Biological Hallmarks and Emerging Strategies to Target STAT3 Signaling in Multiple Myeloma
Abstract
:1. Introduction
2. STAT Signaling and Functions
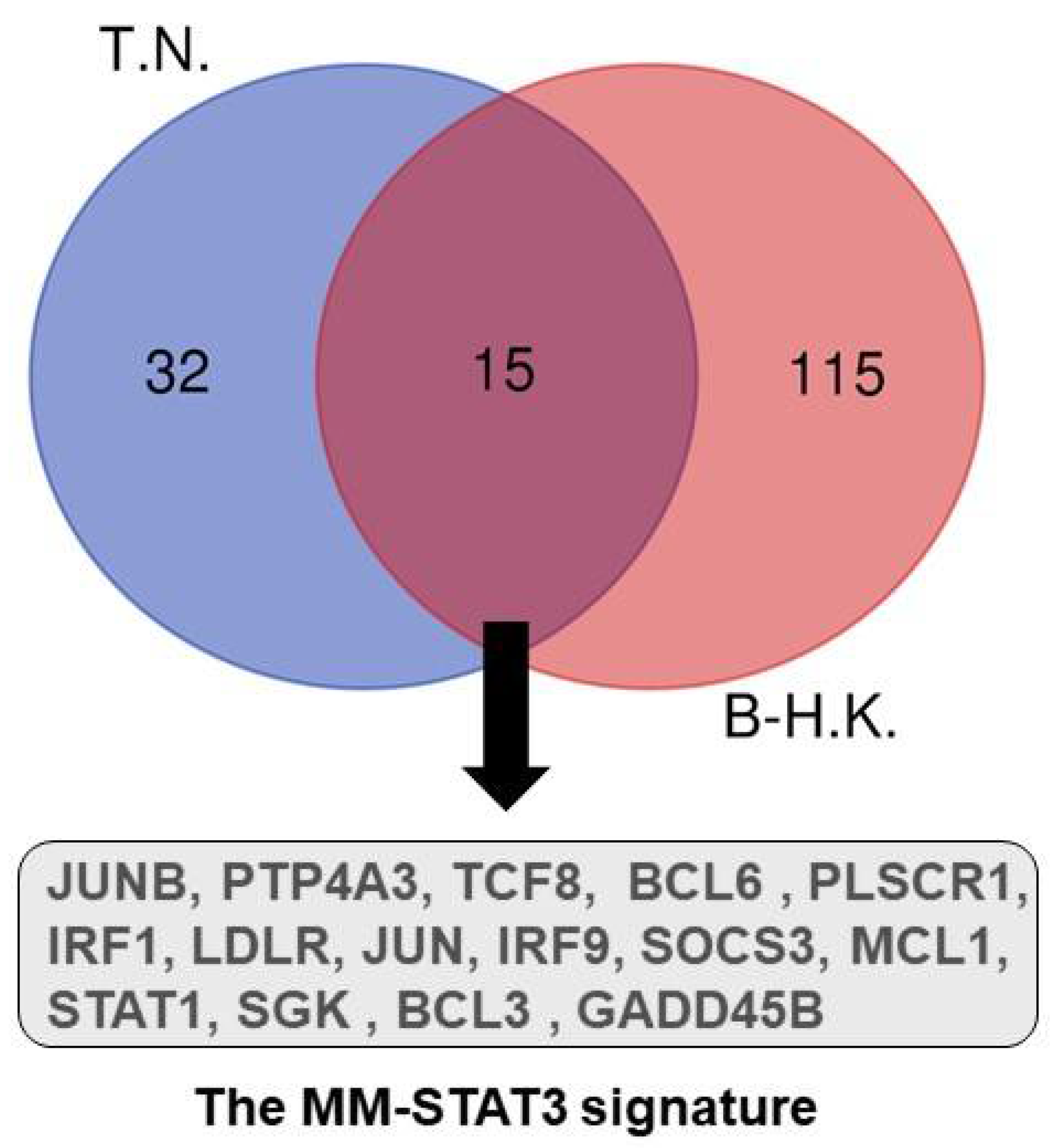
3. STAT3 Sustains MM Cell Survival and Proliferation
4. STAT3 Regulates Tumor Microenvironment
5. STAT3 Induces Immunosuppression
6. Targeting STAT3: Killing Many Birds with One Stone
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef]
- Chng, W.J.; Dispenzieri, A.; Chim, C.S.; Fonseca, R.; Goldschmidt, H.; Lentzsch, S.; Munshi, N.; Palumbo, A.; Miguel, J.S.; Sonneveld, P.; et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014, 28, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Chng, W.J.; Chou, T.; Nawarawong, W.; Hwang, S.Y.; Chim, C.S.; Chen, W.; Durie, B.G.; Lee, J.H. Management of multiple myeloma in Asia: Resource-stratified guidelines. Lancet Oncol. 2013, 14, e571–e581. [Google Scholar] [CrossRef]
- Fuchsl, F.; Krackhardt, A.M. Adoptive Cellular Therapy for Multiple Myeloma Using CAR- and TCR-Transgenic T Cells: Response and Resistance. Cells 2022, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Lancman, G.; Sastow, D.L.; Cho, H.J.; Jagannath, S.; Madduri, D.; Parekh, S.S.; Richard, S.; Richter, J.; Sanchez, L.; Chari, A. Bispecific Antibodies in Multiple Myeloma: Present and Future. Blood Cancer Discov. 2021, 2, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chng, W.J.; Zhou, J. Crosstalk between endoplasmic reticulum stress and oxidative stress: A dynamic duo in multiple myeloma. Cell Mol. Life Sci. 2021, 78, 3883–3906. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.G.; Stojanov, P.; Carter, S.L.; Cruz-Gordillo, P.; Lawrence, M.S.; Auclair, D.; Sougnez, C.; Knoechel, B.; Gould, J.; Saksena, G.; et al. Widespread genetic heterogeneity in multiple myeloma: Implications for targeted therapy. Cancer Cell 2014, 25, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smedt, E.; Lui, H.; Maes, K.; De Veirman, K.; Menu, E.; Vanderkerken, K.; De Bruyne, E. The Epigenome in Multiple Myeloma: Impact on Tumor Cell Plasticity and Drug Response. Front. Oncol. 2018, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Corre, J.; Avet-Loiseau, H. Risk Stratification and Targets in Multiple Myeloma: From Genomics to the Bedside. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Benavides, I.J.; de Ramon, C.; Gutierrez, N.C. Genetic Abnormalities in Multiple Myeloma: Prognostic and Therapeutic Implications. Cells 2021, 10, 336. [Google Scholar] [CrossRef]
- Szalat, R.; Munshi, N.C. Genomic heterogeneity in multiple myeloma. Curr. Opin. Genet. Dev. 2015, 30, 56–65. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Awada, H.; Thapa, B.; Awada, H.; Dong, J.; Gurnari, C.; Hari, P.; Dhakal, B. A Comprehensive Review of the Genomics of Multiple Myeloma: Evolutionary Trajectories, Gene Expression Profiling, and Emerging Therapeutics. Cells 2021, 10, 1961. