Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. The Animals and Housing Conditions
2.2. Study Design
2.3. Novel Cage
2.4. Dark-Light Box
2.5. Rat Exposure Stress
2.6. Home Cage Interaction
2.7. Food Competition Test
2.8. Elevated O-maze
2.9. Modified Forced Swim Test
2.10. Brain Dissection and Tissue Collection
2.11. Quantitative Real-Time PCR (qRT-PCR)
2.12. Statistical Analysis
3. Results
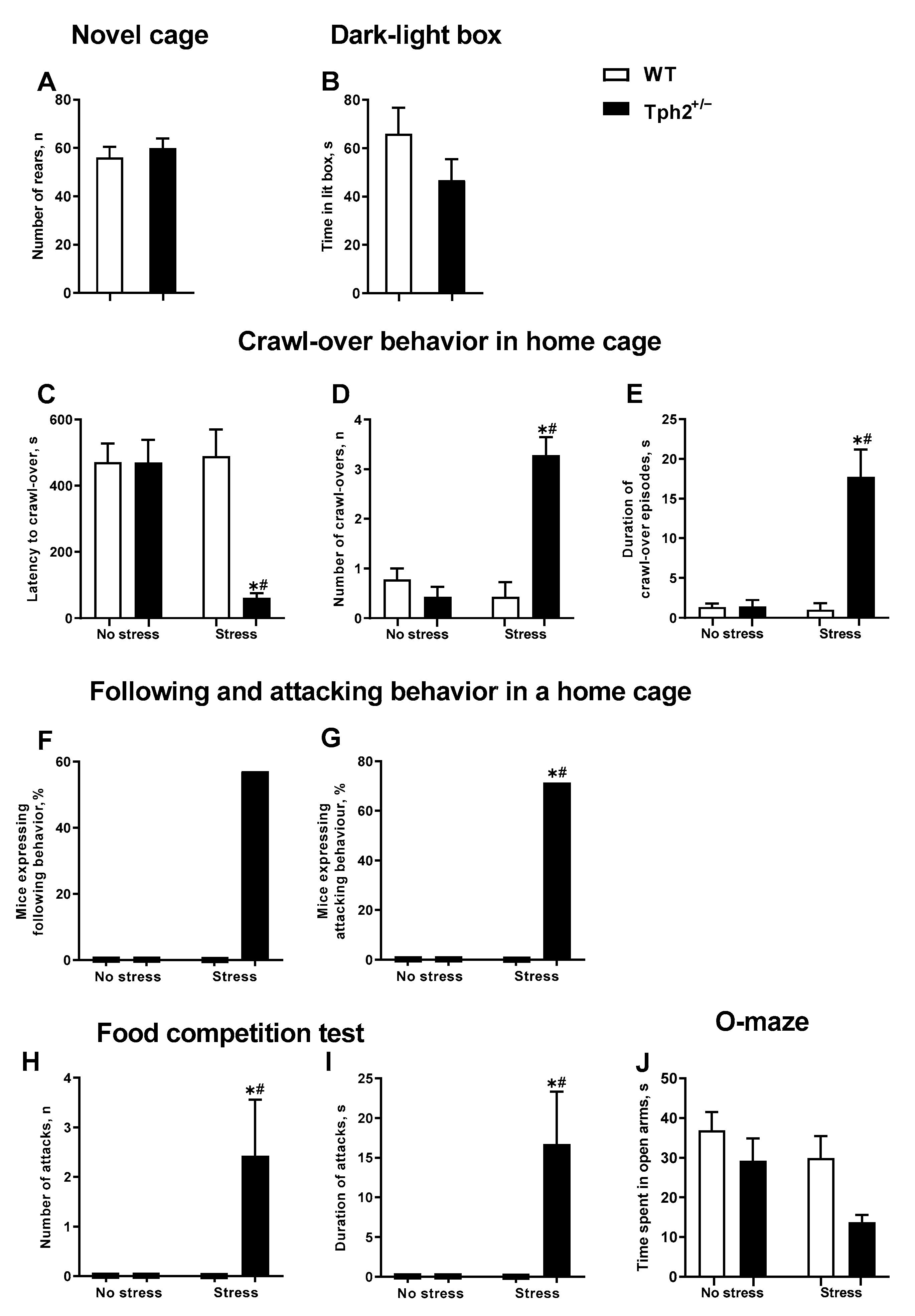
3.1. The Predation Stress Procedure Induces Aggressive and Dominant Behavior in Tph2+/− Females
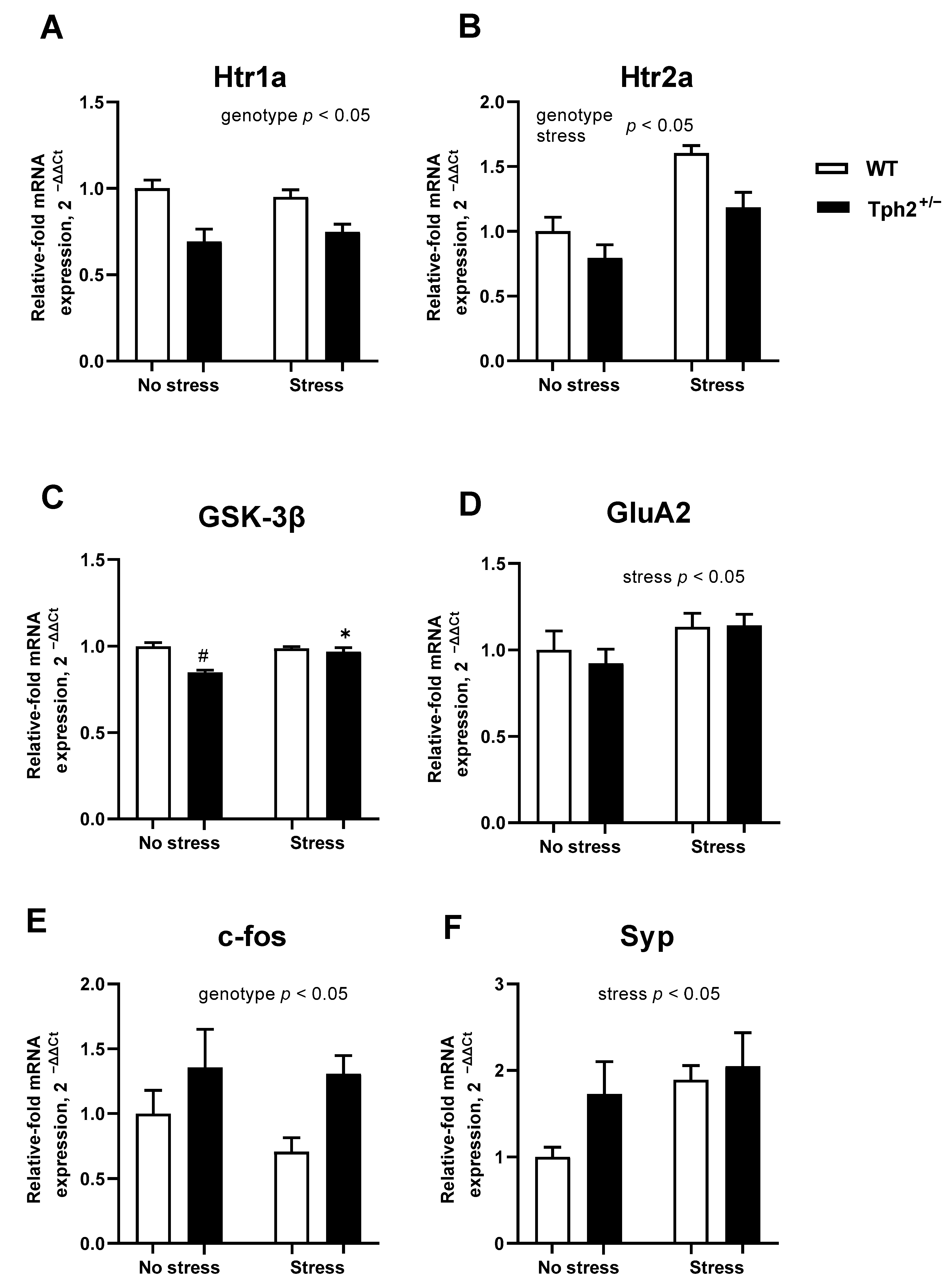
3.2. Altered Gene Expression of Selected Molecular Markers in the Prefrontal Cortex of Stressed Tph2+/− Mice
3.3. Naïve Female Tph2+/− Mice Show Signs of Decreased Learning of Adverse Memories and Helplessness as a Manifestation of Stress Resilience
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Supplementary Methods
| Gene | Primer | Sequence |
|---|---|---|
| Htr1a | Forward | 5′-GACAGGCGGCAACGATACT-3′ |
| Reverse | 5′-CCAAGGAGCCGATGAGATAGTT-3′ | |
| Htr2a | Forward | 5′-TAATGCAATTAGGTGACGACTCG-3′ |
| Reverse | 5′-GCAGGAGAGGTTGGTTCTGTTT-3′ | |
| GSK-3β | Forward | 5′-GCACTCTTCAACTTTACCACTCA-3′ |
| Reverse | 5′-CGAGCATGTGGAGGGATAAG-3′ | |
| GluA2 | Forward | 5′-GCGTGGAAATAGAAAGGGCC-3′ |
| Reverse | 5′-ACTCCAGTACCCAATCTTCCG-3′ | |
| c-fos | Forward | 5′-CGGGTTTCAACGCCGACTA-3′ |
| Reverse | 5′-TTGGCACTAGAGACGGACAGA-3′ | |
| Syp | Forward | 5′-TGTGTTTGCCTTCCTCTACTC-3′ |
| Reverse | 5′-TCAGTGGCCATCTTCACATC-3′ | |
| Plp1 | Forward | 5′-CCAGAATGTATGGTGTTCTCCC-3′ |
| Reverse | 5′-GGCCCATGAGTTTAAGGACG-3′ | |
| Mbp | Forward | 5′-TCACAGCGATCCAAGTACCTG-3′ |
| Reverse | 5′-CCCCTGTCACCGCTAAAGAA-3′ | |
| Mag | Forward | 5′-GGTACATGGCGTCTGGTATTTC-3′ |
| Reverse | 5′-ACTTGTGTGCGGGACTTGAAG-3′ | |
| Mog | Forward | 5′-TCATGCAGCTATGCAGGACAA-3′ |
| Reverse | 5′-TTTCGGTAGAGGTGAACCACT-3′ | |
| Creb | Forward | 5′-CAGGGGTCGCAAGGATTGAAG-3′ |
| Reverse | 5′-ATCGCCTGAGGCAGTGTACT-3′ | |
| Bdnf | Forward | 5′-TGGCTGACACTTTTGAGCAC-3′ |
| Reverse | 5′-AAGTGTACAAGTCCGCGTCC-3′ | |
| Trkb | Forward | 5′-CCTCCACGGATGTTGCTGAC-3′ |
| Reverse | 5′-GCAACATCACCAGCAGGCA-3′ | |
| PSD-95 | Forward | 5′-GACGCCAGCGACGAAGAG-3′ |
| Reverse | 5′-CTCGACCCGCCGTTTG-3′ | |
| GAPDH | Forward | 5′-ATGACCACAGTCCATGCCATC -3′ |
| Reverse | 5′-GAGCTTCCCGTTCAGCTCTG-3′ |
Appendix A.2. Supplementary Results
Daily Food Intake of Tph2+/− Mice

Appendix A.3. Expression of Neurotrophic Factors in the Prefrontal Cortex of Stressed Tph2+/−

Appendix A.4. Tph2+/− Mice Display Reduced Potentiation of Floating in the modFST Paradigm

References
- Vakili, V.; Ziaee, M.; Zarifian, A. Aggression: Is That an Issue for Worrying? Iran. J. Public Health 2015, 44, 1561–1562. [Google Scholar] [PubMed]
- Xiang, C.; Liu, S.; Fan, Y.; Wang, X.; Jia, Y.; Li, L.; Cong, S.; Han, F. Single Nucleotide Polymorphisms, Variable Number Tandem Repeats and Allele Influence on Serotonergic Enzyme Modulators for Aggressive and Suicidal Behaviors: A Review. Pharmacol. Biochem. Behav. 2019, 180, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Freitag, C.M.; Konrad, K.; Stadler, C.; De Brito, S.A.; Popma, A.; Herpertz, S.C.; Herpertz-Dahlmann, B.; Neumann, I.; Kieser, M.; Chiocchetti, A.G.; et al. Conduct Disorder in Adolescent Females: Current State of Research and Study Design of the FemNAT-CD Consortium. Eur. Child Adolesc. Psychiatry 2018, 27, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Denson, T.F.; O’Dean, S.M.; Blake, K.R.; Beames, J.R. Aggression in Women: Behavior, Brain and Hormones. Front. Behav. Neurosci. 2018, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Neumann Aggression and Anxiety: Social Context and Neurobiological Links. Front. Behav. Neurosci. 2010, 4, 12. [CrossRef]
