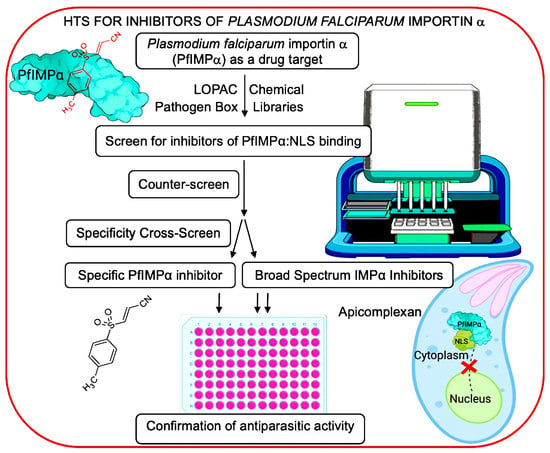
High-Throughput Screening to Identify Inhibitors of Plasmodium falciparum Importin α
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid Construction
2.2. Protein Expression, Purification, and Use in AlphaScreen Assay
2.3. HTS
2.4. Thermostability Assay (TSA)
2.5. P. falciparum Culture and Growth Inhibition Assay
2.6. T. gondii Tachyzoite Culture and Growth Inhibition Assay
2.7. MTT Assay for Host Cell Cytotoxicity
2.8. Statistical Analysis
3. Results
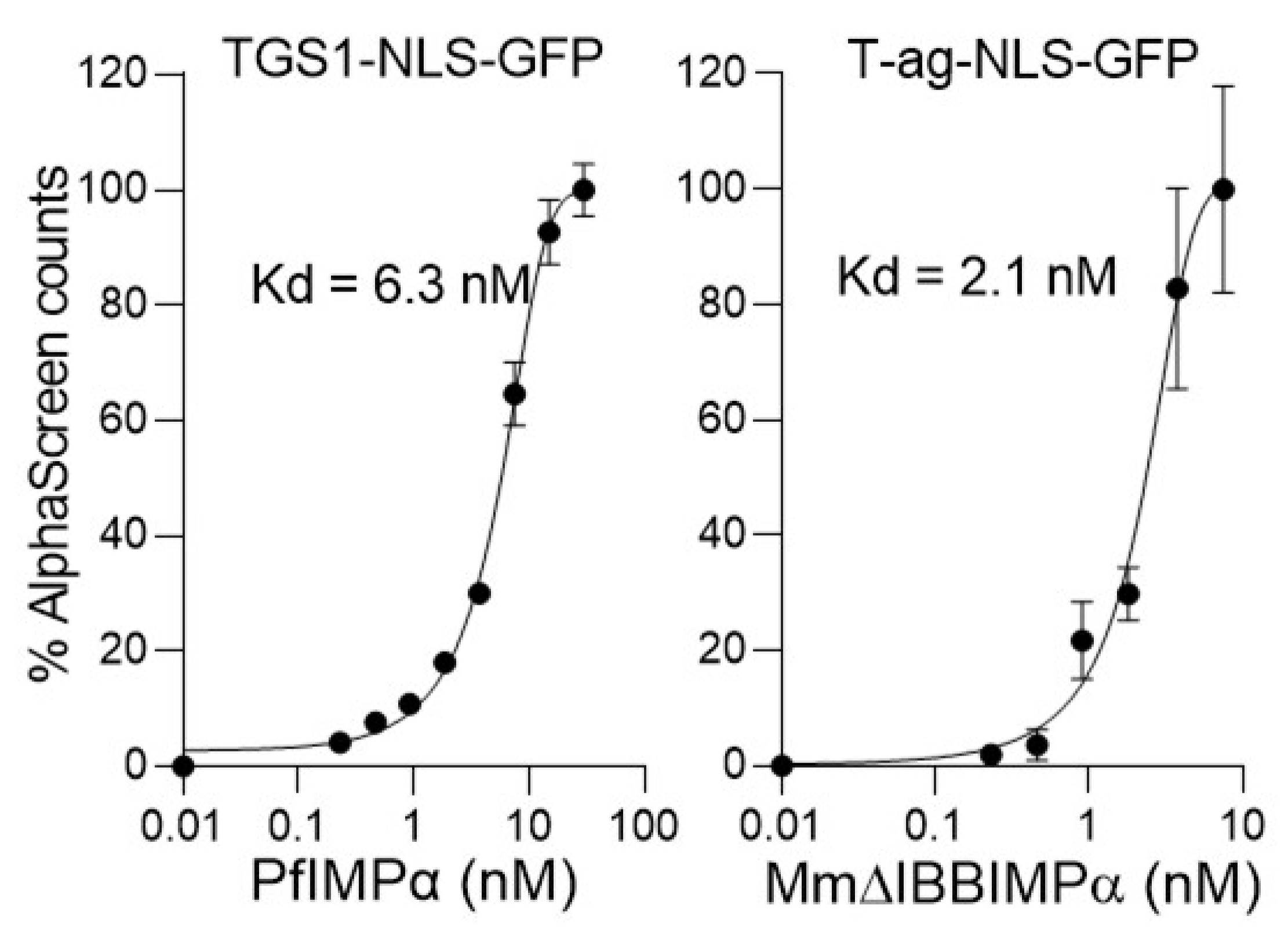
3.1. Optimisation of AlphaScreen Binding Assay for HTS
3.2. Library Screening for Inhibitors of the Interaction between PfIMPα and TGS1-NLS
