Current Methods to Unravel the Functional Properties of Lysosomal Ion Channels and Transporters
Abstract
1. Introduction
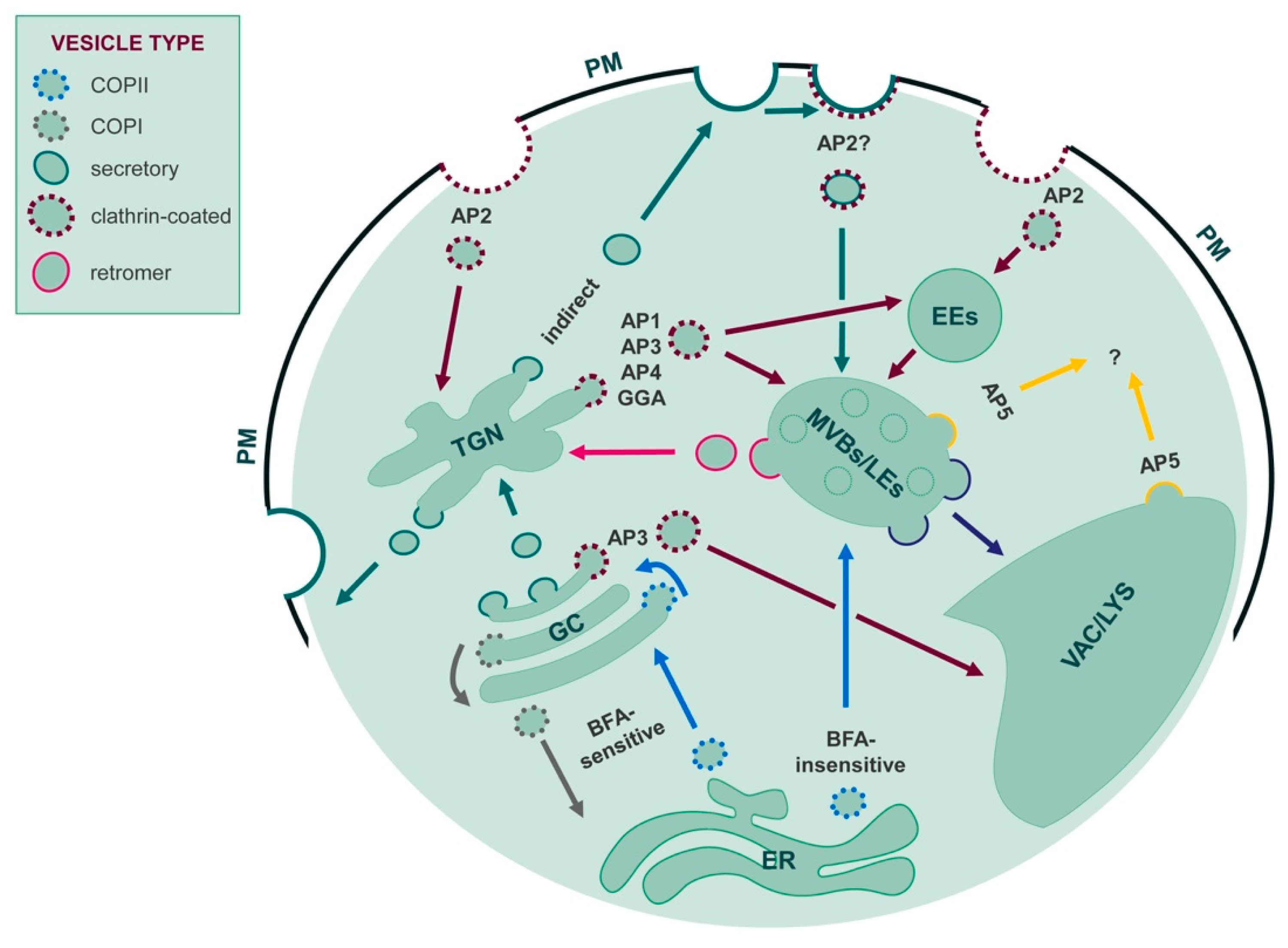
1.1. Main Families of Lysosomal Channels and Transporters
1.2. Summary of the Experimental Methods to Investigate the Functional Properties of Lysosomal Ion Channels and Transporters
2. Approaches Using Purified Proteins or Native Endolysosomal Membranes
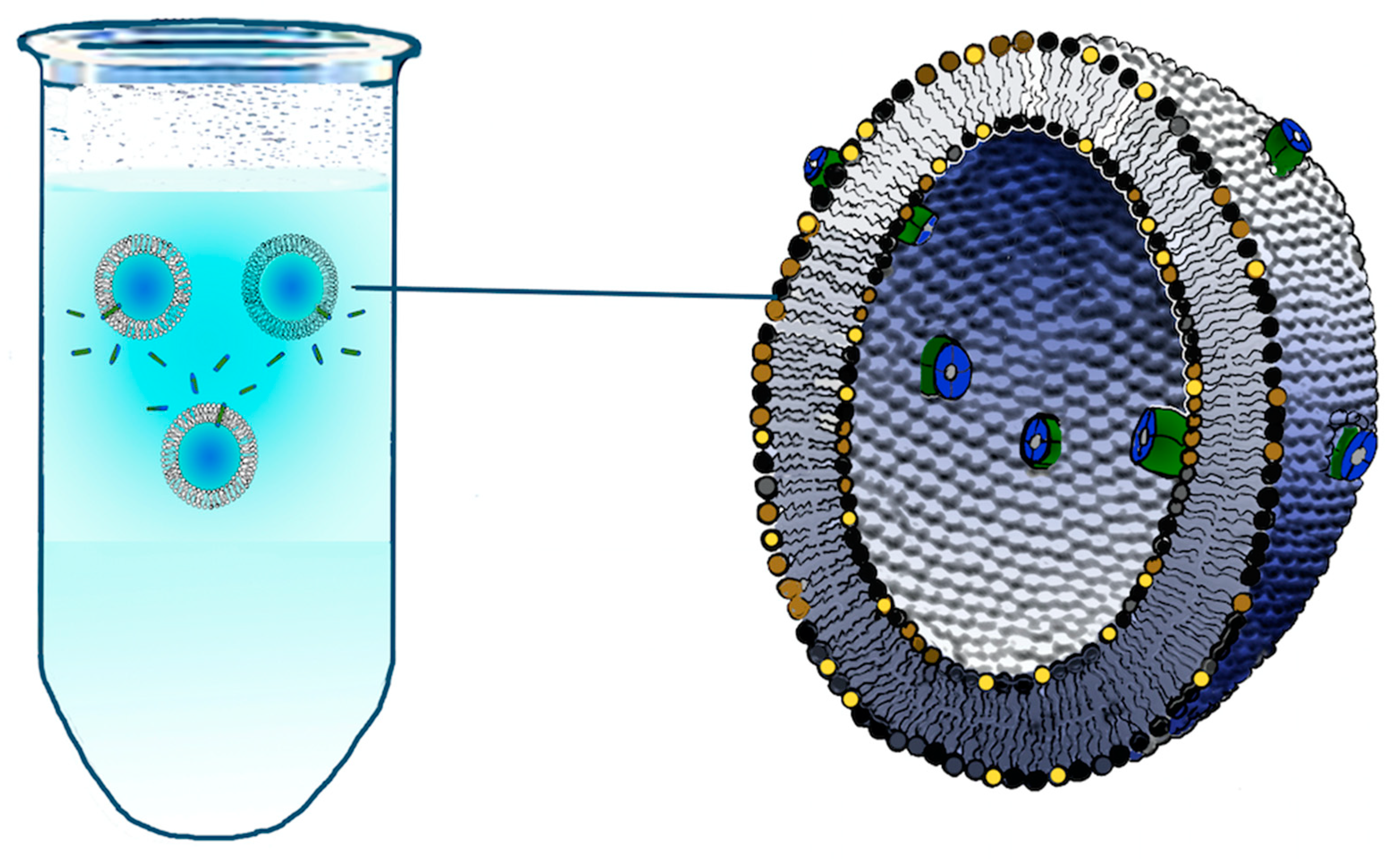
2.1. Incorporation into Artificial Membranes or Liposomes
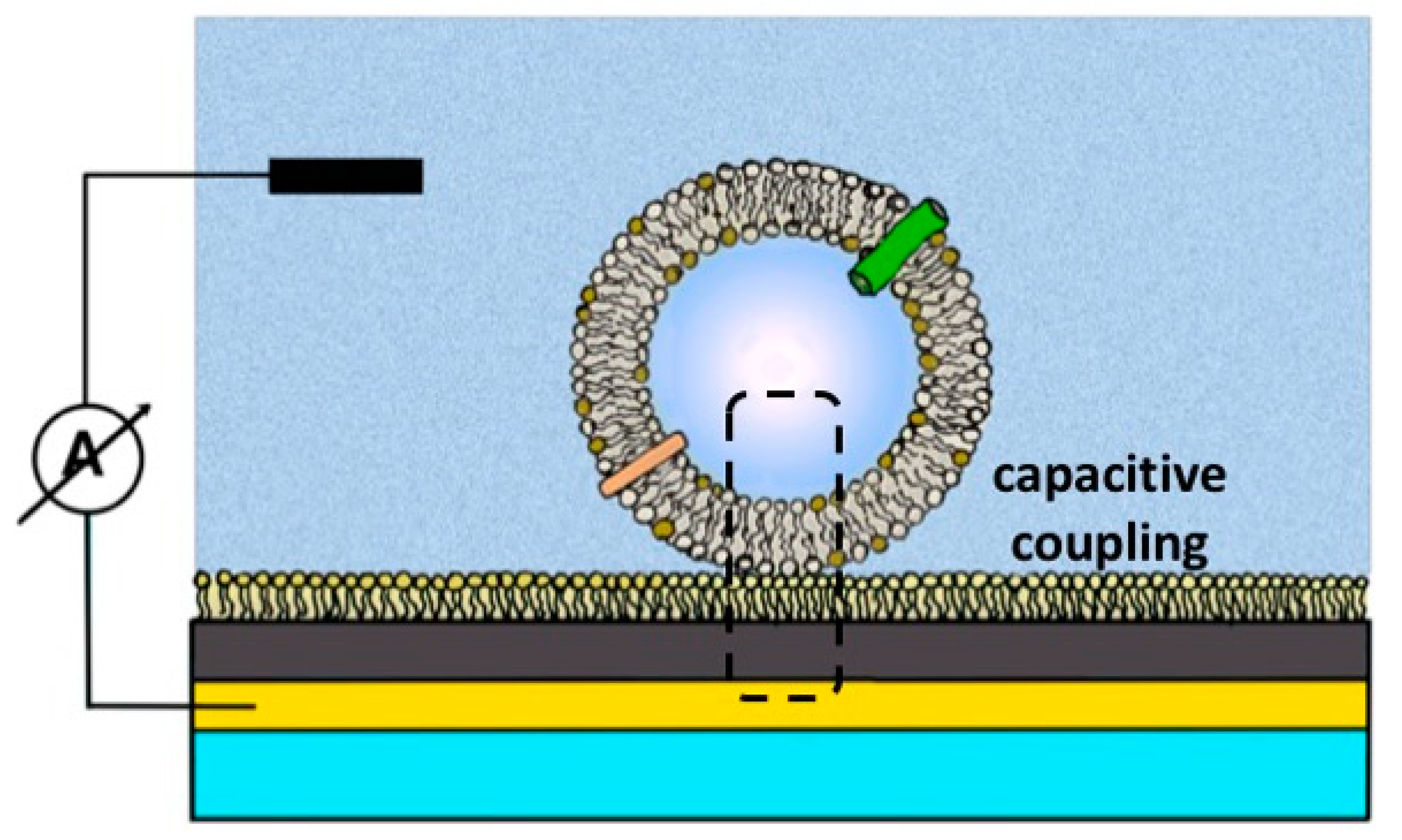
2.2. Solid-Supported Membrane-Based Electrophysiology
2.3. Flux Measurements on Purified Lysosomes
2.4. Patch-Clamp Electrophysiology on Enlarged Lysosomes
3. Approaches Based on Alternative Targeting and Heterologous Expression
3.1. Targeting to the Plasma Membrane upon Manipulation of Sorting Signals
3.2. Nuclear Membrane Electrophysiology
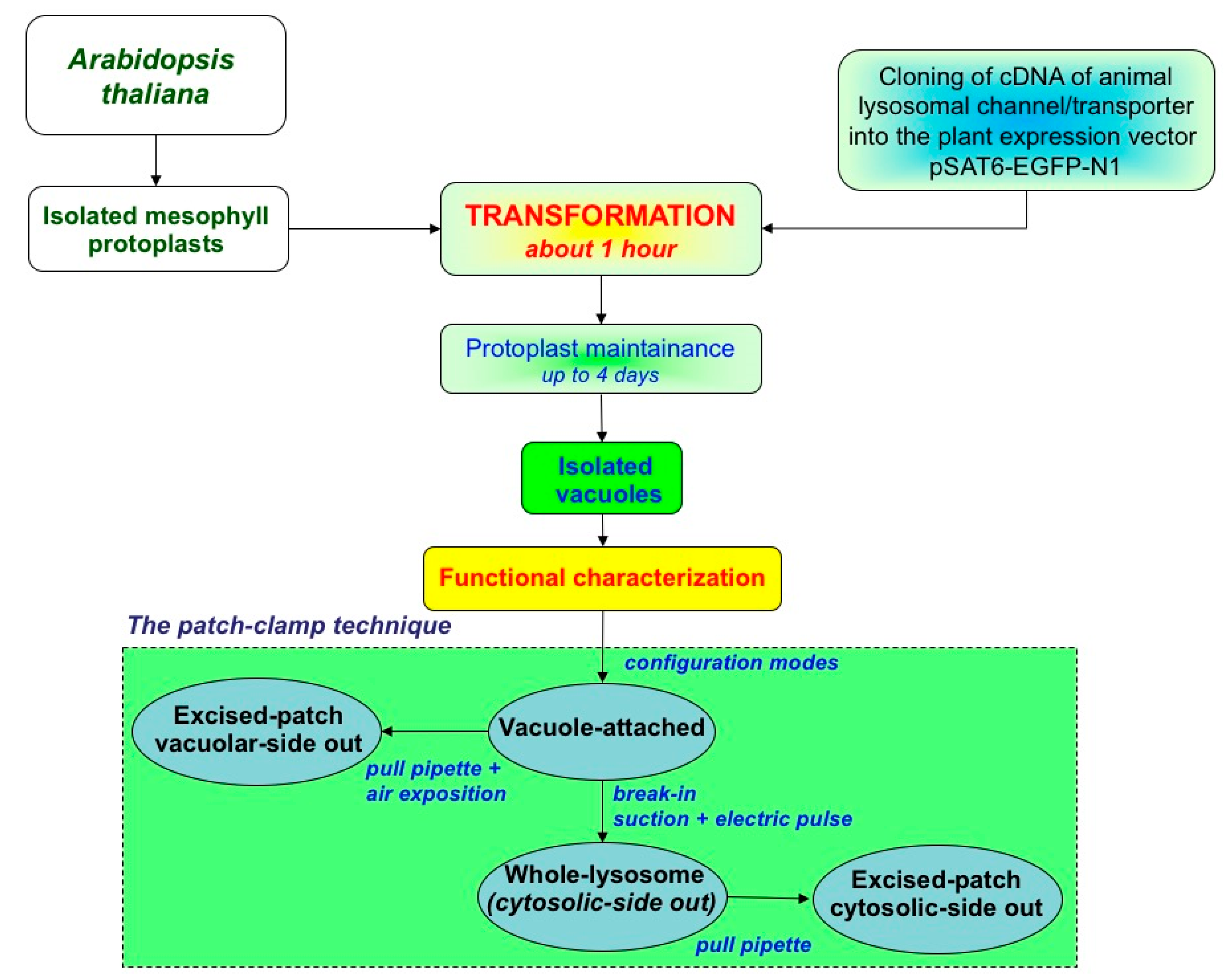
3.3. Patch-Clamp Electrophysiology on Plant Vacuoles
3.3.1. Sorting Routes and Signals to the Tonoplast and the Lysosomal Membrane
3.3.2. The Plant Vacuole as a Heterologous Expression System of Lysosomal Channels and Transporters
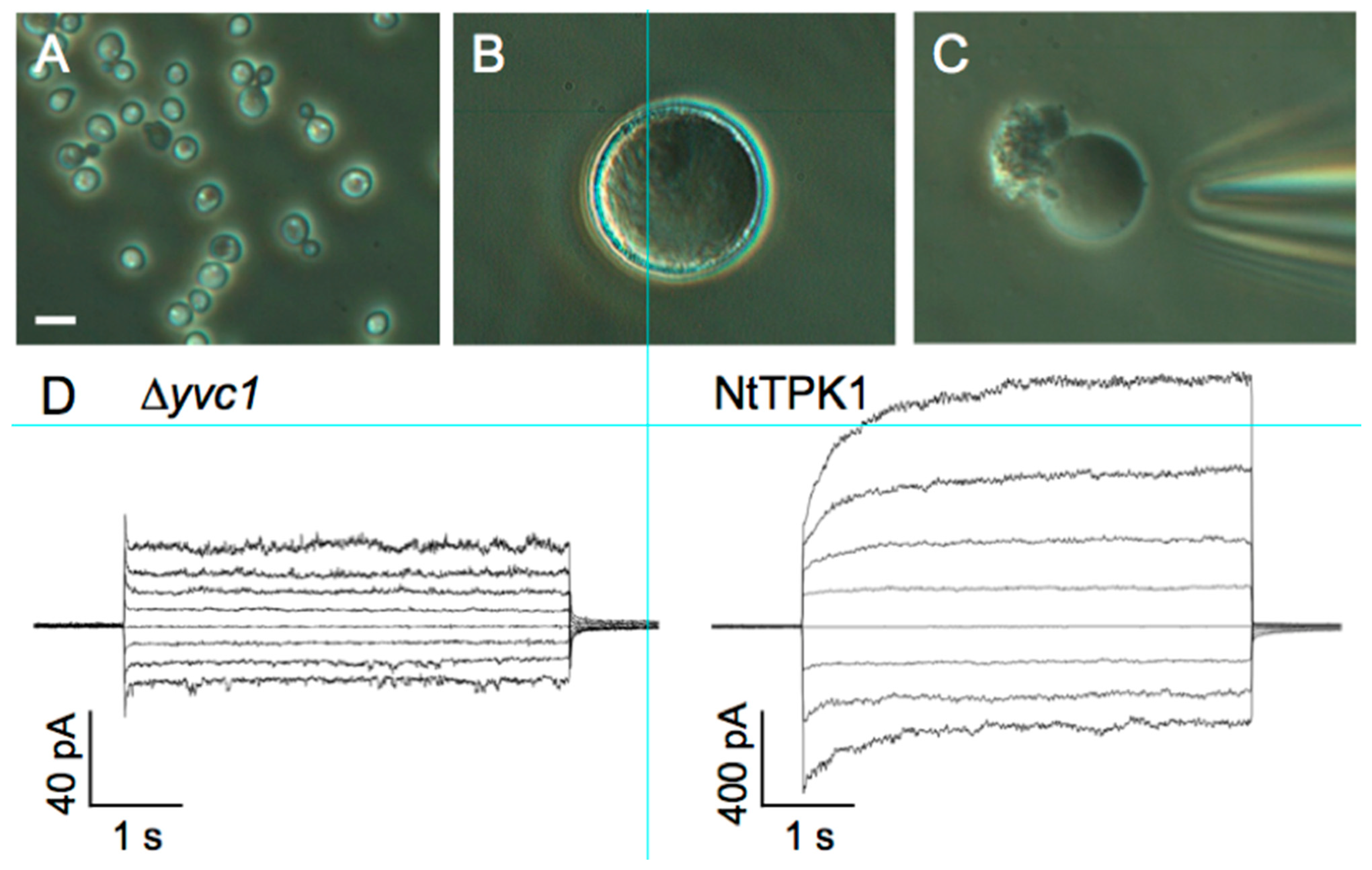
3.4. Patch-Clamp Electrophysiology on Giant Vacuoles from Yeast Cells
4. Outlook on Novel Techniques Complementing Direct Functional Studies
4.1. Cryo-Electron Microscopy
4.2. Molecular Dynamics Simulations
4.3. Genome Editing
4.4. Nanoscopy
5. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| PI(3,5)P2 | phosphatidylinositol-(3,5)-bisphosphate; |
| NAADP | nicotinic acid adenine dinucleotide phosphate; |
| ER | endoplasmic reticulum; |
| LSDs | lysosomal storage disorders; |
| VM | vacuolar membrane; |
References
- Xu, H.; Ren, D. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and Function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Pryor, P.R.; Luzio, J.P. Delivery of Endocytosed Membrane Proteins to the Lysosome. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2009, 1793, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals for the Lysosome: A Control Center for Cellular Clearance and Energy Metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, M.; Wang, P.; Syeda, A.K.R.; Huang, P.; Dong, X.-P. Lysosomal Potassium Channels. Cell Calcium 2022, 102, 102536. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A. Lysosomal Acidification Mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, M.X. Regulation of Lysosomal Ion Homeostasis by Channels and Transporters. Sci. China Life Sci. 2016, 59, 777–791. [Google Scholar] [CrossRef]
- Bagshaw, R.D.; Mahuran, D.J.; Callahan, J.W. A Proteomic Analysis of Lysosomal Integral Membrane Proteins Reveals the Diverse Composition of the Organelle. Mol. Cell. Proteom. MCP 2005, 4, 133–143. [Google Scholar] [CrossRef]
- Schröder, B.; Wrocklage, C.; Pan, C.; Jäger, R.; Kösters, B.; Schäfer, H.; Elsässer, H.-P.; Mann, M.; Hasilik, A. Integral and Associated Lysosomal Membrane Proteins. Traffic Cph. Den. 2007, 8, 1676–1686. [Google Scholar] [CrossRef]
- Repnik, U.; Turk, B. Lysosomal-Mitochondrial Cross-Talk during Cell Death. Mitochondrion 2010, 10, 662–669. [Google Scholar] [CrossRef]
- Lieberman, A.P.; Puertollano, R.; Raben, N.; Slaugenhaupt, S.; Walkley, S.U.; Ballabio, A. Autophagy in Lysosomal Storage Disorders. Autophagy 2012, 8, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Z.; Yang, Y.; Sun, X.; Dong, X.-P. Methods for Monitoring Ca2+ and Ion Channels in the Lysosome. Cell Calcium 2017, 64, 20–28. [Google Scholar] [CrossRef]
- Filippini, A.; D’Amore, A.; Palombi, F.; Carpaneto, A. Could the Inhibition of Endo-Lysosomal Two-Pore Channels (TPCs) by the Natural Flavonoid Naringenin Represent an Option to Fight SARS-CoV-2 Infection? Front. Microbiol. 2020, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin Is a Powerful Inhibitor of SARS-CoV-2 Infection in Vitro. Pharmacol. Res. 2021, 163, 105255. [Google Scholar] [CrossRef] [PubMed]
- Graves, A.R.; Curran, P.K.; Smith, C.L.; Mindell, J.A. The Cl-/H+ Antiporter ClC-7 Is the Primary Chloride Permeation Pathway in Lysosomes. Nature 2008, 453, 788–792. [Google Scholar] [CrossRef]
- Majumdar, A.; Capetillo-Zarate, E.; Cruz, D.; Gouras, G.K.; Maxfield, F.R. Degradation of Alzheimer’s Amyloid Fibrils by Microglia Requires Delivery of ClC-7 to Lysosomes. Mol. Biol. Cell 2011, 22, 1664–1676. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Pusch, M. CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef]
- Bröer, S. The SLC38 Family of Sodium-Amino Acid Co-Transporters. Pflug. Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef]
- Hägglund, M.G.A.; Sreedharan, S.; Nilsson, V.C.O.; Shaik, J.H.A.; Almkvist, I.M.; Bäcklin, S.; Wrange, O.; Fredriksson, R. Identification of SLC38A7 (SNAT7) Protein as a Glutamine Transporter Expressed in Neurons. J. Biol. Chem. 2011, 286, 20500–20511. [Google Scholar] [CrossRef]
- Efeyan, A.; Zoncu, R.; Sabatini, D.M. Amino Acids and MTORC1: From Lysosomes to Disease. Trends Mol. Med. 2012, 18, 524–533. [Google Scholar] [CrossRef]
- Ögmundsdóttir, M.H.; Heublein, S.; Kazi, S.; Reynolds, B.; Visvalingam, S.M.; Shaw, M.K.; Goberdhan, D.C.I. Proton-Assisted Amino Acid Transporter PAT1 Complexes with Rag GTPases and Activates TORC1 on Late Endosomal and Lysosomal Membranes. PLoS ONE 2012, 7, e36616. [Google Scholar] [CrossRef] [PubMed]
- Verdon, Q.; Boonen, M.; Ribes, C.; Jadot, M.; Gasnier, B.; Sagné, C. SNAT7 Is the Primary Lysosomal Glutamine Exporter Required for Extracellular Protein-Dependent Growth of Cancer Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E3602–E3611. [Google Scholar] [CrossRef] [PubMed]
- Donowitz, M.; Ming Tse, C.; Fuster, D. SLC9/NHE Gene Family, a Plasma Membrane and Organellar Family of Na+/H+ Exchangers. Mol. Asp. Med. 2013, 34, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.-T.; et al. NAADP Mobilizes Calcium from Acidic Organelles through Two-Pore Channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Dong, X.-P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC Proteins Are Phosphoinositide- Activated Sodium-Selective Ion Channels in Endosomes and Lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef]