- Takahashi, A.; Miczek, K.A. Neurogenetics of Aggressive Behavior: Studies in Rodents. Curr. Top. Behav. Neurosci. 2014, 17, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, L.; Araragi, N.; Merker, S.; Waider, J.; Sommerlandt, F.M.J.; Mlinar, B.; Baccini, G.; Mayer, U.; Proft, F.; Hamon, M.; et al. Impacts of Brain Serotonin Deficiency Following Tph2 Inactivation on Development and Raphe Neuron Serotonergic Specification. PLoS ONE 2012, 7, e43157. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, L.; Popp, S.; Waider, J.; Sommerlandt, F.M.J.; Göppner, C.; Post, A.; Reif, A.; Van Den Hove, D.; Strekalova, T.; Schmitt, A.; et al. Interaction of Brain 5-HT Synthesis Deficiency, Chronic Stress and Sex Differentially Impact Emotional Behavior in Tph2 Knockout Mice. Psychopharmacology 2015, 232, 2429–2441. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Sykes, C.E.; Shah, M.M.; Francescutti, D.M.; Rosenberg, D.R.; Thomas, D.M.; Kuhn, D.M. Genetic Depletion of Brain 5HT Reveals a Common Molecular Pathway Mediating Compulsivity and Impulsivity. J. Neurochem. 2012, 121, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Weidner, M.T.; Lardenoije, R.; Eijssen, L.; Mogavero, F.; De Groodt, L.P.M.T.; Popp, S.; Palme, R.; Förstner, K.U.; Strekalova, T.; Steinbusch, H.W.M.; et al. Identification of Cholecystokinin by Genome-Wide Profiling as Potential Mediator of Serotonin-Dependent Behavioral Effects of Maternal Separation in the Amygdala. Front. Neurosci. 2019, 13, 460. [Google Scholar] [CrossRef]
- Yang, J.; Lee, M.S.; Lee, S.H.; Lee, B.C.; Kim, S.H.; Joe, S.H.; Jung, I.K.; Choi, I.G.; Ham, B.J. Association between Tryptophan Hydroxylase 2 Polymorphism and Anger-Related Personality Traits among Young Korean Women. Neuropsychobiology 2010, 62, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.J.; Ko, H.C.; Chang, F.M.; Yeh, T.L.; Sun, H.S. Population-Specific Functional Variant of the Tph2 Gene 2755C>A Polymorphism Contributes Risk Association to Major Depression and Anxiety in Chinese Peripartum Women. Arch. Women’s Ment. Health 2009, 12, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Faschingbauer, F.; Goecke, T.W.; Engel, A.; Häberle, L.; Seifert, A.; Voigt, F.; Amann, M.; Rebhan, D.; Burger, P.; et al. Genetic Variants in the Tryptophan Hydroxylase 2 Gene (Tph2) and Depression during and after Pregnancy. J. Psychiatr. Res. 2012, 46, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Anstrom, K.K.; Miczek, K.A.; Budygin, E.A. Increased Phasic Dopamine Signaling in the Mesolimbic Pathway during Social Defeat in Rats. Neuroscience 2009, 161, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Deal, A.L.; Park, J.; Weiner, J.L.; Budygin, E.A. Stress Alters the Effect of Alcohol on Catecholamine Dynamics in the Basolateral Amygdala. Front. Behav. Neurosci. 2021, 15, 640651. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chao, Y.L.; Chang, C.E.; Hsieh, M.H.; Liu, K.T.; Chen, H.C.; Lu, M.L.; Chen, W.Y.; Chen, C.H.; Tsai, M.H.; et al. Transcriptome Changes in Relation to Manic Episode. Front. Psychiatry 2019, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P. Alcohol Dependence and Gene x Environment Interaction in Emotion Regulation: Is Serotonin the Link? Eur. J. Pharmacol. 2005, 526, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Haller, J. The Role of Central and Medial Amygdala in Normal and Abnormal Aggression: A Review of Classical Approaches. Neurosci. Biobehav. Rev. 2017, 85, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, M.; Maercker, A. Associations between Trauma Exposure, Posttraumatic Stress Disorder, and Aggression Perpetrated by Women. A Meta-Analysis. Clin. Psychol. Sci. Pract. 2020, 27, e12322. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Waider, J.; Gorlova, A.; Cespuglio, R.; Kalueff, A.; Pomytkin, I.; Schmitt-Boehrer, A.G.; Lesch, K.P.; Anthony, D.C. Altered Behaviour, Dopamine and Norepinephrine Regulation in Stressed Mice Heterozygous in Tph2 Gene. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 108, 110155. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, A.; Ortega, G.; Waider, J.; Bazhenova, N.; Veniaminova, E.; Proshin, A.; Kalueff, A.V.; Anthony, D.C.; Lesch, K.P.; Strekalova, T. Stress-Induced Aggression in Heterozygous Tph2 Mutant Mice Is Associated with Alterations in Serotonin Turnover and Expression of 5-HT6 and AMPA Subunit 2A Receptors. J. Affect. Disord. 2020, 272, 440–451. [Google Scholar] [CrossRef]
