The Sin3A/MAD1 Complex, through Its PAH2 Domain, Acts as a Second Repressor of Retinoic Acid Receptor Beta Expression in Breast Cancer Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Lines and Culture Media
2.2. Synthetic Peptide Decoys and Generation of SID-Transfected Stable Cell Lines
2.3. Protein Extraction and Western Blotting
2.4. Co-Immunoprecipitation
2.5. Luciferase Assays
2.6. RT-PCR
3. Chromatin Immunoprecipitation (ChIP)
Electrophoretic Mobility Assay (EMSA)
4. Results
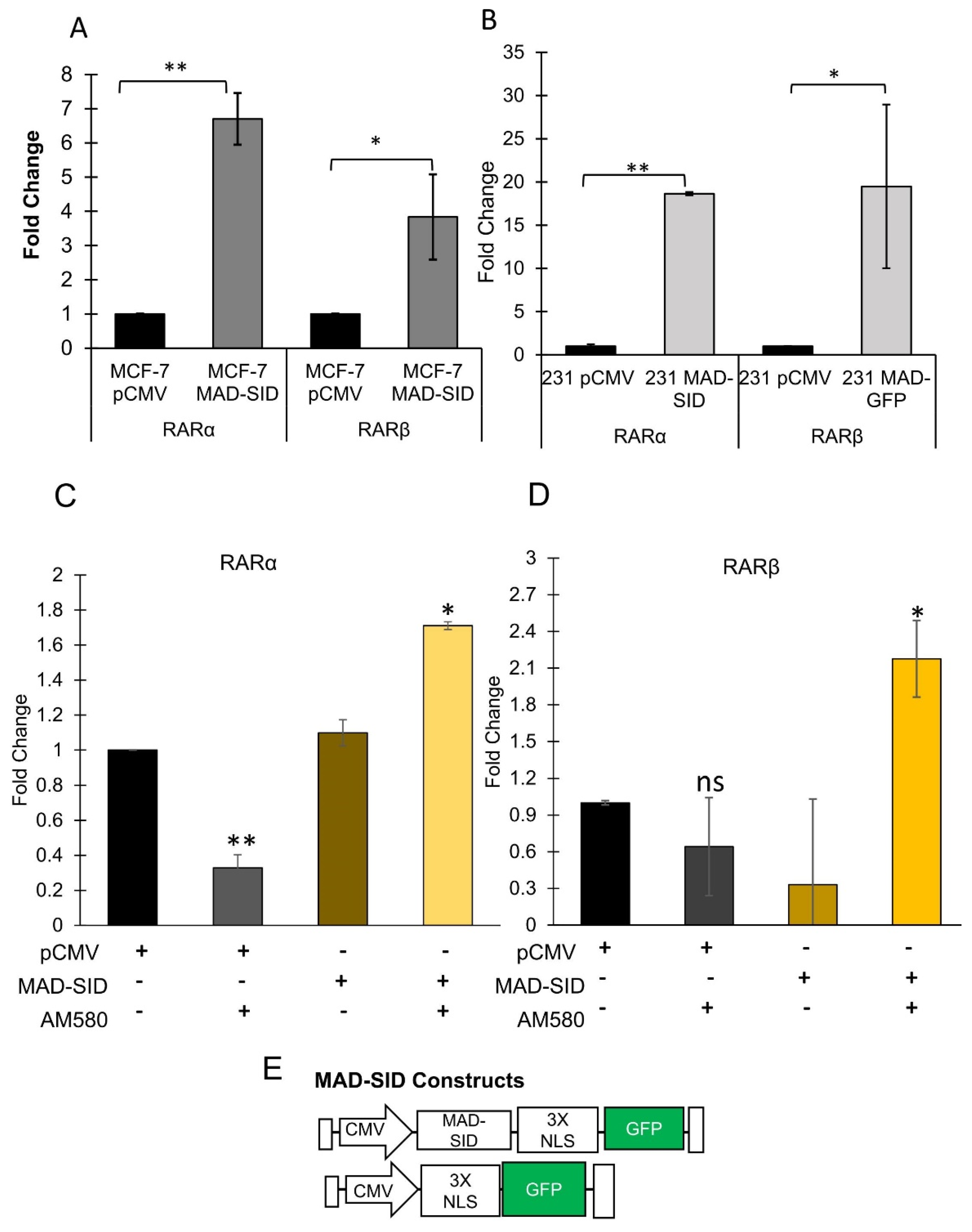
4.1. MAD1 Regulates RARα through the SIN3A PAH2 Domain
4.2. The MAD-SID Peptide Disrupts RARα/RXRα Interaction with Sin3A
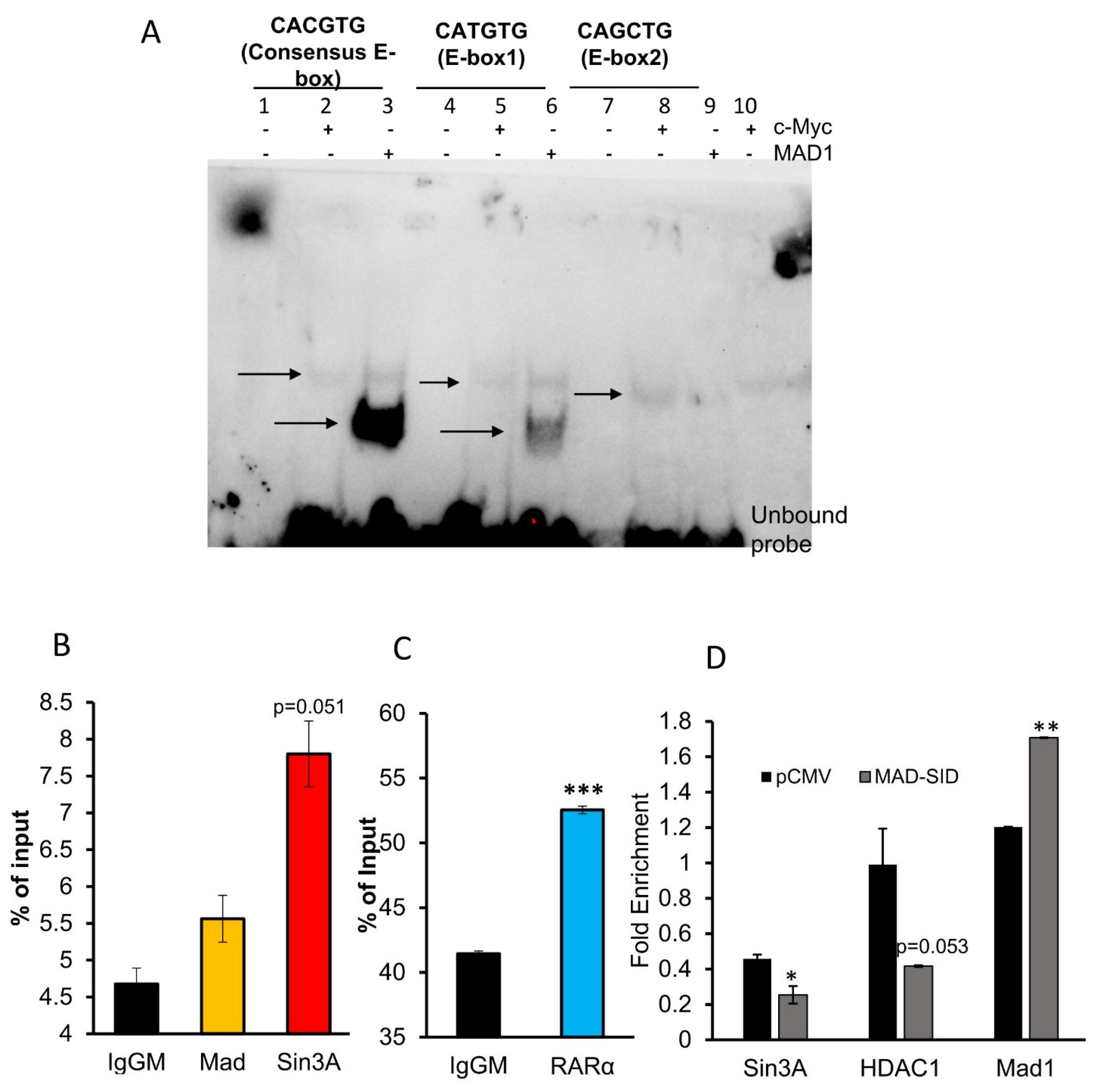
4.3. Putative E-Box in RARβ Promoter Binds with MAD1
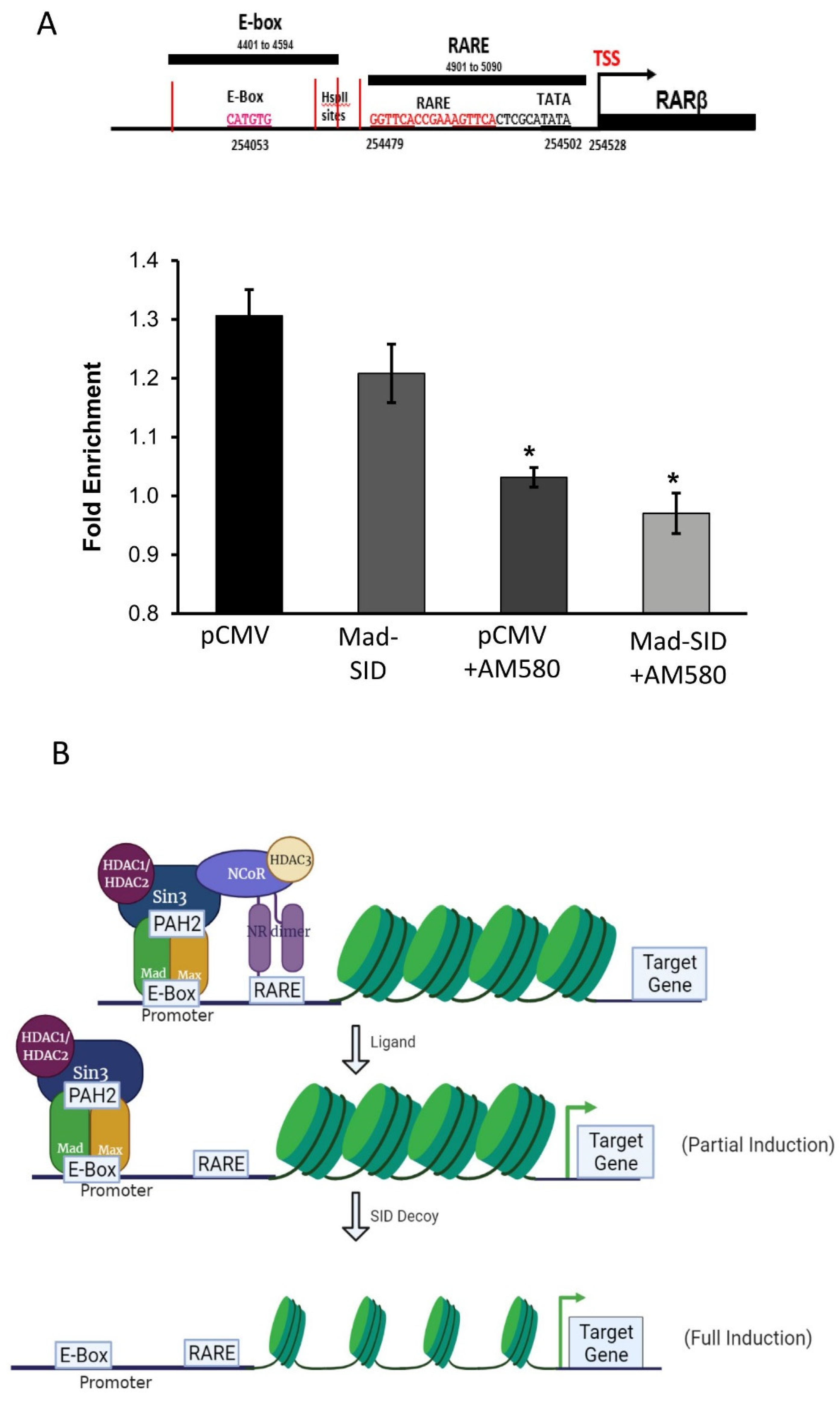
4.4. Members of SIN3A Complex Are Present at the RARβ Promoter
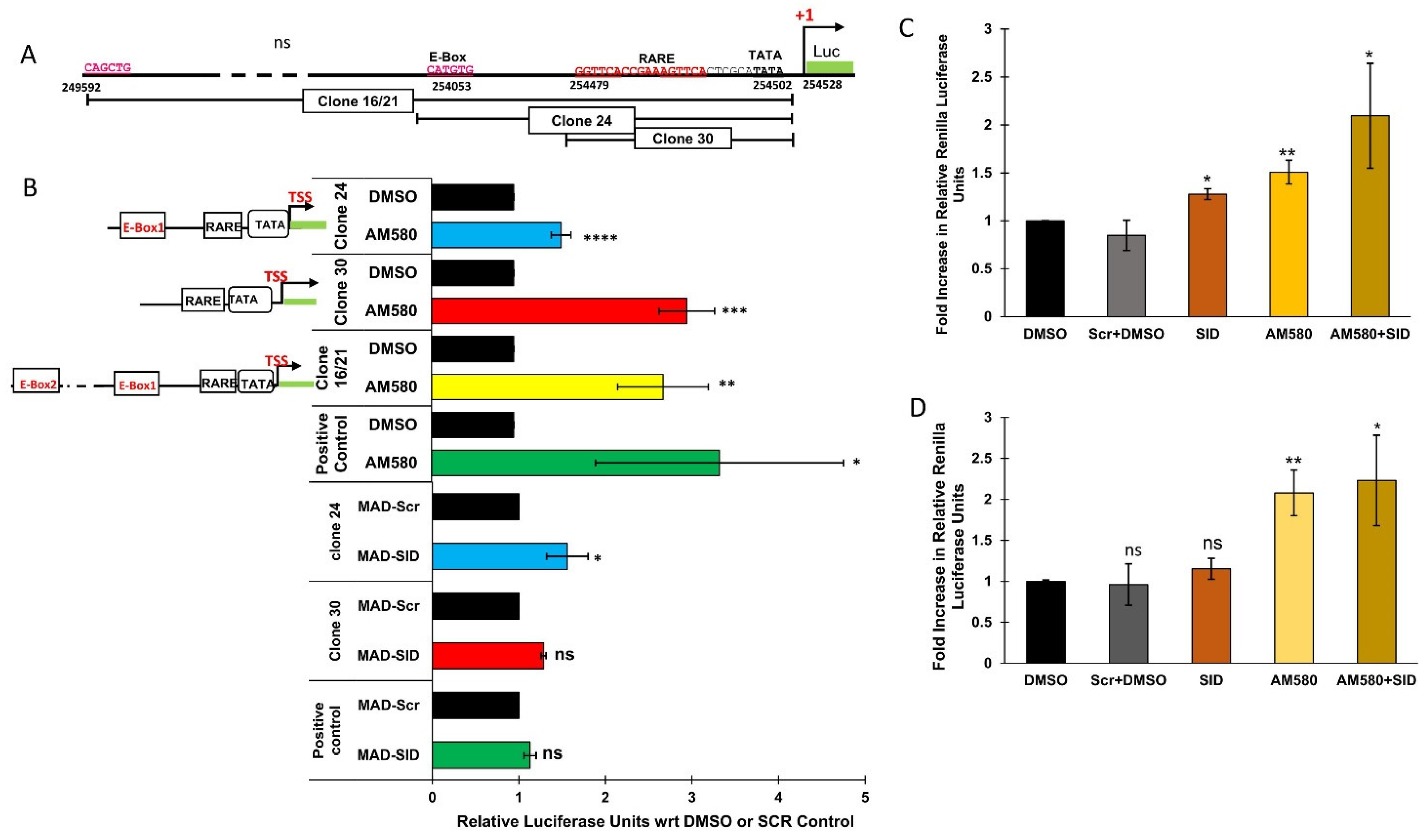
4.5. The RARβ Promoter Responds to AM580 and MAD-SID Treatment
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dilworth, F.J.; Chambon, P. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene 2001, 20, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Xu, W. Nuclear receptor coactivators: The key to unlock chromatin. Biochem. Cell Biol. 2005, 83, 418–428. [Google Scholar] [CrossRef]
- Perissi, V.; Staszewski, L.M.; McInerney, E.M.; Kurokawa, R.; Krones, A.; Rose, D.W.; Lambert, M.H.; Milburn, M.V.; Glass, C.K.; Rosenfeld, M.G. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999, 13, 3198–3208. [Google Scholar] [CrossRef]
- Lotan, R. Retinoids and their receptors in modulation of differentiation, development, and prevention of head and neck cancers. Anticancer Res. 1996, 16, 2415–2419. [Google Scholar]