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.; Wen, Z.; Haspel, R.L.; Zhang, J.J.; Darnell, J.E., Jr. The linker domain of Stat1 is required for gamma interferon-driven transcription. Mol. Cell Biol. 1999, 19, 5106–5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, M.; He, W.; Zhang, J.J. The DNA replication factor MCM5 is essential for Stat1-mediated transcriptional activation. Proc. Natl. Acad. Sci. USA 2005, 102, 14539–14544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciak, J.M.; Martinez-Yamout, M.A.; Dyson, H.J.; Wright, P.E. Structural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domains. EMBO J. 2009, 28, 948–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, A.C.; Shishodia, S.; Reuben, J.M.; Weber, D.; Alexanian, R.; Raj-Vadhan, S.; Estrov, Z.; Talpaz, M.; Aggarwal, B.B. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 2004, 103, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Brocke-Heidrich, K.; Kretzschmar, A.K.; Pfeifer, G.; Henze, C.; Loffler, D.; Koczan, D.; Thiesen, H.J.; Burger, R.; Gramatzki, M.; Horn, F. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood 2004, 103, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, N.; Danjoh, I.; Otsuyama, K.; Obata, M.; Tahara, H.; Ohta, T.; Ishikawa, H. IL-6-induced Bcl6 variant 2 supports IL-6-dependent myeloma cell proliferation and survival through STAT3. Biochem. Biophys. Res. Commun. 2005, 337, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Teoh, P.J.; Chung, T.H.; Chng, P.Y.Z.; Toh, S.H.M.; Chng, W.J. IL6R-STAT3-ADAR1 (P150) interplay promotes oncogenicity in multiple myeloma with 1q21 amplification. Haematologica 2020, 105, 1391–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teoh, P.J.; An, O.; Chung, T.H.; Chooi, J.Y.; Toh, S.H.M.; Fan, S.; Wang, W.; Koh, B.T.H.; Fullwood, M.J.; Ooi, M.G.; et al. Aberrant hyperediting of the myeloma transcriptome by ADAR1 confers oncogenicity and is a marker of poor prognosis. Blood 2018, 132, 1304–1317. [Google Scholar] [CrossRef]
- Abdollahi, P.; Kohn, M.; Borset, M. Protein tyrosine phosphatases in multiple myeloma. Cancer Lett. 2021, 501, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Zhou, J.; Cheong, L.L.; Liu, S.C.; Qian, J.; Guo, T.; Sze, S.K.; Zeng, Q.; Chng, W.J. LEO1 is regulated by PRL-3 and mediates its oncogenic properties in acute myelogenous leukemia. Cancer Res. 2014, 74, 3043–3053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chong, P.S.; Lu, X.; Cheong, L.L.; Bi, C.; Liu, S.C.; Zhou, Y.; Tan, T.Z.; Yang, H.; Chung, T.H.; et al. Phosphatase of regenerating liver-3 is regulated by signal transducer and activator of transcription 3 in acute myeloid leukemia. Exp. Hematol. 2014, 42, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Bardelli, A.; Buckhaults, P.; Velculescu, V.E.; Rago, C.; St Croix, B.; Romans, K.E.; Choti, M.A.; Lengauer, C.; Kinzler, K.W.; et al. A phosphatase associated with metastasis of colorectal cancer. Science 2001, 294, 1343–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasker, N.R.; Rastelli, E.J.; Burnett, J.C.; Sharlow, E.R.; Lazo, J.S.; Wipf, P. Tapping the therapeutic potential of protein tyrosine phosphatase 4A with small molecule inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 2008–2015. [Google Scholar] [CrossRef]