- Veenema, A.H.; Bredewold, R.; Neumann, I.D. Opposite Effects of Maternal Separation on Intermale and Maternal Aggression in C57BL/6 Mice: Link to Hypothalamic Vasopressin and Oxytocin Immunoreactivity. Psychoneuroendocrinology 2007, 32, 437–450. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.E.M.; Neumann, I.D.; de Jong, T.R. Post-Weaning Social Isolation Exacerbates Aggression in Both Sexes and Affects the Vasopressin and Oxytocin System in a Sex-Specific Manner. Neuropharmacology 2019, 156, 107504. [Google Scholar] [CrossRef] [PubMed]
- Vignisse, J.; Sambon, M.; Gorlova, A.; Pavlov, D.; Caron, N.; Malgrange, B.; Shevtsova, E.; Svistunov, A.; Anthony, D.C.; Markova, N.; et al. Thiamine and Benfotiamine Prevent Stress-Induced Suppression of Hippocampal Neurogenesis in Mice Exposed to Predation without Affecting Brain Thiamine Diphosphate Levels. Mol. Cell. Neurosci. 2017, 82, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Veniaminova, E.; Cespuglio, R.; Markova, N.; Mortimer, N.; Wai Cheung, C.; Steinbusch, H.W.; Lesch, K.-P.; Strekalova, T. Behavioral Features of Mice Fed with a Cholesterol-Enriched Diet:Deficient Novelty Exploration and Unaltered Aggressive Behavior. Transl. Neurosci. Clin. 2016, 2, 87. [Google Scholar] [CrossRef]
- Veniaminova, E.; Cespuglio, R.; Cheung, C.W.; Umriukhin, A.; Markova, N.; Shevtsova, E.; Lesch, K.-P.; Anthony, D.C.; Strekalova, T. Autism-Like Behaviours and Memory Deficits Result from a Western Diet in Mice. Neural Plast. 2017, 2017, 9498247. [Google Scholar] [CrossRef]
- Veniaminova, E.; Cespuglio, R.; Chernukha, I.; Schmitt-Boehrer, A.G.; Morozov, S.; Kalueff, A.V.; Kuznetsova, O.; Anthony, D.C.; Lesch, K.P.; Strekalova, T. Metabolic, Molecular, and Behavioral Effects of Western Diet in Serotonin Transporter-Deficient Mice: Rescue by Heterozygosity? Front. Neurosci. 2020, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, A.; Zhao, Z.Q.; Liu, X.Y.; Chen, Z.F. Postnatal Maintenance of the 5-Ht1a-Pet1 Autoregulatory Loop by Serotonin in the Raphe Nuclei of the Brainstem. Mol. Brain 2014, 7, 48. [Google Scholar] [CrossRef]
- Mlinar, B.; Montalbano, A.; Waider, J.; Lesch, K.P.; Corradetti, R. Increased Functional Coupling of 5-HT1A Autoreceptors to GIRK Channels in Tph2−/− Mice. Eur. Neuropsychopharmacol. 2017, 27, 1258–1267. [Google Scholar] [CrossRef]
- Wang, L.R.; Kim, S.H.; Baek, S.S. Effects of Treadmill Exercise on the Anxiety-like Behavior through Modulation of GSK3β/β-Catenin Signaling in the Maternal Separation Rat Pup. J. Exerc. Rehabil. 2019, 15, 206–212. [Google Scholar] [CrossRef]
- Pavlov, D.; Bettendorff, L.; Gorlova, A.; Olkhovik, A.; Kalueff, A.V.; Ponomarev, E.D.; Inozemtsev, A.; Chekhonin, V.; Lesch, K.P.; Anthony, D.C.; et al. Neuroinflammation and Aberrant Hippocampal Plasticity in a Mouse Model of Emotional Stress Evoked by Exposure to Ultrasound of Alternating Frequencies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 90, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Costa-Nunes, J.; Zubareva, O.; Araújo-Correia, M.; Valença, A.; Schroeter, C.A.; Pawluski, J.L.; Vignisse, J.; Steinbusch, H.; Hermes, D.; Phillipines, M.; et al. Altered Emotionality, Hippocampus-Dependent Performance and Expression of NMDA Receptor Subunit MRNAs in Chronically Stressed Mice. Stress 2014, 17, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Costa-Nunes, J.P.; Gorlova, A.; Pavlov, D.; Cespuglio, R.; Gorovaya, A.; Proshin, A.; Umriukhin, A.; Ponomarev, E.D.; Kalueff, A.V.; Strekalova, T.; et al. Ultrasound Stress Compromises the Correlates of Emotional-like States and Brain AMPAR Expression in Mice: Effects of Antioxidant and Anti-Inflammatory Herbal Treatment. Stress 2020, 23, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, A.; Pavlov, D.; Anthony, D.C.; Ponomarev, E.D.; Sambon, M.; Proshin, A.; Shafarevich, I.; Babaevskaya, D.; Lesch, K.P.; Bettendorff, L.; et al. Thiamine and Benfotiamine Counteract Ultrasound-Induced Aggression, Normalize AMPA Receptor Expression and Plasticity Markers, and Reduce Oxidative Stress in Mice. Neuropharmacology 2019, 156, 107543. [Google Scholar] [CrossRef] [PubMed]
- Svirin, E.; Gorlova, A.; Lim, L.W.; Veniaminova, E.; Costa-Nunes, J.; Anthony, D.; Lesch, K.-P.; Strekalova, T. Sexual Bias in the Altered Expression of Myelination Factors in Mice with Partial Genetic Deficiency of Tryptophan Hydroxylase 2 and Pro-Aggressive Effects of Predation Stress. In Proceedings of the IBNS 30th Annual Meeting, Puerto Vallarta, Mexico, 1–5 June 2021. [Google Scholar]