- Altucci, L.; Gronemeyer, H. The promise of retinoids to fight against cancer. Nat. Rev. Cancer 2001, 1, 181–193. [Google Scholar] [CrossRef]
- Wan, H.; Hong, W.K.; Lotan, R. Increased retinoic acid responsiveness in lung carcinoma cells that are nonresponsive despite the presence of endogenous retinoic acid receptor (RAR) β by expression of exogenous retinoid receptors retinoid X receptor α, RARα, and RARγ. Cancer Res. 2001, 61, 556–564. [Google Scholar] [PubMed]
- Wu, Q.; Dawson, M.I.; Zheng, Y.; Hobbs, P.D.; Agadir, A.; Jong, L.; Li, Y.; Liu, R.; Lin, B.; Zhang, X.K. Inhibition of trans-retinoic acid-resistant human breast cancer cell growth by retinoid X receptor-selective retinoids. Mol. Cell Biol. 1997, 17, 6598–6608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.K.; Liu, Y.; Lee, M.O. Retinoid receptors in human lung cancer and breast cancer. Mutat. Res.-Fundam. Mol. Mech. Mutagenesis 1996, 350, 267–277. [Google Scholar] [CrossRef]
- Kupumbati, T.S.; Cattoretti, G.; Marzan, C.; Farias, E.F.; Taneja, R.; Mira-y-Lopez, R. Dominant negative retinoic acid receptor initiates tumor formation in mice. Mol. Cancer 2006, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Marzan, C.V.; Kupumbati, T.S.; Bertran, S.P.; Samuels, T.; Leibovitch, B.; Mira-Y-Lopez, R.; Ossowski, L.; Farias, E.F. Adipocyte derived paracrine mediators of mammary ductal morphogenesis controlled by retinoic acid receptors. Dev. Biol. 2011, 349, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Widschwendter, M.; Berger, J.; Müller, H.M.; Zeimet, A.G.; Marth, C. Epigenetic downregulation of the retinoic acid receptor-β2 gene in breast cancer. J. Mammary Gland. Biol. Neoplasia 2001, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, S.; Jiang, C.; Wang, Y.; Zhu, H.; Wang, X.D. High expression of RARβ is a favorable factor in colorectal cancer. Dis. Markers 2019, 2019, 7138754. [Google Scholar] [CrossRef]
