Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review
Abstract
1. Introduction
1.1. Depression and Gut Microbiota
1.2. The Pathological Role of Gut Dysbiosis in Depression
1.3. Objectives
2. Gut Microbiota
2.1. The Composition, Dynamics, and Functions of Gut Microbiota
2.2. Microbial-Derived Metabolites and Their Physiological Functions
2.3. Gut–Brain Axis or Gut–Microbiota–Brain Axis
3. The Pathological Mechanisms Underlying GD-Associated Depression
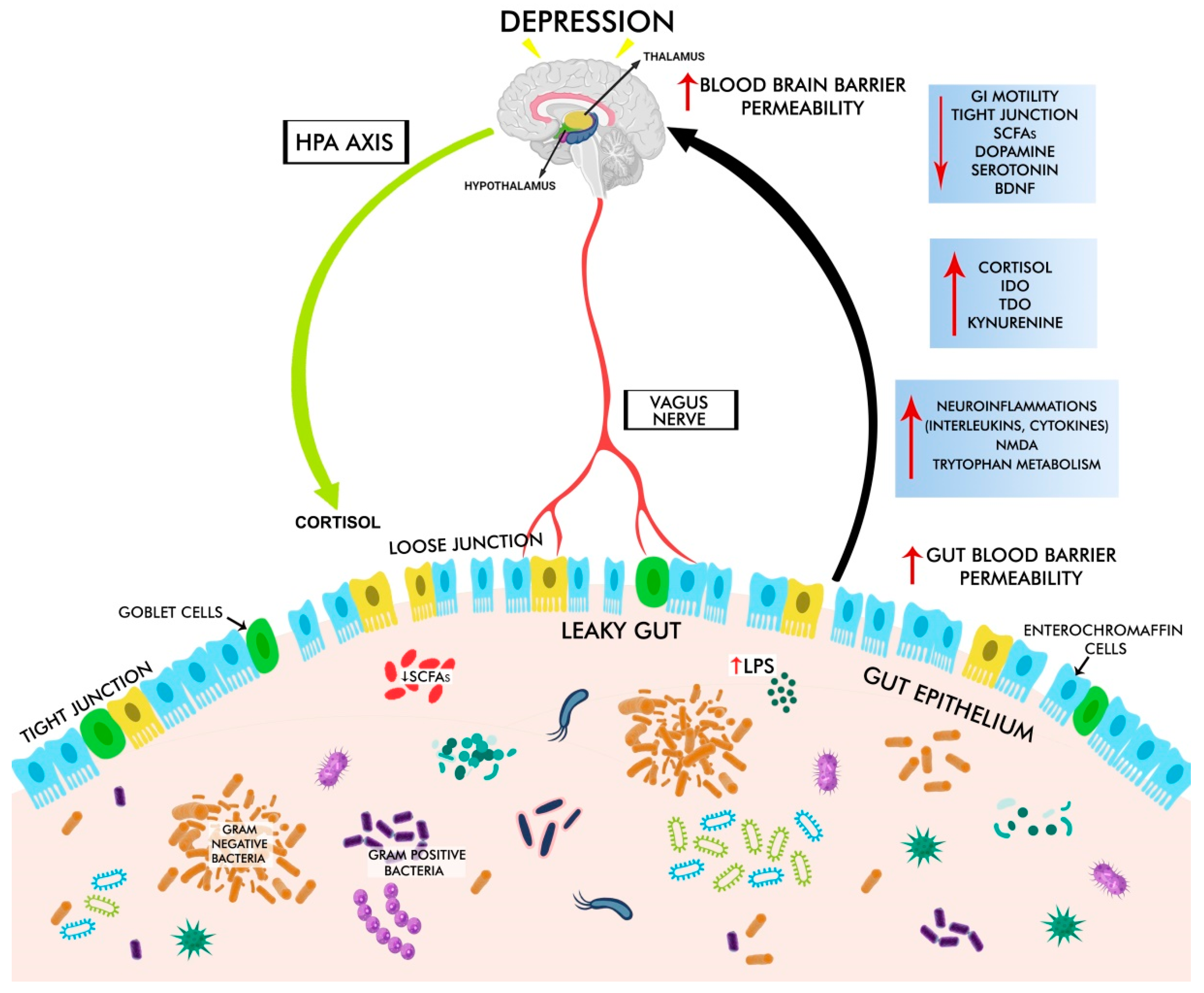
3.1. Gut Dysbiosis in Depression
3.2. GBA Dysregulation in Depression
3.3. HPA Dysregulation in Depression
3.3.1. Stress-Induced Activation of HPA
3.3.2. LPS- and Peptidoglycan (Derived from Pathogens)-Induced Activation of HPA
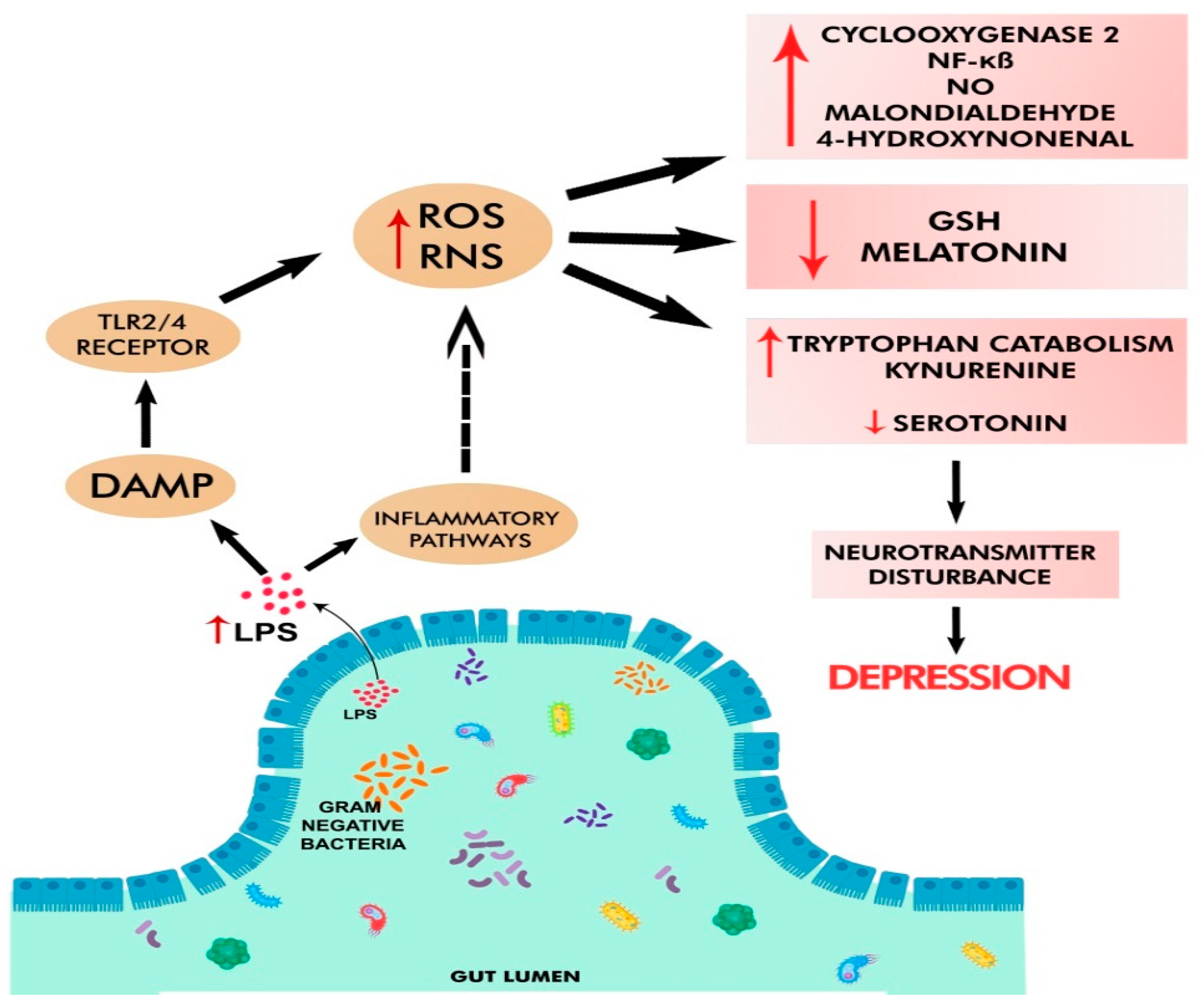
3.4. Chronic Oxidative and Nitrosative Stress in Depression
3.5. Altered Metabolism of Serotonin and Tryptophan in Depression
3.6. Altered Metabolism of Homocysteine in Depression
3.7. Neuroinflammation in Depression
4. Alterations in the Gut Microbial Abundance in Depression
4.1. Preclinical Evidence Using Germ-Free and Specific-Pathogen-Free Animal Models
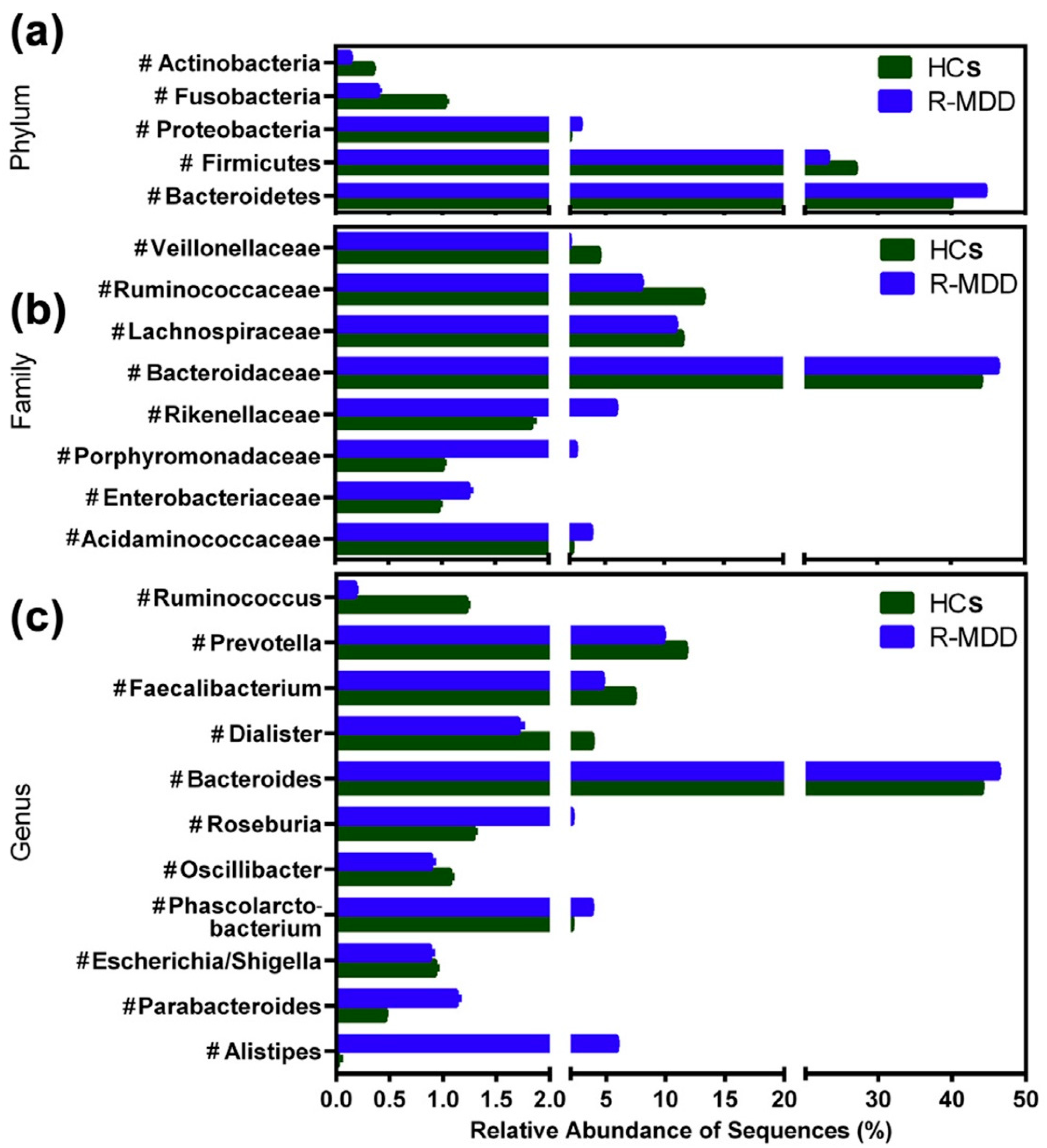
4.2. Clinical Evidence
5. The Alternative Strategies Targeting Gut Dysbiosis-Associated Depression
5.1. Probiotics
5.2. Prebiotics
5.3. Postbiotics
5.4. Synbiotics
5.5. Polyphenols
5.6. Diet Modifications
5.7. Fecal Microbiota Transplantation (FMT)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| BDNF | Brain-Derived Neurotrophic Factor |
| CNS | Central Nervous System |
| ENS | Enteric Nervous System |
| EEC | Enteroendocrine Cells |
| FMT | Fecal Microbial Transplantation |
| GABA | Gamma Aminobutyric Acid |
| GD | Gut Dysbiosis |
| GF | Germ Free |
| GBA | Gut–Brain Axis |
| GI | Gastrointestinal |
| GM | Gut microbiota |
| HPA | Hypothalamus–Pituitary–Adrenal Axis |
| IFN-γ | Interferon-γ |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IBS | Inflammatory Bowel Syndrome |
| IRS | Inflammatory Response System |
| JB-1 | Lactobacillus Rhamnosus |
| LPS | Lipopolysaccharide |
| MAMPs | Microbe Associated With Molecular Patterns |
| MDD | Major Depressive Disorder |
| NOD | Nucleotide-Binding Oligomerization Domain-Containing Protein |
| SCFAs | Short-Chain Fatty Acids |
| TLRs | Toll-like Receptors |
| TNF-α | Tumor Necrosis Factor-A |
References
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Patten, S.B.; Vos, T.; Whiteford, H.A. The Epidemiological Modelling of Major Depressive Disorder: Application for the Global Burden of Disease Study 2010. PLoS ONE 2013, 8, e69637. [Google Scholar] [CrossRef]
- Cao, C.; Liu, M.; Qu, S.; Huang, R.; Qi, M.; Zhu, Z.; Zheng, J.; Chen, Z.; Wang, Z.; Han, Z.; et al. Chinese Medicine Formula Kai-Xin-San Ameliorates Depression-like Behaviours in Chronic Unpredictable Mild Stressed Mice by Regulating Gut Microbiota-Inflammation-Stress System. J. Ethnopharmacol. 2020, 261, 113055. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R. Why Is Depression More Prevalent in Women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed]
- Little, A. Treatment-Resistant Depression. Am. Fam. Physician 2009, 80, 167–172. [Google Scholar] [PubMed]
- Vahia, V.N. Diagnostic and Statistical Manual of Mental Disorders 5: A Quick Glance. Indian J. Psychiatry 2013, 55, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Srivastava, M.; Tiwary, N.K.; Kumar, A. Prevalence of Depression and Anxiety among Children in Rural and Suburban Areas of Eastern Uttar Pradesh: A Cross-Sectional Study. J. Fam. Med. Prim. Care 2018, 7, 21–26. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut Dysbiosis, Defective Autophagy and Altered Immune Responses in Neurodegenerative Diseases: Tales of a Vicious Cycle. Pharmacol. Ther. 2021, 231, 107988. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Karen, C.; Shyu, D.J.H.; Rajan, K.E. Lactobacillus Paracasei Supplementation Prevents Early Life Stress-Induced Anxiety and Depressive-Like Behavior in Maternal Separation Model-Possible Involvement of Microbiota-Gut-Brain Axis in Differential Regulation of MicroRNA124a/132 and Glutamate Receptors. Front. Neurosci. 2021, 15, 719933. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, N.; Zeng, Y.; Bao, M.; Chen, Y.; Liao, J.; Wei, L.; Cao, D.; Huang, S.; Luo, Q.; et al. The Endogenous Alterations of the Gut Microbiota and Feces Metabolites Alleviate Oxidative Damage in the Brain of LanCL1 Knockout Mice. Front. Microbiol. 2020, 11, 557342. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Q.; Jiang, S.; Xu, Z.; Jiang, Y.; Liu, L.; Jiang, J.; Tong, Y.; Wang, P. Crocetin Ameliorates Chronic Restraint Stress-Induced Depression-like Behaviors in Mice by Regulating MEK/ERK Pathways and Gut Microbiota. J. Ethnopharmacol. 2021, 268, 113608. [Google Scholar] [CrossRef] [PubMed]