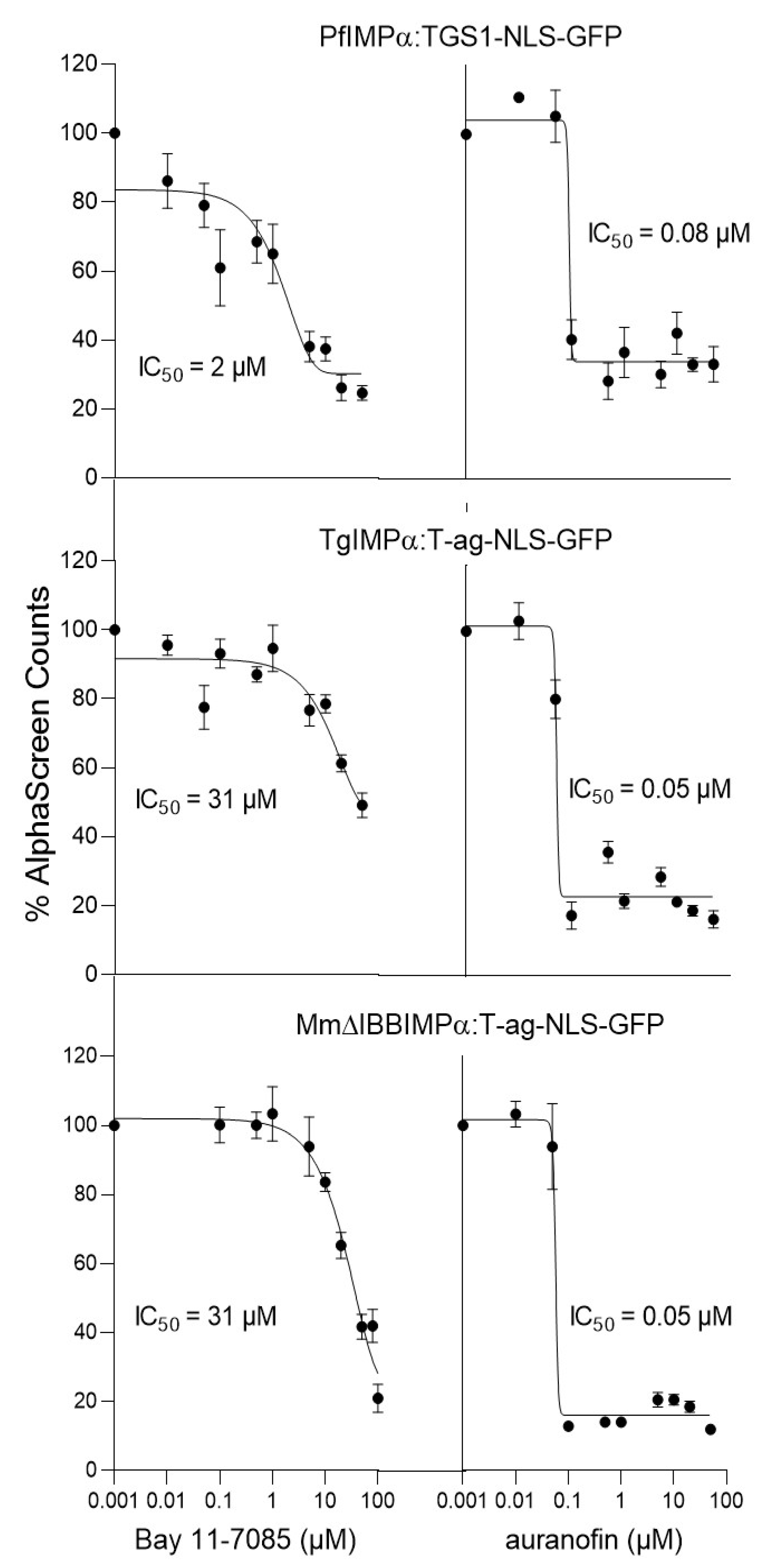
3.3. Cross-Screening to Identify Selective Inhibitors of PfIMPα:TGS1–NLS Binding
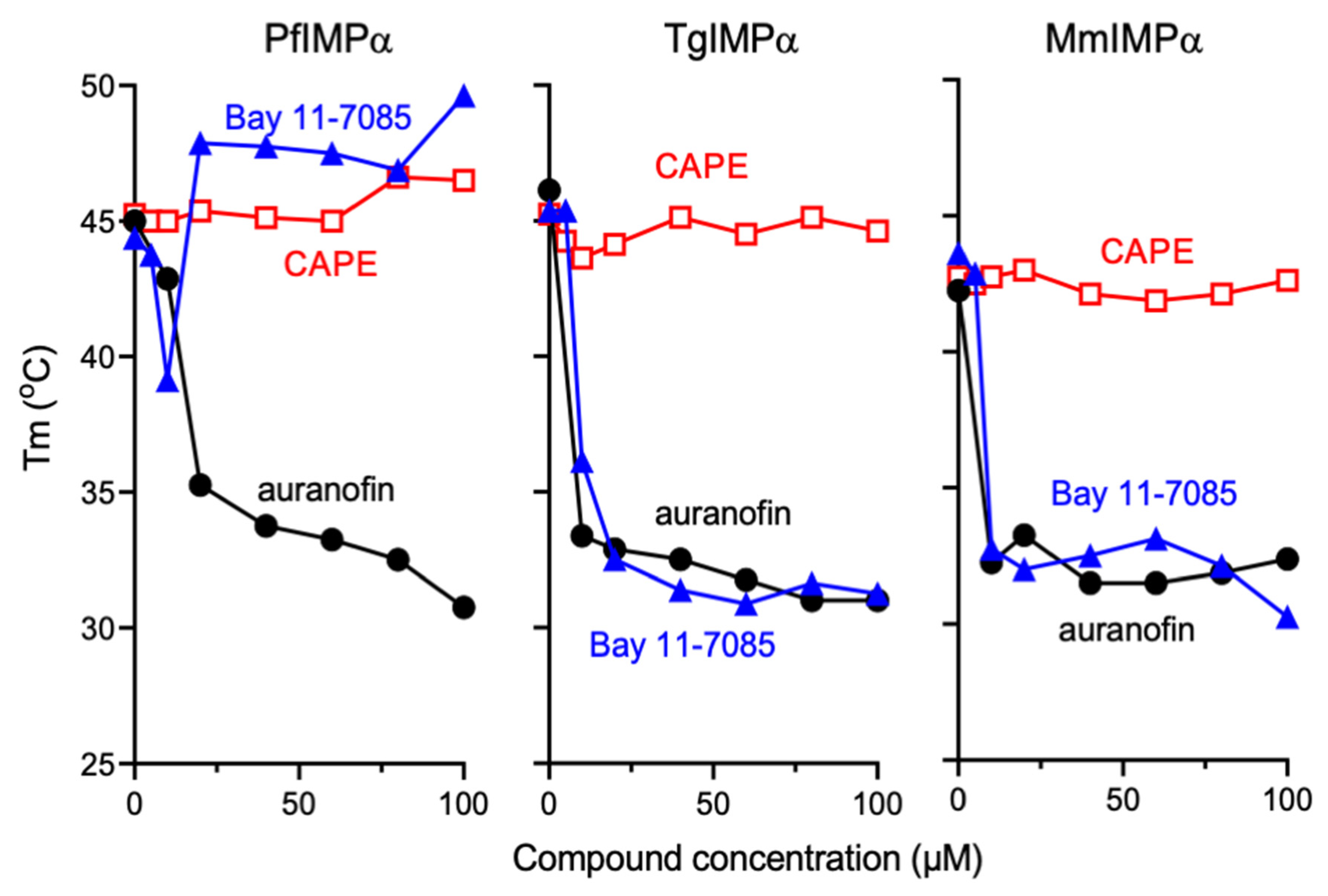
3.4. Bay 11-7085 and Auranofin Appear to Bind Directly to IMPα
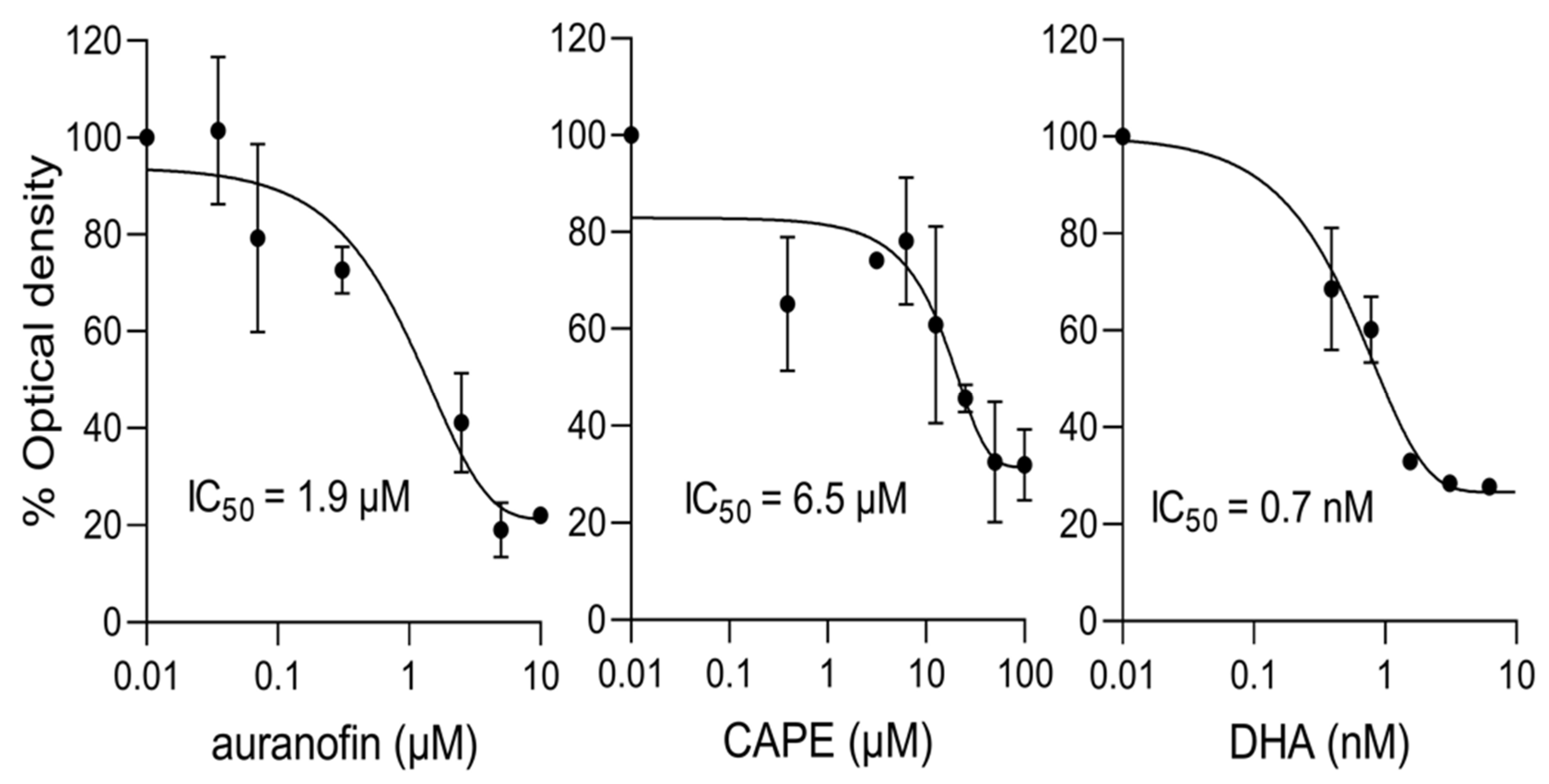
3.5. PfIMPα Inhibitors Limit Proliferation of P. falciparum and T. gondii In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Furtado, J.M.; Smith, J.R.; Belfort, R.; Gattey, D.; Winthrop, K.L. Toxoplasmosis: A global threat. J. Glob. Infect. Dis. 2011, 3, 281–284. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 29 March 2022).