- Boccaccio, A.; Scholz-Starke, J.; Hamamoto, S.; Larisch, N.; Festa, M.; Gutla, P.V.K.; Costa, A.; Dietrich, P.; Uozumi, N.; Carpaneto, A. The Phosphoinositide PI (3, 5) P2 Mediates Activation of Mammalian but Not Plant TPC Proteins: Functional Expression of Endolysosomal Channels in Yeast and Plant Cells. Cell. Mol. Life Sci. 2014, 71, 4275–4283. [Google Scholar] [CrossRef]
- Zong, X.; Schieder, M.; Cuny, H.; Fenske, S.; Gruner, C.; Rötzer, K.; Griesbeck, O.; Harz, H.; Biel, M.; Wahl-Schott, C. The Two-Pore Channel TPCN2 Mediates NAADP-Dependent Ca(2+)-Release from Lysosomal Stores. Pflug. Arch. 2009, 458, 891–899. [Google Scholar] [CrossRef]
- Grimm, C.; Holdt, L.M.; Chen, C.-C.; Hassan, S.; Müller, C.; Jörs, S.; Cuny, H.; Kissing, S.; Schröder, B.; Butz, E.; et al. High Susceptibility to Fatty Liver Disease in Two-Pore Channel 2-Deficient Mice. Nat. Commun. 2014, 5, 4699. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.-C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Ebola Virus. Two-Pore Channels Control Ebola Virus Host Cell Entry and Are Drug Targets for Disease Treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef]
- D’Amore, A.; Gradogna, A.; Palombi, F.; Minicozzi, V.; Ceccarelli, M.; Carpaneto, A.; Filippini, A. The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection. Cells 2021, 10, 1130. [Google Scholar] [CrossRef]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Brailoiu, G.C.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of Lysosomal Morphology by Pathogenic LRRK2 Is Corrected by TPC2 Inhibition. J. Cell Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Favia, A.; Desideri, M.; Gambara, G.; D’Alessio, A.; Ruas, M.; Esposito, B.; Del Bufalo, D.; Parrington, J.; Ziparo, E.; Palombi, F.; et al. VEGF-Induced Neoangiogenesis Is Mediated by NAADP and Two-Pore Channel-2-Dependent Ca2+ Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E4706–E4715. [Google Scholar] [CrossRef] [PubMed]
- Pafumi, I.; Festa, M.; Papacci, F.; Lagostena, L.; Giunta, C.; Gutla, V.; Cornara, L.; Favia, A.; Palombi, F.; Gambale, F.; et al. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Sci. Rep. 2017, 7, 5121. [Google Scholar] [CrossRef]
- Benkerrou, D.; Minicozzi, V.; Gradogna, A.; Milenkovic, S.; Bodrenko, I.V.; Festa, M.; Lagostena, L.; Cornara, L.; D’Amore, A.; Ceccarelli, M.; et al. A Perspective on the Modulation of Plant and Animal Two Pore Channels (TPCs) by the Flavonoid Naringenin. Biophys. Chem. 2019, 254, 106246. [Google Scholar] [CrossRef] [PubMed]
- Padamsey, Z.; McGuinness, L.; Bardo, S.J.; Reinhart, M.; Tong, R.; Hedegaard, A.; Hart, M.L.; Emptage, N.J. Activity-Dependent Exocytosis of Lysosomes Regulates the Structural Plasticity of Dendritic Spines. Neuron 2017, 93, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gala, U.; Zhang, Y.; Shang, W.; Nagarkar Jaiswal, S.; di Ronza, A.; Jaiswal, M.; Yamamoto, S.; Sandoval, H.; Duraine, L.; et al. A Voltage-Gated Calcium Channel Regulates Lysosomal Fusion with Endosomes and Autophagosomes and Is Required for Neuronal Homeostasis. PLoS Biol. 2015, 13, e1002103. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Flockerzi, V. Mammalian Transient Receptor Potential (TRP) Cation Channels. Preface. Handb. Exp. Pharmacol. 2014, 223, v–vi. [Google Scholar]
- Zheng, J. Molecular Mechanism of TRP Channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Wong, C.-O.; Zhu, M.X. The Role of TRPMLs in Endolysosomal Trafficking and Function. Cell Calcium 2015, 58, 48–56. [Google Scholar] [CrossRef]
- Cheng, X.; Shen, D.; Samie, M.; Xu, H. Mucolipins: Intracellular TRPML1-3 Channels. FEBS Lett. 2010, 584, 2013–2021. [Google Scholar] [CrossRef]
- Sun, L.; Hua, Y.; Vergarajauregui, S.; Diab, H.I.; Puertollano, R. Novel Role of TRPML2 in the Regulation of the Innate Immune Response. J. Immunol. 2015, 195, 4922–4932. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Li, L.; Dong, X.; Clapham, D.E. Activating Mutation in a Mucolipin Transient Receptor Potential Channel Leads to Melanocyte Loss in Varitint-Waddler Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 18321–18326. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Cuajungco, M.P.; van Aken, A.F.J.; Schnee, M.; Jörs, S.; Kros, C.J.; Ricci, A.J.; Heller, S. A Helix-Breaking Mutation in TRPML3 Leads to Constitutive Activity Underlying Deafness in the Varitint-Waddler Mouse. Proc. Natl. Acad. Sci. USA 2007, 104, 19583–19588. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Li, Q.; Tjon-Kon-Sang, S.; So, I.; Kiselyov, K.; Muallem, S. Gain-of-Function Mutation in TRPML3 Causes the Mouse Varitint-Waddler Phenotype. J. Biol. Chem. 2007, 282, 36138–36142. [Google Scholar] [CrossRef]
- Nagata, K.; Zheng, L.; Madathany, T.; Castiglioni, A.J.; Bartles, J.R.; García-Añoveros, J. The Varitint-Waddler (Va) Deafness Mutation in TRPML3 Generates Constitutive, Inward Rectifying Currents and Causes Cell Degeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 353–358. [Google Scholar] [CrossRef]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Murrell-Lagnado, R.; Zhu, M.X.; Dong, X.-P. Calcium Release through P2X4 Activates Calmodulin to Promote Endolysosomal Membrane Fusion. J. Cell Biol. 2015, 209, 879–894. [Google Scholar] [CrossRef]
- Cang, C.; Aranda, K.; Seo, Y.; Gasnier, B.; Ren, D. TMEM175 Is an Organelle K+ Channel Regulating Lysosomal Function. Cell 2015, 162, 1101–1112. [Google Scholar] [CrossRef]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Zhang, Z.; Toro, L.; Dong, X.-P. BK Channels Alleviate Lysosomal Storage Diseases by Providing Positive Feedback Regulation of Lysosomal Ca2+ Release. Dev. Cell 2015, 33, 427–441. [Google Scholar] [CrossRef]
- Lee, C.; Guo, J.; Zeng, W.; Kim, S.; She, J.; Cang, C.; Ren, D.; Jiang, Y. The Lysosomal Potassium Channel TMEM175 Adopts a Novel Tetrameric Architecture. Nature 2017, 547, 472–475. [Google Scholar] [CrossRef]
- Jinn, S.; Drolet, R.E.; Cramer, P.E.; Wong, A.H.-K.; Toolan, D.M.; Gretzula, C.A.; Voleti, B.; Vassileva, G.; Disa, J.; Tadin-Strapps, M.; et al. TMEM175 Deficiency Impairs Lysosomal and Mitochondrial Function and Increases α-Synuclein Aggregation. Proc. Natl. Acad. Sci. USA 2017, 114, 2389–2394. [Google Scholar] [CrossRef]
