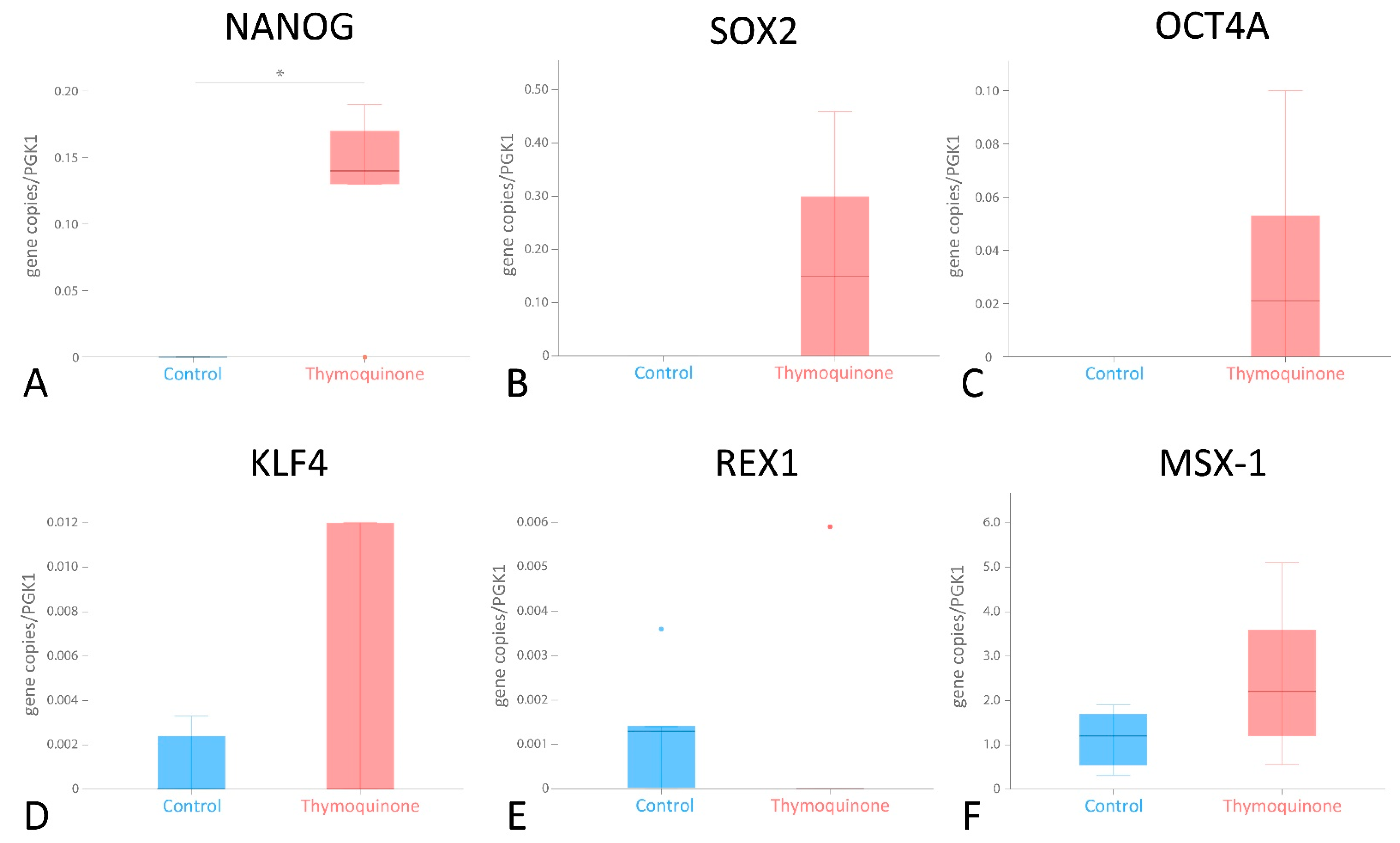
2.7. Differential mRNA Expression of Pluripotency-Associated Genes after TQ Stimulation
2.8. Statistical Analysis
3. Results
3.1. Phase Contrast Inverted Microscopy, CFUs and Analysis of Mesenchymal Stem Cells’ Predefined Surface Markers Using Flow Cytometry
3.2. Multilineage Differentiation Potential of G-MSCs
3.3. Flowcytometric Analysis of TLRs in G-MSCs after Thymoquinone Stimulation
3.4. Differential mRNA Expression of Pluripotency-Associated Genes in G-MSCs following TQ Stimulation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekhemar, M.; Tolle, J.; Dorfer, C.; Fawzy El-Sayed, K. Gingival Mesenchymal Stem/Progenitor Cells. J. Clin. Periodontol. 2020, 47, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dorfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef] [PubMed]
- Mekhemar, M.K.; Adam-Klages, S.; Kabelitz, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. TLR-induced immunomodulatory cytokine expression by human gingival stem/progenitor cells. Cell Immunol. 2018, 326, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.; Graetz, C.; Kohnlein, T.; Mekhemar, M.; Dorfer, C. Effect of total sonicated Aggregatibacter actinomycetemcomitans fragments on gingival stem/progenitor cells. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e569. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Paris, S.; Becker, S.T.; Neuschl, M.; De Buhr, W.; Salzer, S.; Wulff, A.; Elrefai, M.; Darhous, M.S.; El-Masry, M.; et al. Periodontal regeneration employing gingival margin-derived stem/progenitor cells: An animal study. J. Clin. Periodontol. 2012, 39, 861–870. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Elahmady, M.; Adawi, Z.; Aboushadi, N.; Elnaggar, A.; Eid, M.; Hamdy, N.; Sanaa, D.; Dorfer, C.E. The periodontal stem/progenitor cell inflammatory-regenerative cross talk: A new perspective. J. Periodontal. Res. 2019, 54, 81–94. [Google Scholar] [CrossRef]
- Fawzy-El-Sayed, K.; Mekhemar, M.; Adam-Klages, S.; Kabelitz, D.; Dorfer, C. TlR expression profile of human gingival margin-derived stem progenitor cells. Med. Oral Patol. Oral Cir. Buccal 2016, 21, e30. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Boeckler, J.; Dörfer, C.E. TLR expression profile of human alveolar bone proper-derived stem/progenitor cells and osteoblasts. J. Craniomaxillofac. Surg. 2017, 45, 2054–2060. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Klingebiel, P.; Dörfer, C.E. Toll-like Receptor Expression Profile of Human Dental Pulp Stem/Progenitor Cells. J. Endod. 2016, 42, 413–417. [Google Scholar] [CrossRef]
- Zhou, L.; Dorfer, C.E.; Chen, L.; Fawzy El-Sayed, K.M. Porphyromonas gingivalis lipopolysaccharides affect gingival stem/progenitor cells attributes through NF-kappaB, but not Wnt/beta-catenin, pathway. J. Clin. Periodontol. 2017, 44, 1112–1122. [Google Scholar] [CrossRef]