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed]
- Alday, P.H.; Doggett, J.S. Drugs in Development for Toxoplasmosis: Advances, Challenges, and Current Status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Mharakurwa, S.; Ndiaye, D.; Rathod, P.K.; Rosenthal, P.J. Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am. J. Trop. Med. Hyg. 2015, 93, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Frankel, M.B.; Knoll, L.J. The Ins and Outs of Nuclear Trafficking: Unusual Aspects in Apicomplexan Parasites. DNA Cell Biol. 2009, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liang, C.; Li, F.; Wang, L.; Wu, X.; Lu, A.; Xiao, G.; Zhang, G. The rules and functions of nucleocytoplasmic shuttling proteins. Int. J. Mol. Sci. 2018, 19, 1445. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Yamada, K.; Yoneda, Y. Importin α: A Key Molecule in Nuclear Transport and Non-Transport Functions. J. Biochem. 2016, 160, 69–75. [Google Scholar] [CrossRef]
- Loveland, K.L.; Major, A.T.; Butler, R.; Young, J.C.; Jans, D.A.; Miyamoto, Y. Putting things in place for fertilization: Discovering roles for importin proteins in cell fate and spermatogenesis. Asian J. Androl. 2015, 17, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, A.J.; Jans, D.A. Regulation of Nucleocytoplasmic Trafficking of Viral Proteins: An Integral Role in Pathogenesis? Biochim. Biophys. 2011, 1813, 2176–2190. [Google Scholar] [CrossRef]
- Marfori, M.; Mynott, A.; Ellis, J.J.; Mehdi, A.M.; Saunders, N.F.W.; Curmi, P.M.; Forwood, J.K.; Bodén, M.; Kobe, B. Molecular Basis for Specificity of Nuclear Import and Prediction of Nuclear Localization. Biochim. Biophys. Acta 2011, 1813, 1562–1577. [Google Scholar] [CrossRef]
- Kobe, B. Autoinhibition by an internal nuclear localization signal revealed by the crystal structure of mammalian importin alpha. Nat. Struct. Biol. 1999, 6, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Harreman, M.T.; Hodel, M.R.; Fanara, P.; Hodel, A.E.; Corbett, A.H. The Auto-inhibitory Function of Importin α Is Essential in Vivo. J. Biol. Chem. 2003, 278, 5854–5863. [Google Scholar] [CrossRef] [PubMed]
- Chahine, M.N.; Pierce, G.N. Therapeutic Targeting of Nuclear Protein Import in Pathological Cell Conditions. Pharmacol. Rev. 2009, 61, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Kosyna, F.; Depping, R. Controlling the Gatekeeper: Therapeutic Targeting of Nuclear Transport. Cells 2018, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Jans, D.A.; Martin, A.J.; Wagstaff, K.M. Inhibitors of Nuclear Transport. Curr. Opin. Cell Biol. 2019, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Jans, D.A. Antivirals That Target the Host IMPα/Β1-Virus Interface. Biochem. Soc. Trans. 2021, 49, 281–295. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An ALPHAscreen based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen. 2011, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.E.; Watanabe, S.; Wang, C.; Chan, W.K.; Maher, B.; Lopez-Denman, A.; Hick, C.; Wagstaff, K.M.; Mackenzie, J.M.; Sexton, P.M.; et al. A nuclear transport inhibitor that modulates the unfolded protein response and provides in vivo protection against lethal dengue virus infection. J. Infect. Dis. 2014, 210, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R.; Lundberg, L.; Pinkhan, C.; Shechter, S.; Debono, A.; Baell, J.; Wagstaff, K.M.; Hick, C.A.; Kehn-Hall, K.; Jans, D.A. Identification of novel antivirals inhibiting recognition of Venezuelan equine encephalitis virus capsid protein by the Importin α/β1 heterodimer through high-throughput screening. Antivir. Res. 2018, 151, 8–19. [Google Scholar] [CrossRef]
