The Effect of Mineralocorticoid Receptor 3 Antagonists on Anti-Inflammatory and Anti-Fatty Acid Transport Profile in Patients with Heart Failure
Abstract
:1. Introduction
2. Material and Methods
2.1. Human Open-Heart Surgery Samples
2.2. Human Heart Failure Samples
2.3. Adipogenesis Induction for 14 Days
2.4. Cell-Type RNA Transcripts on Fat Biopsies and Adipogenesis Assay
2.5. Immunohistochemistry
2.6. Wound Healing Assay
2.7. Released and Expression ITLN-1 by Epicardial Stromal Cells
2.8. Blood Analytes Measurements
2.9. Statistical Analysis
3. Results
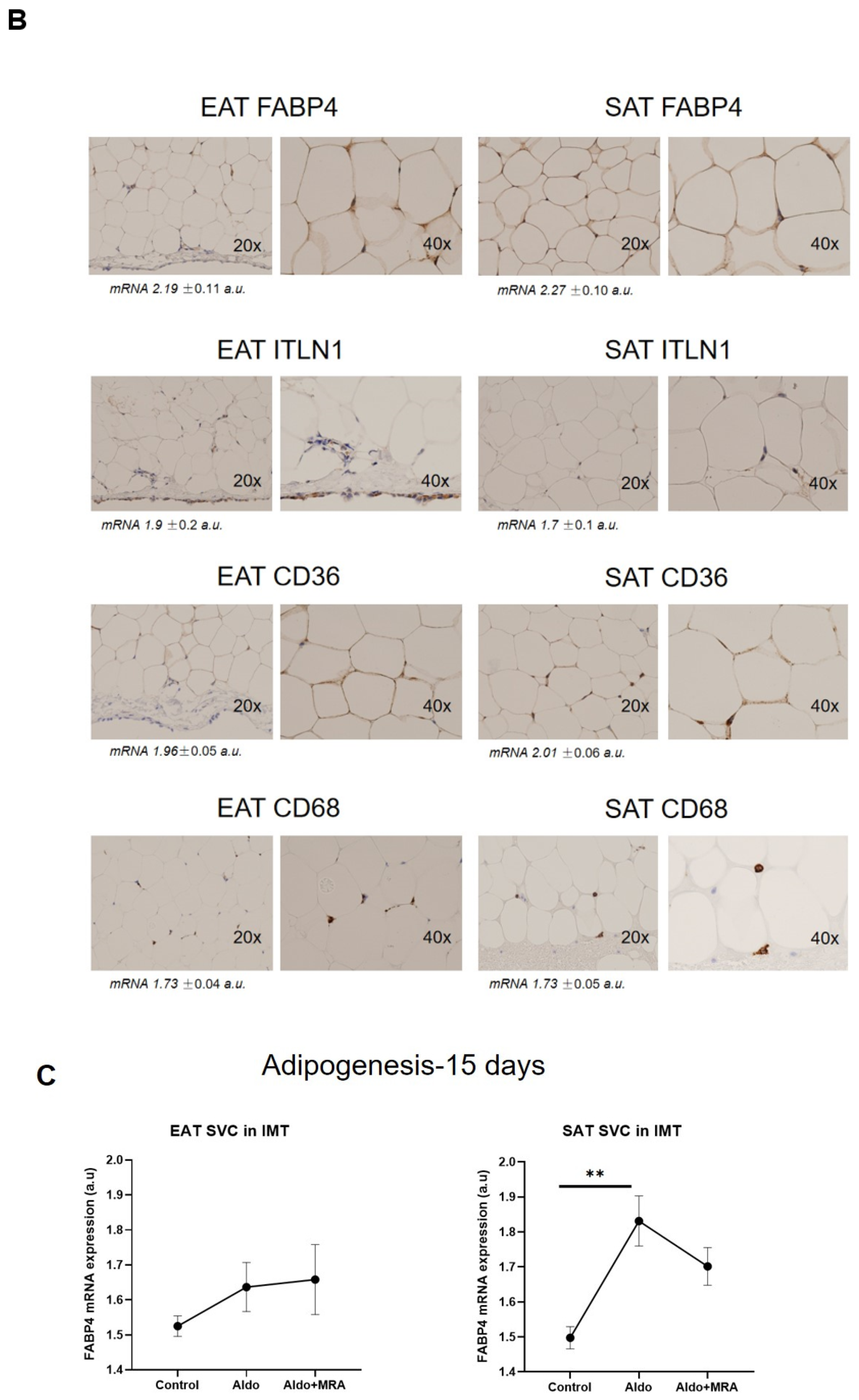
3.1. Stromal Fat Cell Adipogenesis and Cell-Type RNA Transcripts
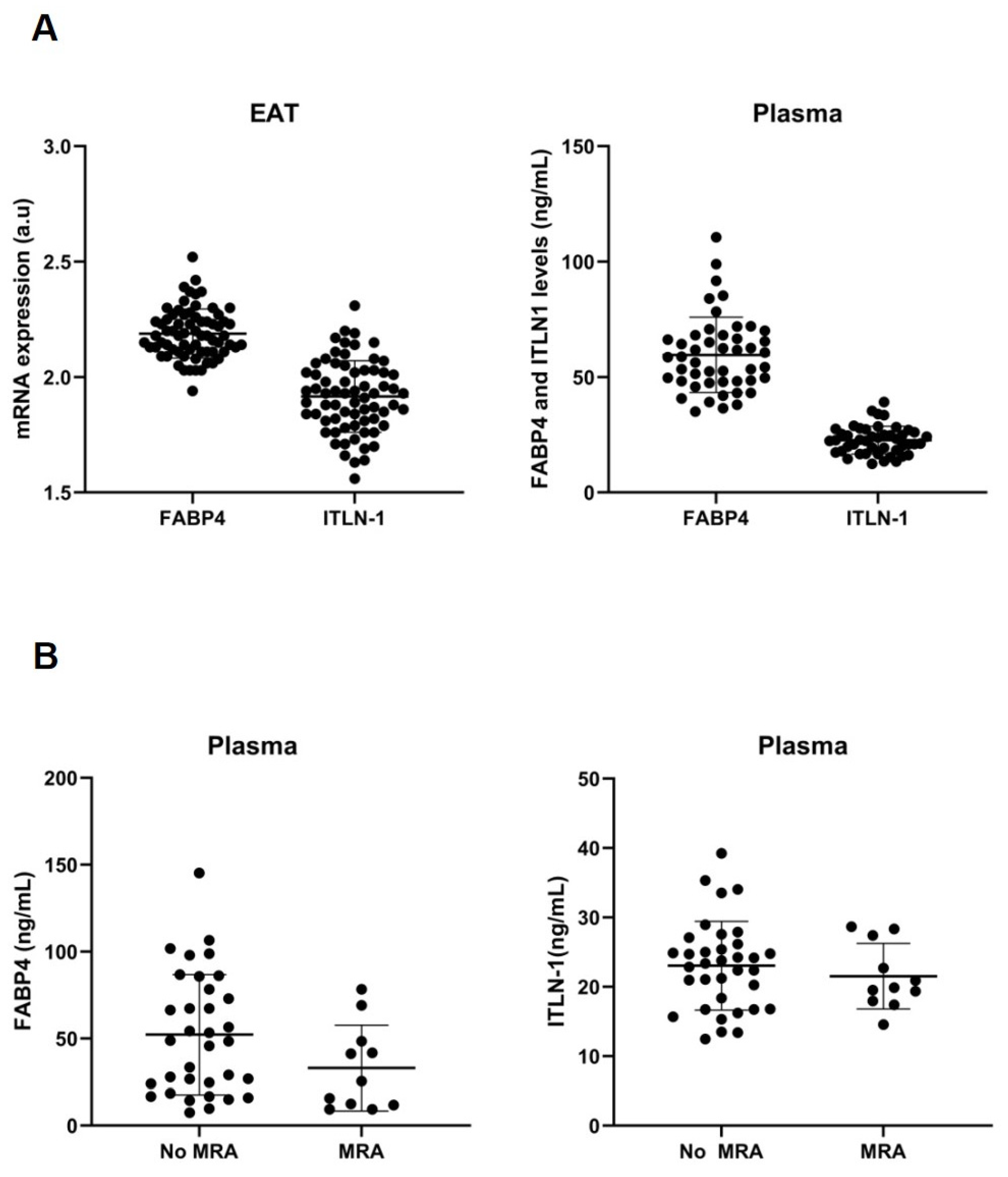
3.2. Cell-Type RNA-Transcripts in Epicardial and Subcutaneous Fat
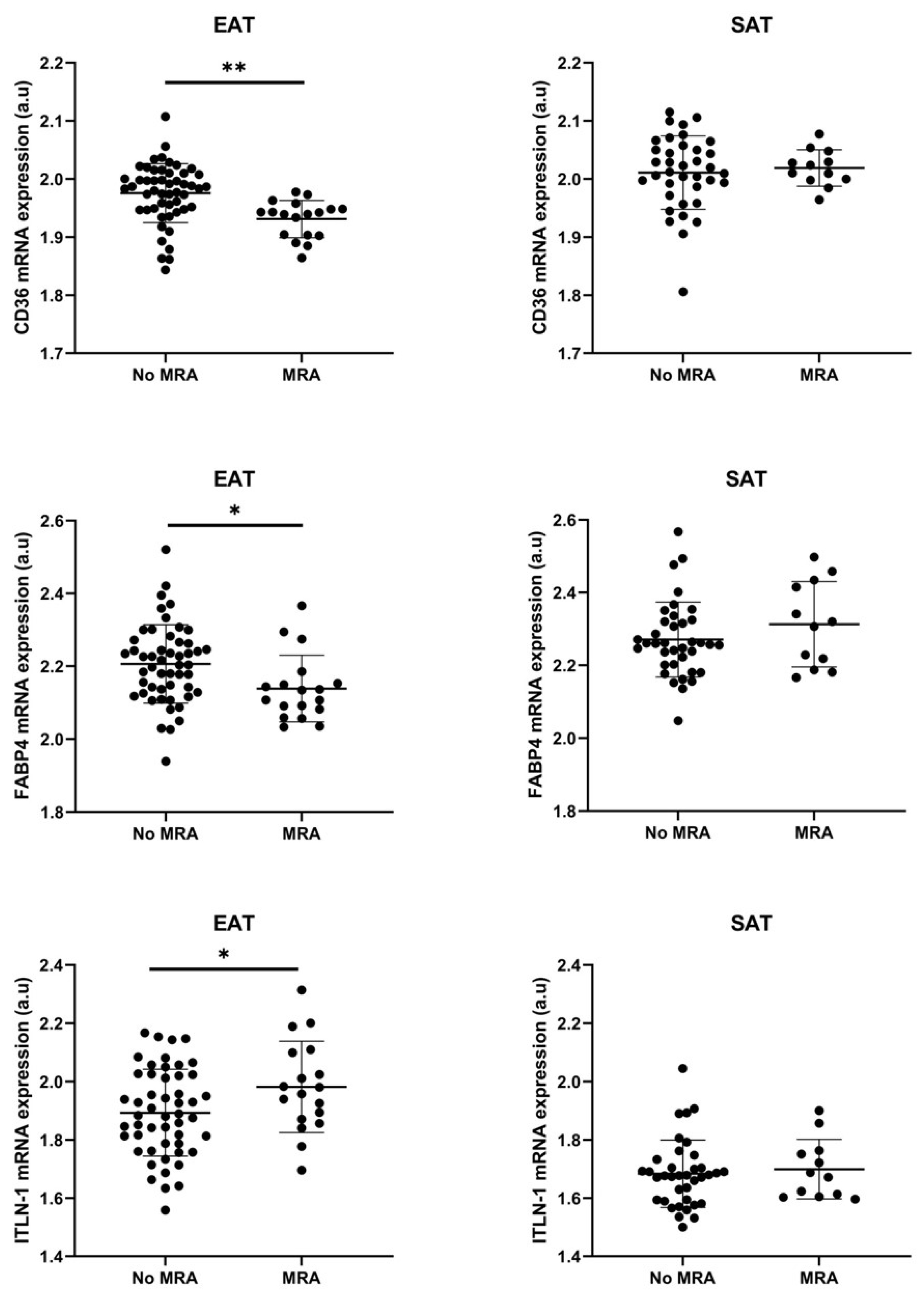
3.3. Cell Types RNA-Transcripts on Epicardial and Subcutaneous Fat with MRA
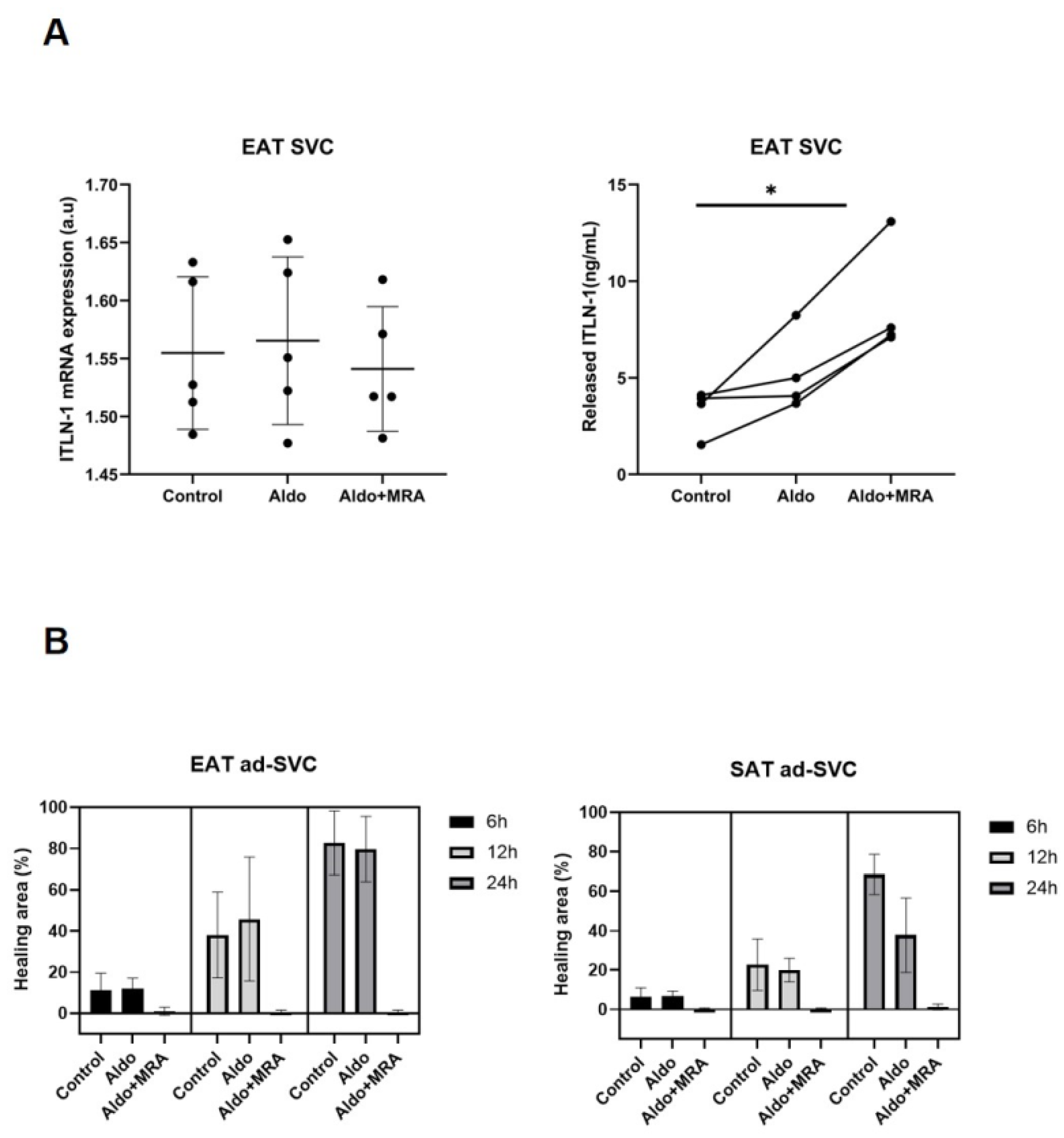
3.4. MRA on Epicardial Stromal Cells and ITLN-1
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaeta, M.; Bandera, F.; Tassinari, F.; Capasso, L.; Cargnelutti, M.; Pelissero, G.; Malavazos, A.E.; Ricci, C. Is epicardial fat depot associated with atrial fibrillation? A systematic review and meta-analysis. Europace 2017, 19, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Yerramasu, A.; Dey, D.; Venuraju, S.; Anand, D.V.; Atwal, S.; Corder, R.; Berman, D.S.; Lahiri, A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012, 220, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Ribaudo, M.C.; Zappaterreno, A.; Iannucci, C.V.; Leonetti, F. Relation between epicardial adipose tissue and left ventricular mass. Am. J. Cardiol. 2004, 94, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Batal, O.; Schoenhagen, P.; Shao, M.; Ayyad, A.E.; Van Wagoner, D.R.; Halliburton, S.S.; Tchou, P.J.; Chung, M.K. Left atrial epicardial adiposity and atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Trasancos, A.; Fandiño-Vaquero, R.; Agra, R.M.; Fernández, A.L.; Viñuela, J.E.; González-Juanatey, J.R.; Eiras, S. Impaired Adipogenesis and Insulin Resistance in Epicardial Fat-Mesenchymal Cells from Patients with Cardiovascular Disease. J. Cell. Physiol. 