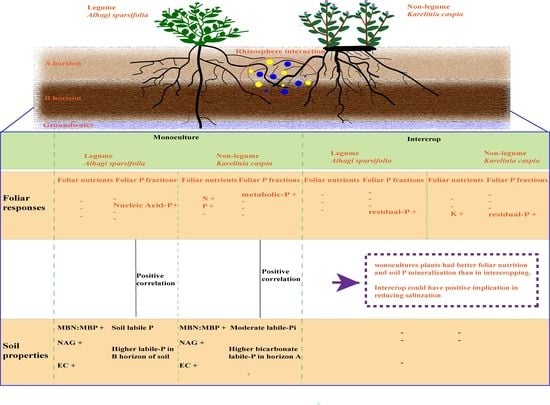
Intercropping of Leguminous and Non-Leguminous Desert Plant Species Does Not Facilitate Phosphorus Mineralization and Plant Nutrition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Plant and Soil Sampling
2.3. Foliar P Fraction Determination
2.4. Soil P Fraction Determination
2.5. Leaf Trait Determination
2.6. Soil Properties Analysis
2.7. Statistical Analysis
3. Results
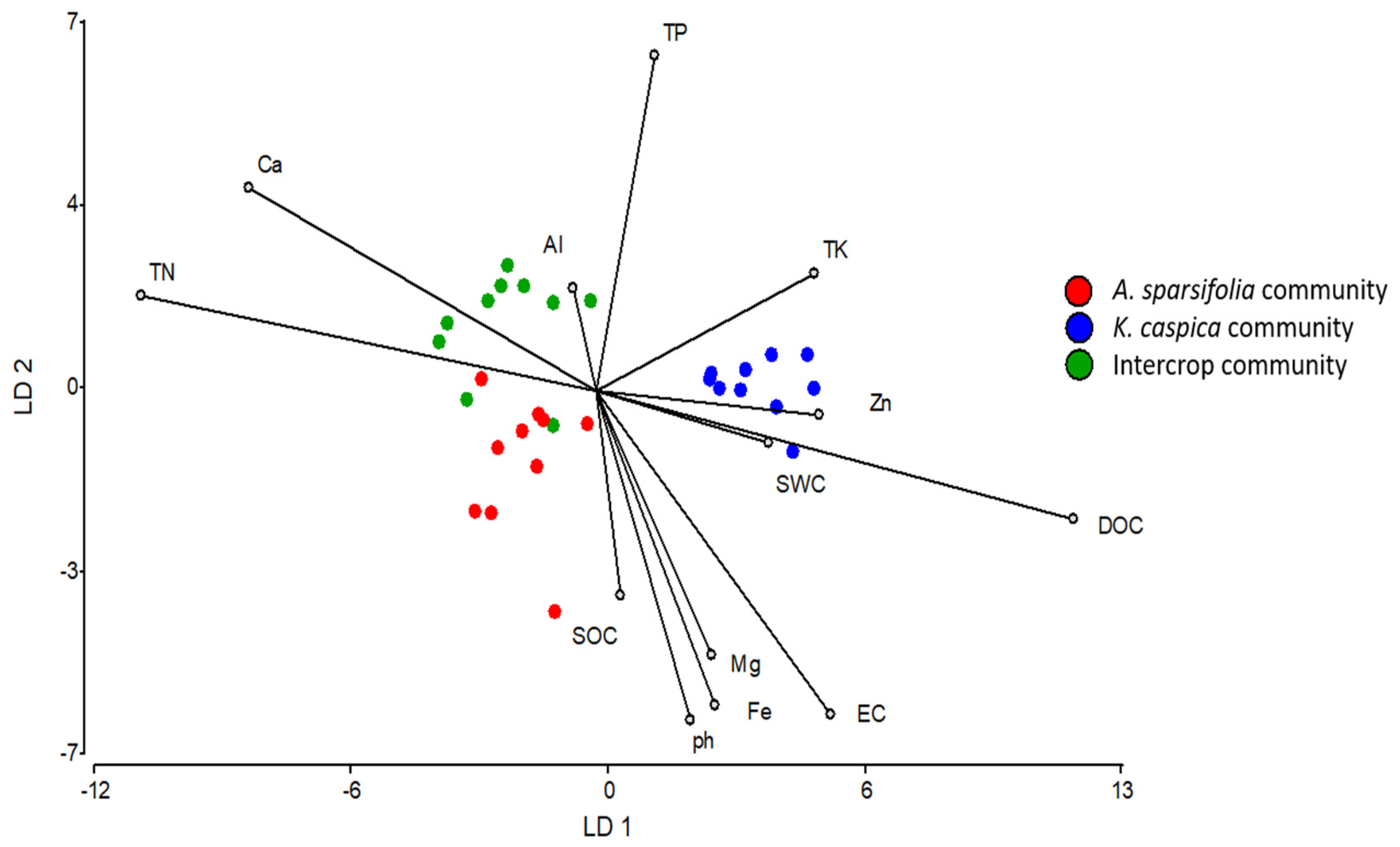
3.1. Responses of Soil Properties to Plant Community
| No Significance | ||||||
|---|---|---|---|---|---|---|
| Soil layer | A horizon | B horizon | ||||
| Planting pattern | A. sparsifolia | K. caspia | Intercrop | A. sparsifolia | K. caspia | Intercrop |
| DOC | 16.82 ± 1.81 | 14.54 ± 0.36 | 12.62 ± 0.99 | 13.33 ± 0.61 | 12.74 ± 0.62 | 13.97 ± 1.25 |
| Zn | 38.45 ± 0.29 | 37.91 ± 1.19 | 38.75 ± 0.94 | 39.43 ± 0.59 | 40.35 ± 1.76 | 37.84 ± 0.83 |
| Ca | 68.24 ± 1.45 | 64.55 ± 1.25 | 67.45 ± 0.58 | 67.61 ± 0.57 | 66.16 ± 0.48 | 66.92 ± 0.33 |
| Planting pattern × soil layer interaction | ||||||
| Soil layer | A horizon | B horizon | ||||
| Planting pattern | A. sparsifolia | K. caspia | Intercrop | A. sparsifolia | K. caspia | Intercrop |
| SWC | 0.43 ± 4.61bc | 0.53 ± 6.03bc | 0.38 ± 5.55c | 0.91 ± 11.45bc | 1.98 ± 49.49a | 1.15 ± 9.31b |
| TN | 0.24 ± 0.00a | 0.18 ± 0.01b | 0.21 ± 0.01ab | 0.24 ± 0.01a | 0.19 ± 0.00b | 0.24 ± 0.01a |
| TK | 17.44 ± 0.08c | 18.18 ± 0.14a | 17.76 ± 0.06b | 17.88 ± 0.05b | 17.73 ± 0.08b | 17.86 ± 0.05b |
| Mg | 14.62 ± 0.17a | 13.33 ± 0.21c | 13.87 ± 0.2bc | 14.34 ± 0.09ab | 13.94 ± 0.15bc | 14.35 ± 0.26ab |
| No interaction | ||||||
| Planting pattern | A. sparsifolia | K. caspia | Intercrop | Soil layer | A horizon | B horizon |
| EC | 98.57 ± 5.52b | 121.35 ± 7.24a | 97.28 ± 8.15b | pH | 9.63 ± 0.02a | 9.57 ± 0.02b |
| SOC | 2.69 ± 0.04a | 2.27 ± 0.06b | 2.51 ± 0.13ab | TP | 0.60 ± 0.00b | 0.61 ± 0.00a |
| Fe | 20.86 ± 0.14a | 20.49 ± 0.17ab | 20.29 ± 0.12b | |||
| Al | 32.07 ± 0.47ab | 30.76 ± 0.53b | 32.88 ± 0.52a | |||
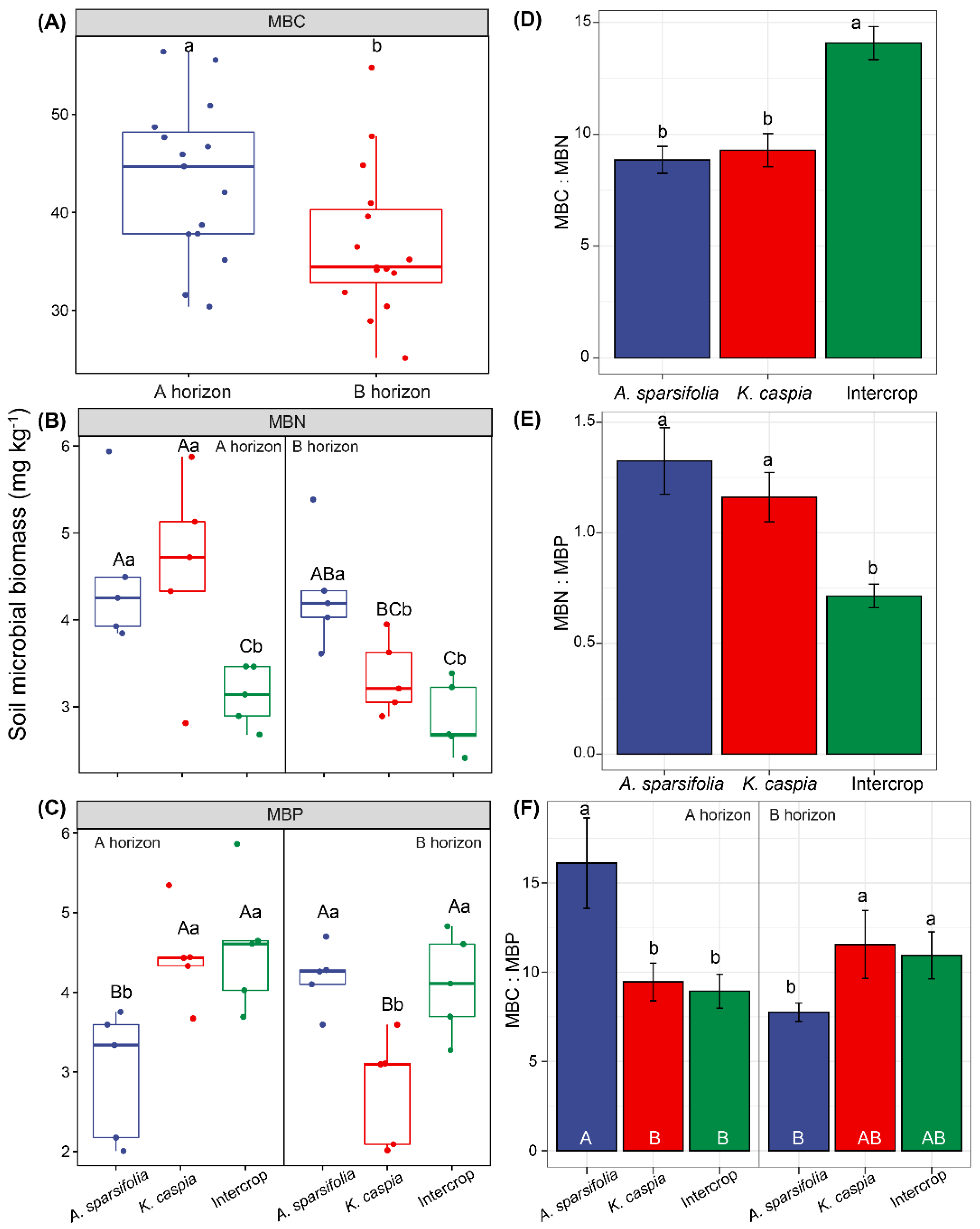
3.2. Soil Microbial Biomass C, N, and P Concentrations and Their Stoichiometry in Distinct Plant Communities
3.3. Soil P Fractions


3.4. Soil Enzyme Activity in Response to Soil Horizons and Plant Community Composition