- Sirchia, S.; Ferguson, A.T.; Sironi, E.; Subramanyan, S.; Orlandi, R.; Sukumar, S.; Sacchi, N. Evidence of epigenetic changes affecting the chromatin state of the retinoic acid receptor β2 promoter in breast cancer cells. Oncogene 2000, 19, 1556–1563. [Google Scholar] [CrossRef]
- Ivanova, T.; Petrenko, A.; Gritsko, T.; Vinokourova, S.; Eshilev, E.; Kobzeva, V.; Kisseljov, F.; Kisseljova, N. Methylation and silencing of the retinoic acid receptor-β2 gene in cervical cancer. BMC Cancer 2002, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Wongwarangkana, C.; Wanlapakorn, N.; Chansaenroj, J.; Poovorawan, Y. Retinoic acid receptor beta promoter methylation and risk of cervical cancer. World J. Virol. 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, D.; Ma, Y.; Liu, H. Association between Retinoic acid receptor-β hypermethylation and NSCLC risk: A meta-analysis and literature review. Oncotarget 2017, 8, 5814–5822. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Widschwendter, M.; Berger, J.; Daxenbichler, G.; Widschwendter, A.; Zeimet, A.; Schröcksnadel, H.; Dapunt, O. Loss of Retinoic Acid Receptor β Expression in Breast Cancer and Morphologically Normal Adjacent Tissue but not in the Normal Breast Tissue Distant from the Cancer. Cancer Res. 1997, 57, 4158–4161. [Google Scholar] [CrossRef]