- Li, P.; Hu, M.; Wang, C.; Feng, X.; Zhao, Z.; Yang, Y.; Sahoo, N.; Gu, M.; Yang, Y.; Xiao, S.; et al. LRRC8 Family Proteins within Lysosomes Regulate Cellular Osmoregulation and Enhance Cell Survival to Multiple Physiological Stresses. Proc. Natl. Acad. Sci. USA 2020, 117, 29155–29165. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W.; Wieczorek, H. The V-Type H+ ATPase: Molecular Structure and Function, Physiological Roles and Regulation. J. Exp. Biol. 2006, 209, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, W.; Lin, B.; Yao, Y.; Li, C.; Hu, W.; Wu, H.; Huang, J.; Zhang, M.; Xue, T.; et al. CLN7 Is an Organellar Chloride Channel Regulating Lysosomal Function. Sci. Adv. 2021, 7, eabj9608. [Google Scholar] [CrossRef] [PubMed]
- Sterea, A.M.; Almasi, S.; El Hiani, Y. The Hidden Potential of Lysosomal Ion Channels: A New Era of Oncogenes. Cell Calcium 2018, 72, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Gaburjakova, J.; Gaburjakova, M. Reconstitution of Ion Channels in Planar Lipid Bilayers: New Approaches. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 147–185. ISBN 978-0-12-815772-5. [Google Scholar]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Reconstitution of Cell Membrane Structure in Vitro and Its Transformation into an Excitable System. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef]
- Oiki, S.; Iwamoto, M. Lipid Bilayers Manipulated through Monolayer Technologies for Studies of Channel-Membrane Interplay. Biol. Pharm. Bull. 2018, 41, 303–311. [Google Scholar] [CrossRef]
- Dalla Serra, M.; Menestrina, G. Liposomes in the Study of Pore-Forming Toxins. Methods Enzymol. 2003, 372, 99–124. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel Methods for Liposome Preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Venturi, E.; Sitsapesan, R. Reconstitution of Lysosomal Ion Channels into Artificial Membranes. Methods Cell Biol. 2015, 126, 217–236. [Google Scholar] [CrossRef]
- Pitt, S.J.; Funnell, T.M.; Sitsapesan, M.; Venturi, E.; Rietdorf, K.; Ruas, M.; Ganesan, A.; Gosain, R.; Churchill, G.C.; Zhu, M.X.; et al. TPC2 Is a Novel NAADP-Sensitive Ca2+ Release Channel, Operating as a Dual Sensor of Luminal PH and Ca2+. J. Biol. Chem. 2010, 285, 35039–35046. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Lam, A.K.M.; Rietdorf, K.; Galione, A.; Sitsapesan, R. Reconstituted Human TPC1 Is a Proton-Permeable Ion Channel and Is Activated by NAADP or Ca2+. Sci. Signal. 2014, 7, ra46. [Google Scholar] [CrossRef] [PubMed]
- Rybalchenko, V.; Ahuja, M.; Coblentz, J.; Churamani, D.; Patel, S.; Kiselyov, K.; Muallem, S. Membrane Potential Regulates Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) Dependence of the PH- and Ca2+-Sensitive Organellar Two-Pore Channel TPC1. J. Biol. Chem. 2012, 287, 20407–20416. [Google Scholar] [CrossRef] [PubMed]
- Raychowdhury, M.K.; González-Perrett, S.; Montalbetti, N.; Timpanaro, G.A.; Chasan, B.; Goldmann, W.H.; Stahl, S.; Cooney, A.; Goldin, E.; Cantiello, H.F. Molecular Pathophysiology of Mucolipidosis Type IV: PH Dysregulation of the Mucolipin-1 Cation Channel. Hum. Mol. Genet. 2004, 13, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Seifert, K.; Fendler, K.; Bamberg, E. Charge Transport by Ion Translocating Membrane Proteins on Solid Supported Membranes. Biophys. J. 1993, 64, 384–391. [Google Scholar] [CrossRef]
- Schulz, P.; Garcia-Celma, J.J.; Fendler, K. SSM-Based Electrophysiology. Methods 2008, 46, 97–103. [Google Scholar] [CrossRef]
- Bazzone, A.; Costa, W.S.; Braner, M.; Călinescu, O.; Hatahet, L.; Fendler, K. Introduction to Solid Supported Membrane Based Electrophysiology. J. Vis. Exp. JoVE 2013, 75, e50230. [Google Scholar] [CrossRef]
- Bazzone, A.; Barthmes, M.; Fendler, K. SSM-Based Electrophysiology for Transporter Research. Methods Enzymol. 2017, 594, 31–83. [Google Scholar] [CrossRef]
- Obrdlik, P.; Diekert, K.; Watzke, N.; Keipert, C.; Pehl, U.; Brosch, C.; Boehm, N.; Bick, I.; Ruitenberg, M.; Volknandt, W.; et al. Electrophysiological Characterization of ATPases in Native Synaptic Vesicles and Synaptic Plasma Membranes. Biochem. J. 2010, 427, 151–159. [Google Scholar] [CrossRef]
- Schulz, P.; Werner, J.; Stauber, T.; Henriksen, K.; Fendler, K. The G215R Mutation in the Cl−/H+-Antiporter ClC-7 Found in ADO II Osteopetrosis Does Not Abolish Function but Causes a Severe Trafficking Defect. PLoS ONE 2010, 5, e12585. [Google Scholar] [CrossRef]
- Garty, H.; Rudy, B.; Karlish, S.J. A Simple and Sensitive Procedure for Measuring Isotope Fluxes through Ion-Specific Channels in Heterogenous Populations of Membrane Vesicles. J. Biol. Chem. 1983, 258, 13094–13099. [Google Scholar] [CrossRef]
- Goldberg, A.F.; Miller, C. Solubilization and Functional Reconstitution of a Chloride Channel from Torpedo Californica Electroplax. J. Membr. Biol. 1991, 124, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The Type IV Mucolipidosis-Associated Protein TRPML1 Is an Endolysosomal Iron Release Channel. Nature 2008, 455, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shen, D.; Wang, X.; Dawson, T.; Li, X.; Zhang, Q.; Cheng, X.; Zhang, Y.; Weisman, L.S.; Delling, M.; et al. PI(3,5)P2 Controls Membrane Trafficking by Direct Activation of Mucolipin Ca2+ Release Channels in the Endolysosome. Nat. Commun. 2010, 1, 38. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Zhou, Y.; Navarro, B.; Seo, Y.-J.; Aranda, K.; Shi, L.; Battaglia-Hsu, S.; Nissim, I.; Clapham, D.E.; Ren, D. MTOR Regulates Lysosomal ATP-Sensitive Two-Pore Na(+) Channels to Adapt to Metabolic State. Cell 2013, 152, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Bekele, B.; Ren, D. The Voltage-Gated Sodium Channel TPC1 Confers Endolysosomal Excitability. Nat. Chem. Biol. 2014, 10, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Cang, C.; Fenske, S.; Butz, E.; Chao, Y.-K.; Biel, M.; Ren, D.; Wahl-Schott, C.; Grimm, C. Patch-Clamp Technique to Characterize Ion Channels in Enlarged Individual Endolysosomes. Nat. Protoc. 2017, 12, 1639–1658. [Google Scholar] [CrossRef]
- Saito, M.; Hanson, P.I.; Schlesinger, P. Luminal Chloride-Dependent Activation of Endosome Calcium Channels: Patch Clamp Study of Enlarged Endosomes. J. Biol. Chem. 2007, 282, 27327–27333. [Google Scholar] [CrossRef]
- Huynh, C.; Andrews, N.W. The Small Chemical Vacuolin-1 Alters the Morphology of Lysosomes without Inhibiting Ca2+-Regulated Exocytosis. EMBO Rep. 2005, 6, 843–847. [Google Scholar] [CrossRef]
- Schieder, M.; Rötzer, K.; Brüggemann, A.; Biel, M.; Wahl-Schott, C. Planar Patch Clamp Approach to Characterize Ionic Currents from Intact Lysosomes. Sci. Signal. 2010, 3, pl3. [Google Scholar] [CrossRef]