- Jha, S.C.; Meltzer-Brody, S.; Steiner, R.J.; Cornea, E.; Woolson, S.; Ahn, M.; Verde, A.R.; Hamer, R.M.; Zhu, H.; Styner, M.; et al. Antenatal Depression, Treatment with Selective Serotonin Reuptake Inhibitors, and Neonatal Brain Structure: A Propensity-Matched Cohort Study. Psychiatry Res.-Neuroimaging 2016, 253, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, M.; Ikawa, D.; Miyamoto, Y.; Yamauchi, J.; Yamamuro, K.; Yamashita, Y.; Toritsuka, M.; Kimoto, S.; Okumura, K.; Yamauchi, T.; et al. Social Isolation Impairs Remyelination in Mice through Modulation of IL-6. FASEB J. 2016, 30, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Rüsch, N.; Weber, M.; Il’yasov, K.A.; Lieb, K.; Ebert, D.; Hennig, J.; van Elst, L.T. Inferior Frontal White Matter Microstructure and Patterns of Psychopathology in Women with Borderline Personality Disorder and Comorbid Attention-Deficit Hyperactivity Disorder. Neuroimage 2007, 35, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Fekete, T.; Siciliano, F.; Biezonski, D.; Greenhill, L.; Pliszka, S.R.; Blader, J.C.; Krain Roy, A.; Leibenluft, E.; Posner, J. Neural Correlates of Aggression in Medication-Naive Children with ADHD: Multivariate Analysis of Morphometry and Tractography. Neuropsychopharmacology 2015, 40, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Dailey, N.S.; Smith, R.; Bajaj, S.; Alkozei, A.; Gottschlich, M.K.; Raikes, A.C.; Satterfield, B.C.; Killgore, W.D.S. Elevated Aggression and Reduced White Matter Integrity in Mild Traumatic Brain Injury: A DTI Study. Front. Behav. Neurosci. 2018, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, G.; Hauser, T.U.; Moutoussis, M.; Bullmore, E.T.; Goodyer, I.M.; Fonagy, P.; Jones, P.B.; Lindenberger, U.; Dolan, R.J. Compulsivity and Impulsivity Traits Linked to Attenuated Developmental Frontostriatal Myelination Trajectories. Nat. Neurosci. 2019, 22, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Markova, N.; Shevtsova, E.; Zubareva, O.; Bakhmet, A.; Steinbusch, H.M.; Bachurin, S.; Lesch, K.-P. Individual Differences in Behavioural Despair Predict Brain GSK-3beta Expression in Mice: The Power of a Modified Swim Test. Neural Plast. 2016, 2016, 5098591. [Google Scholar] [CrossRef] [PubMed]
- Grafman, J.; Schwab, K.; Warden, D.; Pridgen, A.; Brown, H.R.; Salazar, A.M. Frontal Lobe Injuries, Violence, and Aggression: A Report of the Vietnam Head Injury Study. Neurology 1996, 46, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Stress Signalling Pathways That Impair Prefrontal Cortex Structure and Function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Wall, V.L.; Fischer, E.K.; Bland, S.T. Isolation Rearing Attenuates Social Interaction-Induced Expression of Immediate Early Gene Protein Products in the Medial Prefrontal Cortex of Male and Female Rats. Physiol. Behav. 2012, 107, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Nagayasu, K.; Nishitani, N.; Kaneko, S.; Koide, T. Control of Intermale Aggression by Medial Prefrontal Cortex Activation in the Mouse. PLoS ONE 2014, 9, e94657. [Google Scholar] [CrossRef]
- Achterberg, M.; van Duijvenvoorde, A.C.K.; Bakermans-Kranenburg, M.J.; Crone, E.A. Control Your Anger! The Neural Basis of Aggression Regulation in Response to Negative Social Feedback. Soc. Cogn. Affect. Neurosci. 2016, 11, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Markova, N.; Bazhenova, N.; Anthony, D.C.; Vignisse, J.; Svistunov, A.; Lesch, K.P.; Bettendorff, L.; Strekalova, T. Thiamine and Benfotiamine Improve Cognition and Ameliorate GSK-3β-Associated Stress-Induced Behaviours in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T. Optimization of the Chronic Stress Depression Model in C57 BL/6 Mice: Evidences for Improved Validity. In Behavioral Models in Stress Research; LaPorte, J.L., Kalueff, A.V., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2008; Volume I, pp. 95–139. [Google Scholar]
- Strekalova, T.; Steinbusch, H.W.M. Measuring Behavior in Mice with Chronic Stress Depression Paradigm. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial Activation, Increased TNF and SERT Expression in the Prefrontal Cortex Define Stress-Altered Behaviour in Mice Susceptible to Anhedonia. Brain. Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef]