2014, 229, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, A.; Piercecchi-Marti, M.D.; Grino, M.; Leonetti, G.; Alessi, M.C.; Clement, K.; Dadoun, F.; Dutour, A. Epicardial adipose tissue extent: Relationship with age, body fat distribution, and coronaropathy. Obesity 2008, 16, 2424–2430. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Siddique, M.; Antoniades, C. Artificial intelligence in medical imaging: A radiomic guide to precision phenotyping of cardiovascular disease. Cardiovasc. Res. 2020, 116, 2040–2054. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.K.; Desai, M.Y.; Marwan, M.; Kotanidis, C.P.; Antonopoulos, A.S.; Schottlander, D.; Channon, K.M.; Neubauer, S.; Achenbach, S.; Antoniades, C. Perivascular Fat Attenuation Index Stratifies Cardiac Risk Associated with High-Risk Plaques in the CRISP-CT Study. J. Am. Coll. Cardiol. 2020, 76, 755–757. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Long, X.; Gao, Y.; Liu, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ogura, T.; Onodera, S.; et al. Type I collagen inhibits adipogenic differentiation via YAP activation in vitro. J. Cell. Physiol. 2020, 235, 1821–1837. [Google Scholar] [CrossRef]
- Fernández-Trasancos, A.; Guerola-Segura, R.; Paradela-Dobarro, B.; Álvarez, E.; García-Acuña, J.M.; Fernández, A.L.; González-Juanatey, J.R.; Eiras, S. Glucose and Inflammatory Cells Decrease Adiponectin in Epicardial Adipose Tissue Cells: Paracrine Consequences on Vascular Endothelium. J. Cell. Physiol. 2016, 231, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Volat, F.; Sandhow, L.; Galitzky, J.; Nguyen, T.; Esteve, D.; Åström, G.; Mejhert, N.; Ledoux, S.; Thalamas, C.; et al. CD36 Is a Marker of Human Adipocyte Progenitors with Pronounced Adipogenic and Triglyceride Accumulation Potential. Stem Cells 2017, 35, 1799–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boney, C.M.; Fiedorek, F.T.; Paul, S.R.; Gruppuso, P.A. visceral adipose tissue. Endocrinology 1996, 137, 2923–2928. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, K.A.; Kim, J.H.; Hei, S.S. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 2006, 136, 2953–2956. [Google Scholar] [CrossRef] [PubMed]
- Tchoukalova, Y.D.; Sarr, M.G.; Jensen, M.D. Measuring committed preadipocytes in human adipose tissue from severely obese patients by using adipocyte fatty acid binding protein. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1132–R1140. [Google Scholar] [CrossRef] [Green Version]