- Shechter, S.; Thomas, D.R.; Lundberg, L.; Pinkham, C.; Lin, S.-C.; Wagstaff, K.M.; Debono, A.; Kehn-Hall, K.; Jans, D.A. Novel inhibitors targeting Venezuelan equine encephalitis virus capsid protein identified using In Silico Structure-Based-Drug-Design. Sci Rep. 2017, 7, 17705. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved Drug Ivermectin inhibits the replication 1 of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Jans, D.A.; Wagstaff, K.M. The Broad Spectrum Host-Directed Agent Ivermectin as an Antiviral for SARS-CoV-2? Biochem. Biophys. Res. Commun. 2021, 538, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020, 177, 104760. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Y.; Fraser, J.E.; Chan, W.K.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013, 99, 301–306. [Google Scholar] [CrossRef]
- Yang, S.N.Y.; Atkinson, S.C.; Fraser, J.E.; Wang, C.; Maher, B.; Roman, N.; Forwood, J.K.; Wagstaff, K.M.; Borg, N.A.; Jans, D.A. Novel Flavivirus Antiviral That Targets The Host Nuclear Transport Importin α/β1 Heterodimer. Cells 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Wagstaff, K.M.; Jans, D.A. Subcellular trafficking of pathogens: Targeting for therapeutics. Antiviral Res. 2012, 95, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Seeber, F.; Steinfelder, S. Recent Advances in Understanding Apicomplexan Parasites. F1000Research 2016, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the Essential Genes of the Human Malaria Parasite Plasmodium falciparum by Saturation Mutagenesis. Science 2018, 360, 360. [Google Scholar] [CrossRef] [PubMed]
- Sidik, S.M.; Hackett, C.G.; Tran, F.; Westwood, N.J.; Lourido, S. Efficient Genome Engineering of Toxoplasma gondii Using CRISPR/Cas9. PLoS ONE 2014, 9, e100450. [Google Scholar] [CrossRef]
- Bawankar, P.; Shaw, P.J.; Sardana, R.; Babar, P.H.; Patankar, S. 5′ and 3′ End Modifications of Spliceosomal RNAs in Plasmodium falciparum. Mol. Biol. Rep. 2010, 37, 2125–2133. [Google Scholar] [CrossRef]
- Babar, P.H.; Dey, V.; Jaiswar, P.; Patankar, S. An Insertion in the Methyltransferase Domain of P. Falciparum Trimethylguanosine Synthase Harbors a Classical Nuclear Localization Signal. Mol. Biochem. Parasitol. 2016, 210, 58–70. [Google Scholar] [CrossRef]
- Dey, V.; Patankar, S. Molecular Basis for the Lack of Auto-Inhibition of Plasmodium falciparum Importin α. Biochem. Biophys. Res. Commun. 2018, 503, 1792–1797. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Jans, D.A. Intramolecular Masking of Nuclear Localization Signals: Analysis of Importin Binding Using a Novel AlphaScreen-Based Method. Anal. Biochem. 2006, 348, 49–56. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Noedl, H.; Wernsdorfer, W.H.; Miller, R.S.; Wongsrichanalai, C. Histidine-Rich Protein II: A Novel Approach to Malaria Drug Sensitivity Testing. Antimicrob. Agents Chemother. 2002, 46, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Noedl, H.; Bronnert, J.; Yingyuen, K.; Attlmayr, B.; Kollaritsch, H.; Fukuda, M. Simple Histidine-Rich Protein 2 Double-Site Sandwich Enzyme-Linked Immunosorbent Assay for Use in Malaria Drug Sensitivity Testing. Antimicrob. Agents Chemother. 2005, 49, 3575–3577. [Google Scholar] [CrossRef] [PubMed]