- Costa-Nunes, J.P.; Cline, B.H.; Araújo-Correia, M.; Valença, A.; Markova, N.; Dolgov, O.; Kubatiev, A.; Yeritsyan, N.; Steinbusch, H.W.M.; Strekalova, T. Animal Models of Depression and Drug Delivery with Food as an Effective Dosing Method: Evidences from Studies with Celecoxib and Dicholine Succinate. BioMed Res. Int. 2015, 2015, 596126. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Bartsch, D.; Henn, F.A.; Gass, P. Stress-Induced Anhedonia in Mice Is Associated with Deficits in Forced Swimming and Exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Gorenkova, N.; Schunk, E.; Dolgov, O.; Bartsch, D. Selective Effects of Citalopram in a Mouse Model of Stress-Induced Anhedonia with a Control for Chronic Stress. Behav. Pharmacol. 2006, 17, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Clipperton Allen, A.E.; Cragg, C.L.; Wood, A.J.; Pfaff, D.W.; Choleris, E. Agonistic Behavior in Males and Females: Effects of an Estrogen Receptor Beta Agonist in Gonadectomized and Gonadally Intact Mice. Psychoneuroendocrinology 2010, 35, 1008–1022. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terranova, M.L.; Laviola, G.; Alleva, E. Ontogeny of Amicable Social Behavior in the Mouse: Gender Differences and Ongoing Isolation Outcomes. Dev. Psychobiol. 1993, 26, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.M.; Lee, W.; DeCasien, A.R.; Lanham, A.; Romeo, R.D.; Curley, J.P. Social Hierarchy Position in Female Mice Is Associated with Plasma Corticosterone Levels and Hypothalamic Gene Expression. Sci. Rep. 2019, 9, 7324. [Google Scholar] [CrossRef]
- Mackintosh, J.H.; Grant, E.C. A Comparison of the Social Postures of Some Common Laboratory Rodents. Behaviour 1963, 21, 246–259. [Google Scholar] [CrossRef]
- Alleva, E. Assessment of Aggressive Behavior in Rodents. In Methods in Neurosciences; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 1993; Volume 14, pp. 111–137. [Google Scholar]
- Kästner, N.; Richter, S.H.; Urbanik, S.; Kunert, J.; Waider, J.; Lesch, K.P.; Kaiser, S.; Sachser, N. Brain Serotonin Deficiency Affects Female Aggression. Sci. Rep. 2019, 9, 1366. [Google Scholar] [CrossRef]
- Kloke, V.; Jansen, F.; Heiming, R.S.; Palme, R.; Lesch, K.P.; Sachser, N. The Winner and Loser Effect, Serotonin Transporter Genotype, and the Display of Offensive Aggression. Physiol. Behav. 2011, 103, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Trofimov, A.; Markova, N.; Nikolenko, V.; Steinbusch, H.W.; Chekhonin, V.; Schroeter, C.; Lesch, K.P.; Anthony, D.C.; Strekalova, T. Low-Dose Lipopolysaccharide (LPS) Inhibits Aggressive and Augments Depressive Behaviours in a Chronic Mild Stress Model in Mice. J. Neuroinflamm. 2016, 13, 108. [Google Scholar] [CrossRef]
- Pavlov, D.; Markova, N.; Bettendorff, L.; Chekhonin, V.; Pomytkin, I.; Lioudyno, V.; Svistunov, A.; Ponomarev, E.; Lesch, K.P.; Strekalova, T. Elucidating the Functions of Brain GSK3α: Possible Synergy with GSK3β Upregulation and Reversal by Antidepressant Treatment in a Mouse Model of Depressive-like Behaviour. Behav. Brain Res. 2017, 335, 122–127. [Google Scholar] [CrossRef]
- Pavlov, D.; Gorlova, A.; Bettendorff, L.; Kalueff, A.A.; Umriukhin, A.; Proshin, A.; Lysko, A.; Landgraf, R.; Anthony, D.C.; Strekalova, T. Enhanced Conditioning of Adverse Memories in the Mouse Modified Swim Test Is Associated with Neuroinflammatory Changes—Effects That Are Susceptible to Antidepressants. Neurobiol. Learn. Mem. 2020, 172, 107227. [Google Scholar] [CrossRef] [PubMed]