- Holness, C.; Simmons, D. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef] [Green Version]
- Newman, P.J.; Berndt, M.C.; Gorski, J.; White, G.C.; Lyman, S.; Paddock, C.; Muller, W.A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 1990, 247, 1219–1222. [Google Scholar] [CrossRef]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Pang, C.; Gao, Z.; Yin, J.; Zhang, J.; Jia, W.; Ye, J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E313–E322. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Johnson, R.S.; Distel, R.J.; Ellis, R.; Papaioannou, V.E.; Spiegelman, B.M. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996, 274, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Fucho, R.; Görgün, C.Z.; Tuncman, G.; Cao, H.; Hotamisligil, G.S. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Investig. 2008, 118, 2640–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, M.; Fuseya, T.; Murata, M.; Hoshina, K.; Ishimura, S.; Mita, T.; Watanabe, Y.; Omori, A.; Matsumoto, M.; Sugaya, T.; et al. Local production of fatty acid-binding protein 4 in epicardial/perivascular fat and macrophages is linked to coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 825–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, N.; Furuhashi, M.; Koyama, M.; Higashiura, Y.; Akasaka, H.; Tanaka, M.; Moniwa, N.; Ohnishi, H.; Saitoh, S.; Ura, N.; et al. Elevated circulating FABP4 concentration predicts cardiovascular death in a general population: A 12-year prospective study. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Mraz, M.; Cinkajzlova, A.; Lacinova, Z.; Klouckova, J.; Kratochvilova, H.; Lips, M.; Kopecky, P.; Porizka, M.; Lindner, J.; Haluzik, M. The Role of Epicardial Adipose Tissue Lymphocytes in Low-Grade Inflammation and Coronary Artery Disease. Diabetes 2018, 67, 469-P. [Google Scholar] [CrossRef]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Maizels, R.M.; Yazdanbakhsh, M. Immune Regulation by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2003, 3, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Eash, K.J.; Greenbaum, A.M.; Gopalan, P.K.; Link, D.C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Investig. 2010, 120, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Cinkajzlová, A.; Mráz, M.; Haluzík, M. Adipose tissue immune cells in obesity, type 2 diabetes mellitus and cardiovascular diseases. J. Endocrinol. 2021, 252, R1–R22. [Google Scholar] [CrossRef]