- Wang, H.; Vardy, L.A.; Tan, C.P.; Loo, J.M.; Guo, K.; Li, J.; Lim, S.G.; Zhou, J.; Chng, W.J.; Ng, S.B.; et al. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell 2010, 18, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chan, Z.L.; Bi, C.; Lu, X.; Chong, P.S.; Chooi, J.Y.; Cheong, L.L.; Liu, S.C.; Ching, Y.Q.; Zhou, Y.; et al. LIN28B Activation by PRL-3 Promotes Leukemogenesis and a Stem Cell-like Transcriptional Program in AML. Mol. Cancer Res. 2017, 15, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broyl, A.; Hose, D.; Lokhorst, H.; de Knegt, Y.; Peeters, J.; Jauch, A.; Bertsch, U.; Buijs, A.; Stevens-Kroef, M.; Beverloo, H.B.; et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood 2010, 116, 2543–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagerli, U.M.; Holt, R.U.; Holien, T.; Vaatsveen, T.K.; Zhan, F.; Egeberg, K.W.; Barlogie, B.; Waage, A.; Aarset, H.; Dai, H.Y.; et al. Overexpression and involvement in migration by the metastasis-associated phosphatase PRL-3 in human myeloma cells. Blood 2008, 111, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.Y.; Zhou, J.; Lim, J.S.L.; Hee, Y.T.; Chooi, J.Y.; Chung, T.H.; Tan, Z.T.; Zeng, Q.; Waller, D.D.; Sebag, M.; et al. IL6 Promotes a STAT3-PRL3 Feedforward Loop via SHP2 Repression in Multiple Myeloma. Cancer Res. 2019, 79, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Slordahl, T.S.; Abdollahi, P.; Vandsemb, E.N.; Rampa, C.; Misund, K.; Baranowska, K.A.; Westhrin, M.; Waage, A.; Ro, T.B.; Borset, M. The phosphatase of regenerating liver-3 (PRL-3) is important for IL-6-mediated survival of myeloma cells. Oncotarget 2016, 7, 27295–27306. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, W.; Ma, D.; Xiong, J.; Kuang, X.; Zhang, S.; Fang, Q.; Wang, J. Heme oxygenase-1 inhibition mediates Gas6 to enhance bortezomib-sensitivity in multiple myeloma via ERK/STAT3 axis. Aging 2020, 12, 6611–6629. [Google Scholar] [CrossRef]
- Lin, L.; Cao, L.; Liu, Y.; Wang, K.; Zhang, X.; Qin, X.; Zhao, D.; Hao, J.; Chang, Y.; Huang, X.; et al. B7-H3 promotes multiple myeloma cell survival and proliferation by ROS-dependent activation of Src/STAT3 and c-Cbl-mediated degradation of SOCS3. Leukemia 2019, 33, 1475–1486. [Google Scholar] [CrossRef]
- Novak, A.J.; Grote, D.M.; Ziesmer, S.C.; Rajkumar, V.; Doyle, S.E.; Ansell, S.M. A role for IFN-lambda1 in multiple myeloma B cell growth. Leukemia 2008, 22, 2240–2246. [Google Scholar] [CrossRef]
- Gupta, V.A.; Matulis, S.M.; Conage-Pough, J.E.; Nooka, A.K.; Kaufman, J.L.; Lonial, S.; Boise, L.H. Bone marrow microenvironment-derived signals induce Mcl-1 dependence in multiple myeloma. Blood 2017, 129, 1969–1979. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, M.; Honemann, D.; Lentzsch, S.; Bommert, K.; Sers, C.; Herrmann, P.; Mathas, S.; Dorken, B.; Bargou, R.C. In the presence of bone marrow stromal cells human multiple myeloma cells become independent of the IL-6/gp130/STAT3 pathway. Blood 2002, 100, 3311–3318. [Google Scholar] [CrossRef]