- Malatynska, E.; Steinbusch, H.W.M.; Redkozubova, O.; Bolkunov, A.; Kubatiev, A.; Yeritsyan, N.B.; Vignisse, J.; Bachurin, S.; Strekalova, T. Anhedonic-like Traits and Lack of Affective Deficits in 18-Month-Old C57BL/6 Mice: Implications for Modeling Elderly Depression. Exp. Gerontol. 2012, 47, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Bahzenova, N.; Trofimov, A.; Schmitt-Böhrer, A.G.; Markova, N.; Grigoriev, V.; Zamoyski, V.; Serkova, T.; Redkozubova, O.; Vinogradova, D.; et al. Pro-Neurogenic, Memory-Enhancing and Anti-Stress Effects of DF302, a Novel Fluorine Gamma-Carboline Derivative with Multi-Target Mechanism of Action. Mol. Neurobiol. 2018, 55, 335–349. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.; Shafarevich, I.; Liundup, A.; Pavlov, D.; Wolters, E.C.; Gorlova, A.; Veniaminova, E.; Umriukhin, A.; Kalueff, A.; Svistunov, A.; et al. Neuro-Cells Therapy Improves Motor Outcomes and Suppresses Inflammation during Experimental Syndrome of Amyotrophic Lateral Sclerosis in Mice. CNS Neurosci. Ther. 2020, 26, 504–517. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.V.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased Oxidative Stress in the Prefrontal Cortex as a Shared Feature of Depressive- and PTSD-Like Syndromes: Effects of a Standardized Herbal Antioxidant. Front. Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef] [PubMed]
- Veniaminova, E.; Oplatchikova, M.; Bettendorff, L.; Kotenkova, E.; Lysko, A.; Vasilevskaya, E.; Kalueff, A.V.; Fedulova, L.; Umriukhin, A.; Lesch, K.-P.; et al. Prefrontal Cortex Inflammation and Liver Pathologies Accompany Cognitive and Motor Deficits Following Western Diet Consumption in Non-Obese Female Mice. Life Sci. 2020, 241, 117163. [Google Scholar] [CrossRef] [PubMed]
- Audero, E.; Mlinar, B.; Baccini, G.; Skachokova, Z.K.; Corradetti, R.; Gross, C. Suppression of Serotonin Neuron Firing Increases Aggression in Mice. J. Neurosci. 2013, 33, 8678–8688. [Google Scholar] [CrossRef] [PubMed]
- Juárez, P.; Valdovinos, M.G.; May, M.E.; Lloyd, B.P.; Couppis, M.H.; Kennedy, C.H. Serotonin2A/C Receptors Mediate the Aggressive Phenotype of TLX Gene Knockout Mice. Behav. Brain Res. 2013, 256, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Godar, S.C.; Mosher, L.J.; Scheggi, S.; Devoto, P.; Moench, K.M.; Strathman, H.J.; Jones, C.M.; Frau, R.; Melis, M.; Gambarana, C.; et al. Gene-Environment Interactions in Antisocial Behavior Are Mediated by Early-Life 5-HT2A Receptor Activation. Neuropharmacology 2019, 159, 107513. [Google Scholar] [CrossRef] [PubMed]
- Araragi, N.; Mlinar, B.; Baccini, G.; Gutknecht, L.; Lesch, K.-P.; Corradetti, R. Conservation of 5-HT1A Receptor-Mediated Autoinhibition of Serotonin (5-HT) Neurons in Mice with Altered 5-HT Homeostasis. Front. Pharmacol. 2013, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Terranova, J.I.; Song, Z.; Larkin, T.E.; Hardcastle, N.; Norvelle, A.; Riaz, A.; Albers, H.E. Serotonin and Arginine-Vasopressin Mediate Sex Differences in the Regulation of Dominance and Aggression by the Social Brain. Proc. Natl. Acad. Sci. USA 2016, 113, 13233–13238. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.P.; Fink, H. Serotonin Controlling Feeding and Satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Ragnauth, A.K.; Devidze, N.; Moy, V.; Finley, K.; Goodwill, A.; Kow, L.M.; Muglia, L.J.; Pfaff, D.W. Female Oxytocin Gene-Knockout Mice, in a Seminatural Environment, Display Exaggerated Aggressive Behavior. Genes Brain Behav. 2005, 4, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, S.R.; Caldwell, H.K.; Christiansen, M.; Young, W.S. Disruption of the Vasopressin 1b Receptor Gene Impairs the Attack Component of Aggressive Behavior in Mice. Genes Brain Behav. 2007, 6, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Misri, S.; Reebye, P.; Kendrick, K.; Carter, D.; Ryan, D.; Grunau, R.E.; Oberlander, T.F. Internalizing Behaviors in 4-Year-Old Children Exposed in Utero to Psychotropic Medications. Am. J. Psychiatry 2006, 163, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Papsdorf, M.; Brain, U.M.; Misri, S.; Ross, C.; Grunau, R.E. Prenatal Effects of Selective Serotonin Reuptake Inhibitor Antidepressants, Serotonin Transporter Promoter Genotype (SLC6A4), and Maternal Mood on Child Behavior at 3 Years of Age. Arch. Pediatr. Adolesc. Med. 2010, 164, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Reebye, P.; Misri, S.; Papsdorf, M.; Kim, J.; Grunau, R.E. Externalizing and Attentional Behaviors in Children of Depressed Mothers Treated with a Selective Serotonin Reuptake Inhibitor Antidepressant during Pregnancy. Arch. Pediatr. Adolesc. Med. 2007, 161, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.J.R. The Neurobiology of Impulsive Aggression. J. Child Adolesc. Psychopharmacol. 