Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
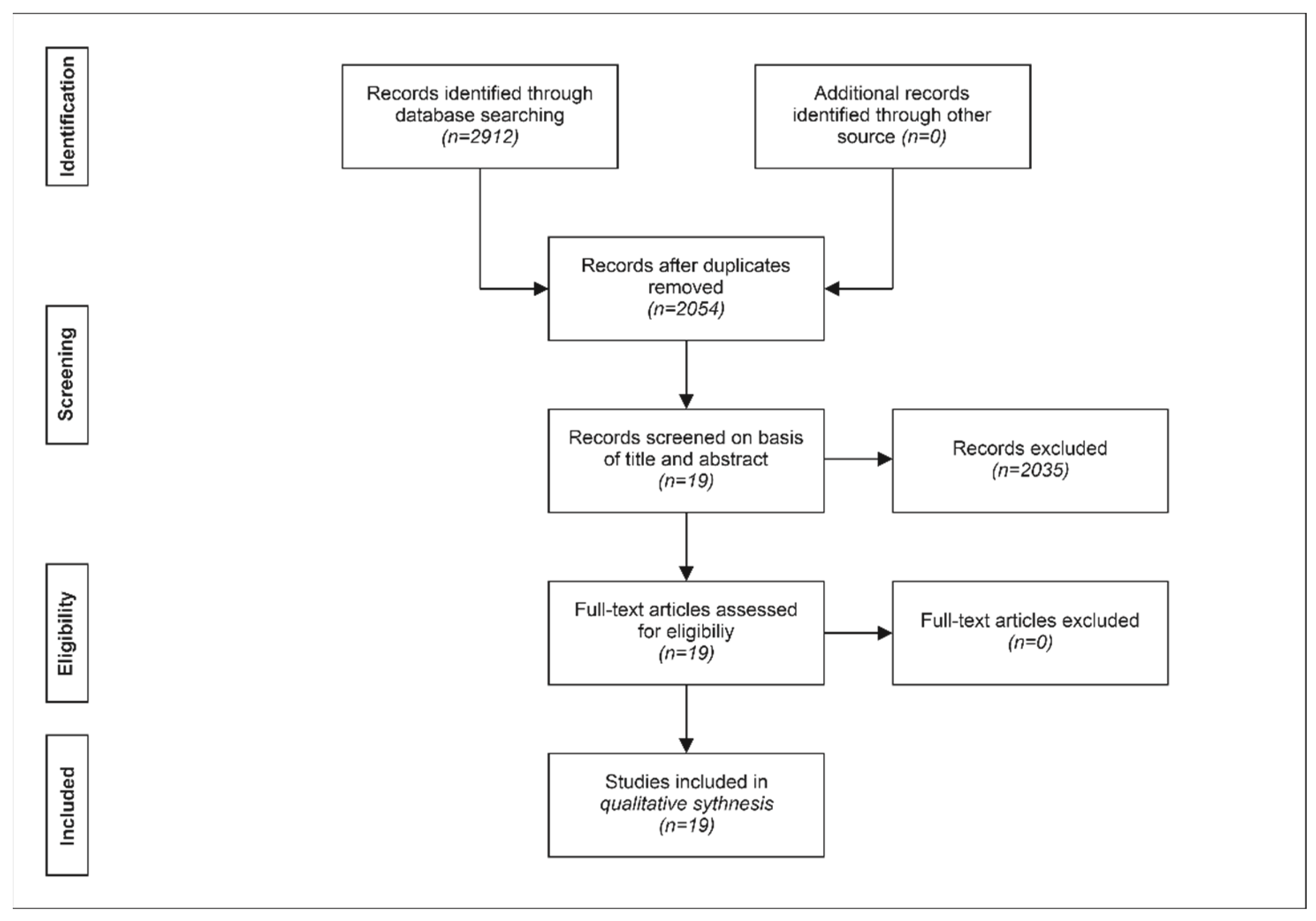
3.1. Assessment of Published Meta-Analyses
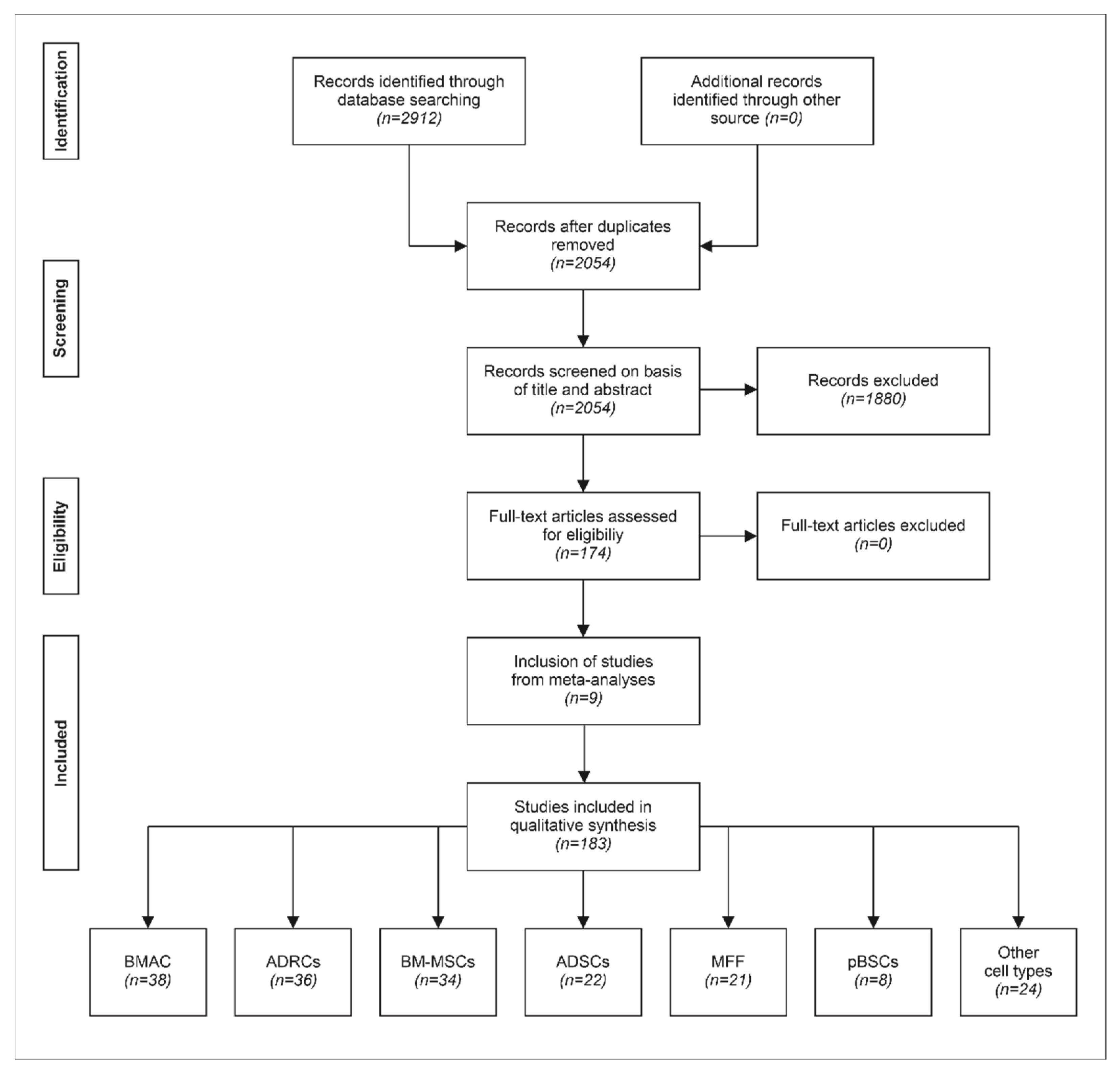
3.2. Systematic Assessment of Clinical Studies on Treatment of Primary Knee Osteoarthritis with Stem Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felson, D.T. Osteoarthritis of the Knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Agaliotis, M.; Bridgett, L.; Mackey, M.G. Hip and knee pain: Role of occupational factors. Best Pract. Res. Clin. Rheumatol. 2011, 25, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Ciapetti, A.; Carotti, M. The sources of pain in osteoarthritis: A pathophysiological review. Reumatismo 2014, 66, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, V. Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Lluch Girbés, E.; Nijs, J.; Torres-Cueco, R.; López Cubas, C. Pain treatment for patients with osteoarthritis and central sensitization. Phys. Ther. 2013, 93, 842–851. [Google Scholar] [CrossRef]
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef]
- Jüni, P.; Hari, R.; Rutjes, A.W.; Fischer, R.; Silletta, M.G.; Reichenbach, S.; da Costa, B.R. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015, 10, CD005328. [Google Scholar] [CrossRef]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy 2016, 32, 495–505. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- He, W.; Kuang, M.; Zhao, J.; Sun, L.; Lu, B.; Wang, Y.; Ma, J.; Ma, X. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: A meta-analysis. Int. J. Surg. 2017, 39, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cherian, J.J.; Parvizi, J.; Bramlet, D.; Lee, K.H.; Romness, D.W.; Mont, M.A. Preliminary results of a phase ii randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing tgf-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr. Cartil. 2015, 23, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone-marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Emadedin, M.; Labibzadeh, N.; Liastani, M.G.; Karimi, A.; Jaroughi, N.; Bolurieh, T.; Hosseini, S.-E.; Baharvand, H.; Aghdami, N. Intra-articular implantation of autologous bone marrow–derived mesenchymal stromal cells to treat knee osteoarthritis: A randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy 2018, 20, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Ha, C.-W.; In, Y.; Cho, S.-D.; Choi, E.-S.; Ha, J.-K.; Lee, J.-H.; Yoo, J.-D.; Bin, S.-I.; Choi, C.-H.; et al. A Multicenter, double-blind, Phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum. Gene Ther. Clin. Dev. 2018, 29, 48–59. [Google Scholar] [CrossRef]
- Kuah, D.; Sivell, S.; Longworth, T.; James, K.; Guermazi, A.; Cicuttini, F.; Wang, Y.; Craig, S.; Comin, G.; Robinson, D.; et al. Safety, tolerability and efficacy of intra-articular progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. J. Transl. Med. 2018, 16, 49. [Google Scholar] [CrossRef]
- Khalifeh Soltani, S.; Forogh, B.; Ahmadbeigi, N.; Hadizadeh Kharazi, H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: A Phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: A double-blinded prospective randomized controlled clinical trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, W.; Liu, H.; Cui, Y.; Mao, Q.; Fei, Z.; Xiang, C. Curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2016, 30, 1472–1477. (In Chinese) [Google Scholar] [PubMed]
- Goncars, V.; Jakobsons, E.; Blums, K.; Briede, I.; Patetko, L.; Erglis, K.; Erglis, M.; Kalnberzs, K.; Muiznieks, I.; Erglis, A. The comparison of knee osteoarthritis treatment with single-dose bone-marrow-derived mononuclear cells vs. hyaluronic acid injections. Medicina 2017, 53, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, Phase IIb clinical trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: Repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized Phase I/II trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Anz, A.W.; Hubbard, R.; Rendos, N.K.; Everts, P.A.; Andrews, J.R.; Hackel, J.G. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: A prospective, randomized trial. Orthop. J. Sports Med. 2020, 8, 232596711990095. [Google Scholar] [CrossRef]
- Dallo, I.; Szwedowski, D.; Mobasheri, A.; Irlandini, E.; Gobbi, A. A prospective study comparing leukocyte-poor platelet-rich plasma combined with hyaluronic acid and autologous microfragmented adipose tissue in patients with early knee osteoarthritis. Stem Cells Dev. 2021, 30, 651–659. [Google Scholar] [CrossRef]
- Kim, Y.S.; Suh, D.S.; Tak, D.H.; Chung, P.K.; Kwon, Y.B.; Kim, T.Y.; Koh, Y.G. Comparative matched-pair cohort analysis of the short-term clinical outcomes of mesenchymal stem cells versus hyaluronic acid treatments through intra-articular injections for knee osteoarthritis. J. Exp. Ortop. 2020, 7, 90. [Google Scholar] [CrossRef]
- Garay-Mendoza, D.; Villarreal-Martínez, L.; Garza-Bedolla, A.; Pérez-Garza, D.M.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Diaz-Hutchinson, C.; Gómez-Almaguer, D.; Jaime-Pérez, J.C.; Mancías-Guerra, C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 140–147. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Zhao, X.; Ruan, J.; Tang, H.; Li, J.; Shi, Y.; Li, M.; Li, S.; Xu, C.; Lu, Q.; Dai, C. Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res. Ther. 2019, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.; Mathias, M.; Andrade, R.; Amaral, R.J.F.C.; Schott, V.; Balduino, A.; Bastos, R.; Miguel Oliveira, J.; Reis, R.L.; Rodeo, S.; et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: A controlled, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Dai, C.; Du, H.; Li, S.; Ye, P.; Zhang, L.; Wang, X.; Song, Y.; Togashi, R.; Vangsness, C.T.; et al. Intra-articular injections of allogeneic human adipose-derived mesenchymal progenitor cells in patients with symptomatic bilateral knee osteoarthritis: A Phase I pilot study. Regen. Med. 2020, 15, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Auregan, J.C.; Dubory, A.; Flouzat-Lachaniette, C.H.; Chevallier, N.; Rouard, H. Subchondral stem cell therapy versus contralateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. Int. Orthop. 2018, 42, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Bouthors, C.; Bastard, C.; Flouzat Lachaniette, C.H.; Rouard, H.; Dubory, A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: What better postpone knee arthroplasty at fifteen years? A randomized study. Int. Orthop. 2021, 45, 391–399. [Google Scholar] [CrossRef]
- Hernigou, P.; Delambre, J.; Quiennec, S.; Poignard, A. Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: A prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int. Orthop. 2021, 45, 365–373. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: A Phase I dose-escalation trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Shin, J.S.; Shim, H.; Yoon, K.S. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A 2-year follow-up study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Quentin, J.; Feirreira, R.; Espinoza, F.; Abdellaoui, N.; Erkilic, N.; Cren, M.; Dufourcq-Lopez, E.; Pullig, O.; Nöth, U.; et al. Injection of adipose-derived stromal cells in the knee of patients with severe osteoarthritis has a systemic effect and promotes an anti-inflammatory phenotype of circulating immune cells. Theranostics 2018, 8, 5519–5528. [Google Scholar] [CrossRef]
- Chahal, J.; Gómez-Aristizábal, A.; Shestopaloff, K.; Bhatt, S.; Chaboureau, A.; Fazio, A.; Chisholm, J.; Weston, A.; Chiovitti, J.; Keating, A.; et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl. Med. 2019, 8, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Estrada, E.; Décima, J.L.; Rodríguez, M.; Di Tomaso, M.; Roberti, J. Patient-reported outcomes after platelet-rich plasma, bone marrow aspirate, and adipose-derived mesenchymal stem cell injections for symptomatic knee osteoarthritis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 117954412093108. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, G.; Manafi Rasi, A.; Baroutkoub, M.; Tavakoli Darestani, R. Effect of administration of mesenchymal stem cells on cartilage recovery and knee function in patients with knee osteoarthritis. Med. Sci. 2020, 24, 1019–1026. [Google Scholar]
- Papalia, R.; Zampogna, B.; Russo, F.; Vasta, S.; Campi, S.; Saccone, L.; Di Giacomo, G.; Vadalà, G.; Denaro, V. Adipose-derived stromal vascular fraction processed with different systems for the treatment of knee osteoarthritis: A pilot study on cell proliferation and clinical results. J. Biol. Regul. Homeost. Agents 2020, 34, 113–119. [Google Scholar] [PubMed]