- Gaborit, B.; Venteclef, N.; Ancel, P.; Pelloux, V.; Gariboldi, V.; Leprince, P.; Amour, J.; Hatem, S.N.; Jouve, E.; Dutour, A.; et al. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc. Res. 2015, 108, 62–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fain, J.N.; Sacks, H.S.; Buehrer, B.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Carter, R.A.; Tichansky, D.S.; Madan, A.K. RNA in human epicardial adipose tissue: Comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int. J. Obes. 2005 2008, 32, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Greulich, S.; Chen, W.J.Y.; Maxhera, B.; Rijzewijk, L.J.; van der Meer, R.W.; Jonker, J.T.; Mueller, H.; de Wiza, D.H.; Floerke, R.-R.; Smiris, K.; et al. Cardioprotective properties of omentin-1 in type 2 diabetes: Evidence from clinical and in vitro studies. PLoS ONE 2013, 8, e59697. [Google Scholar] [CrossRef] [PubMed]
- Agra, R.M.; Varela-Román, A.; González-Ferreiro, R.; Viñuela, J.E.; Castro-Pais, A.; Fernández-Trasancos, Á.; Díaz-Rodríguez, E.; Álvarez, E.; Carreira, M.C.; Casanueva, F.F.; et al. Orosomucoid as prognosis factor associated with inflammation in acute or nutritional status in chronic heart failure. Int. J. Cardiol. 2017, 228, 488–494. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Muir, L.A.; Kiridena, S.; Griffin, C.; DelProposto, J.B.; Geletka, L.; Martinez-Santibañez, G.; Zamarron, B.F.; Lucas, H.; Singer, K.; O’ Rourke, R.W.; et al. Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J. Leukoc. Biol. 2018, 103, 615–628. [Google Scholar] [CrossRef]
- Kazemi, M.R.; McDonald, C.M.; Shigenaga, J.K.; Grunfeld, C.; Feingold, K.R. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1220–1224. [Google Scholar] [CrossRef] [Green Version]
- Matloch, Z.; Kratochvílová, H.; Cinkajzlová, A.; Lipš, M.; Kopecký, P.; Pořízka, M.; Haluzíková, D.; Lindner, J.; Mráz, M.; Kloučková, J.; et al. Changes in omentin levels and its mRNA expression in epicardial adipose tissue in patients undergoing elective cardiac surgery: The influence of type 2 diabetes and coronary heart disease. Physiol. Res. 2018, 67, 881–890. [Google Scholar] [CrossRef]