3.5. Foliar Nutrients and Non-Structural Carbohydrates under Monoculture and Intercropping

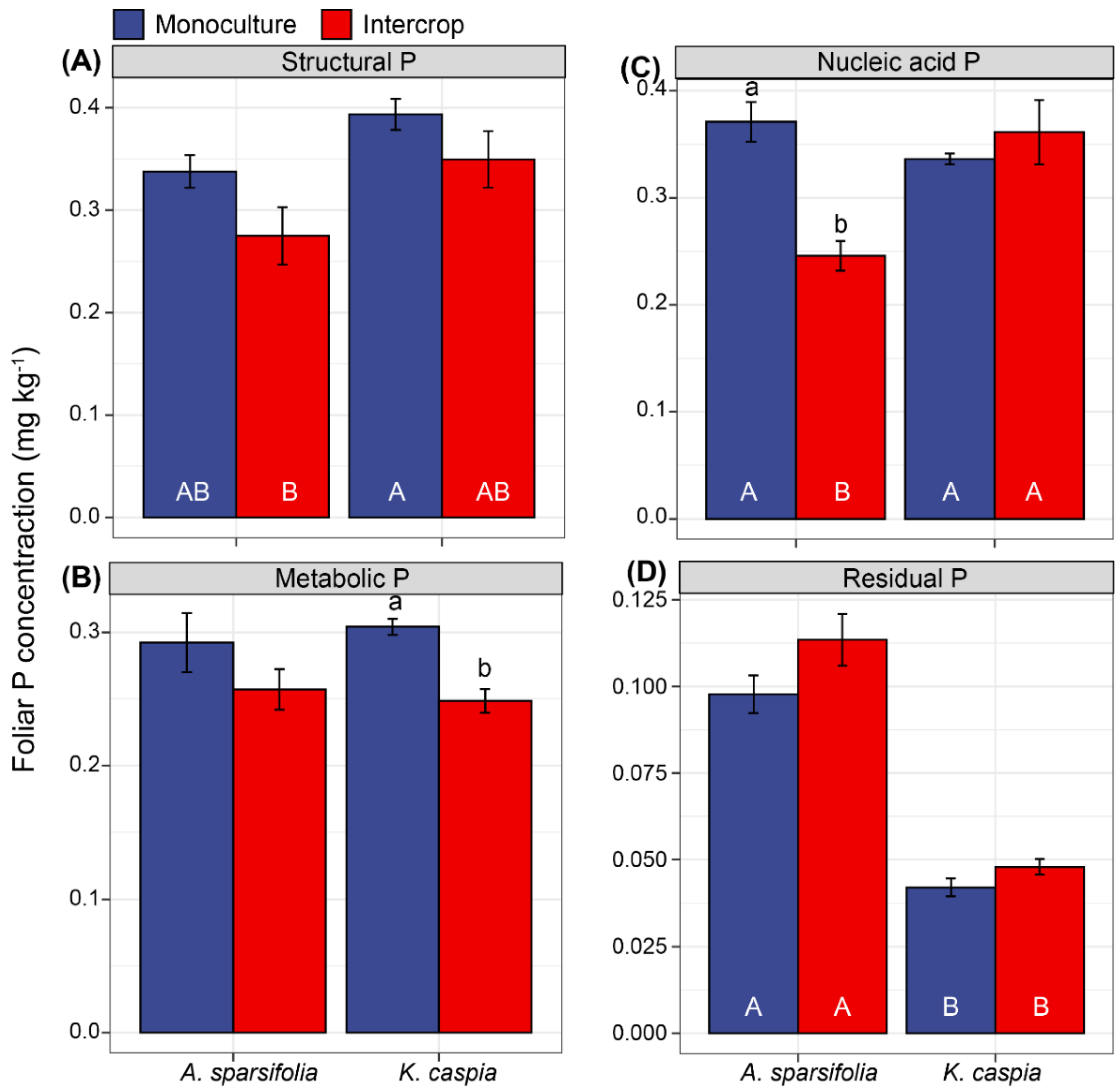
3.6. Foliar P Fractions in Response to Species Identity, and Monoculture and Intercropping Patterns

3.7. Relationships between Foliar P and Soil P Fractions and Soil Properties
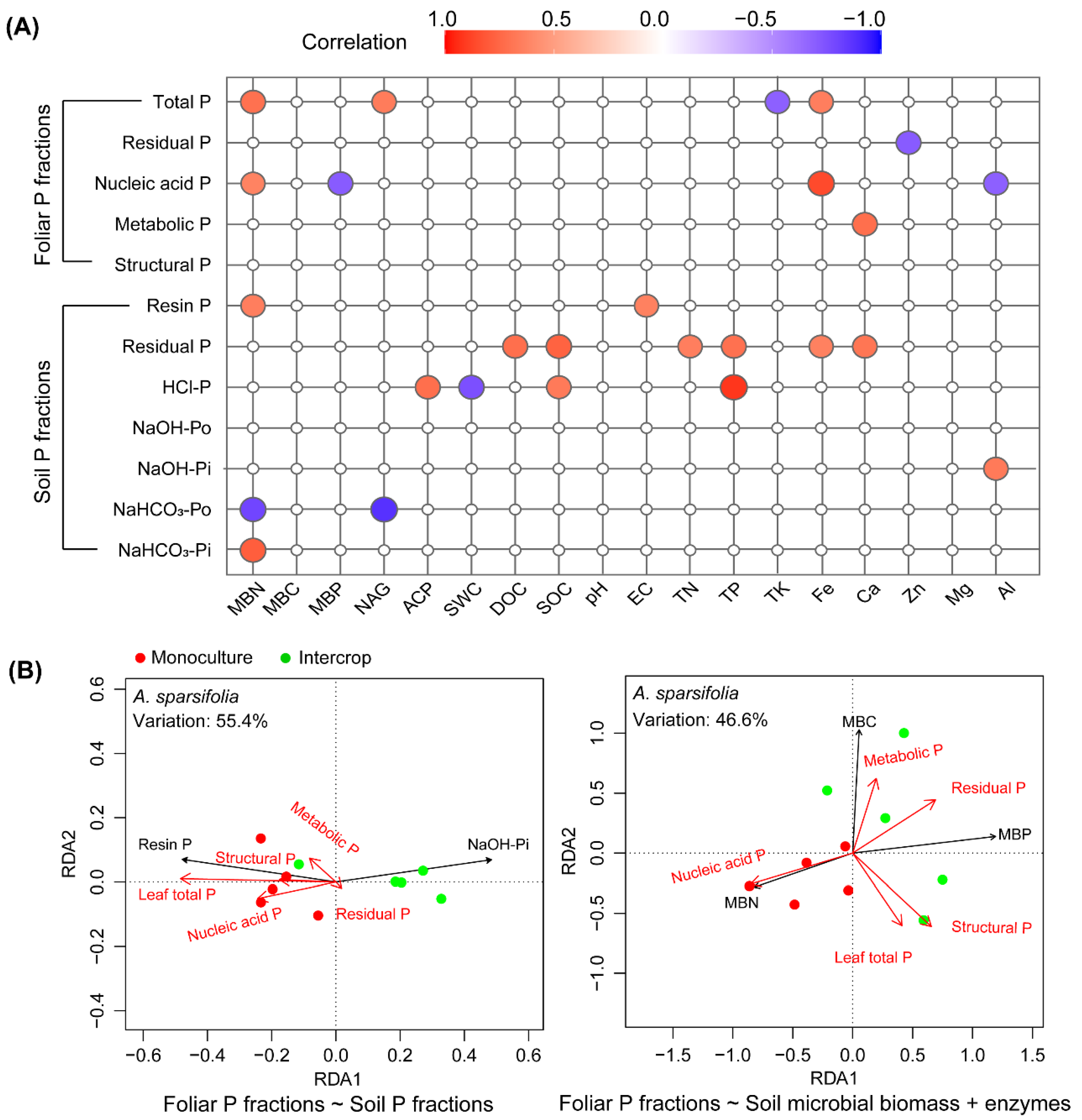
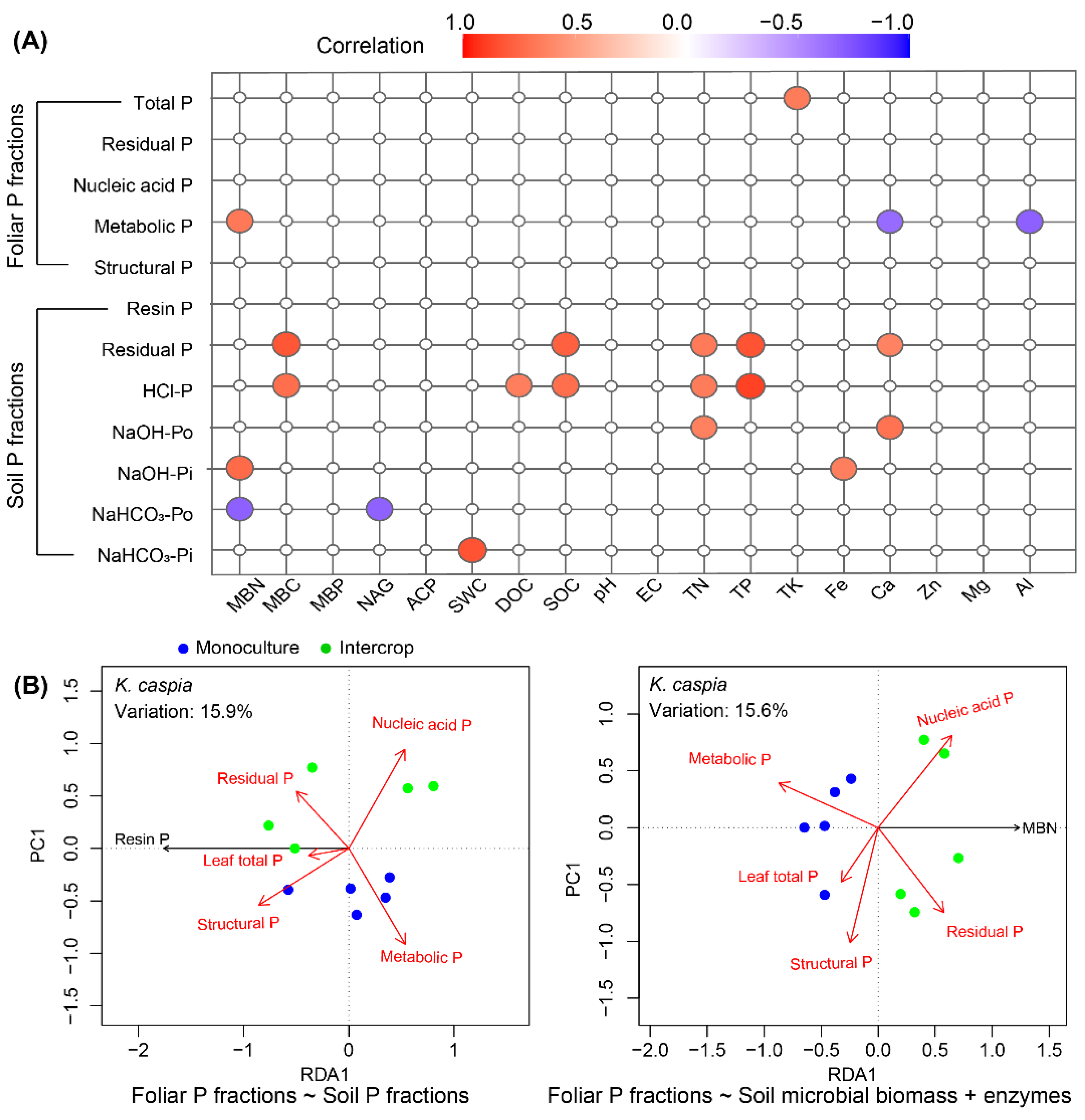
4. Discussion
4.1. Effects of Plant Community Composition on Soil Biochemical Properties and Foliar Nutrition
4.2. Variability of Foliar P Fractions in Response to Monoculture or Intercropping
4.3. Phosphorus Cycling across the Monoculture and Intercropping Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Y.; Li, P.F.; Hou, Z.A.; Yang, X.T. Physiological Characteristics of Drought Resistance in Karelinia caspia and Atriplex tatarica Combining Pressure-Volume Technique. In Advances in Electric and Electronics; Lecture Notes in Electrical Engineering; Hu, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 155. [Google Scholar]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Bui, E.N. Soil salinity: A neglected factor in plant ecology and biogeography. J. Arid. Environ. 2013, 92, 14–25. [Google Scholar] [CrossRef]
- Abdoulaye, A.O.; Lu, H.; Zhu, Y.; Hamoud, Y.A.; Sheteiwy, M. The Global Trend of the Net Irrigation Water Requirement of Maize from 1960 to 2050. Climate 2021, 7, 124. [Google Scholar] [CrossRef]
- Li, M.; Petrie, M.D.; Tariq, A.; Zeng, F. Response of nodulation, nitrogen fxation to salt stress in a desert legume Alhagi sparsifolia. Environ. Exp. Bot. 2021, 183, 104348. [Google Scholar] [CrossRef]
- Liu, R.; Ma, Y.; Zhao, X.; Chen, S.; Mi, Y.; Wang, T. Anatomical structures of Reaumuria soongorica in Alashan desert. Pak. J. Bot. 2018, 50, 2299. [Google Scholar]
- Orians, G.H.; Solbrig, O.T. A cost-income model of leaves and roots with special reference to arid and semi-arid areas. Am. Nat. 1977, 111, 677–690. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Gulías, J.; Figueroa, C.M.; Iñiguez, C.; Clemente-Moreno, M.J.; Nunes-Nesi, A.; Fernie, A.R.; Cavieres, L.A.; Bravo, L.A.; García-Plazaola, J.I.; et al. How do vascular plants perform photosynthesis in extreme environments? An integrative ecophysiological and biochemical story. Plant J. 2020, 101, 979–1000. [Google Scholar] [CrossRef]
