Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. MSC Isolation and Cultivation
2.2. T17b EPC Cultivation and Differentiation
2.3. AV Loop Operation
2.4. Explantation Procedure
2.5. Computer Tomography
2.6. Histological Staining
2.7. Statistical Analysis
3. Results
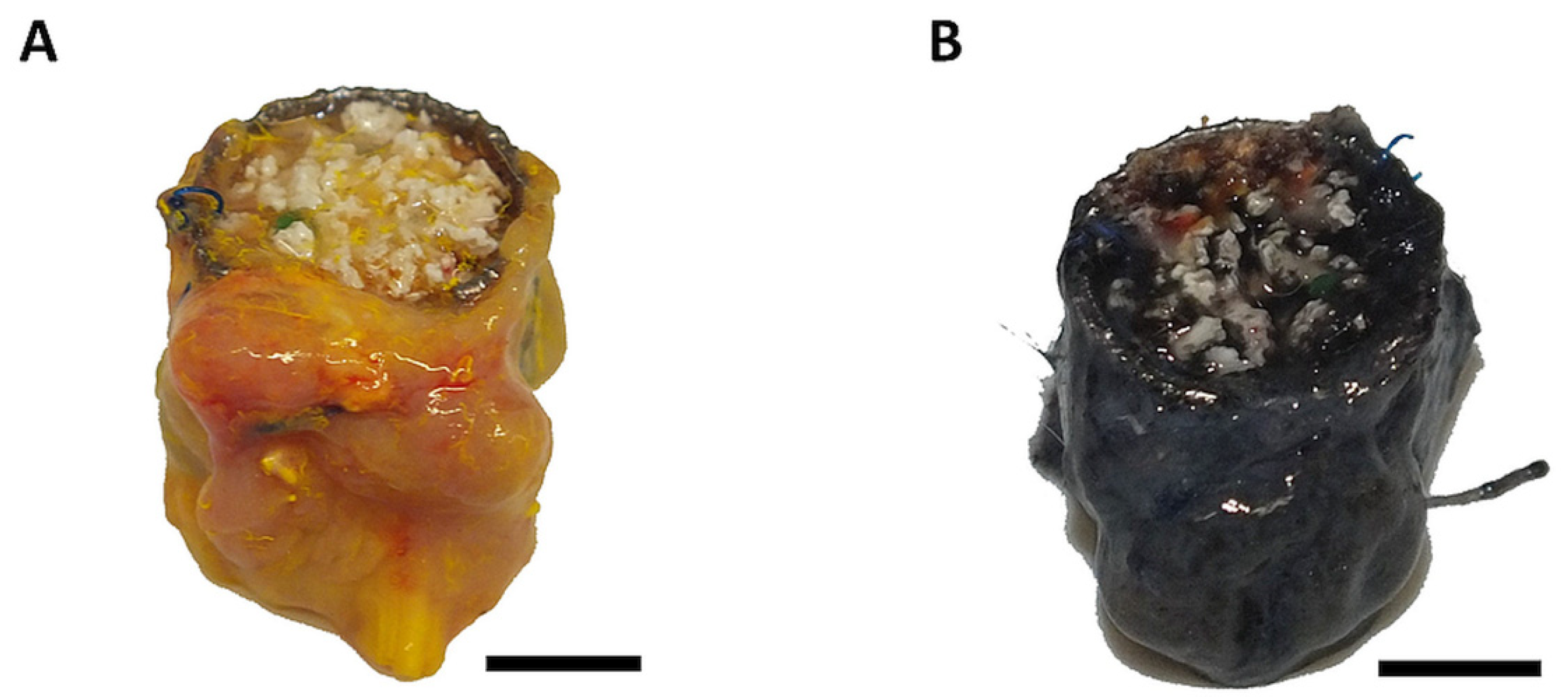
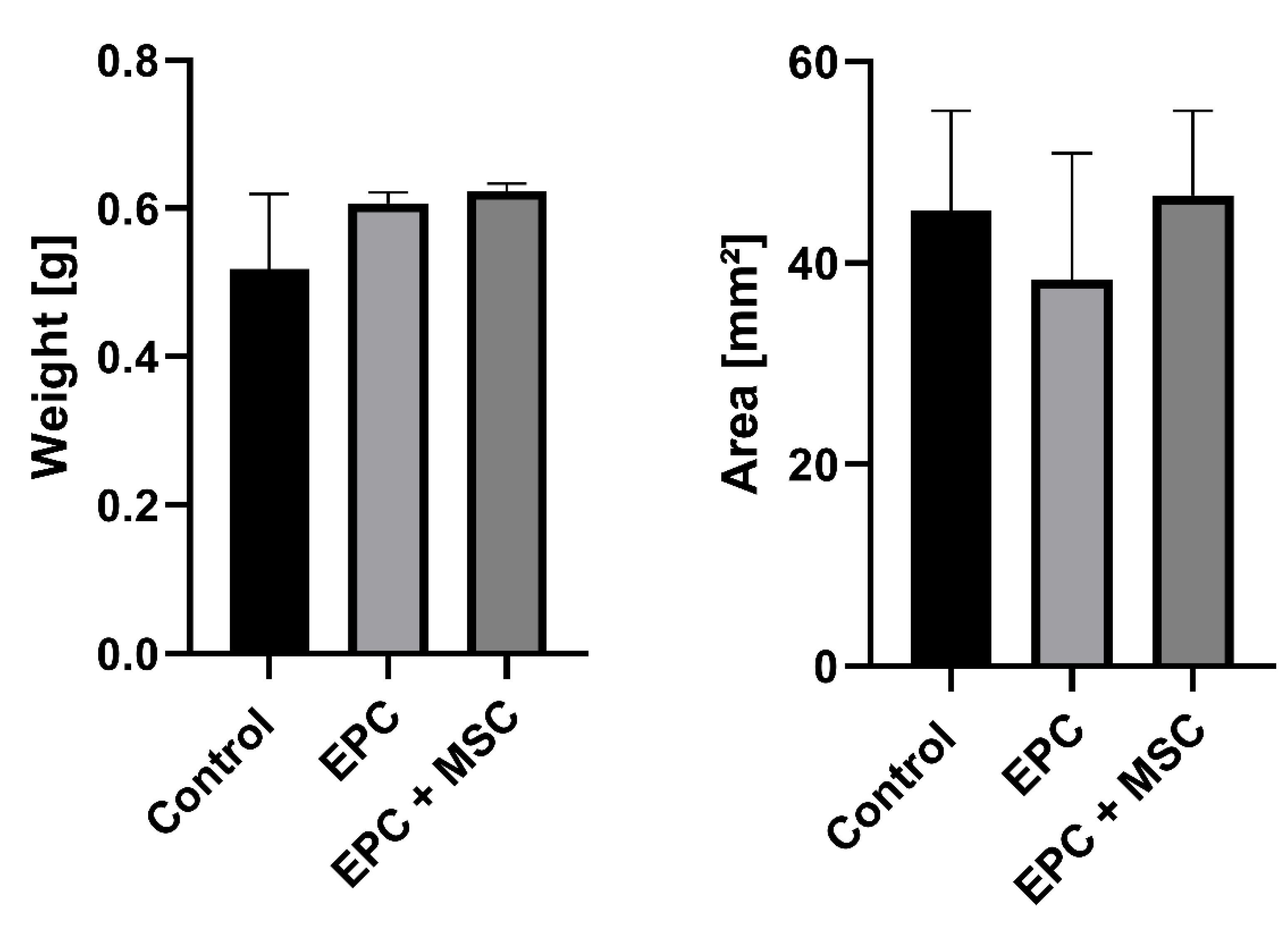
3.1. Surgical Outcome and Macroscopic Appearance
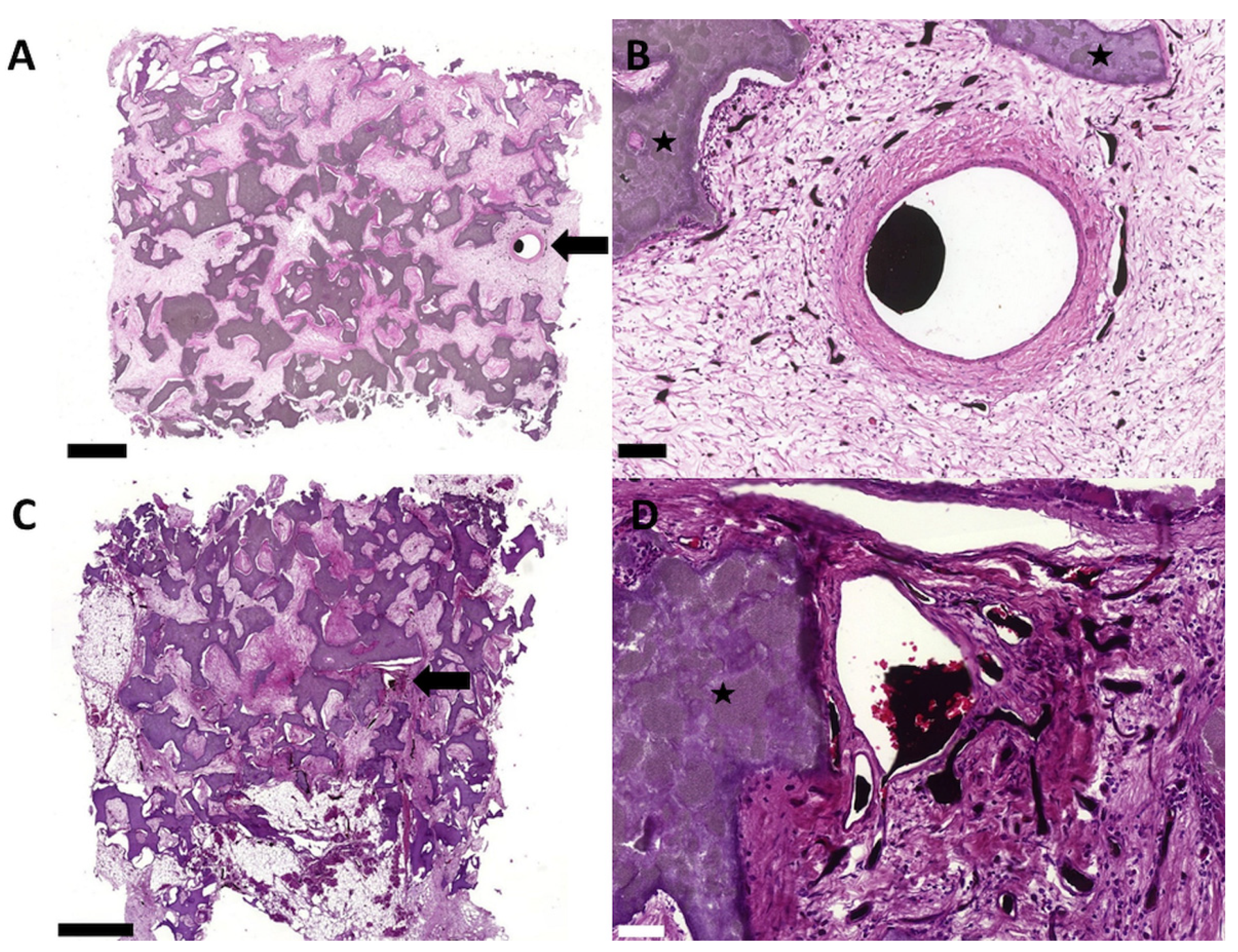
3.2. Biocompatibility and Degradation of the HA/ß–TCP–Fibrin Matrix
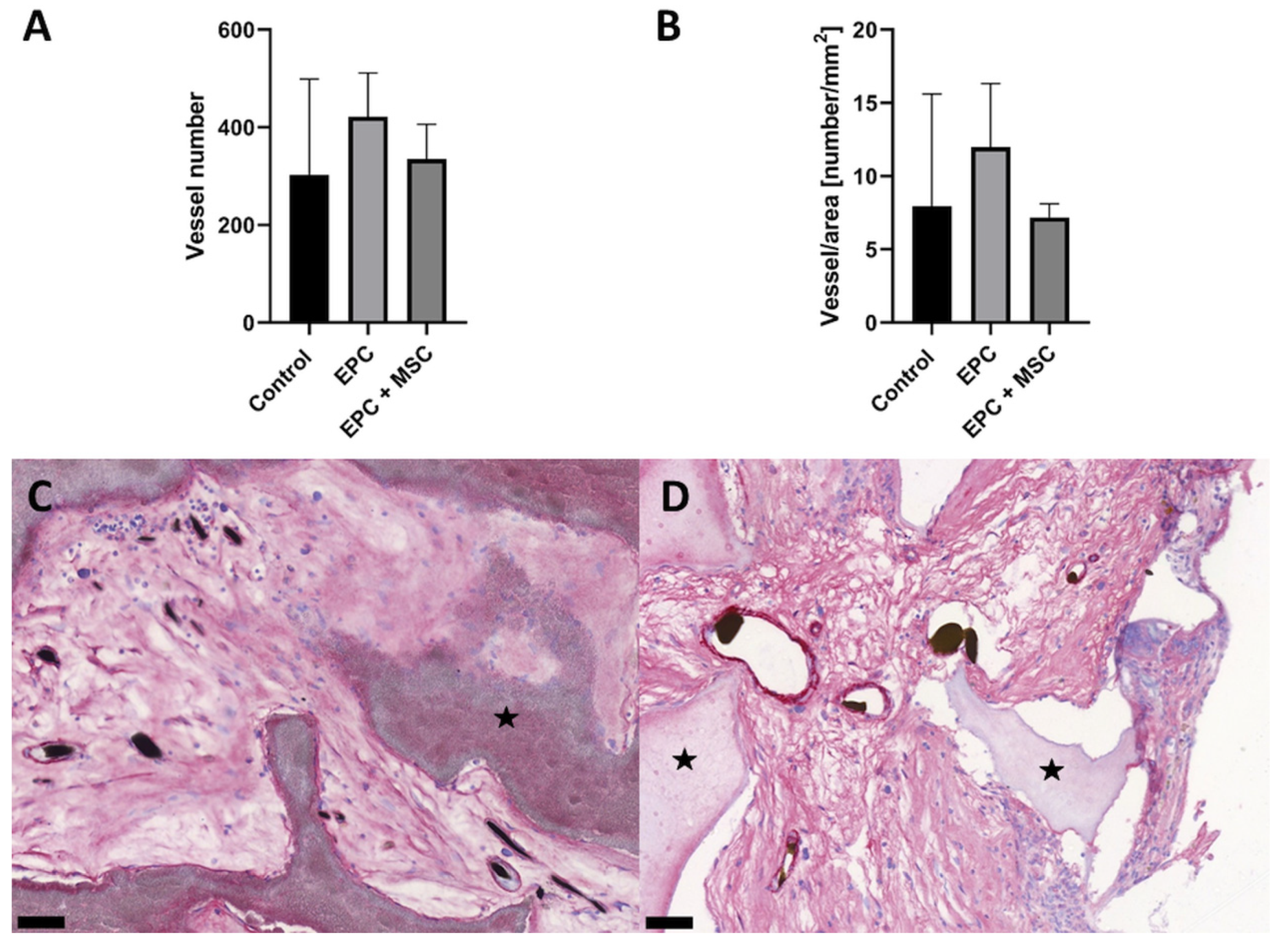
3.3. Vascularization and Bone Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willemot, L.; Stewart, D.; Lawson, R. Reconstruction of an infected midshaft radius and ulna nonunion using a free vascularized fibula and medial femoral condyle flap. Microsurgery 2021, 41, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Adani, R.; Delcroix, L.; Innocenti, M.; Marcoccio, I.; Tarallo, L.; Celli, A.; Ceruso, M. Reconstruction of large posttraumatic skeletal defects of the forearm by vascularized free fibular graft. Microsurgery 2004, 24, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ma, H.; Shujaat, S.; Orhan, K.; Coucke, W.; Amoli, M.S.; Bila, M.; Politis, C.; Jacobs, R. Donor- and recipient-site morbidity of vascularized fibular and iliac flaps for mandibular reconstruction: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1470–1479. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef]
- Chen, C.F.; Chen, Y.C.; Fu, Y.S.; Tsai, S.W.; Wu, P.K.; Chen, C.M.; Chang, M.C.; Chen, W.M. Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 8987. [Google Scholar] [CrossRef]