- Marcondes Ávila, P.R.; Fiorot, M.; Michels, M.; Dominguini, D.; Abatti, M.; Vieira, A.; de Moura, A.B.; Behenck, J.P.; Borba, L.A.; Botelho, M.E.M.; et al. Effects of Microbiota Transplantation and the Role of the Vagus Nerve in Gut–Brain Axis in Animals Subjected to Chronic Mild Stress. J. Affect. Disord. 2020, 277, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Han, S.-K.; Joo, M.-K.; Kim, D.-H. Buspirone Alleviates Anxiety, Depression, and Colitis; and Modulates Gut Microbiota in Mice. Sci. Rep. 2021, 11, 6094. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-W.; Kim, J.-K.; Lee, K.-E.; Oh, Y.J.; Choi, H.-J.; Han, M.J.; Kim, D.-H. A Probiotic Lactobacillus Gasseri Alleviates Escherichia Coli-Induced Cognitive Impairment and Depression in Mice by Regulating IL-1β Expression and Gut Microbiota. Nutrients 2020, 12, 3441. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Moylan, S.; Berk, M.; Dean, O.M.; Samuni, Y.; Williams, L.J.; O’Neil, A.; Hayley, A.C.; Pasco, J.A.; Anderson, G.; Jacka, F.N.; et al. Oxidative & Nitrosative Stress in Depression: Why so Much Stress? Neurosci. Biobehav. Rev. 2014, 45, 46–62. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C. The Gut-Brain Barrier in Major Depression: Intestinal Mucosal Dysfunction with an Increased Translocation of LPS from Gram Negative Enterobacteria (Leaky Gut) Plays a Role in the Inflammatory Pathophysiology of Depression. Neuro Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar] [PubMed]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of Microbiota on Central Nervous System and Neurological Diseases: The Gut-Brain Axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Rathipriya, A.; AM, M.; Sharma, S.; Hediyal, T.; Ray, B.; Sunanda, T.; Rungratanawanich, W.; Kashyap, R.; Qoronfleh, M.; et al. The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells 2022, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Shu, R.; Wu, C.; Tong, Y.; Xiong, Z.; Zhou, J.; Yu, C.; Xie, X.; Fu, Z. Crocin-I Alleviates the Depression-like Behaviors Probably via Modulating “Microbiota-Gut-Brain” Axis in Mice Exposed to Chronic Restraint Stress. J. Affect. Disord. 2020, 276, 476–486. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Guan, N.L.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut Microbiota Fermentation of Prebiotics Increases Satietogenic and Incretin Gut Peptide Production with Consequences for Appetite Sensation and Glucose Response after a Meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.; Abe, F.; Osawa, R. Age-Related Changes in Gut Microbiota Composition from Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Yu, D.-H.; Gadkari, M.; Zhou, Q.; Yu, S.; Gao, N.; Guan, Y.; Schady, D.; Roshan, T.N.; Chen, M.-H.; Laritsky, E.; et al. Postnatal Epigenetic Regulation of Intestinal Stem Cells Requires DNA Methylation and Is Guided by the Microbiome. Genome Biol. 2015, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Kerr, C.; Murphy, K.; O’Sullivan, O.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.P.; O’Toole, P.W.; Cotter, P.D.; Fitzgerald, G.F.; et al. The Individual-Specific and Diverse Nature of the Preterm Infant Microbiota. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F334–F340. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The Regulation of Intestinal Mucin MUC2 Expression by Short-Chain Fatty Acids: Implications for Epithelial Protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Konturek, J.W.; Pawlik, T.; Brzozowski, T. Brain-Gut Axis and Its Role in the Control of Food Intake. J. Physiol. Pharmacol. 2004, 55, 137–154. [Google Scholar]
- Nell, S.; Suerbaum, S.; Josenhans, C. The Impact of the Microbiota on the Pathogenesis of IBD: Lessons from Mouse Infection Models. Nat. Rev. Microbiol. 2010, 8, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty Acid Transport Protein Expression in Human Brain and Potential Role in Fatty Acid Transport across Human Brain Microvessel Endothelial Cells: Fatty Acid Transport Protein Expression in Human Brain. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a Psychobiotic Therapy for Depression: Bifidobacterium breve CCFM1025 Reverses Chronic Stress-Induced Depressive Symptoms and Gut Microbial Abnormalities in Mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.-J.; Bravo, D.M.; Callaghan, B. The Gut as a Sensory Organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-J.; Li, J.-N.; Nie, Y.-Z. Gut Hormones in Microbiota-Gut-Brain Cross-Talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Akita, T.; Kimura, R.; Akaguma, S.; Nagai, M.; Nakao, Y.; Tsugane, M.; Suzuki, H.; Oka, J.; Yamashita, C. Usefulness of Cell-Penetrating Peptides and Penetration Accelerating Sequence for Nose-to-Brain Delivery of Glucagon-like Peptide-2. J. Control. Release 2021, 335, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Myers, B.; Herman, J.P. Role of Central Glucagon-like Peptide-1 in Stress Regulation. Physiol. Behav. 2013, 122, 201–207. [Google Scholar] [CrossRef]
- Gulec, G.; Isbil-Buyukcoskun, N.; Kahveci, N. Effects of Centrally-Injected Glucagon-like Peptide-1 on Pilocarpine-Induced Seizures, Anxiety and Locomotor and Exploratory Activity in Rat. Neuropeptides 2010, 44, 285–291. [Google Scholar] [CrossRef]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The Fasting Polypeptide FGF21 Can Enter Brain from Blood. Peptides 2007, 28, 2382–2386. [Google Scholar] [CrossRef]
- Perry, R.J.; Lee, S.; Ma, L.; Zhang, D.; Schlessinger, J.; Shulman, G.I. FGF1 and FGF19 Reverse Diabetes by Suppression of the Hypothalamic–Pituitary–Adrenal Axis. Nat. Commun. 2015, 6, 6980. [Google Scholar] [CrossRef]