- Doan, T.B.; Cheung, V.; Clyne, C.D.; Hilton, H.N.; Eriksson, N.; Young, M.J.; Funder, J.W.; Muscat, G.E.O.; Fuller, P.J.; Clarke, C.L.; et al. A tumour suppressive relationship between mineralocorticoid and retinoic acid receptors activates a transcriptional program consistent with a reverse Warburg effect in breast cancer. Breast Cancer Res. 2020, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kadamb, R.; Leibovitch, B.A.; Farias, E.F.; Dahiya, N.; Suryawanshi, H.; Bansal, N.; Waxman, S. Invasive phenotype in triple negative breast cancer is inhibited by blocking SIN3A–PF1 interaction through KLF9 mediated repression of ITGA6 and ITGB1. Transl. Oncol. 2022, 16, 101320. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Bosch, A.; Leibovitch, B.; Pereira, L.; Cubedo, E.; Yu, J.; Pierzchalski, K.; Jones, J.W.; Fishel, M.; Kane, M.; et al. Blocking the PAH2 domain of Sin3A inhibits tumorigenesis and confers retinoid sensitivity in triple negative breast cancer. Oncotarget 2016, 7, 43689–43702. [Google Scholar] [CrossRef]
- Farias, E.F.; Petrie, K.; Leibovitch, B.; Murtagh, J.; Chornet, M.B.; Schenk, T.; Zelent, A.; Waxman, S. Interference with Sin3 function induces epigenetic reprogramming and differentiation in breast cancer cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11811–11816. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Xia, L.; Waxman, S. Targeted removal of PML-RARα protein is required prior to inhibition of histone deacetylase for overcoming all-trans retinoic acid differentiation resistance in acute promyelocytic leukemia. Blood 2002, 100, 1008–1013. [Google Scholar] [CrossRef]