- Mekhemar, M.; Hassan, Y.; Dörfer, C. Nigella sativa and Thymoquinone: A Natural Blessing for Periodontal Therapy. Antioxidants 2020, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- WHO. Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013; 76p. [Google Scholar]
- Udalamaththa, V.L.; Jayasinghe, C.D.; Udagama, P.V. Potential role of herbal remedies in stem cell therapy: Proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.C.; Siegel, D. Directing Stem Cell Fate: The Synthetic Natural Product Connection. Chem. Rev. 2017, 117, 12052–12086. [Google Scholar] [CrossRef] [PubMed]
- Mekhemar, M.; Geib, M.; Kumar, M.; Radha Hassan, Y.; Dorfer, C. Salvadora persica: Nature’s Gift for Periodontal Health. Antioxidants 2021, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Alimoradi, E.; Sisakhtnezhad, S.; Akrami, H. Thymoquinone influences the expression of genes involved in self-renewal and immunomodulatory potential of mouse bone marrow-derived mesenchymal stem cells in vitro. Environ. Toxicol. Pharmacol. 2018, 60, 216–224. [Google Scholar] [CrossRef]
- Rezaei, N.; Sardarzadeh, T.; Sisakhtnezhad, S. Thymoquinone promotes mouse mesenchymal stem cells migration in vitro and induces their immunogenicity in vivo. Toxicol. Appl. Pharmacol. 2020, 387, 114851. [Google Scholar] [CrossRef]
- Arslan, A.H.; Tomruk, C.Ö.; Meydanlı, E.G.; Özdemir, İ.; Duygu Çapar, G.; Kütan, E.; Yilmaz, A.; Ülker, G.M.Y. Histopathological evaluation of the effect of systemic thymoquinone administration on healing of bone defects in rat tibia. Biotechnol. Biotechnol. Equip. 2017, 31, 175–181. [Google Scholar] [CrossRef]
- Radwan, R.R.; Mohamed, H.A. Nigella sativa oil modulates the therapeutic efficacy of mesenchymal stem cells against liver injury in irradiated rats. J. Photochem. Photobiol. B Biol. 2018, 178, 447–456. [Google Scholar] [CrossRef]
- El-Sayed, K.M.; Paris, S.; Graetz, C.; Kassem, N.; Mekhemar, M.; Ungefroren, H.; Fandrich, F.; Dorfer, C. Isolation and characterisation of human gingival margin-derived STRO-1/MACS(+) and MACS(-) cell populations. Int. J. Oral Sci. 2015, 7, 80–88. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Delarosa, O.; Dalemans, W.; Lombardo, E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Hans, M.; Hans, V.M. Toll-like receptors and their dual role in periodontitis: A review. J. Oral Sci. 2011, 53, 263–271. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front Immunol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by Mesenchymal Stem Cells: Biological Aspects and Clinical Applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef]