- Maimaiti, A.; Iwanaga, F.; Taniguchi, T.; Hara, N.; Matsuo, N.; Mori, N.; Yunus, Q.; Yamanaka, N. Inorganic and organic osmolytes accumulation in five halophytes growing in saline habitats around the Aiding Lake area in Turpan Basin, Northwest China. Arid. Land Res. Manag. 2016, 30, 421–431. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A.; Zhang, K.; Li, X.; Tan, H.; Gao, Y.; Li, G. Leaf nitrogen and phosphorus of temperate desert plants in response to climate and soil nutrient availability. Sci. Rep. 2014, 4, 6932. [Google Scholar] [CrossRef]
- Zhu, J.-T.; Li, X.-Y.; Zhang, X.-M.; Yu, Q.; Lin, L.-S. Leaf nitrogen allocation and partitioning in three groundwater-dependent herbaceous species in a hyper-arid desert region of North-Western China. Aust. J. Bot. 2012, 60, 61–67. [Google Scholar] [CrossRef][Green Version]
- Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M.; Guo, X.; Elshaikh, N.Z.; Khan, N.U.; Oumarou, A.; Rahim, S.F. Impact of alternative wetting and soil drying and soil clay content on the morphological and physiological traits of rice roots and their relationships to yield and nutrient use-efficiency. Agric. Water Manag. 2019, 223, 105706. [Google Scholar] [CrossRef]
- Yin, H.; Zheng, H.; Zhang, B.; Tariq, A.; Lv, G.; Zeng, F.; Graciano, C. Stoichiometry of C:N:P in the Roots of Alhagi sparsifolia Is More Sensitive to Soil Nutrients Than Aboveground Organs. Front. Plant Sci. 2021, 12, 698961. [Google Scholar] [CrossRef] [PubMed]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant nutrition under climate change and soil carbon sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, Y.A.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.; Chen, S.; Qiu, R.; Elbashier, M.A.A. Effect of Irrigation Regimes and Soil Texture on the Potassium Utilization Efficiency of Rice. Agronomy 2019, 9, 100. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Li, N.; Song, D.; Sun, F.; Wu, X.; Dakhil, M.A.; et al. Impact of phosphorus application on drought resistant responses of Eucalyptus grandis seedlings. Physiol. Plant 2019, 166, 894–908. [Google Scholar] [CrossRef]