- Du, Y.; Ji, Q.; Cai, L.; Huang, F.; Lai, Y.; Liu, Y.; Yu, J.; Han, B.; Zhu, E.; Zhang, J.; et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc. Diabetol. 2016, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef]
- Kauerova, S.; Bartuskova, H.; Muffova, B.; Janousek, L.; Fronek, J.; Petras, M.; Poledne, R.; Kralova Lesna, I. Statins Directly Influence the Polarization of Adipose Tissue Macrophages: A Role in Chronic Inflammation. Biomedicines 2021, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Poledne, R.; Malinska, H.; Kubatova, H.; Fronek, J.; Thieme, F.; Kauerova, S.; Lesna, I.K. Polarization of Macrophages in Human Adipose Tissue is Related to the Fatty Acid Spectrum in Membrane Phospholipids. Nutrients 2019, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Qi, Y.; Li, Y.; Zhang, S.; Guo, S.; Chu, S.; Gao, P.; Zhu, D.; Wu, Z.; Lu, L.; et al. Impact of mineralocorticoid receptor antagonists on changes in cardiac structure and function of left ventricular dysfunction: A meta-analysis of randomized controlled trials. Circ. Heart Fail. 2013, 6, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Johansen, M.L.; Ibarrola, J.; Fernández-Celis, A.; Schou, M.; Sonne, M.P.; Refsgaard Holm, M.; Rasmussen, J.; Dela, F.; Jaisser, F.; Faber, J.; et al. The Mineralocorticoid Receptor Antagonist Eplerenone Suppresses Interstitial Fibrosis in Subcutaneous Adipose Tissue in Patients with Type 2 Diabetes. Diabetes 2021, 70, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. TEM 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Van Harmelen, V.; Röhrig, K.; Hauner, H. Comparison of Proliferation and Differentiation Capacity of Human Adipocyte Precursor Cells from the Omental and Subcutaneous Adipose Tissue Depot of Obese Subjects. Metabolism 2004, 53, 632–637. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Pucéat, M.; et al. Reactivation of the Epicardium at the Origin of Myocardial Fibro-Fatty Infiltration During the Atrial Cardiomyopathy. Circ. Res. 2020, 126, 1330–1342. [Google Scholar] [CrossRef]