- Heinzel, T.; Lavinsky, R.M.; Mullen, T.-M.; Soderstrom, M.; Laherty, C.D.; Torchia, J.A.; Yang, W.-M.; Brard, G.; Ngo, S.D.; Davie, J.; et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 1997, 387, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sucov, H.M.; Murakami, K.K.; Evans, R.M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc. Natl. Acad. Sci. USA 1990, 87, 5392–5396. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gianni, M.; Kopf, E.; Honoré, N.; Chelbi-Alix, M.; Koken, M.; Quignon, F.; Rochette-Egly, C.; de Thé, H. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor α (RARα) and oncogenic RARα fusion proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 14807–14812. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Lee, J.; Liang, X.; Wu, X.; Zhu, T.; Lo, P.-K.; Zhang, X.; Sukumar, S. HOXA5 acts directly downstream of retinoic acid receptor β and contributes to retinoic acid-induced apoptosis and growth inhibition. Cancer Res. 2007, 67, 8007–8013. [Google Scholar] [CrossRef]
- Loinder, K.; Söderström, M. The nuclear receptor corepressor (N-CoR) modulates basal and activated transcription of genes controlled by retinoic acid. J. Steroid Biochem. Mol. Biol. 2003, 84, 15–21. [Google Scholar] [CrossRef]
- Alland, L.; Muhle, R.A.; Hou, H.; Potes, J.; Chin, L.; Schreiber-Agus, N.; Depinho, R.A. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 1997, 387, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, O.; Glass, C.K.; Rosenfeld, M.G. Nuclear receptor coregulators: Multiple modes of modification. Trends Endocrinol. Metab. 2002, 13, 55–60. [Google Scholar] [CrossRef]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Bastien, J.; Rochette-Egly, C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 2004, 328, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Kao, H.-Y.; Chakravarti, D.; Lin, R.J.; Hassig, C.A.; Ayer, D.; Schreiber, S.L.; Evans, R.M. Nuclear Receptor Repression Mediated by a Complex Containing SMRT, mSin3A, and Histone Deacetylase. Cell 1997, 89, 373–380. [Google Scholar] [CrossRef]