- Bistolfi, A.; Roato, I.; Fornelli, G.; Sabatini, L.; Massè, A.; Ferracini, R. Treatment of knee osteoarthritis by intra-articular injection of concentrated autologous adipose tissue: A twenty four month follow-up study. Int. Orthop. 2021, 45, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Mautner, K.; Bowers, R.; Easley, K.; Fausel, Z.; Robinson, R. Functional outcomes following microfragmented adipose tissue versus bone marrow aspirate concentrate injections for symptomatic knee osteoarthritis. Stem Cells Transl. Med. 2019, 8, 1149–1156. [Google Scholar] [CrossRef]
- Yokota, N.; Hattori, M.; Ohtsuru, T.; Otsuji, M.; Lyman, S.; Shimomura, K.; Nakamura, N. Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or noncultured stromal vascular fraction for the treatment of knee osteoarthritis. Am. J. Sports Med. 2019, 47, 2577–2583. [Google Scholar] [CrossRef]
- Li, J.; Shao, Q.; Zhu, X.; Sun, G. Efficacy of autologous bone marrow mesenchymal stem cells in the treatment of knee osteoarthritis and their effects on the expression of serum TNF-α and IL-6. J. Musculoskelet. Neuronal. Interact. 2020, 20, 128–135. [Google Scholar] [PubMed]
- Simunec, D.; Salari, H.; Meyer, J. Treatment of Grade 3 and 4 osteoarthritis with intraoperatively separated adipose tissue-derived stromal vascular fraction: A comparative case series. Cells 2020, 9, 2096. [Google Scholar] [CrossRef]
- Varma, H.S.; Dadarya, B.; Vidyarthi, A. The new avenues in the management of osteo-arthritis of knee—Stem cells. J. Indian Med. Assoc. 2010, 108, 583–585. [Google Scholar]
- Wong, K.L.; Lee, K.B.L.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H.P. Injectable cultured bone marrow–derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: A prospective study. Arthroscopy 2014, 30, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondía, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (Phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Turajane, T.; Chaveewanakorn, U.; Fongsarun, W.; Aojanepong, J.; Papadopoulos, K.I. Avoidance of total knee arthroplasty in early osteoarthritis of the knee with intra-articular implantation of autologous activated peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial with differential effects of growth factor addition. Stem Cells Int. 2017, 2017, 1–10. [Google Scholar]
- Bastos, R.; Mathias, M.; Andrade, R.; Bastos, R.; Balduino, A.; Schott, V.; Rodeo, S.; Espregueira-Mendes, J. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg. Sports. Traumatol. Arthrosc. 2018, 26, 3342–3350. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Lyu, S.J.; Ding, Q.W.; Fan, M.Q.; Tong, P.J. Intraarticular injection of autologous adipose-derived stem cells for knee osteoarthritis: A randomized controlled trial. Zhong Hua Gu Ke Za Zhi 2018, 38, 1426–1434. (In Chinese) [Google Scholar]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Núñez-Córdoba, J.M.; López-Elío, S.; Andreu, E.; Sánchez-Guijo, F.; Aquerreta, J.D.; Bondía, J.M.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Long-term follow up of a multicenter randomized controlled clinical trial (Phase I/II). J. Transl. Med. 2018, 16, 213. [Google Scholar] [CrossRef]
- Peretti, G.M.; Ulivi, M.; De Girolamo, L.; Meroni, V.; Lombardo, M.D.; Mangiavini, L. Evaluation of the use of autologous micro-fragmented adipose tissue in the treatment of knee osteoarthritis: Preliminary results of a randomized controlled trial. J. Biol. Regul. Homeost. Agents 2018, 32, 193–199. [Google Scholar]
- Lamo-Espinosa, J.M.; Blanco, J.F.; Sánchez, M.; Moreno, V.; Granero-Moltó, F.; Sánchez-Guijo, F.; Crespo-Cullel, Í.; Mora, G.; San Vicente, D.D.; Pompei-Fernández, O.; et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J. Transl. Med. 2020, 18, 356. [Google Scholar] [CrossRef]
- Qiao, Z.; Tang, J.; Yue, B.; Wang, J.; Zhang, J.; Xuan, L.; Dai, C.; Li, S.; Li, M.; Xu, C.; et al. Human adipose-derived mesenchymal progenitor cells plus microfracture and hyaluronic acid for cartilage repair: A Phase IIa trial. Regen. Med. 2020, 15, 1193–1214. [Google Scholar] [CrossRef]
- Ruane, J.J.; Ross, A.; Zigmont, V.; McClure, D.; Gascon, G. A Single-blinded randomized controlled trial of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee with active control. J. Stem Cells Regen. Med. 2021, 17, 3–17. [Google Scholar] [PubMed]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.A.; Arthurs, J.R.; Heckman, M.G.; Bestic, J.M.; Kazmerchak, S.E.; Diehl, N.N.; Zubair, A.C.; O’Connor, M.I. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage 2019, 10, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Tran, T.D.-X.; Nguyen, H.T.-N.; Vu, H.T.; Le, P.T.-B.; Phan, N.L.-C.; Vu, N.B.; Phan, N.K.; Van Pham, P. Comparative clinical observation of arthroscopic microfracture in the presence and absence of a stromal vascular fraction injection for osteoarthritis. Stem Cells Transl. Med. 2017, 6, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Koh, Y.G. Comparative matched-pair analysis of open-wedge high tibial osteotomy with versus without an injection of adipose-derived mesenchymal stem cells for varus knee osteoarthritis: Clinical and second-look arthroscopic results. Am. J. Sports Med. 2018, 46, 2669–2677. [Google Scholar] [CrossRef]
- Tran, T.D.X.; Wu, C.-M.; Dubey, N.K.; Deng, Y.-H.; Su, C.-W.; Pham, T.T.; Thi Le, P.B.; Sestili, P.; Deng, W.-P. Time- and Kellgren–Lawrence grade-dependent changes in intra-articularly transplanted stromal vascular fraction in osteoarthritic patients. Cells 2019, 8, 308. [Google Scholar] [CrossRef]
- Ehlers, C.B.; Webb, A.R.; McCormick, B.P.; Wyand, T.J.; Sarna, N.; Povey, K.; Marano, G.; Schainker, L. Standardized platelet rich plasma injections for osteoarthritis of the knee. Cureus 2020, 12, e10900. [Google Scholar] [CrossRef]
- Centeno, C.; Pitts, J.; Al-Sayegh, H.; Freeman, M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Kim, Y.I.; Ryu, J.-S.; Koh, Y.G. Mesenchymal stem cell implantation in osteoarthritic knees: Is fibrin glue effective as a scaffold? Am. J. Sports Med. 2015, 43, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.L.; Kumar, P.P. Role of PRP and stem cell injections in osteoarthritic patients of knee joint. J. Evol. Med. Dent. Sci. 2015, 4, 9468–9474. [Google Scholar]
- Lee, N.-H.; Na, S.-M.; Ahn, H.-W.; Kang, J.-K.; Seon, J.-K.; Song, E.-K. Allogenic human umbilical cord blood-derived mesenchymal stem cells are more effective than bone marrow aspiration concentrate for cartilage regeneration after high tibial osteotomy in medial unicompartmental osteoarthritis of knee. Arthroscopy 2021, 37, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.; Cartier, C.; Stemper, I.; Dodson, E.; Freeman, M.; Azuike, U.; Williams, C.; Hyzy, M.; Silva, O.; Steinmetz, N. The treatment of bone marrow lesions associated with advanced knee osteoarthritis: Comparing intraosseous and intraarticular injections with bone marrow concentrate and platelet products. Pain Physician 2021, 24, E279–E288. [Google Scholar] [PubMed]
- Magnanelli, S.; Screpis, D.; Di Benedetto, P.; Natali, S.; Causero, A.; Zorzi, C. Open-wedge high tibial osteotomy associated with lipogems® intra-articular injection for the treatment of varus knee osteoarthritis—Retrospective study. Acta Bio. Med. Atenei Parm. 2020, 91, e2020022. [Google Scholar]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. preliminary report of four patients: Mesenchymal stem cell therapy for knee osteoarthritis. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pak, J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: A case series. J. Med. Case Rep. 2011, 5, 296. [Google Scholar] [CrossRef]
- Emadedin, M.; Aghdami, N.; Taghiyar, L.; Fazeli, R.; Moghadasali, R.; Jahangir, S.; Farjad, R.; Baghaban Eslaminejad, M. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch. Iran. Med. 2012, 15, 422–428. [Google Scholar]
- Hauser, R.A.; Orlofsky, A. Regenerative injection therapy with whole bone marrow aspirate for degenerative joint disease: A case series. Clin Med. Insights Arthritis Musculoskelet. Disord. 2013, 6, 65–72. [Google Scholar] [CrossRef]