- Hudak, C.S.; Sul, H.S. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. 2013, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Caprio, M.; Antelmi, A.; Chetrite, G.; Muscat, A.; Mammi, C.; Marzolla, V.; Fabbri, A.; Zennaro, M.C.; Fève, B. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: Potential implications for the treatment of metabolic syndrome. Endocrinology 2011, 152, 113–125. [Google Scholar] [CrossRef]
- Biswas, S.; Gao, D.; Altemus, J.B.; Rekhi, U.R.; Chang, E.; Febbraio, M.; Byzova, T.V.; Podrez, E.A. Circulating CD36 is increased in hyperlipidemic mice: Cellular sources and triggers of release. Free Radic. Biol. Med. 2021, 168, 180–188. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Dludla, P.V.; Mkandla, Z.; Mutize, T.; Nyambuya, T.M.; Mxinwa, V.; Nkambule, B.B. Differential expression of glycoprotein IV on monocyte subsets following high-fat diet feeding and the impact of short-term low-dose aspirin treatment. Metab. Open 2020, 7, 100047. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Bakris, G.L.; Pitt, B.; Agarwal, R.; Rossing, P.; Ruilope, L.M.; Butler, J.; Lam, C.S.P.; Kolkhof, P.; Roberts, L.; et al. Finerenone Reduces New-Onset Atrial Fibrillation in Patients with Chronic Kidney Disease and Type 2 Diabetes. J. Am. Coll. Cardiol. 2021, 78, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Liu, P.R.; Shrader, P.; Blanco, R.G.; Allen, L.A.; Fonarow, G.C.; Gersh, B.J.; Kowey, P.R.; Mahaffey, K.W.; Hylek, E.; et al. Mineralocorticoid Receptor Antagonism in Patients with Atrial Fibrillation: Findings from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) Registry. J. Am. Heart Assoc. 2018, 7, e007987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.M.; Sander, S.; Coleman, C.I.; Gallagher, R.; Takata, H.; Humphrey, C.; Henyan, N.; Gillespie, E.L.; Kluger, J. Impact of epicardial anterior fat pad retention on postcardiothoracic surgery atrial fibrillation incidence: The AFIST-III Study. J. Am. Coll. Cardiol. 2007, 49, 298–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, J.; Ollitrault, P.; Fischer, M.-O.; Fellahi, J.-L.; Rozec, B.; Cholley, B.; Dolladille, C.; Chequel, M.; Allouche, S.; Legallois, D.; et al. Spironolactone and perioperative atrial fibrillation occurrence in cardiac surgery patients: Rationale and design of the ALDOCURE trial. Am. Heart J. 2019, 214, 88–96. [Google Scholar] [CrossRef]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertention 2012, 59, 1069–1078. [Google Scholar] [CrossRef]