- Van Dooren, G.G.; Tomova, C.; Agrawal, S.; Humbel, B.M.; Striepen, B. Toxoplasma Gondii Tic20 Is Essential for Apicoplast Protein Import. Proc. Natl. Acad. Sci. USA 2008, 105, 13574–13579. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B. Toxoplasmic encephalitis in children. J. Am. Med. Assoc. 1941, 116, 801–807. [Google Scholar] [CrossRef]
- Hughes, H.P.A.; Hudson, L.; Fleck, D.G. In Vitro Culture of Toxoplasma Gondii in Primary and Established Cell Lines. Int. J. Parasitol. 1986, 16, 317–322. [Google Scholar] [CrossRef]
- Black, M.; Seeber, F.; Soldati, D.; Kim, K.; Boothroyd, J.C. Restriction Enzyme-Mediated Integration Elevates Transformation Frequency and Enables Co-Transfection of Toxoplasma Gondii. Mol. Biochem. Parasitol. 1995, 74, 55–63. [Google Scholar] [CrossRef]
- Gubbels, M.J.; Striepen, B. Studying the Cell Biology of Apicomplexan Parasites Using Fluorescent Proteins. Microsc. Microanal. 2004, 10, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Jacot, D.; Meissner, M.; Sheiner, L.; Soldati-Favre, D.; Striepen, B. Genetic Manipulation of Toxoplasma Gondii. In Toxoplasma Gondii; Weiss, L.M., Kim, K., Eds.; Elsevier Academic Press: Burlington, NJ, USA, 2014; pp. 577–611. [Google Scholar]
- Striepen, B.; Soldati, D. 15—Genetic Manipulation of Toxoplasma gondii. In Toxoplasma Gondii; Louis, M., Weiss, L.M., Kim, K., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 391–418. [Google Scholar]
- Han, Y.; Adeyemi, O.S.; Hazzaz, M.; Kabir, B.; Kato, K. Screening of Compound Libraries for Inhibitors of Toxoplasma Growth and Invasion. Parasitol. Res. 2020, 119, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Belekar, M.A.; Shukla, A.; Tong, J.X.; Sinha, A.; Chu, T.T.T.; Kulkarni, A.S.; Preiser, P.R.; Reddy, D.S.; Tan, K.S.W.; et al. Targeted Phenotypic Screening in Plasmodium falciparum and Toxoplasma gondii Reveals Novel Modes of Action of Medicines for Malaria Venture Malaria Box Molecules. mSphere 2018, 3, e00534-17. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Christopher, P., Austin, Jonathan, B., Thomas, D.Y., Chung, et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Kurth, F.; Pongratz, P.; Bélard, S.; Mordmüller, B.; Kremsner, P.G.; Ramharter, M. In Vitro Activity of Pyronaridine against Plasmodium falciparum and Comparative Evaluation of Anti-Malarial Drug Susceptibility Assays. Malar. J. 2009, 8, 79. [Google Scholar] [CrossRef]
- Van der Ven, A.J.A.M.; Schoondermark-van de Ven, E.M.E.; Camps, W.; Melchers, W.J.G.; Koopmans, P.P.; van der Meer, J.W.M.; Galama, J.M.D. Anti-Toxoplasma Effect of Pyrimethamine, Trimethoprim and Sulphonamides Alone and in Combination: Implications for Therapy. J. Antimicrob. Chemother. 1996, 38, 75–80. [Google Scholar] [CrossRef]