- Najar, M.; Krayem, M.; Meuleman, N.; Bron, D.; Lagneaux, L. Mesenchymal Stromal Cells and Toll-Like Receptor Priming: A Critical Review. Immune Netw. 2017, 17, 89–102. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, D.; Pu, D.; Wang, Y.; Li, L.; He, Y.; Li, Y.; Li, L.; Qiu, Z.; Zhao, S.; et al. The role of Toll-like receptor 3 and 4 in regulating the function of mesenchymal stem cells isolated from umbilical cord. Int. J. Mol. Med. 2015, 35, 1003–1010. [Google Scholar] [CrossRef][Green Version]
- Kadle, R.L.; Abdou, S.A.; Villarreal-Ponce, A.P.; Soares, M.A.; Sultan, D.L.; David, J.A.; Massie, J.; Rifkin, W.J.; Rabbani, P.; Ceradini, D.J. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS ONE. 2018, 13, e0193178. [Google Scholar] [CrossRef]
- Nembo, E.N.; Hescheler, J.; Nguemo, F. Stem cells in natural product and medicinal plant drug discovery—An overview of new screening approaches. Biomed. Pharmacother. 2020, 131, 110730. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ugawa, Y.; Kawamura, M.; Yamashiro, K.; Kochi, S.; Ideguchi, H.; Takashiba, S. Modulation of microenvironment for controlling the fate of periodontal ligament cells: The role of Rho/ROCK signaling and cytoskeletal dynamics. J. Cell Commun. Signal. 2018, 12, 369–378. [Google Scholar] [CrossRef]
- Rahman, M.A.; Saha, S.K.; Rahman, M.S.; Uddin, M.J.; Uddin, M.S.; Pang, M.G.; Rhim, H.; Cho, S.-G. Molecular Insights into Therapeutic Potential of Autophagy Modulation by Natural Products for Cancer Stem Cells. Front. Cell. Dev. Biol. 2020, 8, 283. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Alternat. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef]
- Sallehuddin, N.; Nordin, A.; Bt Hj Idrus, R.; Fauzi, M.B. Nigella sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review. Int. J. Environ. Res. Public Health 2020, 17, 4160. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Hein, D.; Dörfer, C.E. Retinol/inflammation affect stemness and differentiation potential of gingival stem/progenitor cells via Wnt/β-catenin. J. Periodontal. Res. 2019, 54, 413–423. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Bittner, A.; Schlicht, K.; Mekhemar, M.; Enthammer, K.; Höppner, M.; Es-Souni, M.; Schulz, J.; Laudes, M.; Graetz, C.; et al. Ascorbic Acid/Retinol and/or Inflammatory Stimuli’s Effect on Proliferation/Differentiation Properties and Transcriptomics of Gingival Stem/Progenitor Cells. Cells 2021, 10, 3310. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Nguyen, N.; Dorfer, C.E. Ascorbic Acid, Inflammatory Cytokines (IL-1beta/TNF-alpha/IFN-gamma), or Their Combination’s Effect on Stemness, Proliferation, and Differentiation of Gingival Mesenchymal Stem/Progenitor Cells. Stem Cells Int. 2020, 2020, 8897138. [Google Scholar] [CrossRef]