- Steiner, D.; Winkler, S.; Heltmann-Meyer, S.; Trossmann, V.T.; Fey, T.; Scheibel, T.; Horch, R.E.; Arkudas, A. Enhanced vascularization and de novo tissue formation in hydrogels made of engineered RGD-tagged spider silk proteins in the arteriovenous loop model. Biofabrication 2021, 13, 13. [Google Scholar] [CrossRef]
- Steiner, D.; Lingens, L.; Fischer, L.; Kohn, K.; Detsch, R.; Boccaccini, A.R.; Fey, T.; Greil, P.; Weis, C.; Beier, J.P.; et al. Encapsulation of Mesenchymal Stem Cells Improves Vascularization of Alginate-Based Scaffolds. Tissue Eng. Part A 2018, 24, 1320–1331. [Google Scholar] [CrossRef]
- Rottensteiner, U.; Sarker, B.; Heusinger, D.; Dafinova, D.; Rath, S.N.; Beier, J.P.; Kneser, U.; Horch, R.E.; Detsch, R.; Boccaccini, A.R.; et al. In vitro and in vivo Biocompatibility of Alginate Dialdehyde/Gelatin Hydrogels with and without Nanoscaled Bioactive Glass for Bone Tissue Engineering Applications. Materials 2014, 7, 1957–1974. [Google Scholar] [CrossRef]
- Arkudas, A.; Pryymachuk, G.; Hoereth, T.; Beier, J.P.; Polykandriotis, E.; Bleiziffer, O.; Horch, R.E.; Kneser, U. Dose-finding study of fibrin gel-immobilized vascular endothelial growth factor 165 and basic fibroblast growth factor in the arteriovenous loop rat model. Tissue Eng. Part A 2009, 15, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Heltmann-Meyer, S.; Steiner, D.; Muller, C.; Schneidereit, D.; Friedrich, O.; Salehi, S.; Engel, F.B.; Arkudas, A.; Horch, R.E. Gelatin methacryloyl is a slow degrading material allowing vascularization and long-term usein vivo. Biomed. Mater. 2021, 16, 65004. [Google Scholar] [CrossRef] [PubMed]