- Farhana, L.; Dawson, M.I.; Leid, M.; Wang, L.; Moore, D.D.; Liu, G.; Xia, Z.; Fontana, J.A. Adamantyl-Substituted Retinoid-Related Molecules Bind Small Heterodimer Partner and Modulate the Sin3A Repressor. Cancer Res. 2007, 67, 318–325. [Google Scholar] [CrossRef]
- Moehren, U.; Dressel, U.; Reeb, C.A.; Väisänen, S.; Dunlop, T.W.; Carlberg, C.; Baniahmad, A. The highly conserved region of the co-repressor Sin3A functionally interacts with the co-repressor Alien. Nucleic Acids Res. 2004, 32, 2995–3004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cowley, S.M.; Kang, R.S.; Frangioni, J.V.; Yada, J.J.; DeGrand, A.M.; Radhakrishnan, I.; Eisenman, R.N. Functional Analysis of the Mad1-mSin3A Repressor-Corepressor Interaction Reveals Determinants of Specificity, Affinity, and Transcriptional Response. Mol. Cell. Biol. 2004, 24, 2698–2709. [Google Scholar] [CrossRef]
- Kumar, G.S.; Xie, T.; Zhang, Y.; Radhakrishnan, I. Solution structure of the mSin3A PAH2-Pf1 SID1 complex: A Mad1/Mxd1-like interaction disrupted by MRG15 in the Rpd3S/Sin3S complex. J. Mol. Biol. 2011, 408, 987–1000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Zhang, Z.; Demeler, B.; Radhakrishnan, I. Coupled Unfolding and Dimerization by the PAH2 Domain of the Mammalian Sin3A Corepressor. J. Mol. Biol. 2006, 360, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wotton, D.; Knoepfler, P.S.; Laherty, C.D.; Eisenman, R.N.; Massagué, J. The Smad transcriptional corepressor TGIF recruits mSin3. Cell Growth Differ. 2001, 12, 457–463. Available online: http://cgd.aacrjournals.org/cgi/content/full/12/9/457 (accessed on 2 September 2021).