- Koh, Y.-G.; Jo, S.-B.; Kwon, O.-R.; Suh, D.-S.; Lee, S.-W.; Park, S.-H.; Choi, Y.-J. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013, 29, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Turajane, T.; Chaweewannakorn, U.; Larbpaiboonpong, V.; Aojanepong, J.; Thitiset, T.; Honsawek, S.; Fongsarun, J.; Papadopoulos, K.I. Combination of intra-articular autologous activated peripheral blood stem cells with growth factor addition/preservation and hyaluronic acid in conjunction with arthroscopic microdrilling mesenchymal cell stimulation improves quality of life and regenerates articular cartilage in early osteoarthritic knee disease. J. Med. Assoc. Thail. 2013, 96, 580–588. [Google Scholar]
- Ahmad, K.A.; Ibrahim, Y.A.; Saber, N.Z.; Darwish, B.A. MR cartilage imaging in assessment of the regenerative power of autologous peripheral blood stem cell injection in knee osteoarthritis. Egypt. J. Radiol. Nucl. Med. 2014, 45, 787–794. [Google Scholar] [CrossRef][Green Version]
- Van Pham, P.; Bui, K.H.-T.; Duong, T.D.; Nguyen, N.T.; Nguyen, T.D.; Le, V.T.; Mai, V.T.; Phan, N.L.-C.; Le, D.M.; Ngoc, N.K. Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: A clinical study. Biomed. Res. Ther. 2014, 1, 2–8. [Google Scholar] [CrossRef]
- Koh, Y.G.; Choi, Y.J.; Kwon, O.R.; Kim, Y.S. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am. J. Sports Med. 2014, 42, 1628–1637. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two-year follow-up results. Transplantation 2014, 97, e66–e68. [Google Scholar] [CrossRef]
- Centeno, C.J.; Al-Sayegh, H.; Bashir, J.; Goodyear, S.; Freeman, M.D. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 258. [Google Scholar] [CrossRef]
- Emadedin, M.; Ghorbani Liastani, M.; Fazeli, R.; Mohseni, F.; Moghadasali, R.; Mardpour, S.; Hosseini, S.E.; Niknejadi, M.; Moeininia, F.; Aghahossein Fanni, A.; et al. Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch. Iran. Med. 2015, 18, 336–344. [Google Scholar]
- Gibbs, N.; Diamond, R.; Sekyere, E.; Thomas, W. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: A case series. J. Pain Res. 2015, 8, 799–806. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, Y.J.; Koh, Y.G. Mesenchymal stem cell implantation in knee osteoarthritis: An assessment of the factors influencing clinical outcomes. Am. J. Sports Med. 2015, 43, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Choi, Y.-J.; Kwon, S.-K.; Kim, Y.-S.; Yeo, J.-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Sadeghi Abdollahi, B.; Mohyeddin, M.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int. J. Rheum. Dis. 2016, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Fodor, P.B.; Paulseth, S.G. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet. Surg. J. 2016, 36, 229–236. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, Y.J.; Lee, S.W.; Kwon, O.R.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Assessment of clinical and mri outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: A prospective study. Osteoarthr. Cartil. 2016, 24, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Lee, J.H.; Park, K.S.; Jeong, B.C.; Lee, S.H. Regeneration of cartilage in human knee osteoarthritis with autologous adipose tissue-derived stem cells and autologous extracellular matrix. Biores. Open Access 2016, 5, 192–200. [Google Scholar] [CrossRef]
- Sampson, S.; Smith, J.; Vincent, H.; Aufiero, D.; Zall, M.; Botto-van-Bemden, A. Intra-articular bone marrow concentrate injection protocol: Short-term efficacy in osteoarthritis. Regen. Med. 2016, 11, 511–520. [Google Scholar] [CrossRef]
- Soler, R.; Orozco, L.; Munar, A.; Huguet, M.; López, R.; Vives, J.; Coll, R.; Codinach, M.; Garcia-Lopez, J. Final Results of a Phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee 2016, 23, 647–654. [Google Scholar] [CrossRef]
- Al-Najar, M.; Khalil, H.; Al-Ajlouni, J.; Al-Antary, E.; Hamdan, M.; Rahmeh, R.; Alhattab, D.; Samara, O.; Yasin, M.; Abdullah, A.A.; et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: A Phase I/II study. J. Orthop. Surg. Res. 2017, 12, 190. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević-Akmačić, I.; Plečko, M.; et al. The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Pintat, J.; Silvestre, A.; Magalon, G.; Gadeau, A.P.; Pesquer, L.; Perozziello, A.; Peuchant, A.; Mounayer, C.; Dallaudière, B. Intra-articular injection of mesenchymal stem cells and platelet-rich plasma to treat patellofemoral osteoarthritis: Preliminary results of a long-term pilot study. J. Vasc. Interv. Radiol. 2017, 28, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Condello, V.; Madonna, V.; Guerriero, M.; Zorzi, C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J. Exp. Ortop. 2017, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Smyshlyaev, I.A.; Gilfanov, S.I.; Kopylov, V.A.; Gilmutdinov, R.G.; Pulin, I.I.; Korsakov, I.N.; Gilmutdinova, I.R.; Petrikina, A.P.; Eremin, P.S.; Kruchkova, O.V.; et al. Safety and effectiveness of intraarticular administration of adipose-derived stromal vascular fraction for treatment of knee articular cartilage degenerative damage: Preliminary results of a clinical trial. Traumatol. Orthop. Russ. 2017, 23, 17–31. [Google Scholar] [CrossRef]
- Yokota, N.; Yamakawa, M.; Shirata, T.; Kimura, T.; Kaneshima, H. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen. Ther. 2017, 6, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, G.; De Caro, A.; Napoli, F.; Chiapale, D.; Trada, P.; Camera, A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 176. [Google Scholar] [CrossRef]
- Cavallo, M.; Sayyed-Hosseinian, S.-H.; Parma, A.; Buda, R.; Mosca, M.; Giannini, S. Combination of high tibial osteotomy and autologous bone marrow derived cell implantation in early osteoarthritis of knee: A preliminary study. Arch. Bone Jt. Surg. 2018, 6, 112–118. [Google Scholar]
- Rodriguez-Fontan, F.; Piuzzi, N.S.; Kraeutler, M.J.; Pascual-Garrido, C. Early clinical outcomes of intra-articular injections of bone marrow aspirate concentrate for the treatment of early osteoarthritis of the hip and knee: A cohort study. PM&R 2018, 10, 1353–1359. [Google Scholar]
- Russo, A.; Screpis, D.; Di Donato, S.L.; Bonetti, S.; Piovan, G.; Zorzi, C. Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: An update at 3 year follow-up. J. Exp. Ortop. 2018, 5, 52. [Google Scholar] [CrossRef]
- Shaw, B.; Darrow, M.; Derian, A. Short-term outcomes in treatment of knee osteoarthritis with 4 bone marrow concentrate injections. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 117954411878108. [Google Scholar] [CrossRef]
- Spasovski, D.; Spasovski, V.; Baščarević, Z.; Stojiljković, M.; Vreća, M.; Anđelković, M.; Pavlović, S. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J. Gene Med. 2018, 20, e3002. [Google Scholar] [CrossRef] [PubMed]
- Themistocleous, G.S.; Chloros, G.D.; Kyrantzoulis, I.M.; Georgokostas, I.A.; Themistocleous, M.S.; Papagelopoulos, P.J.; Savvidou, O.D. Effectiveness of a single intra-articular bone marrow aspirate concentrate (bmac) injection in patients with Grade 3 and 4 knee osteoarthritis. Heliyon 2018, 4, e00871. [Google Scholar] [CrossRef] [PubMed]
- Borić, I.; Hudetz, D.; Rod, E.; Jeleč, Ž.; Vrdoljak, T.; Skelin, A.; Polašek, O.; Plečko, M.; Trbojević-Akmačić, I.; Lauc, G.; et al. A 24-month follow-up study of the effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes 2019, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Goncars, V.; Kalnberzs, K.; Jakobsons, E.; Enģele, I.; Briede, I.; Blums, K.; Erglis, K.; Erglis, M.; Patetko, L.; Muiznieks, I.; et al. Treatment of knee osteoarthritis with bone marrow–derived mononuclear cell injection: 12-month follow-up. Cartilage 2019, 10, 26–35. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: A prospective study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef]
- Monckeberg, J.E.; Rafols, C.; Apablaza, F.; Gerhard, P.; Rosales, J. Intra-articular administration of peripheral blood stem cells with platelet-rich plasma regenerated articular cartilage and improved clinical outcomes for knee chondral lesions. Knee 2019, 26, 824–831. [Google Scholar] [CrossRef]
- Onoi, Y.; Hiranaka, T.; Nishida, R.; Takase, K.; Fujita, M.; Hida, Y.; Fujishiro, T.; Okamoto, K. Second-look arthroscopic findings of cartilage and meniscus repair after injection of adipose-derived regenerative cells in knee osteoarthrits: Report of two cases. Regen. Ther. 2019, 11, 212–216. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G.; et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: Clinical and histological observations. Int. Orthop. 2019, 43, 15–23. [Google Scholar] [CrossRef]