- Schacht, K.; Jungst, T.; Schweinlin, M.; Ewald, A.; Groll, J.; Scheibel, T. Biofabrication of cell-loaded 3D spider silk constructs. Angew. Chem. Int. Ed. Engl. 2015, 54, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Rottensteiner-Brandl, U.; Detsch, R.; Sarker, B.; Lingens, L.; Kohn, K.; Kneser, U.; Bosserhoff, A.K.; Horch, R.E.; Boccaccini, A.R.; Arkudas, A. Encapsulation of Rat Bone Marrow Derived Mesenchymal Stem Cells in Alginate Dialdehyde/Gelatin Microbeads with and without Nanoscaled Bioactive Glass for In Vivo Bone Tissue Engineering. Materials 2018, 11, 1880. [Google Scholar] [CrossRef]
- Buehrer, G.; Balzer, A.; Arnold, I.; Beier, J.P.; Koerner, C.; Bleiziffer, O.; Brandl, A.; Weis, C.; Horch, R.E.; Kneser, U.; et al. Combination of BMP2 and MSCs significantly increases bone formation in the rat arterio-venous loop model. Tissue Eng. Part A 2015, 21, 96–105. [Google Scholar] [CrossRef]
- Beier, J.P.; Horch, R.E.; Hess, A.; Arkudas, A.; Heinrich, J.; Loew, J.; Gulle, H.; Polykandriotis, E.; Bleiziffer, O.; Kneser, U. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J. Tissue Eng. Regen. Med. 2010, 4, 216–223. [Google Scholar] [CrossRef]
- Arkudas, A.; Pryymachuk, G.; Beier, J.P.; Weigel, L.; Korner, C.; Singer, R.F.; Bleiziffer, O.; Polykandriotis, E.; Horch, R.E.; Kneser, U. Combination of extrinsic and intrinsic pathways significantly accelerates axial vascularization of bioartificial tissues. Plast. Reconstr. Surg. 2012, 129, 55e–65e. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Arkudas, A.; Beier, J.P.; Hess, A.; Greil, P.; Papadopoulos, T.; Kopp, J.; Bach, A.D.; Horch, R.E.; Kneser, U. Intrinsic axial vascularization of an osteoconductive bone matrix by means of an arteriovenous vascular bundle. Plast Reconstr. Surg. 2007, 120, 855–868. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Tjiawi, J.; Euler, S.; Arkudas, A.; Hess, A.; Brune, K.; Greil, P.; Lametschwandtner, A.; Horch, R.E.; Kneser, U. The venous graft as an effector of early angiogenesis in a fibrin matrix. Microvasc. Res. 2008, 75, 25–33. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sung, K.C.; Tsutsumi, A.; Ohba, S.; Ueda, K.; Morrison, W.A. Tissue engineering skin flaps: Which vascular carrier, arteriovenous shunt loop or arteriovenous bundle, has more potential for angiogenesis and tissue generation? Plast. Reconstr. Surg. 2003, 112, 1636–1644. [Google Scholar] [CrossRef]
- Li, Q.; Yu, T.; Wang, F.; Liu, X.; Wang, Z. Endothelial progenitor cells with stem cells enhance osteogenic efficacy. Am. J. Transl. Res. 2020, 12, 2409–2424. [Google Scholar] [PubMed]
- Khojasteh, A.; Fahimipour, F.; Jafarian, M.; Sharifi, D.; Jahangir, S.; Khayyatan, F.; Baghaban Eslaminejad, M. Bone engineering in dog mandible: Coculturing mesenchymal stem cells with endothelial progenitor cells in a composite scaffold containing vascular endothelial growth factor. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, H.; He, Y.; Li, Y.; He, X. Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway. Stem. Cell Res. Ther. 2020, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Hatzopoulos, A.K.; Folkman, J.; Vasile, E.; Eiselen, G.K.; Rosenberg, R.D. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 1998, 125, 1457–1468. [Google Scholar] [CrossRef]
- Wei, J.; Blum, S.; Unger, M.; Jarmy, G.; Lamparter, M.; Geishauser, A.; Vlastos, G.A.; Chan, G.; Fischer, K.D.; Rattat, D.; et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell 2004, 5, 477–488. [Google Scholar] [CrossRef]
- Kupatt, C.; Horstkotte, J.; Vlastos, G.A.; Pfosser, A.; Lebherz, C.; Semisch, M.; Thalgott, M.; Buttner, K.; Browarzyk, C.; Mages, J.; et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005, 19, 1576–1578. [Google Scholar] [CrossRef]