- Arjumand, S.; Shahzad, M.; Shabbir, A.; Yousaf, M.Z. Thymoquinone attenuates rheumatoid arthritis by downregulating TLR2, TLR4, TNF-α, IL-1, and NFκB expression levels. Biomed. Pharmacother. 2019, 111, 958–963. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Kalamegam, G.; Alfakeeh, S.M.; Bahmaid, A.O.; AlHuwait, E.A.; Gari, M.A.; Abbas, M.M.; Ahmed, F.; Abu-Elmagd, M.; Pushparaj, P.N. In Vitro Evaluation of the Anti-inflammatory Effects of Thymoquinone in Osteoarthritis and In Silico Analysis of Inter-Related Pathways in Age-Related Degenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 646. [Google Scholar] [CrossRef]
- Aziz, N.; Son, Y.-J.; Cho, J.Y. Thymoquinone Suppresses IRF-3-Mediated Expression of Type I Interferons via Suppression of TBK1. Int. J. Mol. Sci. 2018, 19, 1355. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Zhao, B.; Li, S.; Zhang, Y.; Pan, S.; Wu, Y.; Wang, J.; Wang, D.; Pan, H.; et al. Thymoquinone induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-κB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol. Rep. 2014, 31, 2063–2070. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.L.; Chen, L.J.; Zhou, T.; Huang, W.K.; Zhou, X.; Shao, L.Q. The role of Toll-like receptors in periodontitis. Oral Dis. 2017, 23, 168–180. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Qi, W.; Yang, X.; Ye, N.; Li, S.; Han, Q.; Huang, J.; Wu, B. TLR4 gene in the regulation of periodontitis and its molecular mechanism. Exp. Ther. Med. 2019, 18, 1961–1966. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, Y.; He, T.; Yang, T.T.; Wu, J.; Cianflone, K.; Lu, H.-L. Anaphylatoxin C5a induces inflammation and reduces insulin sensitivity by activating TLR4/NF-kB/PI3K signaling pathway in 3T3-L1 adipocytes. Biomed. Pharmacother. 2018, 103, 955–964. [Google Scholar] [CrossRef]
- Mori, K.; Yanagita, M.; Hasegawa, S.; Kubota, M.; Yamashita, M.; Yamada, S.; Kitamura, M.; Murakami, S. Necrosis-induced TLR3 Activation Promotes TLR2 Expression in Gingival Cells. J. Dent. Res. 2015, 94, 1149–1157. [Google Scholar] [CrossRef]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000 2020, 82, 93–114. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Zhou, Y.; Li, W. TLR activation inhibits the osteogenic potential of human periodontal ligament stem cells through Akt signaling in a Myd88- or TRIF-dependent manner. J. Periodontol. 2019, 90, 400–415. [Google Scholar] [CrossRef]
- Alvarez, R.; Lee, H.-L.; Wang, C.-Y.; Hong, C. Characterization of the osteogenic potential of mesenchymal stem cells from human periodontal ligament based on cell surface markers. Int. J. Oral Sci. 2015, 7, 213–219. [Google Scholar] [CrossRef]
- Chaikeawkaew, D.; Everts, V.; Pavasant, P. TLR3 activation modulates immunomodulatory properties of human periodontal ligament cells. J. Periodontol. 2020, 91, 1225–1236. [Google Scholar] [CrossRef]
- Tsai, C.C.; Hung, S.C. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle 2012, 11, 3711–3712. [Google Scholar] [CrossRef]