- Schiavone Panni, A.; Vasso, M.; Braile, A.; Toro, G.; De Cicco, A.; Viggiano, D.; Lepore, F. Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: Identification of a subpopulation with greater response. Int. Orthop. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Jung, J.-Y.; Park, H.-S.; Chun, Y.S.; Kim, S.J. Cartilage regeneration in osteoarthritic knees treated with distal femoral osteotomy and intra-lesional implantation of allogenic human umbilical cord blood-derived mesenchymal stem cells: A report of two cases. Knee 2019, 26, 1445–1450. [Google Scholar] [CrossRef]
- Wang, J.; Wright, K.T.; Perry, J.; Tins, B.; Hopkins, T.; Hulme, C.; McCarthy, H.S.; Brown, A.; Richardson, J.B. Combined autologous chondrocyte and bone marrow mesenchymal stromal cell implantation in the knee: An 8-year follow up of two first-in-man cases. Cell Transplant. 2019, 28, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Colberg, R.E.; Jurado Vélez, J.A.; Walsh, K.P.; Fleisig, G. Short-term outcomes after pure bone marrow aspirate injection for severe knee osteoarthritis: A case series. Regen. Med. 2020, 15, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.H.; Canintika, A.F.; Hanitya, A.L.; Pawitan, J.A.; Liem, I.K.; Pandelaki, J. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: A single-arm, open-label study. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Dulic, O.; Lalic, I.; Kecojevic, V.; Gavrilovic, G.; Abazovic, D.; Miskulin, M.; Maric, D.; Bumbasirevic, M. Do knee injection portals affect clinical results of bone marrow aspirate concentrate injection in the treatment of osteoarthritis? a prospective randomized controlled study. Regen. Med. 2020, 15, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Wickham, J.; Shah, K.; Li, D.; Norsworthy, C.; Tenen, A. Mesenchymal stem cell therapy combined with arthroscopic abrasion arthroplasty regenerates cartilage in patients with severe knee osteoarthritis: A case series. Regen. Med. 2020, 15, 1957–1977. [Google Scholar] [CrossRef]
- Heidari, N.; Noorani, A.; Slevin, M.; Cullen, A.; Stark, L.; Olgiati, S.; Zerbi, A.; Wilson, A. Patient-centered outcomes of microfragmented adipose tissue treatments of knee osteoarthritis: An observational, intention-to-treat study at twelve months. Stem Cells Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, J.; Yamagami, R.; Matsumoto, T.; Terao, T.; Inoue, K.; Tsuji, S.; Maenohara, Y.; Matsuzaki, T.; Chijimatsu, R.; Omata, Y.; et al. Associations of clinical outcomes and mri findings in intra-articular administration of autologous adipose-derived stem cells for knee osteoarthritis. Regen. Ther. 2020, 14, 332–340. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, J.-D.; Choi, Y.; Choi, C.H.; Lee, G.W. Intra-articular bone marrow aspirate concentrate injection in patients with knee osteoarthritis. Appl. Sci. 2020, 10, 5945. [Google Scholar] [CrossRef]
- Kim, Y.S.; Suh, D.S.; Tak, D.H.; Chung, P.K.; Koh, Y.G. Mesenchymal stem cell implantation in knee osteoarthritis: Midterm outcomes and survival analysis in 467 patients. Orthop. J. Sports Med. 2020, 8, 232596712096918. [Google Scholar] [CrossRef]
- Lapuente, J.P.; Dos-Anjos, S.; Blázquez-Martínez, A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: Hypothesis on the regulatory role of intra-articular adipose tissue. J. Orthop. Surg. Res. 2020, 15, 137. [Google Scholar] [CrossRef]
- Mehling, B.; Hric, M.; Salatkova, A.; Vetrak, R.; Santora, D.; Ovariova, M.; Mihalyova, R.; Manvelyan, M. A Retrospective study of stromal vascular fraction cell therapy for osteoarthritis. J. Clin. Med. Res. 2020, 12, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Prodromos, C.; Finkle, S. Autologous biologic treatment with fat, bone marrow aspirate and platelet rich plasma is an effective alternative to total knee arthroplasty for patients with moderate knee arthrosis. Medicines 2020, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Jung, J.-Y.; Park, H.-S.; Lee, S.H.; Cho, Y.J.; Kim, S.J. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up. Regen. Ther. 2020, 14, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Park, H.-S.; Choi, N.-H. Human umbilical cord blood-derived mesenchymal stem cell implantation for osteoarthritis of the knee. Arch. Orthop. Trauma Surg. 2020, 140, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-S.; Hong, K.-T.; Kong, C.-G.; Kim, N.-M.; Jung, J.-Y.; Park, H.-S.; Kim, Y.J.; Chang, K.B.; Kim, S.J. High tibial osteotomy with human umbilical cord blood-derived mesenchymal stem cells implantation for knee cartilage regeneration. World J. Stem Cells 2020, 12, 514–526. [Google Scholar] [CrossRef]
- Toan, D.D.; Binh, N.T.; Dung, T.T.; Thuy, L.Q.; Hoa, N.D.; Long, N.H.; Tung, P.S. The effectiveness of knee osteoarthritis treatment by arthroscopic microfracture technique in combination with autologous bone marrow stem cells transplantation. J. Back Musculoskelet. Rehabil. 2020, 33, 397–403. [Google Scholar] [CrossRef]
- Tsubosaka, M.; Matsumoto, T.; Sobajima, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet. Disord. 2020, 21, 207. [Google Scholar] [CrossRef]
- Varady, N.H.; Cate, G.; Barghi, A.; Jobe, N.; Yakin, D.; Ylanan, R.C.; Arnold, C.A. Positive early clinical outcomes of bone marrow aspirate concentrate for osteoarthritis using a novel fenestrated trocar. Knee 2020, 27, 1627–1634. [Google Scholar] [CrossRef]
- Wells, K.; Klein, M.; Hurwitz, N.; Santiago, K.; Cheng, J.; Abutalib, Z.; Beatty, N.; Lutz, G. Cellular and clinical analyses of autologous bone marrow aspirate injectate for knee osteoarthritis: A pilot study. PM&R 2021, 13, 387–396. [Google Scholar]
- Bąkowski, P.; Kaszyński, J.; Baka, C.; Kaczmarek, T.; Ciemniewska-Gorzela, K.; Bąkowska-Żywicka, K.; Piontek, T. Patients with Stage II of the knee osteoarthritis most likely benefit from the intra-articular injections of autologous adipose tissue—From 2 years of follow-up studies. Arch. Orthop. Trauma Surg. 2021. [Google Scholar] [CrossRef]
- Borg, T.-M.; Heidari, N.; Noorani, A.; Slevin, M.; Cullen, A.; Olgiati, S.; Zerbi, A.; Danovi, A.; Wilson, A. Gender-specific response in pain and function to biologic treatment of knee osteoarthritis: A gender-bias-mitigated, observational, intention-to-treat study at two years. Stem Cells Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Burnham, R.; Smith, A.; Hart, D. The safety and effectiveness of bone marrow concentrate injection for knee and hip osteoarthritis: A Canadian cohort. Regen. Med. 2021, 16, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Caforio, M.; Nobile, C. Intra-articular administration of autologous purified adipose tissue associated with arthroscopy ameliorates knee osteoarthritis symptoms. J. Clin. Med. 2021, 10, 2053. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Borg, T.-M.; Olgiati, S.; Slevin, M.; Danovi, A.; Fish, B.; Wilson, A.; Noorani, A. Microfragmented adipose tissue injection (mfat) may be a solution to the rationing of total knee replacement: A prospective, gender-bias mitigated, reproducible analysis at two years. Stem Cells Int. 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Suh, D.S.; Tak, D.H.; Chung, P.K.; Kwon, Y.B.; Kim, T.Y.; Koh, Y.G. Factors influencing clinical and mri outcomes of mesenchymal stem cell implantation with concomitant high tibial osteotomy for varus knee osteoarthritis. Orthop. J. Sports Med. 2021, 9, 232596712097998. [Google Scholar] [CrossRef] [PubMed]
- Malanga, G.A.; Chirichella, P.S.; Hogaboom, N.S.; Capella, T. Clinical evaluation of micro-fragmented adipose tissue as a treatment option for patients with meniscus tears with osteoarthritis: A prospective pilot study. Int. Orthop. 2021, 45, 473–480. [Google Scholar] [CrossRef]
- Santoprete, S.; Marchetti, F.; Rubino, C.; Bedini, M.G.; Nasto, L.A.; Cipolloni, V.; Pola, E. Fresh autologous stromal tissue fraction for the treatment of knee osteoarthritis related pain and disability. Orthop. Rev. 2021, 13, 9161. [Google Scholar] [CrossRef]
- Sekiya, I.; Katano, H.; Mizuno, M.; Koga, H.; Masumoto, J.; Tomita, M.; Ozeki, N. Alterations in cartilage quantification before and after injections of mesenchymal stem cells into osteoarthritic knees. Sci. Rep. 2021, 11, 13832. [Google Scholar] [CrossRef] [PubMed]