| Variables | Mean ± SD | N (Yes/No) | % (Yes/No) |
|---|---|---|---|
| Age (years) | 66 ± 8 | ||
| Gender (male/female) | 14/3 | 82/18 | |
| BMI (kg/m2) | 29 ± 4 | ||
| AHT | 9/8 | 53/47 | |
| T2DM | 2/15 | 12/88 | |
| Dyslipidemia | 9/8 | 53/47 | |
| Smoking | 9/8 | 53/47 | |
| HF | 8/9 | 47/53 | |
| Valvulopathy | 15/2 | 88/12 | |
| CAD | 6/11 | 35/65 | |
| AF | 7/10 | 41/59 | |
| CRF | 1/16 | 6/94 | |
| ACEi | 6/11 | 35/65 | |
| b-blockers | 10/7 | 59/41 | |
| Statin | 9/8 | 53/47 | |
| MRA | 3/14 | 18/82 | |
| Oral antidiabetics | 2/15 | 12/88 |
| Variables | Mean ± SD | N (Yes/No) | % (Yes/No) |
|---|---|---|---|
| Age (years) | 70 ± 8 | ||
| Gender (male/female) | 48/23 | 32/68 | |
| BMI (kg/m2) | 29 ± 4 | ||
| Arterial hypertension | 47/24 | 66/34 | |
| T2DM | 14/57 | 20/80 | |
| Dyslipidemia | 56/15 | 79/21 | |
| Smoking | 26/45 | 37/63 | |
| HF | 42/29 | 59/41 | |
| Valvulopathy | 62/9 | 87/13 | |
| CAD | 48/23 | 34/66 | |
| CRF | 7/64 | 10/90 | |
| ACEi | 19/52 | 27/73 | |
| b-blockers | 48/23 | 68/32 | |
| Statin | 52/19 | 73/27 | |
| MRA | 18/53 | 25/75 | |
| Oral antidiabetics | 14/57 | 20/80 |
| Variables | MRA | No MRA | p |
|---|---|---|---|
| Gender (male/female) | 13 (72%)/5 (28%) | 35 (66%)/18 (34%) | 0.63 |
| AHT (Yes/No) | 14 (78%)/4 (22%) | 33 (62%)/20 (38%) | 0.23 |
| T2DM (Yes/No) | 6 (33%)/12 (67%) | 8 (15%)/45 (85%) | 0.09 |
| Dyslipidemia (Yes/No) | 16 (89%)/2 (11%) | 40 (75%)/13 (25%) | 0.23 |
| Smoking (Yes/No) | 7 (39%)/11 (61%) | 19 (36%)/34 (64%) | 0.82 |
| Heart Failure (Yes/No) | 13 (72%)/5 (28%) | 29 (55%)/24 (45%) | 0.19 |
| Valvulopathy (Yes/No) | 17 (94%)/1 (6%) | 45 (85%)/8 (15%) | 0.29 |
| CAD (Yes/No) | 6 (33%)/12 (67%) | 18 (34%)/35 (66%) | 0.96 |
| CRF (Yes/No) | 3 (17%)/15 (83%) | 4 (7%)/49 (93%) | 0.26 |
| AF (Yes/No) | 12 (67%)/6 (33%) | 20 (38%)/33 (62%) | 0.03 |
| ACEi (Yes/No) | 6 (33%)/12 (67%) | 13 (25%)/40 (75%) | 0.47 |
| b-blockers (Yes/No) | 13 (72%)/5 (28%) | 35 (66%)/18 (34%) | 0.63 |
| Statin (Yes/No) | 16 (89%)/2 (11%) | 36 (68%)/17 (32%) | 0.08 |
| Oral antidiabetics (Yes/No) | 6 (33%)/12 (67%) | 36 (68%)/17 (32%) | 0.09 |
| Variables | Mean ± SD | N/% | MRA (n = 54) | No MRA (n = 12) | p |
|---|---|---|---|---|---|
| Age (years) | 71 ± 11 | 25/62 | 72 ± 12 | 71 ± 11 | 0.80 |