- Hawkins, K.; Joy, S.; McKay, T. Cell signalling pathways underlying induced pluripotent stem cell reprogramming. World J. Stem Cells 2014, 6, 620–628. [Google Scholar] [CrossRef]
- Weidgang, C.E.; Seufferlein, T.; Kleger, A.; Mueller, M. Pluripotency Factors on Their Lineage Move. Stem Cells Int. 2016, 2016, 6838253. [Google Scholar] [CrossRef]
- Noronha, N.d.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Canaider, S.; Pizzuti, V.; Pampanella, L.; Casadei, R.; Facchin, F.; Ventura, C. Herb-Derived Products: Natural Tools to Delay and Counteract Stem Cell Senescence. Stem Cells Int. 2020, 2020, 8827038. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Castelo-Branco, P.; Custódio, L. Marine Natural Products as a Promising Source of Therapeutic Compounds to Target Cancer Stem Cells. Curr. Med. Chem. 2021, 28, 4343–4355. [Google Scholar] [CrossRef]
- Fatfat, Z.; Fatfat, M.; Gali-Muhtasib, H. Therapeutic potential of thymoquinone in combination therapy against cancer and cancer stem cells. World J. Clin. Oncol. 2021, 12, 522–543. [Google Scholar] [CrossRef]
- Yu, P.; Nie, Q.; Tang, C.; Zhang, L. Nanog induced intermediate state in regulating stem cell differentiation and reprogramming. BMC Syst. Biol. 2018, 12, 22. [Google Scholar] [CrossRef]
- Chen, L.; Tong, Q.; Chen, X.; Jiang, P.; Yu, H.; Zhao, Q.; Sun, L.; Liu, C.; Gu, B.; Zheng, Y.; et al. PHC1 maintains pluripotency by organizing genome-wide chromatin interactions of the Nanog locus. Nat. Commun. 2021, 12, 2829. [Google Scholar] [CrossRef]
- Liou, Y.F.; Chen, P.N.; Chu, S.C.; Kao, S.H.; Chang, Y.Z.; Hsieh, Y.S.; Chang, H.-R. Thymoquinone suppresses the proliferation of renal cell carcinoma cells via reactive oxygen species-induced apoptosis and reduces cell stemness. Environ. Toxicol. 2019, 34, 1208–1220. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Upadhyay, P.; Sarker, S.; Basu, A.; Das, S.; Ghosh, A.; Ghosh, S.; Adhikary, A. Combinatorial therapy of Thymoquinone and Emodin synergistically enhances apoptosis, attenuates cell migration and reduces stemness efficiently in breast cancer. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129695. [Google Scholar] [CrossRef]
- Chang, C.C.; Li, H.H.; Tsou, S.H.; Hung, H.C.; Liu, G.Y.; Korolenko, T.A.; Lai, T.-J.; Ho, Y.-J.; Lin, C.-L. The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration. Cells 2020, 9, 1339. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 27–53. [Google Scholar] [CrossRef] [PubMed]
| Donor | Age | Gender |
|---|---|---|
| 1 | 26 | Female |
| 2 | 24 | Female |
| 3 | 41 | Female |
| 4 | 87 | Male |
| 5 | 23 | Male |
| Assay ID | Gene Symbol | Accession ID |
|---|---|---|
| 113380 | RUNX2 Homo sapiens | ENST00000359524 |
| 110607 | PPARɣ Homo sapiens | ENST00000287820 |
| 103448 | ALP Homo sapiens | ENST00000374840 |
| 138057 | ACAN Homo sapiens | ENST00000439576 |