2016, 26, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, T.; del Arco, A.; Karlsgodt, K.H. White Matter Integrity in the Fronto-Striatal Accumbofrontal Tract Predicts Impulsivity. Brain Imaging Behav. 2018, 12, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Antontseva, E.; Bondar, N.; Reshetnikov, V.; Merkulova, T. The Effects of Chronic Stress on Brain Myelination in Humans and in Various Rodent Models. Neuroscience 2020, 441, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhou, Y.; Hu, Z.; Lou, J.; Song, W.; Li, J.; Liang, X.; Chen, C.; Wang, S.; Yang, B.; et al. 24-Hour-Restraint Stress Induces Long-Term Depressive-like Phenotypes in Mice. Sci. Rep. 2016, 6, 32935. [Google Scholar] [CrossRef] [PubMed]
- Ibi, D.; Takuma, K.; Koike, H.; Mizoguchi, H.; Tsuritani, K.; Kuwahara, Y.; Kamei, H.; Nagai, T.; Yoneda, Y.; Nabeshima, T.; et al. Social Isolation Rearing-Induced Impairment of the Hippocampal Neurogenesis Is Associated with Deficits in Spatial Memory and Emotion-Related Behaviors in Juvenile Mice. J. Neurochem. 2008, 105, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, M.; Ikawa, D.; Yamamuro, K.; Yamashita, Y.; Toritsuka, M.; Kimoto, S.; Yamauchi, T.; Okumura, K.; Komori, T.; Fukami, S.; et al. Effects of the Mode of Re-Socialization after Juvenile Social Isolation on Medial Prefrontal Cortex Myelination and Function. Sci. Rep. 2017, 7, 5481. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.A.; Berndt, J.A.; Hudson, L.D.; Armstrong, R.C. Myelin Transcription Factor 1 (Myt1) Modulates the Proliferation and Differentiation of Oligodendrocyte Lineage Cells. Mol. Cell. Neurosci. 2004, 25, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Dreyer, J.L. Viral-Mediated Overexpression of the Myelin Transcription Factor 1 (MyT1) in the Dentate Gyrus Attenuates Anxiety- and Ethanol-Related Behaviors in Rats. Psychopharmacology 2017, 234, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sakurai, T.; Davis, K.L.; Buxbaum, J.D. Linking Oligodendrocyte and Myelin Dysfunction to Neurocircuitry Abnormalities in Schizophrenia. Prog. Neurobiol. 2011, 93, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P. Editorial: Can Dysregulated Myelination Be Linked to ADHD Pathogenesis and Persistence? J. Child Psychol. Psychiatry Allied Discip. 2019, 60, 229–231. [Google Scholar] [CrossRef]
- Waider, J.; Popp, S.; Mlinar, B.; Montalbano, A.; Bonfiglio, F.; Aboagye, B.; Thuy, E.; Kern, R.; Thiel, C.; Araragi, N.; et al. Serotonin Deficiency Increases Context-Dependent Fear Learning Through Modulation of Hippocampal Activity. Front. Neurosci. 2019, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Thome, J.; Pesold, B.; Baader, M.; Hu, M.; Gewirtz, J.C.; Duman, R.S.; Henn, F.A. Stress Differentially Regulates Synaptophysin and Synaptotagmin Expression in Hippocampus. Biol. Psychiatry 2001, 50, 809–812. [Google Scholar] [CrossRef]
- Xu, H.; He, J.; Richardson, J.S.; Li, X.M. The Response of Synaptophysin and Microtubule-Associated Protein 1 to Restraint Stress in Rat Hippocampus and Its Modulation by Venlafaxine. J. Neurochem. 2004, 91, 1380–1388. [Google Scholar] [CrossRef]
- Gammie, S.C.; Nelson, R.J. CFOS and PCREB Activation and Maternal Aggression in Mice. Brain Res. 2001, 898, 232–241. [Google Scholar] [CrossRef]
- Jasnow, A.M.; Shi, C.; Israel, J.E.; Davis, M.; Huhman, K.L. Memory of Social Defeat Is Facilitated by CAMP Response Element-Binding Protein Overexpression in the Amygdala. Behav. Neurosci. 2005, 119, 1125–1130. [Google Scholar] [CrossRef]
- Sen, T.; Gupta, R.; Kaiser, H.; Sen, N. Activation of PERK Elicits Memory Impairment through Inactivation of CREB and Downregulation of PSD95 After Traumatic Brain Injury. J. Neurosci. 2017, 37, 5900–5911. [Google Scholar] [CrossRef]
- Esvald, E.E.; Tuvikene, J.; Sirp, A.; Patil, S.; Bramham, C.R.; Timmusk, T. CREB Family Transcription Factors Are Major Mediators of BDNF Transcriptional Autoregulation in Cortical Neurons. J. Neurosci. 2020, 40, 1405–1426. [Google Scholar] [CrossRef] [PubMed]
- Ozdamar Unal, G.; Asci, H.; Erzurumlu, Y.; Ilhan, I.; Hasseyid, N.; Ozmen, O. Dexpanthenol May Protect the Brain against Lipopolysaccharide Induced Neuroinflammation via Anti-Oxidant Action and Regulating CREB/BDNF Signaling. Immunopharmacol. Immunotoxicol. 2022, 44, 186–193. [Google Scholar] [CrossRef]