- Schieder, M.; Rötzer, K.; Brüggemann, A.; Biel, M.; Wahl-Schott, C.A. Characterization of Two-Pore Channel 2 (TPCN2)-Mediated Ca2+ Currents in Isolated Lysosomes. J. Biol. Chem. 2010, 285, 21219–21222. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Butz, E.S.; Chao, Y.-K.; Grishchuk, Y.; Becker, L.; Heller, S.; Slaugenhaupt, S.A.; Biel, M.; Wahl-Schott, C.; Grimm, C. Small Molecules for Early Endosome-Specific Patch Clamping. Cell Chem. Biol. 2017, 24, 907–916.e4. [Google Scholar] [CrossRef] [PubMed]
- Gerndt, S.; Chen, C.-C.; Chao, Y.-K.; Yuan, Y.; Burgstaller, S.; Scotto Rosato, A.; Krogsaeter, E.; Urban, N.; Jacob, K.; Nguyen, O.N.P.; et al. Agonist-Mediated Switching of Ion Selectivity in TPC2 Differentially Promotes Lysosomal Function. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Ahuja, M.; Patel, S.; Brailoiu, E.; Muallem, S. Convergent Regulation of the Lysosomal Two-Pore Channel-2 by Mg2+, NAADP, PI(3,5)P2 and Multiple Protein Kinases. EMBO J. 2014, 33, 501–511. [Google Scholar] [CrossRef]
- Kelly, B.T.; Owen, D.J. Endocytic Sorting of Transmembrane Protein Cargo. Curr. Opin. Cell Biol. 2011, 23, 404–412. [Google Scholar] [CrossRef]
- Staudt, C.; Puissant, E.; Boonen, M. Subcellular Trafficking of Mammalian Lysosomal Proteins: An Extended View. Int. J. Mol. Sci. 2016, 18, 47. [Google Scholar] [CrossRef]
- Traub, L.M.; Bonifacino, J.S. Cargo Recognition in Clathrin-Mediated Endocytosis. Cold Spring Harb. Perspect. Biol. 2013, 5, a016790. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Traub, L.M. Signals for Sorting of Transmembrane Proteins to Endosomes and Lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar] [CrossRef]
- Jung, N.; Wienisch, M.; Gu, M.; Rand, J.B.; Müller, S.L.; Krause, G.; Jorgensen, E.M.; Klingauf, J.; Haucke, V. Molecular Basis of Synaptic Vesicle Cargo Recognition by the Endocytic Sorting Adaptor Stonin 2. J. Cell Biol. 2007, 179, 1497–1510. [Google Scholar] [CrossRef]
- Robinson, M.S. Forty Years of Clathrin-Coated Vesicles. Traffic Cph. Den. 2015, 16, 1210–1238. [Google Scholar] [CrossRef]
- Ibberson, M.; Uldry, M.; Thorens, B. GLUTX1, a Novel Mammalian Glucose Transporter Expressed in the Central Nervous System and Insulin-Sensitive Tissues. J. Biol. Chem. 2000, 275, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.; Riley, J.; Moley, K.H. GLUT8 Contains a [DE]XXXL[LI] Sorting Motif and Localizes to a Late Endosomal/Lysosomal Compartment. Traffic Cph. Den. 2005, 6, 1196–1212. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Osakabe, Y.; Mizoi, J.; Nakashima, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of an Arabidopsis Thaliana Abiotic Stress-Inducible Facilitated Diffusion Transporter for Monosaccharides. J. Biol. Chem. 2010, 285, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Rahman, T.; Churamani, D.; Prole, D.L.; Brailoiu, G.C.; Hooper, R.; Taylor, C.W.; Patel, S. An NAADP-Gated Two-Pore Channel Targeted to the Plasma Membrane Uncouples Triggering from Amplifying Ca2+ Signals. J. Biol. Chem. 2010, 285, 38511–38516. [Google Scholar] [CrossRef]
- Kirsch, S.A.; Kugemann, A.; Carpaneto, A.; Böckmann, R.A.; Dietrich, P. Phosphatidylinositol-3,5-Bisphosphate Lipid-Binding-Induced Activation of the Human Two-Pore Channel 2. Cell. Mol. Life Sci. CMLS 2018, 75, 3803–3815. [Google Scholar] [CrossRef]
- Larisch, N.; Schulze, C.; Galione, A.; Dietrich, P. An N-Terminal Dileucine Motif Directs Two-Pore Channels to the Tonoplast of Plant Cells. Traffic Cph. Den. 2012, 13, 1012–1022. [Google Scholar] [CrossRef]
- Guo, J.; Zeng, W.; Chen, Q.; Lee, C.; Chen, L.; Yang, Y.; Cang, C.; Ren, D.; Jiang, Y. Structure of the Voltage-Gated Two-Pore Channel TPC1 from Arabidopsis Thaliana. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef]
- Ye, F.; Xu, L.; Li, X.; Zeng, W.; Gan, N.; Zhao, C.; Yang, W.; Jiang, Y.; Guo, J. Voltage-Gating and Cytosolic Ca2+ Activation Mechanisms of Arabidopsis Two-Pore Channel AtTPC1. Proc. Natl. Acad. Sci. USA 2021, 118, e2113946118. [Google Scholar] [CrossRef]
- She, J.; Zeng, W.; Guo, J.; Chen, Q.; Bai, X.-C.; Jiang, Y. Structural Mechanisms of Phospholipid Activation of the Human TPC2 Channel. eLife 2019, 8, e45222. [Google Scholar] [CrossRef]
- Stauber, T.; Jentsch, T.J. Sorting Motifs of the Endosomal/Lysosomal CLC Chloride Transporters. J. Biol. Chem. 2010, 285, 34537–34548. [Google Scholar] [CrossRef]
- Leisle, L.; Ludwig, C.F.; Wagner, F.A.; Jentsch, T.J.; Stauber, T. ClC-7 Is a Slowly Voltage-Gated 2Cl(-)/1H(+)-Exchanger and Requires Ostm1 for Transport Activity. EMBO J. 2011, 30, 2140–2152. [Google Scholar] [CrossRef] [PubMed]
- Cherqui, S.; Kalatzis, V.; Trugnan, G.; Antignac, C. The Targeting of Cystinosin to the Lysosomal Membrane Requires a Tyrosine-Based Signal and a Novel Sorting Motif. J. Biol. Chem. 2001, 276, 13314–13321. [Google Scholar] [CrossRef] [PubMed]
- Kalatzis, V.; Cherqui, S.; Antignac, C.; Gasnier, B. Cystinosin, the Protein Defective in Cystinosis, Is a H(+)-Driven Lysosomal Cystine Transporter. EMBO J. 2001, 20, 5940–5949. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, R.; Bellenchi, G.C.; Chen, X.; Zifarelli, G.; Sagné, C.; Debacker, C.; Pusch, M.; Supplisson, S.; Gasnier, B. Mechanism of Proton/Substrate Coupling in the Heptahelical Lysosomal Transporter Cystinosin. Proc. Natl. Acad. Sci. USA 2012, 109, E210–E217. [Google Scholar] [CrossRef] [PubMed]
- Jézégou, A.; Llinares, E.; Anne, C.; Kieffer-Jaquinod, S.; O’Regan, S.; Aupetit, J.; Chabli, A.; Sagné, C.; Debacker, C.; Chadefaux-Vekemans, B.; et al. Heptahelical Protein PQLC2 Is a Lysosomal Cationic Amino Acid Exporter Underlying the Action of Cysteamine in Cystinosis Therapy. Proc. Natl. Acad. Sci. USA 2012, 109, E3434–E3443. [Google Scholar] [CrossRef]
- Leray, X.; Conti, R.; Li, Y.; Debacker, C.; Castelli, F.; Fenaille, F.; Zdebik, A.A.; Pusch, M.; Gasnier, B. Arginine-Selective Modulation of the Lysosomal Transporter PQLC2 through a Gate-Tuning Mechanism. Proc. Natl. Acad. Sci. USA 2021, 118, e2025315118. [Google Scholar] [CrossRef]
- Neagoe, I.; Stauber, T.; Fidzinski, P.; Bergsdorf, E.-Y.; Jentsch, T.J. The Late Endosomal ClC-6 Mediates Proton/Chloride Countertransport in Heterologous Plasma Membrane Expression. J. Biol. Chem. 2010, 285, 21689–21697. [Google Scholar] [CrossRef]