- Yochum, G.S.; Ayer, D.E. Pf1, a Novel PHD Zinc Finger Protein That Links the TLE Corepressor to the mSin3A-Histone Deacetylase Complex. Mol. Cell. Biol. 2001, 21, 4110–4118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, Q.; Liu, Y.; Zhu, P.; Kang, C.; Xu, H.; Qi, B.; Wang, R.; Dong, Y.; Wu, X.Z. SIN3B promotes integrin αv subunit gene transcription and cell migration of hepatocellular carcinoma. J. Mol. Cell Biol. 2019, 11, 421–432. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahiya, N.R.; Leibovitch, B.A.; Kadamb, R.; Bansal, N.; Waxman, S. The Sin3A/MAD1 Complex, through Its PAH2 Domain, Acts as a Second Repressor of Retinoic Acid Receptor Beta Expression in Breast Cancer Cells. Cells 2022, 11, 1179. https://doi.org/10.3390/cells11071179
Dahiya NR, Leibovitch BA, Kadamb R, Bansal N, Waxman S. The Sin3A/MAD1 Complex, through Its PAH2 Domain, Acts as a Second Repressor of Retinoic Acid Receptor Beta Expression in Breast Cancer Cells. Cells. 2022; 11(7):1179. https://doi.org/10.3390/cells11071179
Chicago/Turabian StyleDahiya, Nisha Rani, Boris A. Leibovitch, Rama Kadamb, Nidhi Bansal, and Samuel Waxman. 2022. "The Sin3A/MAD1 Complex, through Its PAH2 Domain, Acts as a Second Repressor of Retinoic Acid Receptor Beta Expression in Breast Cancer Cells" Cells 11, no. 7: 1179. https://doi.org/10.3390/cells11071179
APA StyleDahiya, N. R., Leibovitch, B. A., Kadamb, R., Bansal, N., & Waxman, S. (2022). The Sin3A/MAD1 Complex, through Its PAH2 Domain, Acts as a Second Repressor of Retinoic Acid Receptor Beta Expression in Breast Cancer Cells. Cells, 11(7), 1179. https://doi.org/10.3390/cells11071179