- Van Genechten, W.; Vuylsteke, K.; Martinez, P.R.; Swinnen, L.; Sas, K.; Verdonk, P. Autologous micro-fragmented adipose tissue (MFAT) to treat symptomatic knee osteoarthritis: Early outcomes of a consecutive case series. J. Clin. Med. 2021, 10, 2231. [Google Scholar] [CrossRef]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 2008, 11, 343–353. [Google Scholar]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells, platelet lysate and dexamethasone. Am. J. Case Rep. 2008, 9, 246–251. [Google Scholar]
- Mehrabani, D.; Mojtahed Jaberi, F.; Zakerinia, M.; Hadianfard, M.J.; Jalli, R.; Tanideh, N.; Zare, S. The healing effect of bone-marrow-derived stem cells in knee osteoarthritis: A case report. World J. Plast. Surg. 2016, 5, 168–174. [Google Scholar] [PubMed]
- Bright, B.; Bright, R.; Bright, P.; Limaye, A. Ankylosing spondylitis, chronic fatigue and depression improved after stromal vascular fraction treatment for osteoarthritis: A case report. J. Med. Case Rep. 2018, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Norsworthy, C.; Wickham, J.; Shah, K.; Tenen, A. High tibial osteotomy in combination with arthroscopic abrasion arthroplasty and autologous adipose-derived mesenchymal stem cell therapy in the treatment of advanced knee osteoarthritis. BMJ Case Rep. 2019, 12, bcr-2018-228003. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Saw, K.-Y.; Anz, A.; Siew-Yoke Jee, C.; Merican, S.; Ching-Soong Ng, R.; Roohi, S.A.; Ragavanaidu, K. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial. Arthroscopy 2013, 29, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Akgun, I.; Unlu, M.C.; Erdal, O.A.; Ogut, T.; Erturk, M.; Ovali, E.; Kantarci, F.; Caliskan, G.; Akgun, Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: A 2-year randomized study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J.; Tak, D.-H. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Shadmanfar, S.; Labibzadeh, N.; Emadedin, M.; Jaroughi, N.; Azimian, V.; Mardpour, S.; Kakroodi, F.A.; Bolurieh, T.; Hosseini, S.E.; Chehrazi, M.; et al. Intra-articular knee implantation of autologous bone marrow–derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled Phase 1/2 clinical trial. Cytotherapy 2018, 20, 499–506. [Google Scholar] [CrossRef]
- de Girolamo, L.; Schönhuber, H.; Viganò, M.; Bait, C.; Quaglia, A.; Thiebat, G.; Volpi, P. Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: Results from a randomized controlled study. J. Clin. Med. 2019, 8, 392. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Nishida, Y.; Takahashi, S.; Nakamura, H.; Mera, H.; Kashiwa, K.; Yoshiya, S.; Inagaki, Y.; Uematsu, K.; Tanaka, Y.; et al. Transplantation of autologous bone-marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: A multicenter prospective randomized control clinical trial. Regen. Ther. 2019, 11, 106–113. [Google Scholar] [CrossRef]
- Olivos-Meza, A.; Pérez Jiménez, F.J.; Granados-Montiel, J.; Landa-Solís, C.; Cortés González, S.; Jiménez Aroche, C.A.; Valdez Chávez, M.; Renán León, S.; Gomez-Garcia, R.; Martínez-López, V.; et al. First clinical application of polyurethane meniscal scaffolds with mesenchymal stem cells and assessment of cartilage quality with T2 mapping at 12 months. Cartilage 2021, 13, 197S–207S. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chung, P.K.; Suh, D.S.; Heo, D.B.; Tak, D.H.; Koh, Y.G. Implantation of mesenchymal stem cells in combination with allogenic cartilage improves cartilage regeneration and clinical outcomes in patients with concomitant high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 544–554. [Google Scholar] [CrossRef]
- Lim, H.-C.; Park, Y.-B.; Ha, C.-W.; Cole, B.J.; Lee, B.-K.; Jeong, H.-J.; Kim, M.-K.; Bin, S.-I.; Choi, C.-H.; Choi, C.H.; et al. Allogeneic umbilical cord blood–derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: A multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthop. J. Sports Med. 2021, 9, 232596712097305. [Google Scholar] [CrossRef]
- Saw, K.-Y.; Anz, A.W.; Ng, R.C.-S.; Jee, C.S.-Y.; Low, S.F.; Dorvault, C.; Johnson, K.B. Arthroscopic subchondral drilling followed by injection of peripheral blood stem cells and hyaluronic acid showed improved outcome compared to hyaluronic acid and physiotherapy for massive knee chondral defects: A randomized controlled trial. Arthroscopy 2021, 37, 2502–2517. [Google Scholar] [CrossRef]
- Gobbi, A.; Chaurasia, S.; Karnatzikos, G.; Nakamura, N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: A nonrandomized prospective trial. Cartilage 2015, 6, 82–97. [Google Scholar] [CrossRef]
- Gobbi, A.; Whyte, G.P. One-stage cartilage repair using a hyaluronic acid–based scaffold with activated bone marrow–derived mesenchymal stem cells compared with microfracture: Five-year follow-up. Am. J. Sports Med. 2016, 44, 2846–2854. [Google Scholar] [CrossRef]
- Martinčič, D.; Leban, J.; Filardo, G.; Busacca, M.; Barlič, A.; Veber, M.; Drobnič, M. Autologous chondrocytes versus filtered bone marrow mesenchymal stem/stromal cells for knee cartilage repair—A prospective study. Int. Orthop. 2021, 45, 931–939. [Google Scholar] [CrossRef]
- Ryu, D.J.; Jeon, Y.S.; Park, J.S.; Bae, G.C.; Kim, J.; Kim, M.K. Comparison of bone marrow aspirate concentrate and allogenic human umbilical cord blood derived mesenchymal stem cell implantation on chondral defect of knee: Assessment of clinical and magnetic resonance imaging outcomes at 2-year follow-up. Cell Transplant. 2020, 29, 963689720943581. [Google Scholar] [CrossRef]
- Kasemkijwattana, C.; Hongeng, S.; Kesprayura, S.; Rungsinaporn, V.; Chaipinyo, K.; Chansiri, K. Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: Two cases report. J. Med. Assoc. Thail. 2011, 94, 395–400. [Google Scholar]
- Skowroński, J.; Skowroński, R.; Rutka, M. Cartilage lesions of the knee treated with blood mesenchymal stem cells—Results. Ortop. Traumatol. Rehabil. 2012, 14, 569–577. [Google Scholar]
- Buda, R.; Vannini, F.; Cavallo, M.; Baldassarri, M.; Luciani, D.; Mazzotti, A.; Pungetti, C.; Olivieri, A.; Giannini, S. One-step arthroscopic technique for the treatment of osteochondral lesions of the knee with bone-marrow-derived cells: Three years results. Musculoskelet. Surg. 2013, 97, 145–151. [Google Scholar] [CrossRef]
- Gobbi, A.; Karnatzikos, G.; Sankineani, S.R. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am. J. Sports Med. 2014, 42, 648–657. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Zhang, Z.; Zhong, X.; Ji, H.; Tang, Z.; Bai, J.; Yao, M.; Hou, J.; Zheng, M.; Wood, D.; et al. Matrix-induced autologous chondrocyte implantation for the treatment of chondral defects of the knees in Chinese patients. Drug Des. Devel. Ther. 2014, 8, 2439–2448. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human cartilage-derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration. Stem Cells Transl. Med. 2016, 5, 733–744. [Google Scholar] [CrossRef]
- Sadlik, B.; Jaroslawski, G.; Gladysz, D.; Puszkarz, M.; Markowska, M.; Pawelec, K.; Boruczkowski, D.; Oldak, T. Knee cartilage regeneration with umbilical cord mesenchymal stem cells embedded in collagen scaffold using dry arthroscopy technique. Adv. Exp. Med. Biol. 2017, 1020, 113–122. [Google Scholar]
- Whitehouse, M.R.; Howells, N.R.; Parry, M.C.; Austin, E.; Kafienah, W.; Brady, K.; Goodship, A.E.; Eldridge, J.D.; Blom, A.W.; Hollander, A.P. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: From in vitro optimization to a first-in-human study. Stem Cells Transl. Med. 2017, 6, 1237–1248. [Google Scholar] [CrossRef]
- Kamei, N.; Ochi, M.; Adachi, N.; Ishikawa, M.; Yanada, S.; Levin, L.S.; Kamei, G.; Kobayashi, T. The safety and efficacy of magnetic targeting using autologous mesenchymal stem cells for cartilage repair. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3626–3635. [Google Scholar] [CrossRef]
- Shetty, A.; Kim, S.; Ahmed, S.; Trattnig, S.; Kim, S.; Jang, H. A cost-effective cell- and matrix-based minimally invasive single-stage chondroregenerative technique developed with validated vertical translation methodology. Ann. R. Coll. Surg. Engl. 2018, 100, 240–246. [Google Scholar] [CrossRef]
- Shimomura, K.; Yasui, Y.; Koizumi, K.; Chijimatsu, R.; Hart, D.A.; Yonetani, Y.; Ando, W.; Nishii, T.; Kanamoto, T.; Horibe, S.; et al. First-in-human pilot study of implantation of a scaffold-free tissue-engineered construct generated from autologous synovial mesenchymal stem cells for repair of knee chondral lesions. Am. J. Sports Med. 2018, 46, 2384–2393. [Google Scholar] [CrossRef]