- Polovitskaya, M.M.; Barbini, C.; Martinelli, D.; Harms, F.L.; Cole, F.S.; Calligari, P.; Bocchinfuso, G.; Stella, L.; Ciolfi, A.; Niceta, M.; et al. A Recurrent Gain-of-Function Mutation in CLCN6, Encoding the ClC-6 Cl−/H+-Exchanger, Causes Early-Onset Neurodegeneration. Am. J. Hum. Genet. 2020, 107, 1062–1077. [Google Scholar] [CrossRef]
- Beitz, E.; Liu, K.; Ikeda, M.; Guggino, W.B.; Agre, P.; Yasui, M. Determinants of AQP6 Trafficking to Intracellular Sites versus the Plasma Membrane in Transfected Mammalian Cells. Biol. Cell 2006, 98, 101–109. [Google Scholar] [CrossRef]
- Ikeda, M.; Beitz, E.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Characterization of Aquaporin-6 as a Nitrate Channel in Mammalian Cells. Requirement of Pore-Lining Residue Threonine 63. J. Biol. Chem. 2002, 277, 39873–39879. [Google Scholar] [CrossRef]
- Pergel, E.; Veres, I.; Csigi, G.I.; Czirják, G. Translocation of TMEM175 Lysosomal Potassium Channel to the Plasma Membrane by Dynasore Compounds. Int. J. Mol. Sci. 2021, 22, 10515. [Google Scholar] [CrossRef] [PubMed]
- Mak, D.-O.D.; Vais, H.; Cheung, K.-H.; Foskett, J.K. Nuclear Patch-Clamp Electrophysiology of Ca2+ Channels. Cold Spring Harb. Protoc. 2013, 2013, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Mak, D.-O.D.; Vais, H.; Cheung, K.-H.; Foskett, J.K. Patch-Clamp Electrophysiology of Intracellular Ca2+ Channels. Cold Spring Harb. Protoc. 2013, 2013, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.-K.; Tong, B.C.-K.; Cheng, C.W.-H.; Hung, H.C.-H.; Cheung, K.-H. Characterization of Two-Pore Channel 2 by Nuclear Membrane Electrophysiology. Sci. Rep. 2016, 6, 20282. [Google Scholar] [CrossRef]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar Transporters and Their Essential Role in Plant Metabolism. J. Exp. Bot. 2007, 58, 83–102. [Google Scholar] [CrossRef]
- Marty, F. Plant Vacuoles. Plant Cell 1999, 11, 587–600. [Google Scholar] [CrossRef]
- Hedrich, R. Technical Approaches to Studying Specific Properties of Ion Channels in Plants. In Single-Channel Recording; Sakmann, B., Neher, E., Eds.; Springer: Boston, MA, USA, 1995; pp. 277–305. ISBN 978-1-4419-1229-9. [Google Scholar]
- Carpaneto, A. Nickel Inhibits the Slowly Activating Channels of Radish Vacuoles. Eur. Biophys. J. EBJ 2003, 32, 60–66. [Google Scholar] [CrossRef]
- Hedrich, R.; Marten, I. 30-Year Progress of Membrane Transport in Plants. Planta 2006, 224, 725–739. [Google Scholar] [CrossRef]
- Rocchetti, A.; Sharma, T.; Wulfetange, C.; Scholz-Starke, J.; Grippa, A.; Carpaneto, A.; Dreyer, I.; Vitale, A.; Czempinski, K.; Pedrazzini, E. The Putative K(+) Channel Subunit AtKCO3 Forms Stable Dimers in Arabidopsis. Front. Plant Sci. 2012, 3, 251. [Google Scholar] [CrossRef]
- Martinoia, E. Vacuolar Transporters—Companions on a Longtime Journey[OPEN]. Plant Physiol. 2018, 176, 1384–1407. [Google Scholar] [CrossRef]
- Gambale, F.; Cantu, A.M.; Carpaneto, A.; Keller, B.U. Fast and Slow Activation of Voltage-Dependent Ion Channels in Radish Vacuoles. Biophys. J. 1993, 65, 1837–1843. [Google Scholar] [CrossRef][Green Version]
- Paganetto, A.; Carpaneto, A.; Gambale, F. Ion Transport and Metal Sensitivity of Vacuolar Channels from the Roots of the Aquatic Plant Eichhornia Crassipes. Plant Cell Environ. 2001, 24, 1329–1336. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Carpaneto, A.; Gambale, F. On the Interaction of Neomycin with the Slow Vacuolar Channel of Arabidopsis Thaliana. J. Gen. Physiol. 2006, 127, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.I.; Martínez-Estévez, M. Regulation of the Fast Vacuolar Channel by Cytosolic and Vacuolar Potassium. Biophys. J. 2003, 84, 977–986. [Google Scholar] [CrossRef]
- Konrad, K.R.; Hedrich, R. The Use of Voltage-Sensitive Dyes to Monitor Signal-Induced Changes in Membrane Potential-ABA Triggered Membrane Depolarization in Guard Cells. Plant J. Cell Mol. Biol. 2008, 55, 161–173. [Google Scholar] [CrossRef]
- Gradogna, A.; Scholz-Starke, J.; Gutla, P.V.K.; Carpaneto, A. Fluorescence Combined with Excised Patch: Measuring Calcium Currents in Plant Cation Channels. Plant J. 2009, 58, 175–182. [Google Scholar] [CrossRef]
- Carpaneto, A.; Boccaccio, A.; Lagostena, L.; Di Zanni, E.; Scholz-Starke, J. The Signaling Lipid Phosphatidylinositol-3,5-Bisphosphate Targets Plant CLC-a Anion/H+ Exchange Activity. EMBO Rep. 2017, 18, 1100–1107. [Google Scholar] [CrossRef]
- Carpaneto, A.; Gradogna, A. Modulation of Calcium and Potassium Permeation in Plant TPC Channels. Biophys. Chem. 2018, 236, 1–7. [Google Scholar] [CrossRef]
- Gradogna, A.; Scholz-Starke, J.; Pardo, J.M.; Carpaneto, A. Beyond the Patch-Clamp Resolution: Functional Activity of Nonelectrogenic Vacuolar NHX Proton/Potassium Antiporters and Inhibition by Phosphoinositides. New Phytol. 2021, 229, 3026–3036. [Google Scholar] [CrossRef]
- Costa, A.; Gutla, P.V.K.; Boccaccio, A.; Scholz-Starke, J.; Festa, M.; Basso, B.; Zanardi, I.; Pusch, M.; Schiavo, F.L.; Gambale, F.; et al. The Arabidopsis Central Vacuole as an Expression System for Intracellular Transporters: Functional Characterization of the Cl-/H+ Exchanger CLC-7. J. Physiol. 2012, 590, 3421–3430. [Google Scholar] [CrossRef]
- Lagostena, L.; Festa, M.; Pusch, M.; Carpaneto, A. The Human Two-Pore Channel 1 Is Modulated by Cytosolic and Luminal Calcium. Sci. Rep. 2017, 7, 43900. [Google Scholar] [CrossRef] [PubMed]
- Festa, M.; Lagostena, L.; Carpaneto, A. Using the Plant Vacuole as a Biological System to Investigate the Functional Properties of Exogenous Channels and Transporters. Biochim. Biophys. Acta 2016, 1858, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, E.; Vitale, A. Protein Biosynthesis and Maturation in the ER. Methods Mol. Biol. Clifton NJ 2018, 1691, 179–189. [Google Scholar] [CrossRef]
- Braulke, T.; Bonifacino, J.S. Sorting of Lysosomal Proteins. Biochim. Biophys. Acta 2009, 1793, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, E.; Komarova, N.Y.; Rentsch, D.; Vitale, A. Traffic Routes and Signals for the Tonoplast. Traffic Cph. Den. 2013, 14, 622–628. [Google Scholar] [CrossRef]
- Schwake, M.; Schröder, B.; Saftig, P. Lysosomal Membrane Proteins and Their Central Role in Physiology. Traffic Cph. Den. 2013, 14, 739–748. [Google Scholar] [CrossRef]
- Janvier, K.; Bonifacino, J.S. Role of the Endocytic Machinery in the Sorting of Lysosome-Associated Membrane Proteins. Mol. Biol. Cell 2005, 16, 4231–4242. [Google Scholar] [CrossRef]