- Radersma, S.; Grierson, P.F. Phosphorus mobilization in agroforestry: Organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant Soil 2004, 259, 209–219. [Google Scholar] [CrossRef]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A. Root decisions. Plant Cell Environ. 2009, 32, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Ndour, P.M.S.; Heulin, T.; Achouak, W. The rhizos heath: From desert plants adaptation to crop breeding. Plant Soil 2020, 456, 1–13. [Google Scholar] [CrossRef]
- Hoover, D.L.; Pfennigwerth, A.A.; Duniway, M.C. Drought resistance and resilience: The role of soil moisture–plant interactions and legacies in a dryland ecosystem. J. Ecol. 2021, 109, 3280–3294. [Google Scholar] [CrossRef]
- Li, L.; Li, S.-M.; Sun, J.-H.; Zhou, L.-L.; Bao, X.-G.; Zhang, H.-G.; Zhang, F.-S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xia, H.; Christie, P.; Zhang, Z.; Li, L.; Tang, C. Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: A critical review. Ann. Bot. 2016, 117, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A.; Högberg, P.; Huss-Danell, K.; Mulder, C. Interspecific and spatial differences in nitrogen uptake in monocultures and two-species mixtures in north European grasslands. Funct. Ecol. 2002, 16, 454–461. [Google Scholar] [CrossRef]
- Gao, Y.J.; Tariq, A.; Zeng, F.J.; Graciano, C.; Zhang, Z.; Sardans, J.; Penualas, J. Allocation of foliar-P fraction of Alhagi sparsifolia and its relationship with soil-P fractions and soil properties in a hyperarid desert ecosystem. Geoderma 2022, 407, 115546. [Google Scholar] [CrossRef]
- Nunes, R.; de Sousa, D.M.G.; Goedert, W.J.; de Oliveira, L.E.Z.; Pavinato, P.S.; Pinheiro, T.D. Distribution of soil phosphorus fractions as a function of long-term soil tillage and phosphate fertilization management. Front. Earth Sci. 2020, 8, 350. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil-phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Helfenstein, J.; Tamburini, F.; Sperber, C.V.; Massey, M.S.; Pistocchi, C.; Chadwick, O.A.; Vitousek, P.M.; Kretzschmar, R.; Frossard, E. Combining spectroscopic and isotopic techniques gives a dynamic view of phosphorus cycling in soil. Nat. Commun. 2018, 9, 3226. [Google Scholar] [CrossRef]