- Gobbi, A.; Whyte, G.P. Long-term clinical outcomes of one-stage cartilage repair in the knee with hyaluronic acid–based scaffold embedded with mesenchymal stem cells sourced from bone marrow aspirate concentrate. Am. J. Sports Med. 2019, 47, 1621–1628. [Google Scholar] [CrossRef]
- Sekiya, I.; Koga, H.; Otabe, K.; Nakagawa, Y.; Katano, H.; Ozeki, N.; Mizuno, M.; Horie, M.; Kohno, Y.; Katagiri, K.; et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: A case report. Cell Transplant. 2019, 28, 1445–1454. [Google Scholar] [CrossRef]
- Ciemniewska-Gorzela, K.; Bąkowski, P.; Naczk, J.; Jakob, R.; Piontek, T. Complex meniscus tears treated with collagen matrix wrapping and bone marrow blood injection: Clinical effectiveness and survivorship after a minimum of 5 years’ follow-up. Cartilage 2021, 13, 228S–238S. [Google Scholar] [CrossRef]
- Freitag, J.; Shah, K.; Wickham, J.; Li, D.; Norsworthy, C.; Tenen, A. Evaluation of autologous adipose-derived mesenchymal stem cell therapy in focal chondral defects of the knee: A pilot case series. Regen. Med. 2020, 15, 1703–1717. [Google Scholar] [CrossRef]
- Veber, M.; Vogler, J.; Knežević, M.; Barlič, A.; Drobnič, M. Combination of filtered bone marrow aspirate and biomimetic scaffold for the treatment of knee osteochondral lesions: Cellular and early clinical results of a single centre case series. Tissue Eng. Regen. Med. 2020, 17, 375–386. [Google Scholar] [CrossRef]
- Chung, Y.-W.; Yang, H.-Y.; Kang, S.-J.; Song, E.-K.; Seon, J.-K. Allogeneic umbilical cord blood-derived mesenchymal stem cells combined with high tibial osteotomy: A retrospective study on safety and early results. Int. Orthop. 2021, 45, 481–488. [Google Scholar] [CrossRef]
- Liu, H.-C.; Liu, T.-S.T.; Liu, Y.-L.; Wang, J.-H.; Chang, C.-H.; Shih, T.T.-F.; Lin, F.-H. Atelocollagen-embedded chondrocyte precursors as a treatment for grade-4 cartilage defects of the femoral condyle: A case series with up to 9-year follow-up. Biomolecules 2021, 11, 942. [Google Scholar] [CrossRef]
- Saris, T.F.F.; de Windt, T.S.; Kester, E.C.; Vonk, L.A.; Custers, R.J.H.; Saris, D.B.F. Five-Year Outcome of 1-stage cell-based cartilage repair using recycled autologous chondrons and allogenic mesenchymal stromal cells: A first-in-human clinical trial. Am. J. Sports Med. 2021, 49, 941–947. [Google Scholar] [CrossRef]
- Adachi, N.; Ochi, M.; Deie, M.; Ito, Y. Transplant of mesenchymal stem cells and hydroxyapatite ceramics to treat severe osteochondral damage after septic arthritis of the knee. J. Rheumatol. 2005, 32, 1615–1618. [Google Scholar]
- Kuroda, R.; Ishida, K.; Matsumoto, T.; Akisue, T.; Fujioka, H.; Mizuno, K.; Ohgushi, H.; Wakitani, S.; Kurosaka, M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthr. Cartil. 2007, 15, 226–231. [Google Scholar] [CrossRef]
- Broyles, J.E.; O’Brien, M.A.; Stagg, M.P. Microdrilling surgery augmented with intra-articular bone marrow aspirate concentrate, platelet-rich plasma, and hyaluronic acid: A technique for cartilage repair in the knee. Arthrosc. Tech. 2017, 6, e201–e206. [Google Scholar] [CrossRef][Green Version]
- Freitag, J.; Shah, K.; Wickham, J.; Boyd, R.; Tenen, A. The effect of autologous adipose derived mesenchymal stem cell therapy in the treatment of a large osteochondral defect of the knee following unsuccessful surgical intervention of osteochondritis dissecans—A case study. BMC Musculoskelet. Disord. 2017, 18, 298. [Google Scholar] [CrossRef]
- Freitag, J.; Li, D.; Wickham, J.; Shah, K.; Tenen, A. Effect of autologous adipose-derived mesenchymal stem cell therapy in the treatment of a post-traumatic chondral defect of the knee. BMJ Case Rep. 2017, 2017, bcr-2017-220852. [Google Scholar] [CrossRef]
- Leigheb, M.; Bosetti, M.; De Consoli, A.; Borrone, A.; Cannas, M.; Grassi, F. Chondral tissue engineering of the reumatoid knee with collagen matrix autologous chondrocytes implant. Acta Biomed. 2017, 88, 107–113. [Google Scholar]
- Chen, H.H.; Chen, Y.C.; Yu, S.N.; Lai, W.L.; Shen, Y.S.; Shen, P.C.; Lin, S.H.; Chang, C.H.; Lee, S.M. Infrapatellar fat pad-derived mesenchymal stromal cell product for treatment of knee osteoarthritis: A first-in-human study with evaluation of the potency marker. Cytotherapy 2022, 24, 72–85. [Google Scholar] [CrossRef]
- Furia, J.P.; Lundeen, M.; Hurd, J.L.; Pearce, D.A.; Alt, C.; Alt, E.U.; Schmitz, C.; Maffulli, N. Why and how to use the body’s own stem cells for regeneration in musculoskeletal disorders: A primer. J. Orthop. Surg. Res. 2022, 17, 36. [Google Scholar] [CrossRef]
- Lopa, S.; Colombini, A.; Moretti, M.; de Girolamo, L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: From mechanisms of action to current clinical evidences. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2003–2020. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Jiang, P.; Mao, L.; Qiao, L.; Lei, X.; Zheng, Q.; Li, D. Efficacy and safety of mesenchymal stem cell injections for patients with osteoarthritis: A meta-analysis and review of RCTs. Arch. Orthop. Trauma Surg. 2021, 141, 1241–1251. [Google Scholar] [CrossRef]
- Qu, H.; Sun, S. Efficacy of mesenchymal stromal cells for the treatment of knee osteoarthritis: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2021, 16, 11. [Google Scholar] [CrossRef]
- Ma, W.; Liu, C.; Wang, S.; Xu, H.; Sun, H.; Fan, X. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Medicine 2020, 99, e23343. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Xu, H.; Lin, Z.; Chang, H.; Liu, W.; Kong, L. Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J. Orthop. Translat. 2020, 24, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, W.; Zhao, Y.; Yang, F.; Xu, M. Clinical efficacy and safety of stem cell therapy for knee osteoarthritis: A meta-analysis. Medicine 2020, 99, e19434. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, Y.; Gao, F.; Wu, R.; Dou, P.; Wang, W.; Li, Q. Mesenchymal stem cells: A new choice for nonsurgical treatment of oa? results from a bayesian network meta-analysis. Biomed. Res. Int. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Wei, Z.-J.; Wang, Q.-Q.; Cui, Z.-G.; Inadera, H.; Jiang, X.; Wu, C.-A. Which is the most effective one in knee osteoarthritis treatment from mesenchymal stem cells obtained from different sources?—A systematic review with conventional and network meta-analyses of randomized controlled trials. Ann. Transl. Med. 2021, 9, 452. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Zhang, Y.; Huang, L.; Shi, Q. Mesenchymal stem cells—A promising strategy for treating knee osteoarthritis: A meta-analysis. Bone Joint Res. 2020, 9, 719–728. [Google Scholar] [CrossRef]
- Bolia, I.K.; Bougioukli, S.; Hill, W.J.; Trasolini, N.A.; Petrigliano, F.A.; Lieberman, J.R.; Weber, A.E. Clinical efficacy of bone marrow aspirate concentrate versus stromal vascular fraction injection in patients with knee osteoarthritis: A systematic review and meta-analysis. Am. J. Sports Med. 2021, 036354652110145. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Ganie, P.A. Does the source of mesenchymal stem cell have an effect in the management of osteoarthritis of the knee? meta-analysis of randomized controlled trials. Cartilage 2021, 13, 1532S–1547S. [Google Scholar] [CrossRef]
- Han, X.; Yang, B.; Zou, F.; Sun, J. Clinical therapeutic efficacy of mesenchymal stem cells derived from adipose or bone marrow for knee osteoarthritis: A meta-analysis of randomized controlled trials. J. Comp. Eff. Res. 2020, 9, 361–374. [Google Scholar] [CrossRef]
- Agarwal, N.; Mak, C.; Bojanic, C.; To, K.; Khan, W. Meta-analysis of adipose tissue derived cell-based therapy for the treatment of knee osteoarthritis. Cells 2021, 10, 1365. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, J.; Yang, W.; Han, Y.; Zeng, L.; Liang, G.; Liu, J. Intra-articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: A systematic review and network meta-analysis. Arthroscopy 2021, 37, 2298–2314. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.S.; Kwan, Y.T.; Neo, W.J.; Chong, J.Y.; Kuek, T.Y.J.; See, J.Z.F.; Wong, K.L.; Toh, W.S.; Hui, J.H.P. Intra-articular injections of mesenchymal stem cells without adjuvant therapies for knee osteoarthritis: A systematic review and meta-analysis. Am. J. Sports Med. 2021, 49, 3113–3124. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Djaja, Y.P.; Park, Y.-B.; Park, J.-G.; Ko, Y.-B.; Ha, C.-W. Intra-articular injection of culture-expanded mesenchymal stem cells without adjuvant surgery in knee osteoarthritis: A systematic review and meta-analysis. Am. J. Sports Med. 2020, 48, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xu, Y.; Zhang, Y.; Li, A.; Qiu, X.; Wen, H.; Tan, H. Efficacy and safety of intra-articular cell-based therapy for osteoarthritis: Systematic review and network meta-analysis. Cartilage 2021, 13, 104S–115S. [Google Scholar] [CrossRef]