- Baudy, A.R.; Saxena, N.; Gordish, H.; Hoffman, E.P.; Nagaraju, K. A Robust In Vitro Screening Assay to Identify NF-ΚB Inhibitors for Inflammatory Muscle Diseases. Int. Immunopharmacol. 2009, 9, 1209–1214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berger, N.; Ben Bassat, H.; Klein, B.Y.; Laskov, R. Cytotoxicity of NF-ΚB Inhibitors Bay 11-7085 and Caffeic Acid Phenethyl Ester to Ramos and Other Human B-Lymphoma Cell Lines. Exper. Hematol. 2007, 35, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R D 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sannella, A.R.; Casini, A.; Gabbiani, C.; Messori, L.; Bilia, A.R.; Vincieri, F.F.; Majori, G.; Severini, C. New Uses for Old Drugs. Auranofin, a Clinically Established Antiarthritic Metallodrug, Exhibits Potent Antimalarial Effects In Vitro: Mechanistic and Pharmacological Implications. FEBS Lett. 2008, 582, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.M.; Chaparro, J.D.; Capparelli, E.; Reed, S.L. Auranofin Is Highly Efficacious against Toxoplasma gondii In Vitro and in an In Vivo Experimental Model of Acute Toxoplasmosis. PLoS Negl. Trop. Dis. 2014, 8, e2973. [Google Scholar] [CrossRef] [PubMed]



| Hit Compound x | % Inhibition of IMPα-NLS Interaction * | |||
|---|---|---|---|---|
| PfIMPα | TgIMPα # | MmΔIBBIMPα | MmIMPα/β | |
| (A) General Inhibitors | ||||
| MMV688978 (auranofin) | 94 | 90 | 91 | 100 |
| Daphnetin | 81 | 70 | 81 | 83 |
| Chelerythrine Cl | 81 | 67 | 88 | 94 |
| AC-93253 iodide | 82 | 72 | 67 | 62 |
| MMV003270 | 92 | 83 | 82 | NT $ |
| 6-Fluoronorepinephrine HCl | 77 | 67 | 60 | 51 |
| (B) Inhibitors Showing Selectivity | ||||
| (B1) Selective for PfIMPα | ||||
| Bay 11-7085 | 75 | 0 | 9 | 14 |
| (B2) Selective for IMPαs | ||||
| Caffeic acid phenethyl ester | 84 | 62 | 92 | 14 |
| (B3) Others | ||||
| MMV030734 | 99 | 44 | 43 | 68 |
| MMV024937 | 73 | 26 | 70 | 24 |
| (−)-Epinephrine bitartrate | 85 | 23 | 34 | 94 |
| (±)-Epinephrine HCl | 98 | 24 | 35 | 95 |
| MMV676512 | 100 | 48 | 54 | 100 |
| IC50(μΜ) * | ||||
|---|---|---|---|---|
| Binding Interaction | Bay 11-7085 | CAPE | Auranofin | MMV003270 |
| PfIMPα:TGS1-NLS-GFP | 1.9 ± 0.1 | 1.8 ± 0.3 | 0.07 ± 0.01 | 0.3 ± 0.2 |
| TgIMPα:T-ag-NLS-GFP | 30.4 ± 0.6 | 2.9 ± 0.9 | 0.08 ± 0.01 | 0.2 ± 0.1 |
| MmΔIBBIMPα:T-ag-NLS | 23.4 ± 2.9 | 0.7 ± 0.1 | 0.06 ± 0.01 | 0.4 ± 0.2 |
| Compound | IC50 (µM) * | |
|---|---|---|
| P. falciparum | T. gondii | |
| auranofin | 1.9 + 0.5 | 2.3 ± 0.8 |
| CAPE | 7.5 ± 1.1 | >10 # |
| Bay 11-7085 | >10 # | 2.7 ± 0.7 |
| DHA | 0.001 + 0.0005 | NT x |
| pyrimethamine | NT x | 0.46 ± 0.14 ^ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walunj, S.B.; Dias, M.M.; Kaur, C.; Wagstaff, K.M.; Dey, V.; Hick, C.; Patankar, S.; Jans, D.A. High-Throughput Screening to Identify Inhibitors of Plasmodium falciparum Importin α. Cells 2022, 11, 1201. https://doi.org/10.3390/cells11071201
Walunj SB, Dias MM, Kaur C, Wagstaff KM, Dey V, Hick C, Patankar S, Jans DA. High-Throughput Screening to Identify Inhibitors of Plasmodium falciparum Importin α. Cells. 2022; 11(7):1201. https://doi.org/10.3390/cells11071201
Chicago/Turabian StyleWalunj, Sujata B., Manisha M. Dias, Chhaminder Kaur, Kylie M. Wagstaff, Vishakha Dey, Caroline Hick, Swati Patankar, and David A. Jans. 2022. "High-Throughput Screening to Identify Inhibitors of Plasmodium falciparum Importin α" Cells 11, no. 7: 1201. https://doi.org/10.3390/cells11071201
APA StyleWalunj, S. B., Dias, M. M., Kaur, C., Wagstaff, K. M., Dey, V., Hick, C., Patankar, S., & Jans, D. A. (2022). High-Throughput Screening to Identify Inhibitors of Plasmodium falciparum Importin α. Cells, 11(7), 1201. https://doi.org/10.3390/cells11071201