- Pfosser, A.; El-Aouni, C.; Pfisterer, I.; Dietz, M.; Globisch, F.; Stachel, G.; Trenkwalder, T.; Pinkenburg, O.; Horstkotte, J.; Hinkel, R.; et al. NF kappaB activation in embryonic endothelial progenitor cells enhances neovascularization via PSGL-1 mediated recruitment: Novel role for LL37. Stem Cells 2010, 28, 376–385. [Google Scholar] [CrossRef]
- Kupatt, C.; Hinkel, R.; Lamparter, M.; von Bruhl, M.L.; Pohl, T.; Horstkotte, J.; Beck, H.; Muller, S.; Delker, S.; Gildehaus, F.J.; et al. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: Role of phosphatidylinositol 3-kinase/AKT kinase. Circulation 2005, 112, I117–I122. [Google Scholar] [CrossRef]
- Vielreicher, M.; Gellner, M.; Rottensteiner, U.; Horch, R.E.; Arkudas, A.; Friedrich, O. Multiphoton microscopy analysis of extracellular collagen I network formation by mesenchymal stem cells. J. Tissue Eng. Regen Med. 2017, 11, 2104–2115. [Google Scholar] [CrossRef]
- Bertram, U.; Steiner, D.; Poppitz, B.; Dippold, D.; Kohn, K.; Beier, J.P.; Detsch, R.; Boccaccini, A.R.; Schubert, D.W.; Horch, R.E.; et al. Vascular Tissue Engineering: Effects of Integrating Collagen into a PCL Based Nanofiber Material. Biomed. Res. Int 2017, 2017, 9616939. [Google Scholar] [CrossRef]
- Winkler, S.; Mutschall, H.; Biggemann, J.; Fey, T.; Greil, P.; Korner, C.; Weisbach, V.; Meyer-Lindenberg, A.; Arkudas, A.; Horch, R.E.; et al. Human Umbilical Vein Endothelial Cell Support Bone Formation of Adipose-Derived Stem Cell-Loaded and 3D-Printed Osteogenic Matrices in the Arteriovenous Loop Model. Tissue Eng. Part A 2021, 27, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Lang, G.; Fischer, L.; Winkler, S.; Fey, T.; Greil, P.; Scheibel, T.; Horch, R.E.; Arkudas, A. Intrinsic