- Sonne, D.P.; van Nierop, F.S.; Kulik, W.; Soeters, M.R.; Vilsbøll, T.; Knop, F.K. Postprandial Plasma Concentrations of Individual Bile Acids and FGF-19 in Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3002–3009. [Google Scholar] [CrossRef]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, J.; Yan, X. Cross-Talk between Bile Acids and Intestinal Microbiota in Host Metabolism and Health. J. Zhejiang Univ. Sci. B 2015, 16, 436–446. [Google Scholar] [CrossRef]
- Thomas, C.; Auwerx, J.; Schoonjans, K. Bile Acids and the Membrane Bile Acid Receptor TGR5—Connecting Nutrition and Metabolism. Thyroid 2008, 18, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.L.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in Anti-Inflammatory Gut Bacteria Are Associated with Depression in a Sample of Young Adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef]
- Nair, A.T.; Ramachandran, V.; Joghee, N.M.; Antony, S.; Ramalingam, G. Gut Microbiota Dysfunction as Reliable Non-Invasive Early Diagnostic Biomarkers in the Pathophysiology of Parkinson’s Disease: A Critical Review. J. Neurogastroenterol. Motil. 2018, 24, 30–42. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal Pathways for Microbiome-Brain-Gut Axis Communication. Adv. Exp. Med. Biol. 2014, 817, 115–133. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Gut Microbiota: Microbiota and Neuroimmune Signalling-Metchnikoff to Microglia. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 494–496. [Google Scholar] [CrossRef]
- Sherwin, E.; Rea, K.; Dinan, T.G.; Cryan, J.F. A Gut (Microbiome) Feeling about the Brain. Curr. Opin. Gastroenterol. 2016, 32, 96–102. [Google Scholar] [CrossRef]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M.; Frank, M.; Maier, S.F. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 2017, 42, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 Inflamasomes Are Required for Atherogenesis and Activated by Cholesterol Crystals That Form Early in Disease. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, F.; Liu, S.; Liu, G.; Yang, X.; Gao, W.; Fan, K.; Zhao, H.; Ma, J. Significance of Gastrointestinal Tract in the Therapeutic Mechanisms of Exercise in Depression: Synchronism between Brain and Intestine through GBA. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 103, 109971. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of Peptidoglycan from the Microbiota by Nod1 Enhances Systemic Innate Immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Simeonova, D.; Stoyanov, D.; Leunis, J. Upregulation of the Nitrosylome in Bipolar Disorder Type 1 (BP1) and Major Depression, but Not BP2: Increased IgM Antibodies to Nitrosylated Conjugates Are Associated with Indicants of Leaky Gut. Nitric Oxide 2019, 91, 67–76. [Google Scholar] [CrossRef]
- Sandes, S.; Figueiredo, N.; Pedroso, S.; Sant’Anna, F.; Acurcio, L.; Abatemarco Junior, M.; Barros, P.; Oliveira, F.; Cardoso, V.; Generoso, S.; et al. Weissella Paramesenteroides WpK4 Plays an Immunobiotic Role in Gut-Brain Axis, Reducing Gut Permeability, Anxiety-like and Depressive-like Behaviors in Murine Models of Colitis and Chronic Stress. Food Res. Int. 2020, 137, 109741. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Collyer, R.; Clancy, A.; Borody, T. Faecal Microbiota Transplantation Alleviates Symptoms of Depression in Individuals with Irritable Bowel Syndrome: A Case Series. Med. Microecol. 2020, 6, 100029. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile Is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N. Microbiome, HPA Axis and Production of Endocrine Hormones in the Gut. Adv. Exp. Med. Biol 2014, 817, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–Pituitary–Adrenal Axis, Neuroendocrine Factors and Stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Collins, S.M.; Bercik, P. The Relationship between Intestinal Microbiota and the Central Nervous System in Normal Gastrointestinal Function and Disease. Gastroenterology 2009, 136, 2003–2014. [Google Scholar] [CrossRef]
- Forsythe, P.; Sudo, N.; Dinan, T.; Taylor, V.H.; Bienenstock, J. Mood and Gut Feelings. Brain Behav. Immun. 2010, 24, 9–16. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial Genes, Brain & Behaviour—Epigenetic Regulation of the Gut-Brain Axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef]
- Jedema, H.P.; Grace, A.A. Corticotropin-Releasing Hormone Directly Activates Noradrenergic Neurons of the Locus Ceruleus Recorded in Vitro. J. Neurosci. 2004, 24, 9703–9713. [Google Scholar] [CrossRef]
- Nirmal, J.; Babu, C.S.; Harisudhan, T.; Ramanathan, M. Evaluation of Behavioural and Antioxidant Activity of Cytisus Scoparius Link in Rats Exposed to Chronic Unpredictable Mild Stress. BMC Complement. Altern. Med. 2008, 8, 15. [Google Scholar] [CrossRef]