| Gender (male) (n/%) | 19/35 | 6/50 | 0.34 | ||
| BMI (kg/m2) | 32 ± 8 | 31±7 | 29 ± 6 | 0,52 | |
| AHT (n/%) | 53/80 | 44/81 | 10/83 | 0.88 | |
| T2DM (n/%) | 33/50 | 26/48 | 7/58 | 0.52 | |
| Dyslipidemia (n/%) | 39/59 | 29/54 | 10/83 | 0.06 | |
| Smoking (n/%) | 16/24 | 14/26 | 2/17 | 0.48 | |
| CAD (n/%) | 15/23 | 13/24 | 2/17 | 0.58 | |
| LVEF ≥ 50% | 18/27 | 12/22 | 6/50 | 0.05 | |
| AF (n/%) | 2/ | 2/4 | 0/0 | 0.50 | |
| ACEi (n/%) | 43/65 | 36/67 | 7/58 | 0.58 | |
| b-blockers (n/%) | 55/83 | 46/85 | 9/75 | 0.39 | |
| Statin (n/%) | 41/62 | 33/61 | 8/67 | 0.72 | |
| Oral antidiabetics (n/%) | 24/36 | 17/31 | 7/58 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Almenglo, C.; Fernandez, Á.L.; Martínez-Cereijo, J.M.; Iglesias-Alvarez, D.; Duran-Muñoz, D.; García-Caballero, T.; Gonzalez-Juanatey, J.R.; Rodriguez-Mañero, M.; Eiras, S. The Effect of Mineralocorticoid Receptor 3 Antagonists on Anti-Inflammatory and Anti-Fatty Acid Transport Profile in Patients with Heart Failure. Cells 2022, 11, 1264. https://doi.org/10.3390/cells11081264
Fu X, Almenglo C, Fernandez ÁL, Martínez-Cereijo JM, Iglesias-Alvarez D, Duran-Muñoz D, García-Caballero T, Gonzalez-Juanatey JR, Rodriguez-Mañero M, Eiras S. The Effect of Mineralocorticoid Receptor 3 Antagonists on Anti-Inflammatory and Anti-Fatty Acid Transport Profile in Patients with Heart Failure. Cells. 2022; 11(8):1264. https://doi.org/10.3390/cells11081264
Chicago/Turabian StyleFu, Xiaoran, Cristina Almenglo, Ángel Luis Fernandez, José Manuel Martínez-Cereijo, Diego Iglesias-Alvarez, Darío Duran-Muñoz, Tomás García-Caballero, Jose Ramón Gonzalez-Juanatey, Moises Rodriguez-Mañero, and Sonia Eiras. 2022. "The Effect of Mineralocorticoid Receptor 3 Antagonists on Anti-Inflammatory and Anti-Fatty Acid Transport Profile in Patients with Heart Failure" Cells 11, no. 8: 1264. https://doi.org/10.3390/cells11081264
APA StyleFu, X., Almenglo, C., Fernandez, Á. L., Martínez-Cereijo, J. M., Iglesias-Alvarez, D., Duran-Muñoz, D., García-Caballero, T., Gonzalez-Juanatey, J. R., Rodriguez-Mañero, M., & Eiras, S. (2022). The Effect of Mineralocorticoid Receptor 3 Antagonists on Anti-Inflammatory and Anti-Fatty Acid Transport Profile in Patients with Heart Failure. Cells, 11(8), 1264. https://doi.org/10.3390/cells11081264