Vascularization of Recombinant eADF4(C16) Spider Silk Matrices in the Arteriovenous Loop Model. Tissue Eng. Part A 2019, 25, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Boos, A.M.; Loew, J.S.; Weigand, A.; Deschler, G.; Klumpp, D.; Arkudas, A.; Bleiziffer, O.; Gulle, H.; Kneser, U.; Horch, R.E.; et al. Engineering axially vascularized bone in the sheep arteriovenous-loop model. J. Tissue Eng. Regen. Med. 2013, 7, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Usami, K.; Mizuno, H.; Okada, K.; Narita, Y.; Aoki, M.; Kondo, T.; Mizuno, D.; Mase, J.; Nishiguchi, H.; Kagami, H.; et al. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J. Biomed. Mater. Res. A 2009, 90, 730–741. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Kahling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto β-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Pang, H.; Wu, X.H.; Fu, S.L.; Luo, F.; Zhang, Z.H.; Hou, T.Y.; Li, Z.Q.; Chang, Z.Q.; Yu, B.; Xu, J.Z. Prevascularisation with endothelial progenitor cells improved restoration of the architectural and functional properties of newly formed bone for bone reconstruction. Int. Orthop. 2013, 37, 753–759. [Google Scholar] [CrossRef][Green Version]
- Steiner, D.; Kohn, K.; Beier, J.P.; Sturzl, M.; Horch, R.E.; Arkudas, A. Cocultivation of Mesenchymal Stem Cells and Endothelial Progenitor Cells Reveals Antiapoptotic and Proangiogenic Effects. Cells Tissues Organs 2017, 204, 218–227. [Google Scholar] [CrossRef]
- Abe, Y.; Ozaki, Y.; Kasuya, J.; Yamamoto, K.; Ando, J.; Sudo, R.; Ikeda, M.; Tanishita, K. Endothelial progenitor cells promote directional three-dimensional endothelial network formation by secreting vascular endothelial growth factor. PLoS ONE 2013, 8, e82085. [Google Scholar] [CrossRef]