| 113230 | LPL Homo sapiens | ENST00000311322 |
| 148147 | Nanog Homo sapiens | ENST00000229307 |
| 137021 | MSX1Homo sapiens | ENST00000382723 |
| 111867 | Sox2 Homo sapiens | ENST00000325404 |
| 125775 | KLF4Homo sapiens | ENST00000358094 |
| 122584 | REXO1Homo sapiens | ENST00000170168 |
| 113034 | Oct4A Homo sapiens | ENST00000259915 |
| 102083 | PGK1 Homo sapiens | ENST00000373316 |
| Toll-like Receptor | Unstimulated G-MSCs (Control) | 1 µg/mL TQ-Stimulated G-MSCs | p-Value |
|---|---|---|---|
| TLR 1 | 21.8163, 18.3802/31.1601 | 17.6136, 11.6273/19.6647 | 0.043 |
| TLR 2 | 39.0383, 32.4885/57.5765 | 37.6595, 28.2930/54.8602 | >0.05 |
| TLR 3 | 15.7353, 6.6038/31.1184 | 24.1686, 10.0862/32.1546 | 0.043 |
| TLR 4 | 18.9917, 14.8641/42.0839 | 32.6764, 12.5569/69.0827 | >0.05 |
| TLR 5 | 0.3003, 0.0033/0.8241 | 4.8510, 0.1363/18.1387 | >0.05 |
| TLR 6 | 1.8360, 0.0330/5.8871 | 8.0203, 2.7385/19.6100 | >0.05 |
| TLR 7 | 0.0575, 0. 0067/8.6946 | 0.0656, 0.0000/1.9158 | >0.05 |
| TLR 8 | 80.8476, 76.8108/92.7699 | 83.7606, 64.4336/90.5349 | >0.05 |
| TLR 9 | 0.8045, 0.4643/2.8881 | 0.4037, 0.0160/3.4859 | >0.05 |
| TLR 10 | 7.9431, 5.5215/18.0287 | 25.6538, 6.8429/54.3215 | >0.05 |
| Stemness Transcriptional Factor | Unstimulated G-MSCs (Control) | 1 µg/mL TQ-Stimulated G-MSCs | p-Value |
|---|---|---|---|
| NANOG | No expression | 0.1400, 0.0650/0.1800 | 0.043 |
| SOX2 | No expression | 0.1500, 0.0004/0.3800 | >0.05 |
| OCT4 | No expression | 0.0210, 0.0000/0.0765 | >0.05 |
| MSX1 | 1.2000, 0.4200/1.8000 | 2.2000, 0.8750/4.3500 | >0.05 |
| REX1 | 0.0013, 0.0025/0.0000 | 0.0000, 0.0000/0.0030 | >0.05 |
| KLF4 | 0.0000, 0.0000/0.0029 | 0.0000, 0.0000/0.0120 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekhemar, M.; Tölle, J.; Hassan, Y.; Dörfer, C.; El-Sayed, K.F. Thymoquinone-Mediated Modulation of Toll-like Receptors and Pluripotency Factors in Gingival Mesenchymal Stem/Progenitor Cells. Cells 2022, 11, 1452. https://doi.org/10.3390/cells11091452
Mekhemar M, Tölle J, Hassan Y, Dörfer C, El-Sayed KF. Thymoquinone-Mediated Modulation of Toll-like Receptors and Pluripotency Factors in Gingival Mesenchymal Stem/Progenitor Cells. Cells. 2022; 11(9):1452. https://doi.org/10.3390/cells11091452
Chicago/Turabian StyleMekhemar, Mohamed, Johannes Tölle, Yasmine Hassan, Christof Dörfer, and Karim Fawzy El-Sayed. 2022. "Thymoquinone-Mediated Modulation of Toll-like Receptors and Pluripotency Factors in Gingival Mesenchymal Stem/Progenitor Cells" Cells 11, no. 9: 1452. https://doi.org/10.3390/cells11091452
APA StyleMekhemar, M., Tölle, J., Hassan, Y., Dörfer, C., & El-Sayed, K. F. (2022). Thymoquinone-Mediated Modulation of Toll-like Receptors and Pluripotency Factors in Gingival Mesenchymal Stem/Progenitor Cells. Cells, 11(9), 1452. https://doi.org/10.3390/cells11091452