- Jiang, M.K.; Caldararu, S.; Zaehle, S.; Ellworth, D.S.; Medlyn, B.E. Towards a more physiological representation of vegetation phosphorus processes in land surface models. New Phytol. 2019, 222, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Ishihara, H.; Schlereth, A.; Cawthray, G.R.; Encke, B.; Giavalisco, P.; Ivakov, A.; Arrivault, S.; Jost, R.; Krohn, N.; et al. Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ. 2014, 37, 1276–1298. [Google Scholar] [CrossRef]
- Hayes, P.E.; Clode, P.L.; Oliveira, R.S.; Lambers, H. Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: An adaptation improving phosphorus-use efficiency. Plant Cell Environ. 2018, 41, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Tiziani, R.; Mimmo, T.; Valentinuzzi, F.; Pii, Y.; Celletti, S.; Cesco, S. Root handling affects carboxylates exudation and phosphate uptake of white lupin roots. Front. Plant Sci. 2020, 11, 584568. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173195. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- George, T.S.; Richardson, A.E.; Hadobas, P.A.; Simpson, R.J. Characterization of transgenic Trifolium subterraneum L. which expresses phyA and releases extracellular phytase: Growth and P nutrition in laboratory media and soil. Plant Cell Environ. 2004, 27, 1351–1361. [Google Scholar] [CrossRef]
- Hutchings, M.J.; John, E.A. The effects of environmental heterogeneity on root growth and root/shoot partitioning. Ann. Bot. 2004, 94, 1–8. [Google Scholar] [CrossRef]
- Hodge, A. Plastic plants and patchy soils. J. Exp. Bot. 2006, 52, 401–411. [Google Scholar] [CrossRef]
- Ho, M.; Sotomayor, J.C.R.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef]
- Ding, G.C.; Piceno, Y.M.; Heuer, H.; Weinert, N.; Dohrmann, A.B.; Carrillo, A.; Andersen, G.L.; Castellanos, T.; Tebbe, C.C.; Smalla, K. Changes of soil bacterial diversity as a consequence of agricultural land use in a semi-arid ecosystem. PLoS ONE 2013, 8, e59497. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Wu, P.; He, J.; Chen, X.; Gao, Y.; Cao, X. Radiation interception and utilization by wheat/maize strip intercropping systems. Agric. For. Meteorol. 2015, 204, 58–66. [Google Scholar] [CrossRef]
- Gong, X.; Liu, C.; Li, J.; Luo, Y.; Yang, Q.; Zhang, W. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Till. Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Solanki, M.K.; Wang, F.Y.; Wang, Z.; Li, C.N.; Lan, T.J.; Singh, R.K. Rhizospheric and endospheric diazotrophs mediated soil fertility intensification in sugarcane-legume intercropping systems. J. Soils Sediments 2019, 19, 1911–1927. [Google Scholar] [CrossRef]