- Duffy, G.P.; Ahsan, T.; O’Brien, T.; Barry, F.; Nerem, R.M. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng. Part A 2009, 15, 2459–2470. [Google Scholar] [CrossRef]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. NANOmetric BIO-Banked MSC-Derived Exosome (NANOBIOME) as a Novel Approach to Regenerative Medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.W.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.M.; Guo, S.C.; Lang, H.L.; Zhang, C.Q.; Wang, Y.; et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Cao, J.; Ju, Z.; Ma, D.; Liu, Y.; Zhang, J. Coculture of peripheral blood CD34+ cell and mesenchymal stem cell sheets increase the formation of bone in calvarial critical-size defects in rabbits. Br. J. Oral. Maxillofac. Surg. 2014, 52, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiao, Y.; Zhou, W.; Bai, S.; Feng, Z.; Dong, Y.; Liu, Q.; Feng, X.; Zhao, Y. Endothelial progenitor cells improve the therapeutic effect of mesenchymal stem cell sheets on irradiated bone defect repair in a rat model. J. Transl. Med. 2018, 16, 137. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: An in vitro study. Arch. Med. Res. 2013, 44, 504–513. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, H.; Liu, Y.; Liu, Q.; Wang, A.; Ding, Y.; Jin, Z. Evaluation of BMMSCs-EPCs sheets for repairing alveolar bone defects in ovariectomized rats. Sci. Rep. 2017, 7, 16568. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.; Liu, C.; Peng, Z.; Li, J.; Jin, Y.; Wang, Y.; Guo, J.; Zhu, L. Cotransplantation of mesenchymal stem cells and endothelial progenitor cells for treating steroid-induced osteonecrosis of the femoral head. Stem Cells Transl. Med. 2021, 10, 781–796. [Google Scholar] [CrossRef]