- Gasber, A.; Klaumann, S.; Trentmann, O.; Trampczynska, A.; Clemens, S.; Schneider, S.; Sauer, N.; Feifer, I.; Bittner, F.; Mendel, R.R.; et al. Identification of an Arabidopsis Solute Carrier Critical for Intracellular Transport and Inter-Organ Allocation of Molybdate. Plant Biol. Stuttg. Ger. 2011, 13, 710–718. [Google Scholar] [CrossRef]
- Maîtrejean, M.; Vitale, A. How Are Tonoplast Proteins Degraded? Plant Signal. Behav. 2011, 6, 1809–1812. [Google Scholar] [CrossRef]
- Wolfenstetter, S.; Wirsching, P.; Dotzauer, D.; Schneider, S.; Sauer, N. Routes to the Tonoplast: The Sorting of Tonoplast Transporters in Arabidopsis Mesophyll Protoplasts. Plant Cell 2012, 24, 215–232. [Google Scholar] [CrossRef]
- Bottanelli, F.; Foresti, O.; Hanton, S.; Denecke, J. Vacuolar Transport in Tobacco Leaf Epidermis Cells Involves a Single Route for Soluble Cargo and Multiple Routes for Membrane Cargo. Plant Cell 2011, 23, 3007–3025. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Chrispeels, M.J. Tonoplast and Soluble Vacuolar Proteins Are Targeted by Different Mechanisms. Plant Cell 1993, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Viotti, C. ER and Vacuoles: Never Been Closer. Front. Plant Sci. 2014, 5, 20. [Google Scholar] [CrossRef]
- Viotti, C.; Krüger, F.; Krebs, M.; Neubert, C.; Fink, F.; Lupanga, U.; Scheuring, D.; Boutté, Y.; Frescatada-Rosa, M.; Wolfenstetter, S.; et al. The Endoplasmic Reticulum Is the Main Membrane Source for Biogenesis of the Lytic Vacuole in Arabidopsis. Plant Cell 2013, 25, 3434–3449. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Y.; Wang, H.; Zeng, Y.; Zhuang, X.; Li, B.; Jiang, L. Trans-Golgi Network-Located AP1 Gamma Adaptins Mediate Dileucine Motif-Directed Vacuolar Targeting in Arabidopsis. Plant Cell 2014, 26, 4102–4118. [Google Scholar] [CrossRef]
- Müdsam, C.; Wollschläger, P.; Sauer, N.; Schneider, S. Sorting of Arabidopsis NRAMP3 and NRAMP4 Depends on Adaptor Protein Complex AP4 and a Dileucine-Based Motif. Traffic Cph. Den. 2018, 19, 503–521. [Google Scholar] [CrossRef]
- Hirst, J.; Edgar, J.R.; Esteves, T.; Darios, F.; Madeo, M.; Chang, J.; Roda, R.H.; Dürr, A.; Anheim, M.; Gellera, C.; et al. Loss of AP-5 Results in Accumulation of Aberrant Endolysosomes: Defining a New Type of Lysosomal Storage Disease. Hum. Mol. Genet. 2015, 24, 4984–4996. [Google Scholar] [CrossRef]
- Dunkel, M.; Latz, A.; Schumacher, K.; Müller, T.; Becker, D.; Hedrich, R. Targeting of Vacuolar Membrane Localized Members of the TPK Channel Family. Mol. Plant 2008, 1, 938–949. [Google Scholar] [CrossRef]
- Nakano, A.; Luini, A. Passage through the Golgi. Curr. Opin. Cell Biol. 2010, 22, 471–478. [Google Scholar] [CrossRef]
- Brandizzi, F.; Barlowe, C. Organization of the ER-Golgi Interface for Membrane Traffic Control. Nat. Rev. Mol. Cell Biol. 2013, 14, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, E.; Caprera, A.; Fojadelli, I.; Stella, A.; Rocchetti, A.; Bassin, B.; Martinoia, E.; Vitale, A. The Arabidopsis Tonoplast Is Almost Devoid of Glycoproteins with Complex N-Glycans, Unlike the Rat Lysosomal Membrane. J. Exp. Bot. 2016, 67, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, S.; Damme, M. The Lysosomal Membrane-Export of Metabolites and Beyond. FEBS J. 2021, 288, 4168–4182. [Google Scholar] [CrossRef] [PubMed]
- Stühmer, W. Electrophysiologic Recordings from Xenopus Oocytes. Methods Enzymol. 1998, 293, 280–300. [Google Scholar]
- Porée, F.; Wulfetange, K.; Naso, A.; Carpaneto, A.; Roller, A.; Natura, G.; Bertl, A.; Sentenac, H.; Thibaud, J.-B.; Dreyer, I. Plant K(in) and K(out) Channels: Approaching the Trait of Opposite Rectification by Analyzing More than 250 KAT1-SKOR Chimeras. Biochem. Biophys. Res. Commun. 2005, 332, 465–473. [Google Scholar] [CrossRef]
- Nicastro, G.; Orsomando, G.; Ferrari, E.; Manconi, L.; Desario, F.; Amici, A.; Naso, A.; Carpaneto, A.; Pertinhez, T.A.; Ruggieri, S. Solution Structure of the Phytotoxic Protein PcF: The First Characterized Member of the Phytophthora PcF Toxin Family. Protein Sci. 2009, 18, 1786–1791. [Google Scholar] [CrossRef]
- Carpaneto, A.; Koepsell, H.; Bamberg, E.; Hedrich, R.; Geiger, D. Sucrose-and H+-Dependent Charge Movements Associated with the Gating of Sucrose Transporter ZmSUT1. PLoS ONE 2010, 5, e12605. [Google Scholar] [CrossRef]
- Derrer, C.; Wittek, A.; Bamberg, E.; Carpaneto, A.; Dreyer, I.; Geiger, D. Conformational Changes Represent the Rate-Limiting Step in the Transport Cycle of Maize Sucrose Transporter1. Plant Cell 2013, 25, 3010–3021. [Google Scholar] [CrossRef][Green Version]
- Yoo, S.-D.; Cho, Y.-H.; Sheen, J. Arabidopsis Mesophyll Protoplasts: A Versatile Cell System for Transient Gene Expression Analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Neher, E.; Sakmann, B. Single-Channel Currents Recorded from Membrane of Denervated Frog Muscle Fibres. Nature 1976, 260, 799–802. [Google Scholar] [CrossRef]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved Patch-Clamp Techniques for High-Resolution Current Recording from Cells and Cell-Free Membrane Patches. Pflug. Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef]
- Carpaneto, A.; Geiger, D.; Bamberg, E.; Sauer, N.; Fromm, J.; Hedrich, R. Phloem-Localized, Proton-Coupled Sucrose Carrier ZmSUT1 Mediates Sucrose Efflux under the Control of the Sucrose Gradient and the Proton Motive Force. J. Biol. Chem. 2005, 280, 21437–21443. [Google Scholar] [CrossRef] [PubMed]
- Carpaneto, A.; Dalla Serra, M.; Menestrina, G.; Fogliano, V.; Gambale, F. The Phytotoxic Lipodepsipeptide Syringopeptin 25A from Pseudomonas Syringae Pv Syringae Forms Ion Channels in Sugar Beet Vacuoles. J. Membr. Biol. 2002, 188, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gutla, P.V.K.; Boccaccio, A.; De Angeli, A.; Gambale, F.; Carpaneto, A. Modulation of Plant TPC Channels by Polyunsaturated Fatty Acids. J. Exp. Bot. 2012, 63, 6187–6197. [Google Scholar] [CrossRef] [PubMed]
- Carpaneto, A.; Accardi, A.; Pisciotta, M.; Gambale, F. Chloride Channels Activated by Hypotonicity in N2A Neuroblastoma Cell Line. Exp. Brain Res. 1999, 124, 193–199. [Google Scholar] [CrossRef]
- Carpaneto, A.; Naso, A.; Paganetto, A.; Cornara, L.; Pesce, E.-R.; Gambale, F. Properties of Ion Channels in the Protoplasts of the Mediterranean Seagrass Posidonia Oceanica. Plant Cell Environ. 2004, 27, 279–292. [Google Scholar] [CrossRef]