- de Weerth, C. Do Bacteria Shape Our Development? Crosstalk between Intestinal Microbiota and HPA Axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471. [Google Scholar] [CrossRef]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Daugé, V.; Naudon, L.; Rabot, S. Absence of the Gut Microbiota Enhances Anxiety-like Behavior and Neuroendocrine Response to Acute Stress in Rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, B.; Zeng, L.; Du, X.; Li, B.; Huo, R.; Liu, L.; Wang, H.; Dong, M.; Pan, J.; et al. Gut Microbiota Regulates Mouse Behaviors through Glucocorticoid Receptor Pathway Genes in the Hippocampus. Transl. Psychiatry 2018, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic–Pituitary–Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, R.; Fröhlich, E.E.; Reichmann, F.; Farzi, A.; Kogelnik, N.; Fröhlich, E.; Sattler, W.; Holzer, P. Diverse Action of Lipoteichoic Acid and Lipopolysaccharide on Neuroinflammation, Blood-Brain Barrier Disruption, and Anxiety in Mice. Brain Behav. Immun. 2017, 60, 174–187. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced Glycation End Products (AGEs) and Other Adducts in Aging-Related Diseases and Alcohol-Mediated Tissue Injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Leunis, J.-C.; Berk, M.; Geffard, M.; Bosmans, E. In Depression, Bacterial Translocation May Drive Inflammatory Responses, Oxidative and Nitrosative Stress (O&NS), and Autoimmune Responses Directed against O&NS-Damaged Neoepitopes. Acta Psychiatr. Scand. 2013, 127, 344–354. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Oxidative/Nitrosative Stress and Immuno-Inflammatory Pathways in Depression: Treatment Implications. Curr. Pharm. Des. 2014, 20, 3812–3847. [Google Scholar] [CrossRef]
- Saravana Babu, C.; Sathiya, S.; Anbarasi, C.; Prathyusha, N.; Ramakrishnan, G.; Kalaivani, P.; Jyothi Priya, R.; Selvarajan Kesavanarayanan, K.; Verammal Mahadevan, M.; Thanikachalam, S. Polyphenols in Madhumega Chooranam, a Siddha Medicine, Ameliorates Carbohydrate Metabolism and Oxidative Stress in Type II Diabetic Rats. J. Ethnopharmacol. 2012, 142, 331–336. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C.; Chuang, H.L. Effect of Intestinal Microbiota on Exercise Performance in Mice. J. Strength Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef]
- Kaster, M.P.; Budni, J.; Gazal, M.; Cunha, M.P.; Santos, A.R.S.; Rodrigues, A.L.S. The Antidepressant-like Effect of Inosine in the FST Is Associated with Both Adenosine A1 and A2A Receptors. Purinergic Signal. 2013, 9, 481–486. [Google Scholar] [CrossRef]
- Chan, E.D.; Riches, D.W. IFN-Gamma + LPS Induction of INOS Is Modulated by ERK, JNK/SAPK, and P38(Mapk) in a Mouse Macrophage Cell Line. Am. J. Physiol. Cell Physiol. 2001, 280, C441–C450. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll Like Receptor (TLR) Radical Cycle in Chronic Inflammation: Possible Treatments Targeting the TLR4 Pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased Levels of Glutathione, the Major Brain Antioxidant, in Post-Mortem Prefrontal Cortex from Patients with Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, E.; Agrawal, R.; Nath, C.; Shukla, R. Effect of Melatonin on Neuroinflammation and Acetylcholinesterase Activity Induced by LPS in Rat Brain. Eur. J. Pharmacol. 2010, 640, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, C.; Klabnik, J.J.; O’Donnell, J.M. Novel Therapeutic Targets in Depression and Anxiety: Antioxidants as a Candidate Treatment. Curr. Neuropharmacol. 2014, 12, 108–119. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Camilleri, M. Serotonin: A Mediator of The Brain–Gut Connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Müller, N.; Schwarz, M.J. The Immune-Mediated Alteration of Serotonin and Glutamate: Towards an Integrated View of Depression. Mol. Psychiatry 2007, 12, 988–1000. [Google Scholar] [CrossRef]
- Turner, E.H.; Loftis, J.M.; Blackwell, A.D. Serotonin a La Carte: Supplementation with the Serotonin Precursor 5-Hydroxytryptophan. Pharmacol. Ther. 2006, 109, 325–338. [Google Scholar] [CrossRef]
- Evrensel, A.; Ünsalver, B.Ö.; Ceylan, M.E. Neuroinflammation, Gut-Brain Axis and Depression. Psychiatry Investig. 2020, 17, 2–8. [Google Scholar] [CrossRef]
- Sperner-Unterweger, B.; Kohl, C.; Fuchs, D. Immune Changes and Neurotransmitters: Possible Interactions in Depression? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gainetdinov, R.R.; Beaulieu, J.-M.; Sotnikova, T.D.; Burch, L.H.; Williams, R.B.; Schwartz, D.A.; Krishnan, K.R.R.; Caron, M.G. Loss-of-Function Mutation in Tryptophan Hydroxylase-2 Identified in Unipolar Major Depression. Neuron 2005, 45, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.-E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Co.omposition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ghia, J.-E.; Wang, H.; McClemens, J.; Cote, F.; Suehiro, Y.; Mallet, J.; Khan, W.I. Serotonin Activates Dendritic Cell Function in the Context of Gut Inflammation. Am. J. Pathol. 2011, 178, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Arango, V.; Huang, Y.; Underwood, M.D.; Mann, J.J. Genetics of the Serotonergic System in Suicidal Behavior. J. Psychiatr. Res. 2003, 37, 375–386. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609, 609.e1-3. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced Anxiety-like Behavior and Central Neurochemical Change in Germ-Free Mice. Neurogastroenterol. Motil. 2011, 23, 255-e119. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The Probiotic Bifidobacteria Infantis: An Assessment of Potential Antidepressant Properties in the Rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Purton, T.; Staskova, L.; Lane, M.M.; Dawson, S.L.; West, M.; Firth, J.; Clarke, G.; Cryan, J.F.; Berk, M.; O’Neil, A.; et al. Prebiotic and Probiotic Supplementation and the Tryptophan-Kynurenine Pathway: A Systematic Review and Meta Analysis. Neurosci. Biobehav. Rev. 2021, 123, 1–13. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.; Reynolds, E.H. Homocysteine, Folate, Methylation, and Monoamine Metabolism in Depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Stanger, O.; Herrmann, W.; Pietrzik, K.; Fowler, B.; Geisel, J.; Dierkes, J.; Weger, M. Clinical Use and Rational Management of Homocysteine, Folic Acid, and B Vitamins in Cardiovascular and Thrombotic Diseases. Z. Kardiol. 2004, 93, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Zinno, P.; Motta, V.; Guantario, B.; Natella, F.; Roselli, M.; Bello, C.; Comitato, R.; Carminati, D.; Tidona, F.; Meucci, A.; et al. Supplementation with Dairy Matrices Impacts on Homocysteine Levels and Gut Microbiota Composition of Hyperhomocysteinemic Mice. Eur. J. Nutr. 2020, 59, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.; Liu, T.; Peter, I.; Buel, J.; Arsenault, L.; Scott, T.; Qiu, W.W. The Homocysteine Hypothesis of Depression. Am. J. Psychiatry 2007, 7, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Tell, G.S.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Folate, Vitamin B12, Homocysteine, and the MTHFR 677C→T Polymorphism in Anxiety and Depression: The Hordaland Homocysteine Study. Arch. Gen. Psychiatry 2003, 60, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W. Folic Acid. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 262–269. ISBN 978-0-12-384885-7. [Google Scholar]
- Pérez-Dueñas, B.; Ormazábal, A.; Toma, C.; Torrico, B.; Cormand, B.; Serrano, M.; Sierra, C.; De Grandis, E.; Marfa, M.P.; García-Cazorla, A.; et al. Cerebral Folate Deficiency Syndromes in Childhood: Clinical, Analytical, and Etiologic Aspects. Arch. Neurol. 2011, 68, 615–621. [Google Scholar] [CrossRef]
- Permoda-Osip, A.; Dorszewska, J.; Skibinska, M.; Chlopocka-Wozniak, M.; Rybakowski, J.K. Hyperhomocysteinemia in Bipolar Depression: Clinical and Biochemical Correlates. Neuropsychobiology 2013, 68, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Liu, S.; Liu, H.; He, X.; Sun, L.; Chen, L.; Wei, M.; Gao, F.; Jiang, H. Homocysteine Aggravates Intestinal Epithelial Barrier Dysfunction in Rats with Experimental Uremia. Kidney Blood Press. Res. 2018, 43, 1516–1528. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the Microbiota-Gut-Brain Axis: Diet, Microbiome, and Neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic Analysis of Gut Microbiome Reveals the Role of Bacterial Folate and Homocysteine Metabolism in Parkinson’s Disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Abdalla Saad, M.J. Influence of Gut Microbiota on Subclinical Inflammation and Insulin Resistance. Mediat. Inflamm. 2013, 2013, e986734. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Kopschina Feltes, P.; Doorduin, J.; Klein, H.C.; Juárez-Orozco, L.E.; Dierckx, R.A.J.O.; Moriguchi-Jeckel, C.M.; de Vries, E.F.J. Anti-Inflammatory Treatment for Major Depressive Disorder: Implications for Patients with an Elevated Immune Profile and Non-Responders to Standard Antidepressant Therapy. J. Psychopharmacol. 2017, 31, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a Microglial Disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- Wong, M.-L.; Inserra, A.; Lewis, M.; Mastronardi, C.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.; et al. Inflammasome Signaling Affects Anxiety- and Depressive-like Behavior and Gut Microbiome Composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. The Link between Gut Dysbiosis and Neuroinflammation in Parkinson’s Disease. Neuroscience 2020, 432, 160–173. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Melancholic Microbes: A Link between Gut Microbiota and Depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Gur, T.L.; Shay, L.; Palkar, A.V.; Fisher, S.; Varaljay, V.A.; Dowd, S.; Bailey, M.T. Prenatal Stress Affects Placental Cytokines and Neurotrophins, Commensal Microbes, and Anxiety-like Behavior in Adult Female Offspring. Brain Behav. Immun. 2017, 64, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Hong, G.; Pang, M.; Xu, H.; Li, H.; Tian, F.; Fang, R.; Yao, Y.; Liu, J. Antidepressant-like Effects of Sodium Butyrate and Its Possible Mechanisms of Action in Mice Exposed to Chronic Unpredictable Mild Stress. Neurosci. Lett. 2016, 618, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult Microbiota-Deficient Mice Have Distinct Dendritic Morphological Changes: Differential Effects in the Amygdala and Hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and Host Determinants of Behavioural Phenotype in Maternally Separated Mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.-R. Obese-Type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Wilson, S.J.; Shrout, M.R.; Madison, A.A.; Andridge, R.; Peng, J.; Malarkey, W.B.; Bailey, M.T. The Gut Reaction to Couples’ Relationship Troubles: A Route to Gut Dysbiosis through Changes in Depressive Symptoms. Psychoneuroendocrinology 2021, 125, 105132. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q.; et al. Lactobacillus plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering 2020, 7, 376–385. [Google Scholar] [CrossRef]
- De Palma, G.; Lynch, M.D.J.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pigrau Pastor, M.; Sidani, S.; et al. Transplantation of Fecal Microbiota from Patients with Irritable Bowel Syndrome Alters Gut Function and Behavior in Recipient Mice. Sci. Transl. Med. 2017, 9, eaaf6397. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Palsson, O.; Jones, K.R. Systematic Review of the Comorbidity of Irritable Bowel Syndrome with Other Disorders: What Are the Causes and Implications? Gastroenterology 2002, 122, 1140–1156. [Google Scholar] [CrossRef]