- Chatterjee, M.; Stuhmer, T.; Herrmann, P.; Bommert, K.; Dorken, B.; Bargou, R.C. Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells. Blood 2004, 104, 3712–3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hideshima, T.; Mitsiades, C.; Ikeda, H.; Chauhan, D.; Raje, N.; Gorgun, G.; Hideshima, H.; Munshi, N.C.; Richardson, P.G.; Carrasco, D.R.; et al. A proto-oncogene BCL6 is up-regulated in the bone marrow microenvironment in multiple myeloma cells. Blood 2010, 115, 3772–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, S.R.; Foley, N.; Schroeder, M.A. Daratumumab for the treatment of multiple myeloma. Drugs Today 2021, 57, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Ogiya, D.; Liu, J.; Ohguchi, H.; Kurata, K.; Samur, M.K.; Tai, Y.T.; Adamia, S.; Ando, K.; Hideshima, T.; Anderson, K.C. The JAK-STAT pathway regulates CD38 on myeloma cells in the bone marrow microenvironment: Therapeutic implications. Blood 2020, 136, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2019, 56, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosen, N. Integrins in multiple myeloma. Inflamm. Regen. 2020, 40, 4. [Google Scholar] [CrossRef] [PubMed]
- Shain, K.H.; Yarde, D.N.; Meads, M.B.; Huang, M.; Jove, R.; Hazlehurst, L.A.; Dalton, W.S. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: Implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009, 69, 1009–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meads, M.B.; Fang, B.; Mathews, L.; Gemmer, J.; Nong, L.; Rosado-Lopez, I.; Nguyen, T.; Ring, J.E.; Matsui, W.; MacLeod, A.R.; et al. Targeting PYK2 mediates microenvironment-specific cell death in multiple myeloma. Oncogene 2016, 35, 2723–2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nefedova, Y.; Huang, M.; Kusmartsev, S.; Bhattacharya, R.; Cheng, P.; Salup, R.; Jove, R.; Gabrilovich, D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 2004, 172, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Lee, Y.K.; Lee, H.J.; Choi, N.R.; Vo, M.C.; Hoang, M.D.; Lim, M.S.; Nguyen-Pham, T.N.; Kim, H.J.; Lee, J.J. Dendritic cells loaded with myeloma cells pretreated with a combination of JSI-124 and bortezomib generate potent myeloma-specific cytotoxic T lymphocytes in vitro. Exp. Hematol. 2014, 42, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Parrinello, N.L.; Simeon, V.; Puglisi, F.; La Cava, P.; Bellofiore, C.; Giallongo, C.; Camiolo, G.; D’Auria, F.; Grieco, V.; et al. High-density neutrophils in MGUS and multiple myeloma are dysfunctional and immune-suppressive due to increased STAT3 downstream signaling. Sci. Rep. 2020, 10, 1983. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.T.; Sloane, H.S.; Kester, M.; Kelly, K.A. Formation and role of exosomes in cancer. Cell Mol. Life Sci. 2015, 72, 659–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, S.; Sun, K.; Chng, W.J. The emerging roles of exosomes in leukemogeneis. Oncotarget 2016, 7, 50698–50707. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; De Veirman, K.; Faict, S.; Frassanito, M.A.; Ribatti, D.; Vacca, A.; Menu, E. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J. Pathol. 2016, 239, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Uchida, T.; Terui, Y.; Hayakawa, F.; Kobayashi, Y.; Taniwaki, M.; Takamatsu, Y.; Naoe, T.; Tobinai, K.; Munakata, W.; et al. Phase I study of OPB-51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies. Cancer Sci. 2015, 106, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Demartis, A.; Bernassola, F.; Savino, R.; Melino, G.; Ciliberto, G. Interleukin 6 receptor superantagonists are potent inducers of human multiple myeloma cell death. Cancer Res. 1996, 56, 4213–4218. [Google Scholar] [PubMed]