- Costa, A.; Carpaneto, A.; Varotto, S.; Formentin, E.; Marin, O.; Barizza, E.; Terzi, M.; Gambale, F.; Lo Schiavo, F. Potassium and Carrot Embryogenesis: Are K+ Channels Necessary for Development? Plant Mol. Biol. 2004, 54, 837–852. [Google Scholar] [CrossRef]
- Bregante, M.; Yang, Y.; Formentin, E.; Carpaneto, A.; Schroeder, J.I.; Gambale, F.; Lo Schiavo, F.; Costa, A. KDC1, a Carrot Shaker-like Potassium Channel, Reveals Its Role as a Silent Regulatory Subunit When Expressed in Plant Cells. Plant Mol. Biol. 2008, 66, 61–72. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Naso, A.; Carpaneto, A. A Perspective on the Slow Vacuolar Channel in Vacuoles from Higher Plant Cells. J. Chem. Inf. Model. 2005, 45, 1502–1506. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Gambale, F.; Carpaneto, A. Modulation of Plant Ion Channels by Oxidizing and Reducing Agents. Arch. Biochem. Biophys. 2005, 434, 43–50. [Google Scholar] [CrossRef]
- Hedrich, R.; Marten, I. TPC1-SV Channels Gain Shape. Mol. Plant 2011, 4, 428–441. [Google Scholar] [CrossRef]
- Bertl, A.; Slayman, C.L. Cation-Selective Channels in the Vacuolar Membrane of Saccharomyces: Dependence on Calcium, Redox State, and Voltage. Proc. Natl. Acad. Sci. USA 1990, 87, 7824–7828. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Okuda, N.; Saitoh, N.; Hiyama, T.; Terasaki, Y.; Anazawa, H.; Hirata, A.; Mogi, T.; Kusaka, I.; Tsuchiya, T.; et al. Patch Clamp Studies on Ion Pumps of the Cytoplasmic Membrane of Escherichia Coli. Formation, Preparation, and Utilization of Giant Vacuole-like Structures Consisting of Everted Cytoplasmic Membrane. J. Biol. Chem. 1998, 273, 16897–16904. [Google Scholar] [CrossRef] [PubMed]
- Yabe, I.; Horiuchi, K.; Nakahara, K.; Hiyama, T.; Yamanaka, T.; Wang, P.C.; Toda, K.; Hirata, A.; Ohsumi, Y.; Hirata, R.; et al. Patch Clamp Studies on V-Type ATPase of Vacuolar Membrane of Haploid Saccharomyces Cerevisiae. Preparation and Utilization of a Giant Cell Containing a Giant Vacuole. J. Biol. Chem. 1999, 274, 34903–34910. [Google Scholar] [CrossRef][Green Version]
- Gaxiola, R.A.; Rao, R.; Sherman, A.; Grisafi, P.; Alper, S.L.; Fink, G.R. The Arabidopsis Thaliana Proton Transporters, AtNhx1 and Avp1, Can Function in Cation Detoxification in Yeast. Proc. Natl. Acad. Sci. USA 1999, 96, 1480–1485. [Google Scholar] [CrossRef]
- Liu, L.-H.; Ludewig, U.; Gassert, B.; Frommer, W.B.; von Wirén, N. Urea Transport by Nitrogen-Regulated Tonoplast Intrinsic Proteins in Arabidopsis. Plant Physiol. 2003, 133, 1220–1228. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Apse, M.P.; Shi, H.; Blumwald, E. Topological Analysis of a Plant Vacuolar Na+/H+ Antiporter Reveals a Luminal C Terminus That Regulates Antiporter Cation Selectivity. Proc. Natl. Acad. Sci. USA 2003, 100, 12510–12515. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Yabe, I.; Maeshima, M. Patch Clamp Analysis of a H+ Pump Heterologously Expressed in Giant Yeast Vacuoles. J. Biochem. 2003, 134, 615–623. [Google Scholar] [CrossRef]
- Hamamoto, S.; Marui, J.; Matsuoka, K.; Higashi, K.; Igarashi, K.; Nakagawa, T.; Kuroda, T.; Mori, Y.; Murata, Y.; Nakanishi, Y.; et al. Characterization of a Tobacco TPK-Type K+ Channel as a Novel Tonoplast K+ Channel Using Yeast Tonoplasts. J. Biol. Chem. 2008, 283, 1911–1920. [Google Scholar] [CrossRef]
- Hamamoto, S.; Yabe, I.; Uozumi, N. Electrophysiological Properties of NtTPK1 Expressed in Yeast Tonoplast. Biosci. Biotechnol. Biochem. 2008, 72, 2785–2787. [Google Scholar] [CrossRef]
- Hamamoto, S.; Mori, Y.; Yabe, I.; Uozumi, N. In Vitro and in Vivo Characterization of Modulation of the Vacuolar Cation Channel TRPY1 from Saccharomyces Cerevisiae. FEBS J. 2018, 285, 1146–1161. [Google Scholar] [CrossRef]
- Palmer, C.P.; Zhou, X.L.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP Homolog in Saccharomyces Cerevisiae Forms an Intracellular Ca(2+)-Permeable Channel in the Yeast Vacuolar Membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Schlenstedt, G.; Flockerzi, V.; Beck, A. Properties of the Intracellular Transient Receptor Potential (TRP) Channel in Yeast, Yvc1. FEBS Lett. 2010, 584, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Hamamoto, S.; Miyanoiri, Y.; Takeda, M.; Kainosho, M.; Yabe, I.; Uozumi, N.; Yamashita, A. Molecular Bases of Multimodal Regulation of a Fungal Transient Receptor Potential (TRP) Channel. J. Biol. Chem. 2013, 288, 15303–15317. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Grigorieff, N.; Penczek, P.A.; Walz, T. A Primer to Single-Particle Cryo-Electron Microscopy. Cell 2015, 161, 438–449. [Google Scholar] [CrossRef]
- Earl, L.A.; Falconieri, V.; Milne, J.L.S.; Subramaniam, S. Cryo-EM: Beyond the Microscope. Curr. Opin. Struct. Biol. 2017, 46, 71–78. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.K.; Benvin, N.M.; Zhou, X.; Su, D.; Li, H.; Wang, S.; Michailidis, I.E.; Tong, L.; Li, X.; et al. Structural Basis of Ca2+/PH Dual Regulation of the Endolysosomal TRPML1 Channel. Nat. Struct. Mol. Biol. 2017, 24, 205–213. [Google Scholar] [CrossRef]
- Zhou, X.; Li, M.; Su, D.; Jia, Q.; Li, H.; Li, X.; Yang, J. Cryo-EM Structures of the Human Endolysosomal TRPML3 Channel in Three Distinct States. Nat. Struct. Mol. Biol. 2017, 24, 1146–1154. [Google Scholar] [CrossRef]
- Schrecker, M.; Korobenko, J.; Hite, R.K. Cryo-EM Structure of the Lysosomal Chloride-Proton Exchanger CLC-7 in Complex with OSTM1. eLife 2020, 9, e59555. [Google Scholar] [CrossRef]
- Khalili-Araghi, F.; Gumbart, J.; Wen, P.-C.; Sotomayor, M.; Tajkhorshid, E.; Schulten, K. Molecular Dynamics Simulations of Membrane Channels and Transporters. Curr. Opin. Struct. Biol. 2009, 19, 128–137. [Google Scholar] [CrossRef]
- Maffeo, C.; Bhattacharya, S.; Yoo, J.; Wells, D.; Aksimentiev, A. Modeling and Simulation of Ion Channels. Chem. Rev. 2012, 112, 6250–6284. [Google Scholar] [CrossRef]
- Milenkovic, S.; Bodrenko, I.V.; Lagostena, L.; Gradogna, A.; Serra, G.; Bosin, A.; Carpaneto, A.; Ceccarelli, M. The Mechanism and Energetics of a Ligand-Controlled Hydrophobic Gate in a Mammalian Two Pore Channel. Phys. Chem. Chem. Phys. PCCP 2020, 22, 15664–15674. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, S.; Bodrenko, I.V.; Carpaneto, A.; Ceccarelli, M. The Key Role of the Central Cavity in Sodium Transport through Ligand-Gated Two-Pore Channels. Phys. Chem. Chem. Phys. 2021, 23, 18461–18474. [Google Scholar] [CrossRef] [PubMed]