- Huang, J.; Ji, M.; Wang, S.; He, Y.; Ran, J. Global semi-arid climate change over last 60 years. Clim. Dyn. 2015, 46, 1131–1150. [Google Scholar] [CrossRef]
- Scholes, R.J. The future of semi-arid regions: A weak fabric unravels. Climate 2020, 8, 43. [Google Scholar] [CrossRef]
- Dai, S.Y.; Lei, J.Q.; Zhao, J.F.; Fan, J.L.; Fan, D.D.; Zeng, F.J.; Ye, K.Q. Groundwater characteristics and their effects to eco-environmental of desert-oasis transitional zone in western Qira. J. Arid. Land Res. Environ. 2009, 23, 99–103. [Google Scholar]
- Niederberger, J.; Kohler, M.; Bauhus, J. Distribution of phosphorus fractions with different plant availability in German forest soils and their relationship with common soil properties and foliar P contents. Soil 2019, 5, 189–204. [Google Scholar] [CrossRef]
- Hou, E.Q.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Q.; Li, K.; Gong, Y.; Liu, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- Zhang, Z.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, B. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef]
- Ning, L.H.; Du, W.K.; Song, H.N.; Shao, H.B.; Qi, W.C.; Sheteiwy, M.S.A.; Yu, D.Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Env. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Tang, G.L.; Li, X.Y.; Lin, L.S. Impact of phloem girdling on water status in desert plants Alhagi sparsifolia Shap. (Fabaceae) and Karelinia caspia (Pall.) Less. (Asteraceae). Braz. J. Bot. 2015, 38, 717–728. [Google Scholar] [CrossRef]
- Thomas, F.; Arndt, S.; Bruelheide, H.; Foetzki, A.; Gries, D.; Huang, J.; Runge, M. Ecological basis for a sustainable management of the indigenous vegetation in a Central-Asian desert: Presentation and first results. J. Appl. Bot. 2000, 74, 212–219. [Google Scholar]
- Zhang, X.; Liao, M.; Chang, D. Comparative transcriptome analysis of the Asteraceae halophyte Karelinia caspia under salt stress. BMC Res. Notes 2014, 7, 927. [Google Scholar] [CrossRef] [PubMed]
- Gui, D. Characteristics of the clonal propagation of Alhagi sparsifolia Shap. (Fabaceae) under different groundwater depths in Xinjiang, China. Rangeland J. 2013, 35, 355–362. [Google Scholar] [CrossRef]