- Savino, R.; Lahm, A.; Salvati, A.L.; Ciapponi, L.; Sporeno, E.; Altamura, S.; Paonessa, G.; Toniatti, C.; Ciliberto, G. Generation of interleukin-6 receptor antagonists by molecular-modeling guided mutagenesis of residues important for gp130 activation. EMBO J. 1994, 13, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Tassone, P.; Neri, P.; Burger, R.; Savino, R.; Shammas, M.; Catley, L.; Podar, K.; Chauhan, D.; Masciari, S.; Gozzini, A.; et al. Combination therapy with interleukin-6 receptor superantagonist Sant7 and dexamethasone induces antitumor effects in a novel SCID-hu In vivo model of human multiple myeloma. Clin. Cancer Res. 2005, 11, 4251–4258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassone, P.; Galea, E.; Forciniti, S.; Tagliaferri, P.; Venuta, S. The IL-6 receptor super-antagonist Sant7 enhances antiproliferative and apoptotic effects induced by dexamethasone and zoledronic acid on multiple myeloma cells. Int. J. Oncol. 2002, 21, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Tassone, P.; Forciniti, S.; Galea, E.; Savino, R.; Turco, M.C.; Iacopino, P.; Tagliaferri, P.; Morrone, G.; Ciliberto, G.; Venuta, S. Synergistic induction of growth arrest and apoptosis of human myeloma cells by the IL-6 super-antagonist Sant7 and Dexamethasone. Cell Death Differ. 2000, 7, 327–328. [Google Scholar] [CrossRef] [Green Version]
- Petrucci, M.T.; Ricciardi, M.R.; Ariola, C.; Gregorj, C.; Ribersani, M.; Savino, R.; Ciliberto, G.; Tafuri, A. Cell cycle regulation and induction of apoptosis by IL-6 variants on the multiple myeloma cell line XG-1. Ann. Hematol. 1999, 78, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Honemann, D.; Chatterjee, M.; Savino, R.; Bommert, K.; Burger, R.; Gramatzki, M.; Dorken, B.; Bargou, R.C. The IL-6 receptor antagonist SANT-7 overcomes bone marrow stromal cell-mediated drug resistance of multiple myeloma cells. Int. J. Cancer 2001, 93, 674–680. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.B.; Fook-Alves, V.L.; Eugenio, A.I.P.; Fernando, R.C.; Sanson, L.F.G.; de Carvalho, M.F.; Braga, W.M.T.; Davies, F.E.; Colleoni, G.W.B. Anti-myeloma effects of ruxolitinib combined with bortezomib and lenalidomide: A rationale for JAK/STAT pathway inhibition in myeloma patients. Cancer Lett. 2017, 403, 206–215. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Sanchez, E.; Soof, C.M.; Bujarski, S.; Ng, N.; Cao, J.; Hekmati, T.; Zahab, B.; Nosrati, J.D.; et al. JAK1/2 pathway inhibition suppresses M2 polarization and overcomes resistance of myeloma to lenalidomide by reducing TRIB1, MUC1, CD44, CXCL12, and CXCR4 expression. Br. J. Haematol. 2020, 188, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Ng, N.; Yu, E.; Bujarski, S.; Yin, Z.; Wen, M.; Hekmati, T.; Field, D.; Wang, J.; et al. Ruxolitinib reverses checkpoint inhibition by reducing programmed cell death ligand-1 (PD-L1) expression and increases anti-tumour effects of T cells in multiple myeloma. Br. J. Haematol. 