- Been, L.E.; Moore, K.M.; Kennedy, B.C.; Meisel, R.L. Metabotropic Glutamate Receptor and Fragile x Signaling in a Female Model of Escalated Aggression. Biol. Psychiatry 2016, 79, 685–692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dohare, P.; Cheng, B.; Ahmed, E.; Yadala, V.; Singla, P.; Thomas, S.; Kayton, R.; Ungvari, Z.; Ballabh, P. Glycogen Synthase Kinase-3β Inhibition Enhances Myelination in Preterm Newborns with Intraventricular Hemorrhage, but Not Recombinant Wnt3A. Neurobiol. Dis. 2018, 118, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Butt, A.M. GSK3β Negatively Regulates Oligodendrocyte Differentiation and Myelination in Vivo. Glia 2011, 59, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Zhang, X.; Rodriguiz, R.M.; Sotnikova, T.D.; Cools, M.J.; Wetsel, W.C.; Gainetdinov, R.R.; Caron, M.G. Role of GSK3β in Behavioral Abnormalities Induced by Serotonin Deficiency. Proc. Natl. Acad. Sci. USA 2008, 105, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Graeff, F.G.; Guimarães, F.S.; De Andrade, T.G.C.S.; Deakin, J.F.W. Role of 5-HT in Stress, Anxiety, and Depression. Pharmacol. Biochem. Behav. 1996, 54, 129–141. [Google Scholar] [CrossRef]
- Albert, P.R. Transcriptional Regulation of the 5-HT1A Receptor: Implications for Mental Illness. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2402–2415. [Google Scholar] [CrossRef] [PubMed]
- Marchisella, F.; Paladini, M.S.; Guidi, A.; Begni, V.; Brivio, P.; Spero, V.; Calabrese, F.; Molteni, R.; Riva, M.A. Chronic Treatment with the Antipsychotic Drug Blonanserin Modulates the Responsiveness to Acute Stress with Anatomical Selectivity. Psychopharmacology 2020, 237, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Jang, Y.C.; Hwang, D.J.; Um, H.S.; Lee, N.H.; Jung, J.H.; Cho, J.Y. Treadmill Exercise Produces Neuroprotective Effects in a Murine Model of Parkinson’s Disease by Regulating the TLR2/MyD88/NF-ΚB Signaling Pathway. Neuroscience 2017, 356, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Parise, E.M.; Parise, L.F.; Sial, O.K.; Cardona-Acosta, A.M.; Gyles, T.M.; Juarez, B.; Chaudhury, D.; Han, M.H.; Nestler, E.J.; Bolaños-Guzmán, C.A. The Resilient Phenotype Induced by Prophylactic Ketamine Exposure During Adolescence Is Mediated by the Ventral Tegmental Area–Nucleus Accumbens Pathway. Biol. Psychiatry 2021, 90, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.J.; Smith, M.; Ramakrishnan, A.; Cates, H.M.; Bagot, R.C.; Kronman, H.G.; Patel, B.; Chang, A.B.; Purushothaman, I.; Dudley, J.; et al. Early Life Stress Alters Transcriptomic Patterning across Reward Circuitry in Male and Female Mice. Nat. Commun. 2019, 10, 5098. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Du, S.; Lei, T.; Xie, X.; Wang, Y. Glycogen Synthase Kinase 3β in Tumorigenesis and Oncotherapy (Review). Oncol. Rep. 2020, 44, 2373–2385. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svirin, E.; Veniaminova, E.; Costa-Nunes, J.P.; Gorlova, A.; Umriukhin, A.; Kalueff, A.V.; Proshin, A.; Anthony, D.C.; Nedorubov, A.; Tse, A.C.K.; et al. Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes. Cells 2022, 11, 1036. https://doi.org/10.3390/cells11061036
Svirin E, Veniaminova E, Costa-Nunes JP, Gorlova A, Umriukhin A, Kalueff AV, Proshin A, Anthony DC, Nedorubov A, Tse ACK, et al. Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes. Cells. 2022; 11(6):1036. https://doi.org/10.3390/cells11061036
Chicago/Turabian StyleSvirin, Evgeniy, Ekaterina Veniaminova, João Pedro Costa-Nunes, Anna Gorlova, Aleksei Umriukhin, Allan V. Kalueff, Andrey Proshin, Daniel C. Anthony, Andrey Nedorubov, Anna Chung Kwan Tse, and et al. 2022. "Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes" Cells 11, no. 6: 1036. https://doi.org/10.3390/cells11061036
APA StyleSvirin, E., Veniaminova, E., Costa-Nunes, J. P., Gorlova, A., Umriukhin, A., Kalueff, A. V., Proshin, A., Anthony, D. C., Nedorubov, A., Tse, A. C. K., Walitza, S., Lim, L. W., Lesch, K.-P., & Strekalova, T. (2022). Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes. Cells, 11(6), 1036. https://doi.org/10.3390/cells11061036