- Bangsgaard Bendtsen, K.M.; Krych, L.; Sørensen, D.B.; Pang, W.; Nielsen, D.S.; Josefsen, K.; Hansen, L.H.; Sørensen, S.J.; Hansen, A.K. Gut Microbiota Composition Is Correlated to Grid Floor Induced Stress and Behavior in the BALB/c Mouse. PLoS ONE 2012, 7, e46231. [Google Scholar] [CrossRef]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-Induced Microbiota Perturbation Causes Gut Endocannabinoidome Changes, Hippocampal Neuroglial Reorganization and Depression in Mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Rajabi, S.; Mohammadshahi, M. Effect of Synbiotic and Probiotic Supplementation on Serum Brain-Derived Neurotrophic Factor Level, Depression and Anxiety Symptoms in Hemodialysis Patients: A Randomized, Double-Blinded, Clinical Trial. Nutr. Neurosci. 2021, 24, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2018, 1420, 5–25. [Google Scholar] [CrossRef]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human Gut Microbiota: The Links with Dementia Development. Protein Cell 2017, 8, 90–102. [Google Scholar] [CrossRef]
- Dinan, T.G.; Quigley, E.M. Probiotics in the Treatment of Depression: Science or Science Fiction? Aust. N. Z. J. Psychiatry 2011, 45, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Katano, Y.; Fujinami, S.; Kawakoshi, A.; Nakazawa, H.; Oji, S.; Iino, T.; Oguchi, A.; Ankai, A.; Fukui, S.; Terui, Y.; et al. Complete Genome Sequence of Oscillibacter valericigenes Sjm18-20T (=NBRC 101213T). Stand. Genom. Sci. 2012, 6, 406–414. [Google Scholar] [CrossRef]
- Khom, S.; Baburin, I.; Timin, E.; Hohaus, A.; Trauner, G.; Kopp, B.; Hering, S. Valerenic Acid Potentiates and Inhibits GABA(A) Receptors: Molecular Mechanism and Subunit Specificity. Neuropharmacology 2007, 53, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Saravana Babu, C.; Kesavanarayanan, K.S.; Kalaivani, P.; Ranju, V.; Ramanathan, M. A Simple Densitometric Method for the Quantification of Inhibitory Neurotransmitter Gamma-Aminobutyric Acid (GABA) in Rat Brain Tissue. Chromatogr. Res. Int. 2011, 2011, e732409. [Google Scholar] [CrossRef][Green Version]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a Translational Psychobiotic: Modulation of Stress, Electrophysiology and Neurocognition in Healthy Volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Balanzá Martínez, V. Nutritional Supplements in Psychotic Disorders. Actas Esp. De Psiquiatr. 2017, 45, 16–25. [Google Scholar]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic Administration Normalizes Lipopolysaccharide (LPS)-Induced Anxiety and Cortical 5-HT2A Receptor and IL1-β Levels in Male Mice. Brain Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Chiaruttini, C.; Vicario, A.; Li, Z.; Baj, G.; Braiuca, P.; Wu, Y.; Lee, F.S.; Gardossi, L.; Baraban, J.M.; Tongiorgi, E. Dendritic Trafficking of BDNF MRNA Is Mediated by Translin and Blocked by the G196A (Val66Met) Mutation. Proc. Natl. Acad. Sci. USA 2009, 106, 16481–16486. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017, 79, 920–926. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Nakaita, Y.; Kaneda, H.; Shigyo, T. Heat-Killed Lactobacillus brevis SBC8803 Induces Serotonin Release from Intestinal Cells. Food Nutr. Sci. 2013, 4, 767–771. [Google Scholar] [CrossRef][Green Version]
- Liu, W.-H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of Behavior and Monoamine Levels Attributable to Lactobacillus plantarum PS128 in Germ-Free Mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of Consuming a Milk Drink Containing a Probiotic on Mood and Cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei Strain Shirota Relieves Stress-associated Symptoms by Modulating the Gut–Brain Interaction in Human and Animal Models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The Immunomodulatory Properties of Probiotic Microorganisms beyond Their Viability (Ghost Probiotics: Proposal of Paraprobiotic Concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef]
- Maehata, H.; Kobayashi, Y.; Mitsuyama, E.; Kawase, T.; Kuhara, T.; Xiao, J.-Z.; Tsukahara, T.; Toyoda, A. Heat-Killed Lactobacillus helveticus Strain MCC1848 Confers Resilience to Anxiety or Depression-like Symptoms Caused by Subchronic Social Defeat Stress in Mice. Biosci. Biotechnol. Biochem. 2019, 83, 1239–1247. [Google Scholar] [CrossRef]
- Kambe, J.; Watcharin, S.; Makioka-Itaya, Y.; Inoue, R.; Watanabe, G.; Yamaguchi, H.; Nagaoka, K. Heat-Killed Enterococcus fecalis (EC-12) Supplement Alters the Expression of Neurotransmitter Receptor Genes in the Prefrontal Cortex and Alleviates Anxiety-like Behavior in Mice. Neurosci. Lett. 2020, 720, 134753. [Google Scholar] [CrossRef]
- Murata, M.; Kondo, J.; Iwabuchi, N.; Takahashi, S.; Yamauchi, K.; Abe, F.; Miura, K. Effects of Paraprobiotic Lactobacillus paracasei MCC1849 Supplementation on Symptoms of the Common Cold and Mood States in Healthy Adults. Benef. Microbes 2018, 9, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Kleniewska, P.; Pawliczak, R. Influence of Synbiotics on Selected Oxidative Stress Parameters. Oxidative Med. Cell. Longev. 2017, 2017, e9315375. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Dören, M.; Schneider, B.; Oettel, M. Are the Antioxidative Effects of 17β-Estradiol Modified by Concomitant Administration of a Progestin? Maturitas 1996, 25, 133–139. [Google Scholar] [CrossRef]