2021, 192, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; To, J.; Spektor, T.M.; Martinez, D.; Turner, C.; Sanchez, A.; Ghermezi, M.; Eades, B.M.; Swift, R.A.; Schwartz, G.; et al. A Phase I Study of Ruxolitinib, Lenalidomide, and Steroids for Patients with Relapsed/Refractory Multiple Myeloma. Clin. Cancer Res. 2020, 26, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; McCulloch, S.; Mahe, E.; Shafey, M.; Rashid-Kolvear, F.; Khan, F.; Prajapati, D.; Neri, P.; Duggan, P.; Tay, J.; et al. Anti-myeloma potential of ruxolitinib in co-existing JAK2V617F-positive smouldering myeloma and polycythaemia vera. Br. J. Haematol. 2020, 189, e114–e118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, R.; Le Gouill, S.; Tai, Y.T.; Shringarpure, R.; Tassone, P.; Neri, P.; Podar, K.; Catley, L.; Hideshima, T.; Chauhan, D.; et al. Janus kinase inhibitor INCB20 has antiproliferative and apoptotic effects on human myeloma cells in vitro and in vivo. Mol. Cancer Ther. 2009, 8, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scuto, A.; Krejci, P.; Popplewell, L.; Wu, J.; Wang, Y.; Kujawski, M.; Kowolik, C.; Xin, H.; Chen, L.; Wang, Y.; et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia 2011, 25, 538–550. [Google Scholar] [CrossRef]
- Li, J.; Favata, M.; Kelley, J.A.; Caulder, E.; Thomas, B.; Wen, X.; Sparks, R.B.; Arvanitis, A.; Rogers, J.D.; Combs, A.P.; et al. INCB16562, a JAK1/2 selective inhibitor, is efficacious against multiple myeloma cells and reverses the protective effects of cytokine and stromal cell support. Neoplasia 2010, 12, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Monaghan, K.A.; Khong, T.; Burns, C.J.; Spencer, A. The novel JAK inhibitor CYT387 suppresses multiple signalling pathways, prevents proliferation and induces apoptosis in phenotypically diverse myeloma cells. Leukemia 2011, 25, 1891–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santo, L.; Hideshima, T.; Cirstea, D.; Bandi, M.; Nelson, E.A.; Gorgun, G.; Rodig, S.; Vallet, S.; Pozzi, S.; Patel, K.; et al. Antimyeloma activity of a multitargeted kinase inhibitor, AT9283, via potent Aurora kinase and STAT3 inhibition either alone or in combination with lenalidomide. Clin. Cancer Res. 2011, 17, 3259–3271. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Chng, W.-J. Biological Hallmarks and Emerging Strategies to Target STAT3 Signaling in Multiple Myeloma. Cells 2022, 11, 941. https://doi.org/10.3390/cells11060941
Zhou J, Chng W-J. Biological Hallmarks and Emerging Strategies to Target STAT3 Signaling in Multiple Myeloma. Cells. 2022; 11(6):941. https://doi.org/10.3390/cells11060941
Chicago/Turabian StyleZhou, Jianbiao, and Wee-Joo Chng. 2022. "Biological Hallmarks and Emerging Strategies to Target STAT3 Signaling in Multiple Myeloma" Cells 11, no. 6: 941. https://doi.org/10.3390/cells11060941
APA StyleZhou, J., & Chng, W.-J. (2022). Biological Hallmarks and Emerging Strategies to Target STAT3 Signaling in Multiple Myeloma. Cells, 11(6), 941. https://doi.org/10.3390/cells11060941