- Kedrowski, K.A. Extraction and analysis of nitrogen, phosphorus and carbon fractions in plant material. J. Plant Nutr. 1983, 6, 989–1011. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J. Ecol. 2011, 99, 849–857. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, W.; Zou, B.; Neher, D.A.; Fu, S.; Xia, H.; Li, Z. Seedling growth and soil nutrient availability in exotic and native tree species: Implications for afforestation in southern China. Plant Soil. 2013, 364, 207–218. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, S.; Jin, T. Development and application of the intellectual instrument for field whole-machine balancing. Chin. J. Sci. Instrum. 2003, 24, 127. [Google Scholar]
- Liu, C.; Wang, Y.; Pan, K.; Zhu, T.; Li, W.; Zhang, L. Carbon and Nitrogen Metabolism in Leaves and Roots of Dwarf Bamboo (Fargesia denudata Yi) Subjected to Drought for Two Consecutive Years During Sprouting Period. J. Plant Growth Regul. 2014, 33, 243–255. [Google Scholar] [CrossRef]
- Nelson, D.W.L.; Sommers, A.L.; Page, R.H.; Miller, D.R. Total carbon, organic carbon, and organic matter. Methods Soil Anal. 1982, 9, 539–552. [Google Scholar]
- He, X.R.; Mulvaney, R.L.; Banwart, W. A Rapid Method for Total Nitrogen Analysis Using Microwave Digestion. Soil Sci. Soc. Am. J. 1990, 54, 1625–1629. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hou, M.T.; Dan, M.A.; Han, X.H.; Zhang, R.Y.; Zhang, X.Z. Determination of Total Phosphorus in Soil by Alkali Fusion-Mo-Sb Anti-Spectrophotometric Method; China Standardization: Gansu, China, 2018. [Google Scholar]
- Turner, B.L.; Joseph Wright, S. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Wang, M.; Xue, J.; Horswell, J.; Kimberley, M.O.; Huang, Z. Long-term biosolids application alters the composition of soil microbial groups and nutrient status in a pine plantation. Biol. Fert. Soils 2017, 53, 799–809. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Torsten, H.B.; Peter, W.F. Simultaneous Inference in General Parametric Models. Biomet. J. 2008, 50, 346–363. [Google Scholar]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2018. [Google Scholar]
- Kassambara, A. Package ‘ggcorrplot’. R Package Version 0.1.3. 2019. Available online: https://cran.r-project.org/web/packages/ggcorrplot/ggcorrplot.pdf (accessed on 19 May 2019).