- Miene, C.; Weise, A.; Glei, M. Impact of Polyphenol Metabolites Produced by Colonic Microbiota on Expression of COX-2 and GSTT2 in Human Colon Cells (LT97). Nutr. Cancer 2011, 63, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ballway, J.W.; Song, B.-J. Translational Approaches with Antioxidant Phytochemicals against Alcohol-Mediated Oxidative Stress, Gut Dysbiosis, Intestinal Barrier Dysfunction, and Fatty Liver Disease. Antioxidants 2021, 10, 384. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front. Microbiol 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Rozan, P.; Hidalgo, S.; Nejdi, A.; Bisson, J.-F.; Lalonde, R.; Messaoudi, M. Preventive Antioxidant Effects of Cocoa Polyphenolic Extract on Free Radical Production and Cognitive Performances after Heat Exposure in Wistar Rats. J. Food Sci. 2007, 72, S203–S206. [Google Scholar] [CrossRef]
- Spohr, L.; Soares, M.S.P.; Oliveira, P.S.; da Silveira de Mattos, B.; Bona, N.P.; Pedra, N.S.; Teixeira, F.C.; do Couto, C.A.T.; Chaves, V.C.; Reginatto, F.H.; et al. Combined Actions of Blueberry Extract and Lithium on Neurochemical Changes Observed in an Experimental Model of Mania: Exploiting Possible Synergistic Effects. Metab. Brain Dis. 2019, 34, 605–619. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the Inhibition of Monoamine Oxidase A by Bioactive Coffee Compounds Protecting Serotonin Degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Z.; Wang, Y.; Xie, K.; Zhang, Q.; Luan, Q.; Chen, W.; Liu, D. Antidepressant-like Effects of Curcumin in Chronic Mild Stress of Rats: Involvement of Its Anti-Inflammatory Action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 47, 33–39. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Yuan, L.; Wang, S.; Liu, L.; Yang, X.; Li, G.; Liu, D. The Effects of Curcumin on Depressive-like Behavior in Mice after Lipopolysaccharide Administration. Behav. Brain Res. 2014, 274, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of Curcumin in Brain Disorders. BioFactors 2019, 45, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sharma, A.; Kumari, A.; Kulurkar, P.M.; Raj, R.; Gulati, A.; Padwad, Y.S. Consumption of Green Tea Epigallocatechin-3-Gallate Enhances Systemic Immune Response, Antioxidative Capacity and HPA Axis Functions in Aged Male Swiss Albino Mice. Biogerontology 2017, 18, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-L.; Shi, H.; Wei, Y.-M.; Wang, S.-J.; Sun, C.-Y.; Ding, Z.-B.; Lu, L. Green Tea Polyphenols Produce Antidepressant-like Effects in Adult Mice. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2011, 65, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liu, L.; Liu, H.; Liu, S.; Xue, H.; Wang, X.; Yuan, L.; Wang, Z.; Liu, D. Resveratrol Abrogates Lipopolysaccharide-Induced Depressive-like Behavior, Neuroinflammatory Response, and CREB/BDNF Signaling in Mice. Eur. J. Pharmacol. 2015, 768, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Cao, L.; Wu, F.; Wang, L.; Wang, G.; Yu, Y.; Zhang, M.; Chen, L.; Wang, W.; Lv, W.; et al. The Effect of Trans-Resveratrol on Post-Stroke Depression via Regulation of Hypothalamus-Pituitary-Adrenal Axis. Neuropharmacology 2015, 97, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, C.; Wang, C.; Zhang, J.-F.; Zhou, W.-H.; Cui, W.-G.; Ye, F.; Xu, Y. Chronic Resveratrol Treatment Exerts Antihyperalgesic Effect and Corrects Co-Morbid Depressive like Behaviors in Mice with Mononeuropathy: Involvement of Serotonergic System. Neuropharmacology 2014, 85, 131–141. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Wu, J.; de Theije, C.G.M.; da Silva, S.L.; Abbring, S.; van der Horst, H.; Broersen, L.M.; Willemsen, L.; Kas, M.; Garssen, J.; Kraneveld, A.D. Dietary Interventions That Reduce MTOR Activity Rescue Autistic-like Behavioral Deficits in Mice. Brain Behav. Immun. 2017, 59, 273–287. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 Polyunsaturated Fatty Acids Critically Regulate Behaviour and Gut Microbiota Development in Adolescence and Adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; Kelly, P.; Ariffin, N.; Cryan, J.F.; Clarke, G.; Dinan, T.G. N-3 PUFAs Have Beneficial Effects on Anxiety and Cognition in Female Rats: Effects of Early Life Stress. Psychoneuroendocrinology 2015, 58, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of Fecal Microbiota Transplant on Symptoms of Psychiatric Disorders: A Systematic Review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Shi, X.; Yuan, L.-Z.; Tang, D.; Wang, F. Fecal Microbiota Transplantation in an Elderly Patient with Mental Depression. Int. Psychogeriatr. 2019, 31, 1525–1526. [Google Scholar] [CrossRef]
| Strains (Probiotics/Prebiotics/Postbiotics) | Outcome | References |
|---|---|---|
| Lactobacillus helveticus and Bifidobacterium longum | Normalizes hippocampal BDNF levels and inflammation | [111,150,159] |
| Fructo-oligosaccharides and B-immuno galacto-oligosaccharide | Stimulate the growth of beneficial bacteria, i.e., B. longum, which leads to reduction of stress-induced activation of the HPA axis, corticosterone levels, and pro-inflammatory cytokines, and increased BDNF | [22,146,157,160,161] |