- Arkudas, A.; Beier, J.P.; Pryymachuk, G.; Hoereth, T.; Bleiziffer, O.; Polykandriotis, E.; Hess, A.; Gulle, H.; Horch, R.E.; Kneser, U. Automatic quantitative micro-computed tomography evaluation of angiogenesis in an axially vascularized tissue-engineered bone construct. Tissue Eng. Part. C Methods 2010, 16, 1503–1514. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, Z.; Zhao, Y.; Tang, Y.; Zhou, S.; Sun, Y.; Chen, X. In Vitro and In Vivo Study of a Novel Nanoscale Demineralized Bone Matrix Coated PCL/β-TCP Scaffold for Bone Regeneration. Macromol. Biosci. 2021, 21, e2000336. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steiner, D.; Reinhardt, L.; Fischer, L.; Popp, V.; Körner, C.; Geppert, C.I.; Bäuerle, T.; Horch, R.E.; Arkudas, A. Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds. Cells 2022, 11, 926. https://doi.org/10.3390/cells11060926
Steiner D, Reinhardt L, Fischer L, Popp V, Körner C, Geppert CI, Bäuerle T, Horch RE, Arkudas A. Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds. Cells. 2022; 11(6):926. https://doi.org/10.3390/cells11060926
Chicago/Turabian StyleSteiner, Dominik, Lea Reinhardt, Laura Fischer, Vanessa Popp, Carolin Körner, Carol I. Geppert, Tobias Bäuerle, Raymund E. Horch, and Andreas Arkudas. 2022. "Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds" Cells 11, no. 6: 926. https://doi.org/10.3390/cells11060926
APA StyleSteiner, D., Reinhardt, L., Fischer, L., Popp, V., Körner, C., Geppert, C. I., Bäuerle, T., Horch, R. E., & Arkudas, A. (2022). Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds. Cells, 11(6), 926. https://doi.org/10.3390/cells11060926