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2, Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Olatunji, O.A.; Luo, H.; Pan, K.; Tariq, A.; Sun, X.; Chen, W.; Wu, X.; Zhang, L.; Xiong, C.; Li, Z.; et al. Influence of phosphorus application and water deficit on the soil microbiota of N2-fixing and non-nitrogen fixing tree. Ecosphere 2018, 9, e02276. [Google Scholar] [CrossRef]
- Wang, J.; Gu, W.; Xia, L.; Li, B.; Guo, W. Genetic variation and selection of wood property of Alnus cremastogyne provenance and family. For. Res. 2001, 14, 362–368. [Google Scholar]
- Kramer, C.; Gleixner, G. Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol. Biochem. 2006, 38, 3267–3278. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, L.; Ma, S.; Fang, F.; Schmid, B.; Xu, L.; Zhu, J.; Li, P.; Losapio, G.; Jing, X.; et al. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in China. Sci. Total Environ. 2017, 607–608, 1367–1375. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.B.; Plaxton, W.C.; Lambers, H.; Siebers, M.; Marambe, B.; Wasaki, J. Molecular mechanisms underpinning phosphorus-use efficiency in rice. Plant Cell Environ. 2018, 41, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Li, Z.; Sayer, E.J.; Lambers, H.; Li, Y.; Zou, B.; Tang, J.; Heskel, M.; Ding, Y.; Wang, F. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct. Ecol. 2018, 33, 503–513. [Google Scholar] [CrossRef]
- Lambers, H.; Cawthray, G.R.; Giavalisco, P.; Kuo, J.; Laliberte, E.; Pearse, S.J.; Turner, B.L. Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol. 2012, 196, 1098–1108. [Google Scholar] [CrossRef]
- Schlüter, U.; Colmsee, C.; Scholz, U.; Brautigam, A.; Weber, A.P.; Zellerhoff, N.; Sonnewald, U. Adaptation of maize source leaf metabolism to stress related disturbances in carbon, nitrogen and phosphorus balance. BMC Genom. 2013, 14, 442. [Google Scholar] [CrossRef]
- Blecker, S.W.; Ippolito, J.A.; Barrett, J.E.; Wall, D.H.; Virginia, R.A.; Norvell, K.L. Phosphorus fractions in soils of Taylor Valley, Antarctica. Soil Sci. Soc. Am. J. 2006, 70, 806–815. [Google Scholar] [CrossRef]
- Bate, D.B.; Barrett, J.E.; Poage, M.A.; Virginia, R.A. Soil phosphorus cycling in an Antarctic polar desert. Geoderma 2008, 144, 21–31. [Google Scholar] [CrossRef]
- Costa, M.; Gama-Rodrigues, A.; Gonçalves, J.; Gama-Rodrigues, E.; Sales, M.; Aleixo, S. Labile and Non-Labile Fractions of Phosphorus and Its Transformations in Soil under Eucalyptus Plantations, Brazil. Forests 2016, 7, 15. [Google Scholar] [CrossRef]
- Zhang, X.; Gagnon, B.; Ziadi, N.; Cambouris, A.N.; Alotaibi, K.D.; Hu, Z. Soil Phosphorus Fractionation as Affected by Paper Mill Biosolids Applied to Soils of Contrasting Properties. Front. Environ. Sci. 2020, 8, 38. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.W.; Jin, Y.; Tang, J.; Lin, F.; Olatunji, O.A. Perennial cover crop biomass contributes to regulating soil P availability more than rhizosphere P-mobilizing capacity in rubber-based agroforestry systems. Geoderma 2021, 401, 115218. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, J.; Gale, W.J.; Yang, H.; Zhang, F. Soil aggregation and aggregate-associated organic carbon under typical natural halophyte communities in arid saline areas of Northwest China. Pedosphere 2016, 30, 236–243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, A.; Sardans, J.; Peñuelas, J.; Zhang, Z.; Graciano, C.; Zeng, F.; Olatunji, O.A.; Ullah, A.; Pan, K. Intercropping of Leguminous and Non-Leguminous Desert Plant Species Does Not Facilitate Phosphorus Mineralization and Plant Nutrition. Cells 2022, 11, 998. https://doi.org/10.3390/cells11060998
Tariq A, Sardans J, Peñuelas J, Zhang Z, Graciano C, Zeng F, Olatunji OA, Ullah A, Pan K. Intercropping of Leguminous and Non-Leguminous Desert Plant Species Does Not Facilitate Phosphorus Mineralization and Plant Nutrition. Cells. 2022; 11(6):998. https://doi.org/10.3390/cells11060998
Chicago/Turabian StyleTariq, Akash, Jordi Sardans, Josep Peñuelas, Zhihao Zhang, Corina Graciano, Fanjiang Zeng, Olusanya Abiodun Olatunji, Abd Ullah, and Kaiwen Pan. 2022. "Intercropping of Leguminous and Non-Leguminous Desert Plant Species Does Not Facilitate Phosphorus Mineralization and Plant Nutrition" Cells 11, no. 6: 998. https://doi.org/10.3390/cells11060998
APA StyleTariq, A., Sardans, J., Peñuelas, J., Zhang, Z., Graciano, C., Zeng, F., Olatunji, O. A., Ullah, A., & Pan, K. (2022). Intercropping of Leguminous and Non-Leguminous Desert Plant Species Does Not Facilitate Phosphorus Mineralization and Plant Nutrition. Cells, 11(6), 998. https://doi.org/10.3390/cells11060998