| Bifidobacterium infantis 35624 | Reduces depressive-like behavior via alleviating 5-HT | [82,109,162] |
| Bifidobacterium breve | Stimulates 5-HT receptors in intestinal cells of rats | [163] |
| Bifidobacterium longum PS128 | Produces beneficial metabolites (SCFAs) and improves the locomotor activity in depression | [164] |
| Lactobacillus plantarum | Decreases stress-induced anxiety-like behavior | [164] |
| Lactobacillus rhamnosus (JB-1) | Reduces GABA Aα2 mRNA and corticosterone | [23] |
| Lactobacillus farciminis | Prevents gut barrier leakiness and reverses psychological stress-induced HPA axis activation | [149,165]. |
| Lactobacillus casei strain Shirota | Reduces anxiety scores in patients with chronic fatigue syndrome and increases abundance of Lactobacillus and Bifidobacterium in fecal samples | [166,167] |
| Source | Main Compound | Outcome | References |
|---|---|---|---|
| Cocoa | Catechins Anthocyanins Proanthocyanins Flavanols Epicatechin | Prevents neuroinflammation in the dorsal vagal complex | [180] |
| Blueberry | Anthocyanins | Significantly increases brain activity with improved working memory and depression-like behavior | [181] |
| Coffee | Flavanols Caffeic Acid, Chlorogenic Acid | Markedly increases cognitive performance, psychomotor control, and working memory | [182] |
| Strawberry | Fisetin (polyphenol) | Suppresses proinflammatory markers such as TNF-α | [121] |
| Peanuts, red grape, wine | Polyphenol | Increases monoamine and BDNF levels | [121] |
| Curcumin | Polyphenol | Elevates serotonin, Noradrenaline, and dopamine levels via altering MAO activity | [183,184] |
| Green tea/ epigallocatechin | Epigallocatechin-3-gallate (EGCG). | Free radical scavenging and antioxidative properties Green tea treatment can reduce HPA axis hyperactivity in response to stress | [185,186,187] |
| Resveratrol | Polyphenol | Elevates 5-HT and nor epinephrine levels in pre-frontal cortex (PFC) and upregulates BDNF levels | [188,189,190] |
| Interventions | Disease Conditions | Phase | Status | Clinical Trials.gov Identifier |
|---|---|---|---|---|
| Combination of Bifidobacterium Longum 35624® and 1714™ Probiotics | IBS and depression, anxiety | Phase 2 | Completed | NCT04422327 |
| Diet modifications | IBS and depression | - | Completed | NCT00788658 |
| Kynurenine pathway metabolites as novel translational biological markers (observational study) | Depression, gut, IBS | - | Unknown | NCT01304355 |
| Integrative treatment model, conventional treatment | Anxiety and depression | - | Completed | NCT01631500 |
| Transcutaneous vagus nerve stimulation | Anxiety and IBS | - | Completed | NCT03440255 |
| Dietary fiber supplementation | Anxiety and IBS | - | Not yet recruiting | NCT04619095 |
| Linaclotide | Anxiety and IBS | - | Not yet recruiting | NCT03342287 |
| Rifaximin | Gut microbiota manipulation, anxiety, and depression | Phase 2 | Not yet recruiting | NCT04302402 |
| Specific CBT program (PASCET-PI) | Psychological problems and IBS | - | Unknown | NCT02265588 |
| SAMe (S-adenosyl-L-methionine) and probiotic Lactobacillus plantarum | Depression and IBS | - | Completed | NCT03932474 |
| Selegiline | Anxiety, depression, and IBS | Phase 3 | Completed | NCT01912391 |
| Multi-strain probiotic product (DSF) | Anxiety, depression, and IBS | - | Not yet recruiting | NCT04006977 |
| Relaxing music | GI abnormalities, anxiety | - | Recruiting | NCT04671628 |
| Information on the microbiome with anxiety | Gut flora in anxiety | No intervention | Recruiting | NCT04211376 |
| Psychotherapy | Depression and IBS | - | Not yet recruiting | NCT04639141 |
| Galacto-oligosaccharides, maltodextrin | Microbiota–gut–brain axis in brain development and mental health | - | Recruiting | NCT03835468 |
| Observational study | Role of gut flora in depression | - | Recruiting | NCT04211467 |
| Lactobacillus Plantarum 299v supplementation | Depression, anxiety disorder | Phase 2 | Completed | NCT02469545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonali, S.; Ray, B.; Ahmed Tousif, H.; Rathipriya, A.G.; Sunanda, T.; Mahalakshmi, A.M.; Rungratanawanich, W.; Essa, M.M.; Qoronfleh, M.W.; Chidambaram, S.B.; et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells 2022, 11, 1362. https://doi.org/10.3390/cells11081362
Sonali S, Ray B, Ahmed Tousif H, Rathipriya AG, Sunanda T, Mahalakshmi AM, Rungratanawanich W, Essa MM, Qoronfleh MW, Chidambaram SB, et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells. 2022; 11(8):1362. https://doi.org/10.3390/cells11081362
Chicago/Turabian StyleSonali, Sharma, Bipul Ray, Hediyal Ahmed Tousif, Annan Gopinath Rathipriya, Tuladhar Sunanda, Arehally M. Mahalakshmi, Wiramon Rungratanawanich, Musthafa Mohamed Essa, M. Walid Qoronfleh, Saravana Babu Chidambaram, and et al. 2022. "Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review" Cells 11, no. 8: 1362. https://doi.org/10.3390/cells11081362
APA StyleSonali, S., Ray, B., Ahmed Tousif, H., Rathipriya, A. G., Sunanda, T., Mahalakshmi, A. M., Rungratanawanich, W., Essa, M. M., Qoronfleh, M. W., Chidambaram, S. B., & Song, B.-J. (2022). Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells, 11(8), 1362. https://doi.org/10.3390/cells11081362