- Isaka, Y.; Ekimoto, T.; Kokabu, Y.; Yamato, I.; Murata, T.; Ikeguchi, M. Rotation Mechanism of Molecular Motor V1-ATPase Studied by Multiscale Molecular Dynamics Simulation. Biophys. J. 2017, 112, 911–920. [Google Scholar] [CrossRef]
- Santos-Pereira, C.; Rocha, J.F.; Fernandes, H.S.; Rodrigues, L.R.; Côrte-Real, M.; Sousa, S.F. The Milk-Derived Lactoferrin Inhibits V-ATPase Activity by Targeting Its V1 Domain. Int. J. Biol. Macromol. 2021, 186, 54–70. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Kelu, J.J.; Webb, S.E.; Parrington, J.; Galione, A.; Miller, A.L. Ca2+ Release via Two-Pore Channel Type 2 (TPC2) Is Required for Slow Muscle Cell Myofibrillogenesis and Myotomal Patterning in Intact Zebrafish Embryos. Dev. Biol. 2017, 425, 109–129. [Google Scholar] [CrossRef]
- Westphal, V.; Rizzoli, S.O.; Lauterbach, M.A.; Kamin, D.; Jahn, R.; Hell, S.W. Video-Rate Far-Field Optical Nanoscopy Dissects Synaptic Vesicle Movement. Science 2008, 320, 246–249. [Google Scholar] [CrossRef]


| Channel/Transporter | Transported Ion(s) |
|---|---|
| CLC-6 | Cl−, H+ |
| CLC-7 | |
| SLC38A7 | Na+, aminoacids |
| SLC38A9 | |
| NHE3 | Na+, H+ |
| NHE5 | |
| NHE6 | |
| TPC1 | Na+, Ca2+ |
| TPC2 | |
| VGCCs | Ca2+ |
| TRPML1 | Na+, Ca2+, Fe2+, Zn2+, cations |
| TRPML2 | |
| TRPML3 | |
| P2X4 | |
| BK | K+ |
| TMEM175 | |
| LRRC8 | Cl−, organic anions |
| V-ATPase | H+ |
| CLN7 | Cl− |
| Method | Advantages | Disadvantages | Lysosomal Channels/Transporters |
|---|---|---|---|
| Incorporation into artificial membranes or liposomes | Low level of background current noise | Protein amount Channel removed from native environment Impurities | TPC1, TPC2, TRPLM1 |
| Solid-supported membrane-based electrophysiology | Native environment Automation Suitable for screening | No control of membrane potential No control of luminal solution | CLC-7, V-ATPase |
| Flux measurements on purified lysosomes | Native environment Large number of lysosomes tested | No control of membrane potential No control of luminal solution | CLC-7 |
| Patch-clamp electrophysiology on enlarged lysosomes | Native environment Direct Robust | Insufficient resolution to detect the activity of low turnover rate transporters Interference by endogenous channels and transporters Need of trained electrophysiologist | TPCs, TRPML1, BK, LRRC8, TMEM175, LRRC8, CLN7 |
| Targeting to the plasma membrane upon manipulation of sorting signals | Well-characterized expression systems can be used Easy to perform, even if sorting/retention signals are not known | Different lipid environment may affect activity Modification of protein by mutation or tag Not applicable to all intracellular transmembrane proteins | CLC-6, CLC-7, TPC2, GLUT8, LRRC8, CLN7 |
| Nuclear membrane electrophysiology | Easy access to cytoplasmic and luminal sides Ligand conditions rigorously controlled High temporal resolution Simple protocol High signal-to-noise ratio | Not tolerant to high Vapp Low quality and stability of giga-ohm seals High background current Difficult excised nuclear patches | hTPC2 |
| Patch-clamp electrophysiology on plant vacuoles | Good knowledge of vacuolar endogenous channels Large size Ease of isolation Low noise Possibility of different patch configurations Eukaryotic post-translational modifications | Long protoplasting procedure Need of trained electrophysiologist Fused fluorescent protein could impair functionality Differences with mammalian post translational modifications | CLC-7, hTPC1, hTPC2 |
| Patch-clamp electrophysiology on giant vacuoles from yeast cells | Very high signal-to-noise ratio | Need of preparation of giant cells | hTPC2 |
| Transport Protein | Name | Origin | Localization | Expression System |
|---|---|---|---|---|
| Monosaccharide facilitator/glucose transporter | GLUT8 | Mammalia | Late endosomes, lysosomes | Xenopus oocytes |
| Cystine/proton symporter | Cystinosin | Mammalia | Lysosomes | Xenopus oocytes; COS cells |
| Aquaporin | AQP6 | Mammalia | Acidic vesicles | HEK293 cells; Madin–Darby canine kidney cells |
| Sialic acid/proton symporter | Sialin | Mammalia | Late endosomes, lysosomes | HEK293 cells |
| Nucleoside transporter | ENT3 | Mammalia | Late endosomes, lysosomes | Xenopus oocytes |
| Monosaccharide facilitator/glucose transporter | ESL1 | Plant | Vacuole | Tobacco BY2 cells |
| Two-pore cation channel | TPC2 | Mammalia | Late endosomes, lysosomes | HEK293 cells; Xenopus oocytes |
| Chloride/proton antiporter | CLC-6 | Mammalia | Late endosomes | Xenopus oocytes; CHO cells |
| Chloride/proton antiporter | CLC-7 | Mammalia | Lysosomes | Xenopus oocytes; HeLa cells |
| Cationic amino acid transporter | PQLC2 | Mammalia | Lysosomes | Xenopus oocytes |
| Two-pore cation channel | TPC1 | Arabidopsis | Vacuole | HEK293 cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Festa, M.; Minicozzi, V.; Boccaccio, A.; Lagostena, L.; Gradogna, A.; Qi, T.; Costa, A.; Larisch, N.; Hamamoto, S.; Pedrazzini, E.; et al. Current Methods to Unravel the Functional Properties of Lysosomal Ion Channels and Transporters. Cells 2022, 11, 921. https://doi.org/10.3390/cells11060921
Festa M, Minicozzi V, Boccaccio A, Lagostena L, Gradogna A, Qi T, Costa A, Larisch N, Hamamoto S, Pedrazzini E, et al. Current Methods to Unravel the Functional Properties of Lysosomal Ion Channels and Transporters. Cells. 2022; 11(6):921. https://doi.org/10.3390/cells11060921
Chicago/Turabian StyleFesta, Margherita, Velia Minicozzi, Anna Boccaccio, Laura Lagostena, Antonella Gradogna, Tianwen Qi, Alex Costa, Nina Larisch, Shin Hamamoto, Emanuela Pedrazzini, and et al. 2022. "Current Methods to Unravel the Functional Properties of Lysosomal Ion Channels and Transporters" Cells 11, no. 6: 921. https://doi.org/10.3390/cells11060921
APA StyleFesta, M., Minicozzi, V., Boccaccio, A., Lagostena, L., Gradogna, A., Qi, T., Costa, A., Larisch, N., Hamamoto, S., Pedrazzini, E., Milenkovic, S., Scholz-Starke, J., Ceccarelli, M., Vitale, A., Dietrich, P., Uozumi, N., Gambale, F., & Carpaneto, A. (2022). Current Methods to Unravel the Functional Properties of Lysosomal Ion Channels and Transporters. Cells, 11(6), 921. https://doi.org/10.3390/cells11060921