- Maheshwer, B.; Polce, E.M.; Paul, K.; Williams, B.T.; Wolfson, T.S.; Yanke, A.; Verma, N.N.; Cole, B.J.; Chahla, J. Regenerative potential of mesenchymal stem cells for the treatment of knee osteoarthritis and chondral defects: A systematic review and meta-analysis. Arthroscopy 2021, 37, 362–378. [Google Scholar] [CrossRef]
- Dai, W.; Leng, X.; Wang, J.; Shi, Z.; Cheng, J.; Hu, X.; Ao, Y. Intra-articular mesenchymal stromal cell injections are no different from placebo in the treatment of knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Arthroscopy 2021, 37, 340–358. [Google Scholar] [CrossRef]
- Han, S.-B.; Seo, I.-W.; Shin, Y.-S. Intra-articular injections of hyaluronic acid or steroids associated with better outcomes than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: A network meta-analysis. Arthroscopy 2021, 37, 292–306. [Google Scholar] [CrossRef]
- Vangsness, C.T.; Farr, J.; Boyd, J.; Dellaero, D.T.; Mills, C.R.; LeRoux-Williams, M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: A randomized, double-blind, controlled study. J. Bone Joint Surg. 2014, 96, 90–98. [Google Scholar] [CrossRef]
- Bhattacharya, N. Clinical use of amniotic fluid in osteoarthritis: A source of cell therapy. Transplantation 2011, 90, 395–403. [Google Scholar]
- Lv, X.X.; Huang, C.; Yin, Z.; Hong, B.G.; Jiang, H.J.; Huang, X.J. Effectiveness of autologous bone marrow mesenchymal stem cell transplant for knee osteoarthritis. Chin. J. Cell Stem. Cell. 2015, 5, 28–32. [Google Scholar]
- Ha, C.Z.; Li, W.; Ren, S.D.; Zhou, C.H.; Chen, S.F.; Wang, D.W.; Liu, C. Effect of platelet rich plasma combined with mesenchymal stem cells in treatment of knee osteoarthritis. Chin. J. Joint Surg. (Electron. Ed.) 2018, 12, 644–652. [Google Scholar]
- Dieppe, P.; Goldingay, S.; Greville-Harris, M. The power and value of placebo and nocebo in painful osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. The powerful placebo effect in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 118–123. [Google Scholar] [PubMed]
- Previtali, D.; Merli, G.; Di Laura Frattura, G.; Candrian, C.; Zaffagnini, S.; Filardo, G. The long-lasting effects of “placebo injections” in knee osteoarthritis: A meta-analysis. Cartilage 2021, 13, 185S–196S. [Google Scholar] [CrossRef]
- Winnier, G.E.; Valenzuela, N.; Peters-Hall, J.; Kellner, J.; Alt, C.; Alt, E.U. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS ONE 2019, 14, e0221457. [Google Scholar] [CrossRef]
- Alt, E.U.; Winnier, G.; Haenel, A.; Rothoerl, R.; Solakoglu, O.; Alt, C.; Schmitz, C. Towards a comprehensive understanding of ua-adrcs (uncultured, autologous, fresh, unmodified, adipose derived regenerative cells, isolated at point of care) in regenerative medicine. Cells 2020, 9, 1097. [Google Scholar] [CrossRef]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef]
- Srivastava, M.; Ahlawat, N.; Srivastava, A. Amniotic fluid stem cells: A new era in regenerative medicine. J. Obstet. Gynecol. India 2018, 68, 15–19. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Heitjan, D.F. Ignorability and bias in clinical trials. Statist. Med. 1999, 18, 2421–2434. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (cx601) for complex perianal fistulas in crohn’s disease: A Phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Álvaro-Gracia, J.M.; Jover, J.A.; García-Vicuña, R.; Carreño, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martínez-Taboada, V.M.; Taylor, P.; Martín-Martín, C.; et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled Phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2017, 76, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Haack-Sørensen, M.; Juhl, M.; Harary Søndergaard, R.; Follin, B.; Drozd Lund, L.; Mønsted Johansen, E.; Ali Qayyum, A.; Bruun Mathiasen, A.; Jørgensen, E.; et al. Cryopreserved off-the-shelf allogeneic adipose-derived stromal cells for therapy in patients with ischemic heart disease and heart failure—A safety study. Stem Cells Transl. Med. 2017, 6, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.W.; Witten, C.M.; Califf, R.M. Clarifying stem-cell therapy’s benefits and risks. N. Engl. J. Med. 2017, 376, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Eder, C.; Schmidt-Bleek, K.; Geissler, S.; Sass, F.A.; Maleitzke, T.; Pumberger, M.; Perka, C.; Duda, G.N.; Winkler, T. Mesenchymal stromal cell and bone marrow concentrate therapies for musculoskeletal indications: A concise review of current literature. Mol. Biol. Rep. 2020, 47, 4789–4814. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.C.; Wang, S.C.; Han, Y.H.; Wen, Y. Recent advance in source, property, differentiation, and applications of infrapatellar fat pad adipose-derived stem cells. Stem Cells Int. 2020, 2020, 2560174. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, P.; Moghaddamshahabi, R.; Webster, T.J.; Calikoglu Koyuncu, A.C.; Ahmadian, E.; Khan, W.S.; Jimale Mohamed, A.; Eftekhari, A. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: A review. Int. J. Mol. Sci. 2021, 22, 9215. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Piccione, M.; Belluzzi, E.; Petrelli, L.; Pozzuoli, A.; Ramonda, R.; Rossato, M.; Favero, M.; Ruggieri, P.; et al. Infrapatellar fat pad stem cells responsiveness to microenvironment in osteoarthritis: From morphology to function. Front. Cell Dev. Biol. 2019, 7, 323. [Google Scholar] [CrossRef]
| C | Nall | Description |
|---|---|---|
| I | 8 | Intra-articular injection of stem cells as the sole treatment (not considering rehabilitation) compared with i.a. injection of saline or sham treatment as control. |
| II | 6 | Intra-articular injection of stem cells as the sole treatment (not considering rehabilitation) compared with i.a. injection of, respectively, PRP, CS or HA as control. |
| III | 10 | Intra-articular injection of stem cells as the sole treatment (not considering rehabilitation) compared with other treatments than those in Categories I and II as control. |
| IV | 12 | Combinations of i.a. injection of stem cells with other modalities compared with sham treatment or other treatments as control. |
| V | 13 | Combinations of i.a. injection of stem cells with or without other modalities without control group (case series or case reports). |
| VI | 4 | Treatment of focal chondral, osteochondral, or meniscal chondral lesions with stem cells as the sole treatment (not considering rehabilitation) or combinations of stem cells and other modalities with or without other treatments as control. |
| VII | 1 | Treatments that did not comprise stem cells. |
| VIII | 2 | Study not listed in PubMed, Embase, Web of Science, Cochrane Library, or Google Scholar. |
| Sum | 56 |
| Type | Nall | NpkOA | Description |
|---|---|---|---|
| a | 29 | 25 | Randomized controlled trials (RCTs) |
| b | 3 | 3 | RCTs with the contralateral knee as internal control |
| c | 7 | 7 | Prospective cohort studies |
| d | 2 | 2 | Retrospective cohort studies |
| e | 13 | 13 | Case series with more than one subject |
| f | 0 | 0 | Case reports with only one subject |
| N | 2 | 2 | Study not listed in PubMed, Embase, Web of Science, Cochrane Library, or Google Scholar |
| Sum | 56 | 52 |
| Cell Type | O | Nall | NpkOA | Description |
|---|---|---|---|---|
| ADRCs | Auto | 9 | 9 | Autologous, adipose-derived regenerative cells |
| ADSCs | Auto | 11 | 11 | Autologous, adipose-derived stem cells (obtained by culturing ADRCs) |
| ADSCs | Allo | 1 | 1 | Allogeneic, adipose-derived stem cells |
| MFF | Auto | 4 | 4 | Autologous, micro-fragmented fat (from liposuction) |
| CLL | Auto | 1 | 1 | Autologous, centrifuged liposuction liquid |
| BMAC | Auto | 7 | 7 | Autologous bone marrow concentrate |
| BM-MSCs | Auto | 10 | 9 | Autologous, bone-marrow-derived mesenchymal stem cells |
| BM-MSCs | Allo | 3 | 2 | Allogeneic, bone-marrow-derived mesenchymal stromal cells |
| S-MSCs | Auto | 1 | 0 | Autologous, matrix-induced MSCs from synovia |
| Ch-TGFβ | Allo | 2 | 2 | Allogeneic chondrocytes that overexpress transforming growth factor beta |
| hUC-MSCs | Allo | 2 | 2 | Allogeneic, human-umbilical-cord-derived MSCs |
| P-MSCs | Allo | 1 | 1 | Allogeneic, placental MSCs |
| pBMCs | Auto | 1 | 0 | Autologous, activated peripheral blood stem cells |
| No cells | Allo | 1 | 1 | Allogeneic amniotic fluid |
| Sum | 54 | 50 |
| C | N | Description |
|---|---|---|
| 1 | 12 | Meta-analysis of studies in which treatment of pkOA with stem cells was compared with placebo treatment (or studies in which treatment of pkOA with stem cells plus concomitant therapy was compared with the concomitant therapy alone, respectively). |
| 2 | 4 | Meta-analysis in which only endpoints of the same patients before and after treatment were compared. |
| 3 | 3 | Network meta-analysis that included only a small number of studies on the treatment of pkOA with stem cells and a much higher number of studies on the treatment of pkOA without stem cells. |
| Sum | 19 |
| No. | Na | Nr [%] | |
|---|---|---|---|
| 1 | At least 2 different clinical studies were included. | 141 | 89.8 |
| 2 | Only clinical studies on pkOA were included. | 141 | 89.8 |
| 3 | Only clinical studies in which stem cells were applied were included. | 155 | 98.7 |
| 4 | Only randomized controlled trials were included. | 133 | 84.7 |
| 5 | Only clinical studies were included in which application of stem cells was compared with placebo treatment or in which application of stem cells plus concomitant therapy (including arthroscopic debridement, high tibial osteotomy, injection of hyaluronic acid, etc.) was compared with the concomitant therapy alone, respectively. | 55 | 35.0 |
| 6 | Only clinical studies using, respectively, autologous or allogeneic stem cells were included. | 64 | 40.8 |
| 7 | Only clinical studies in which, respectively, cultured or uncultured cells were applied were included. | 133 | 84.7 |
| 8 | Clinical studies in which more than 1 dose of stem cells was applied were only considered once in the corresponding meta-analysis. | 100 | 63.7 |
| C | Nall | Description |
|---|---|---|
| I | 8 | Treatment of pkOA with i.a. injection of stem cells as the sole treatment (not considering rehabilitation) compared with i.a. injection of saline or sham treatment as control. |
| II | 8 | Treatment of pkOA with i.a. injection of stem cells as the sole treatment (not considering rehabilitation) compared with i.a. injection of, respectively, PRP, CS or HA as control. |
| III | 22 | Treatment of pkOA with i.a. injection of stem cells as the sole treatment (not considering rehabilitation) compared with other treatments than those in Categories I and II as control. |
| IV | 27 | Treatment of pkOA with combinations of stem cells and other modalities compared with sham treatment or other treatments as control. |
| V | 78 | Treatment of pkOA with combinations of stem cells with or without other modalities without control group (case series or case reports). |
| VI | 40 | Treatment of focal chondral, osteochondral, or meniscal chondral lesions with stem cells as the sole treatment (not considering rehabilitation) or combinations of stem cells and other modalities with or without other treatments as control. |
| Sum | 183 |
| Type | Nall | NpkOA | Description |
|---|---|---|---|
| a | 44 | 33 | Randomized controlled trials (RCTs) |
| b | 6 | 6 | RCTs with the contralateral knee as internal control |
| c | 18 | 15 | Prospective cohort studies |
| d | 12 | 11 | Retrospective cohort studies |
| e | 92 | 73 | Case series with more than one subject |
| f | 11 | 5 | Case reports with only one subject |
| Sum | 183 | 143 |
| Cell Type | Nall | NpkOA | Description |
|---|---|---|---|
| ADRCs | 36 | 35 | Adipose-derived regenerative cells |
| ADSCs | 22 | 18 | Adipose-derived stem cells (obtained by culturing ADRCs) |
| MFF | 21 | 21 | Micro-fragmented fat (from liposuction) |
| CLL | 1 | 1 | Centrifuged liposuction liquid |
| BMA | 1 | 1 | Bone marrow aspirate |
| BMAC | 38 | 26 | Bone marrow aspirate concentrate |
| BM-MSCs | 34 | 25 | Bone-marrow-derived mesenchymal stem cells |
| Cs/CPs | 2 | 0 | Chondrocytes and chondrocyte precursors |
| CSCs | 1 | 0 | Cartilage stem cells |
| MACI | 1 | 0 | Matrix-induced, autologous chondrocyte implant |
| S-MSCs | 4 | 1 | Matrix-induced MSCs from synovia |
| Ch-TGFβ | 2 | 2 | Chondrocytes that overexpress transforming growth factor beta |
| hUC-MSCs | 4 | 3 | Human-umbilical-cord-derived MSCs |
| hUCB-MSCs | 7 | 6 | Human-umbilical-cord-blood-derived MSCs |
| P-MSCs | 1 | 1 | Placental MSCs |
| pBSCs | 8 | 4 | Activated peripheral blood stem cells |
| Sum | 183 | 143 |
| Category | Type of Study | ADRCs | ADSCs | MFF | CLL | BMA | BMAC | BM-MSCs | Cs/CPs | CSCs | MACI | S-MSCs | Ch-TGFβ | hUC-MSCs | hUCB-MSCs | P-MSCs | pBSCs | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | a | 1 | 2 | 1 | 2 | 1 | 7 | |||||||||||
| b | 1 | 1 | ||||||||||||||||
| c | 0 | |||||||||||||||||
| d | 0 | |||||||||||||||||
| II | a | 1 | 1 | 2 | 1 | 2 | 7 | |||||||||||
| b | 0 | |||||||||||||||||
| c | 0 | |||||||||||||||||
| d | 1 | 1 | ||||||||||||||||
| III | a | 4 | 1 | 1 | 6 | |||||||||||||
| b | 3 | 3 | ||||||||||||||||
| c | 1 | 5 | 2 | 1 | 9 | |||||||||||||
| d | 2 | 1 | 1 | 4 | ||||||||||||||
| IV | a | 2 | 1 | 1 | 1 | 7 | 1 | 13 | ||||||||||
| b | 1 | 1 | 2 | |||||||||||||||
| c | 5 | 1 | 6 | |||||||||||||||
| d | 1 | 1 | 4 | 6 | ||||||||||||||
| V | e | 20 | 3 | 15 | 1 | 1 | 13 | 11 | 1 | 1 | 5 | 2 | 74 | |||||
| f | 1 | 1 | 2 | 4 | ||||||||||||||
| VI | a | 1 | 1 | 1 | 3 | 1 | 1 | 3 | 11 | |||||||||
| b | 0 | |||||||||||||||||
| c | 3 | 3 | ||||||||||||||||
| d | 1 | 1 | ||||||||||||||||
| e | 1 | 6 | 4 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 19 | |||||||
| f | 2 | 1 | 2 | 1 | 6 | |||||||||||||
| NpkOA | 1568 | 346 | 1489 | 20 | 3 | 2588 | 385 | 8 | 128 | 75 | 186 | 10 | 54 | 6860 | ||||
| NCL | 40 | 22 | 317 | 83 | 13 | 15 | 15 | 18 | * | 166 | 129 | 818 |
| R | C | T | First Author | Y | Cell Type | Treatment | Control |
|---|---|---|---|---|---|---|---|
| Autologous, uncultured cells | |||||||
| [19] | 1 | a | Garza | 2020 | ADRCs | C | RS |
| [52] | 4 | a | Koh | 2014 | ADRCs | C + AD + HTO + PRP | AD + HTO + PRP |
| [58] | 4 | a | Peretti | 2018 | MFF | C + AD | AD |
| Autologous, cultured cells | |||||||
| [18] | 1 | a | Lee | 2019 | ADSCs | C | Sa |
| [30] | 3 | a | Freitag | 2019 | ADSCs | C | CM |
| [56] | 4 | a | Zhang | 2018 | ADSCs | C + HA | C |
| [60] | 4 | a | Qiao | 2020 | ADSCs | C + AD + MF + HA | MF or MF + HA |
| [14] | 1 | a | Emamedin | 2018 | BM-MSCs | C | Sa |
| [50] | 4 | a | Varma | 2010 | BM-MSCs | C + AD | AD |
| [51] | 4 | a | Wong | 2013 | BM-MSCs | C + MF + HTO + HA | MF + HA + HTO |
| [53] | 4 | a | Lamo-Espinosa | 2016 | BM-MSCs | C + HA | HA |
| [55] | 4 | a | Bastos | 2018 | BM-MSCs | C + PRP | C |
| [57] | 4 | a | Lamo-Espinosa | 2018 | BM-MSCs | C + HA | HA |
| [59] | 4 | a | Lamo-Espinosa | 2020 | BM-MSCs | C + PRP | PRP |
| Allogeneic, cultured cells | |||||||
| [12] | 1 | a | Cherian | 2015 | Ch-TGFß | C | Sa |
| [13] | 4 | a | Gupta | 2016 | BM-MSCs | C + HA | Sa + HA |
| [15] | 1 | a | Kim | 2018 | Ch-TGFß | C | Sa |
| [16] | 1 | a | Kuah | 2018 | ADSCs | C | Sa |
| [17] | 1 | a | Khalifeh Soltani | 2019 | P-MSCs | C | Sa |
| R | FA | Reported Outcome |
|---|---|---|
| Autologous ADRCs | ||
| [19] | Garza |
|
| [52] | Koh |
|
| Autologous ADSCs | ||
| [18] | Lee |
|
| [30] | Freitag |
|
| [56] | Zhang |
|
| [60] | Qiao |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitz, C.; Alt, C.; Pearce, D.A.; Furia, J.P.; Maffulli, N.; Alt, E.U. Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review. Cells 2022, 11, 965. https://doi.org/10.3390/cells11060965
Schmitz C, Alt C, Pearce DA, Furia JP, Maffulli N, Alt EU. Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review. Cells. 2022; 11(6):965. https://doi.org/10.3390/cells11060965
Chicago/Turabian StyleSchmitz, Christoph, Christopher Alt, David A. Pearce, John P. Furia, Nicola Maffulli, and Eckhard U. Alt. 2022. "Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review" Cells 11, no. 6: 965. https://doi.org/10.3390/cells11060965
APA StyleSchmitz, C., Alt, C., Pearce, D. A., Furia, J. P., Maffulli, N., & Alt, E. U. (2022). Methodological Flaws in Meta-Analyses of Clinical Studies on the Management of Knee Osteoarthritis with Stem Cells: A Systematic Review. Cells, 11(6), 965. https://doi.org/10.3390/cells11060965








