Activation of Calpain Contributes to Mechanical Ventilation-Induced Depression of Protein Synthesis in Diaphragm Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Institutional Approval
2.2. Experimental Design
2.3. Experimental Protocol
2.3.1. Surgical Protocol for AAV-CAST Administration
2.3.2. Acutely Anesthetized Control Animals
2.3.3. Mechanical Ventilation
2.3.4. Western Blot
2.3.5. Measurement of In Vivo Protein Synthesis via the SUnSET Technique
2.4. Statistical Analysis
3. Results
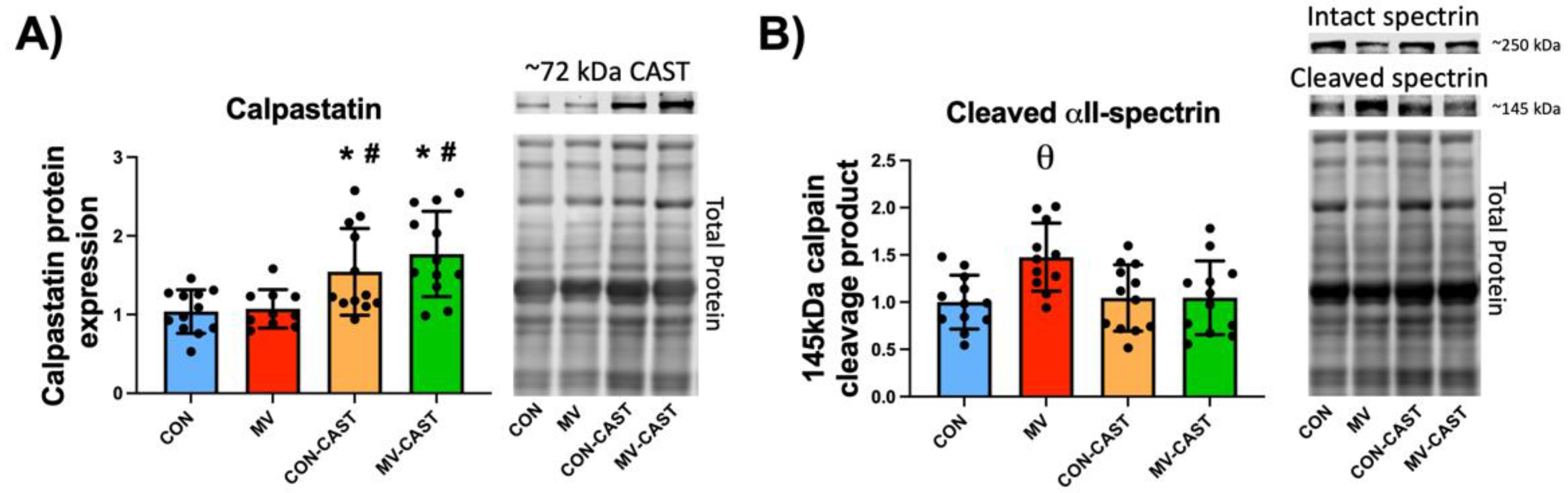
3.1. Calpastatin Overexpression Prevents MV-Induced Calpain Activation in Diaphragm Muscle
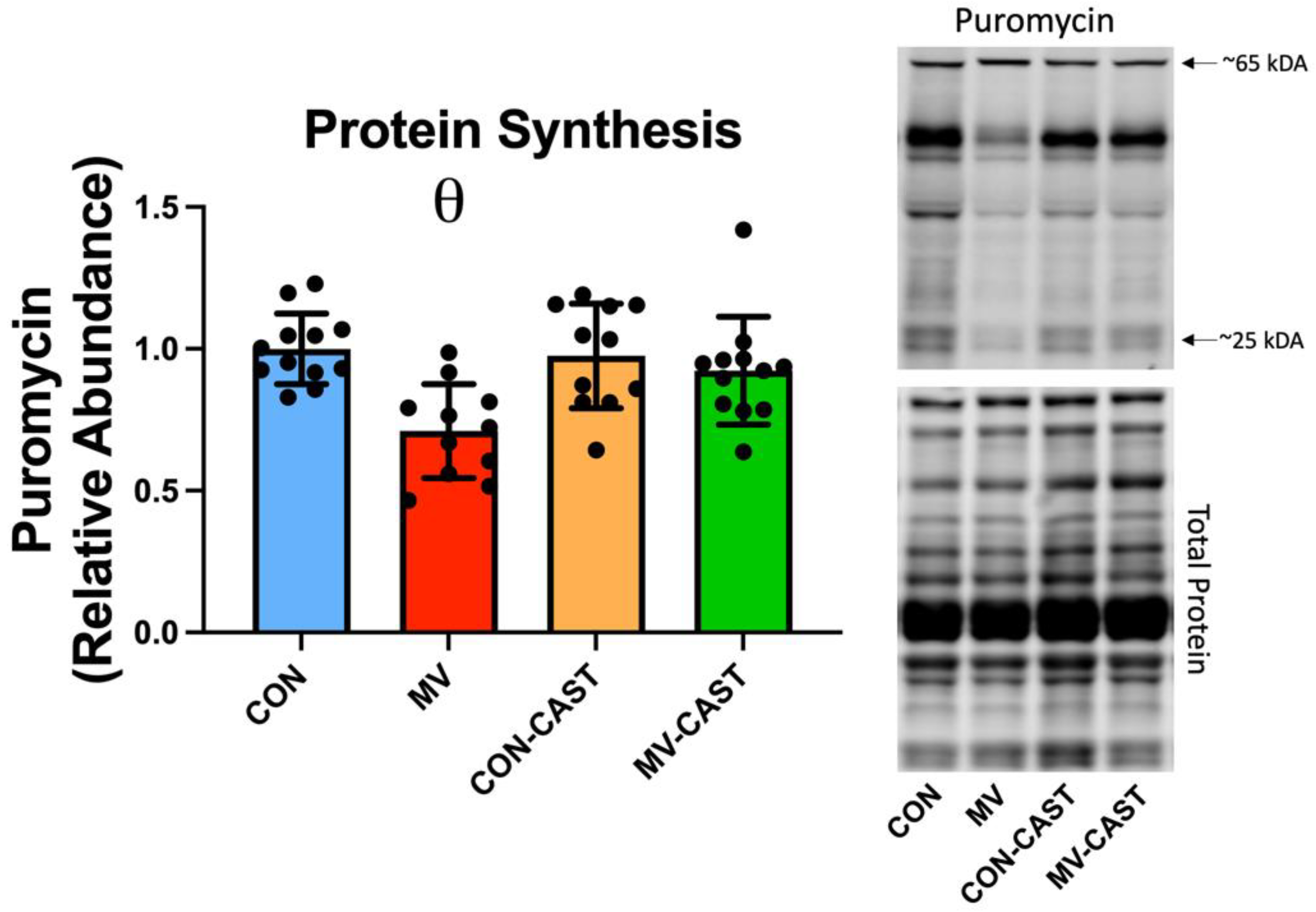
3.2. Prevention of Calpain Activation in the Diaphragm Averted the MV-Induced Depression of Protein Synthesis
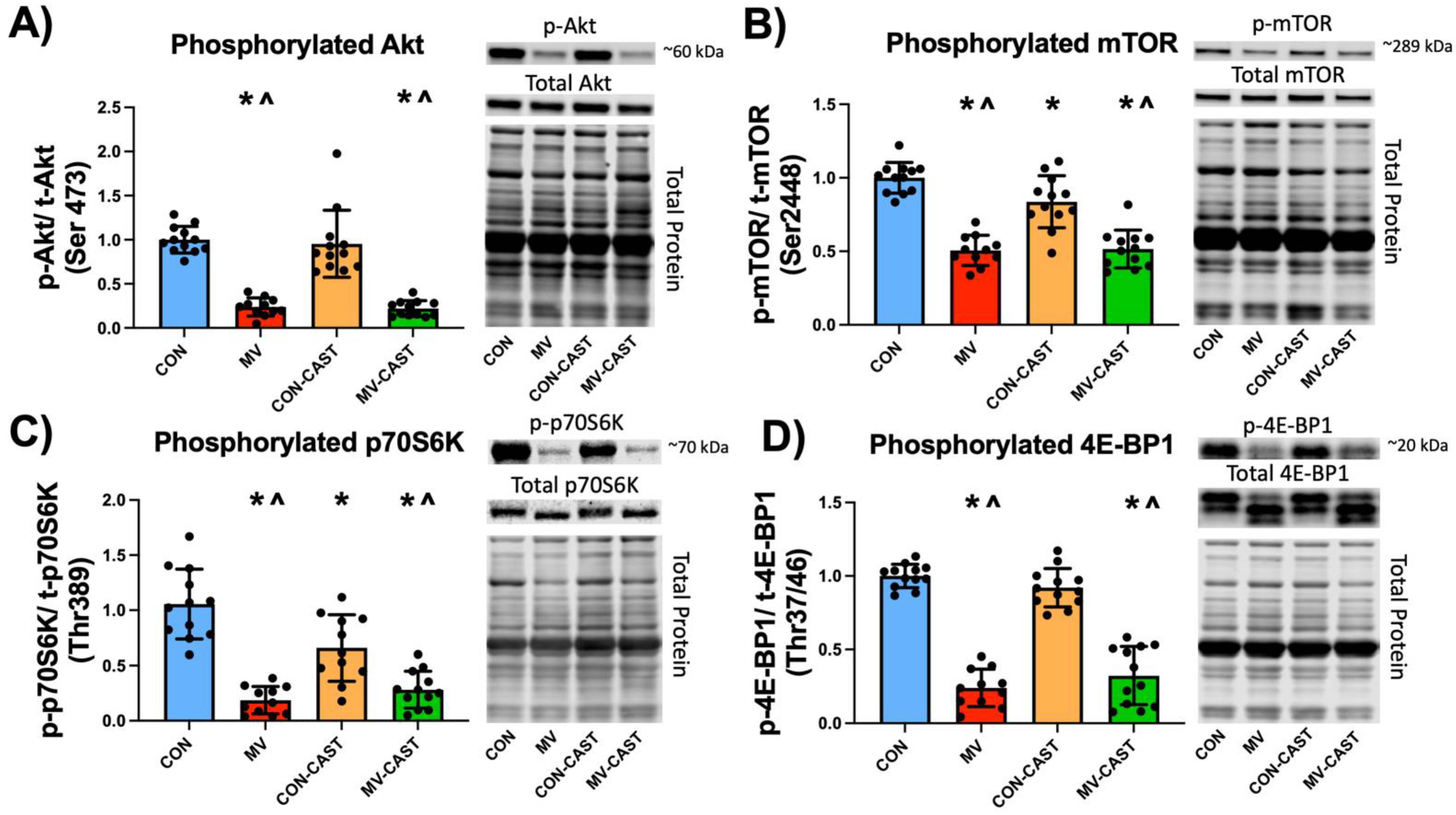
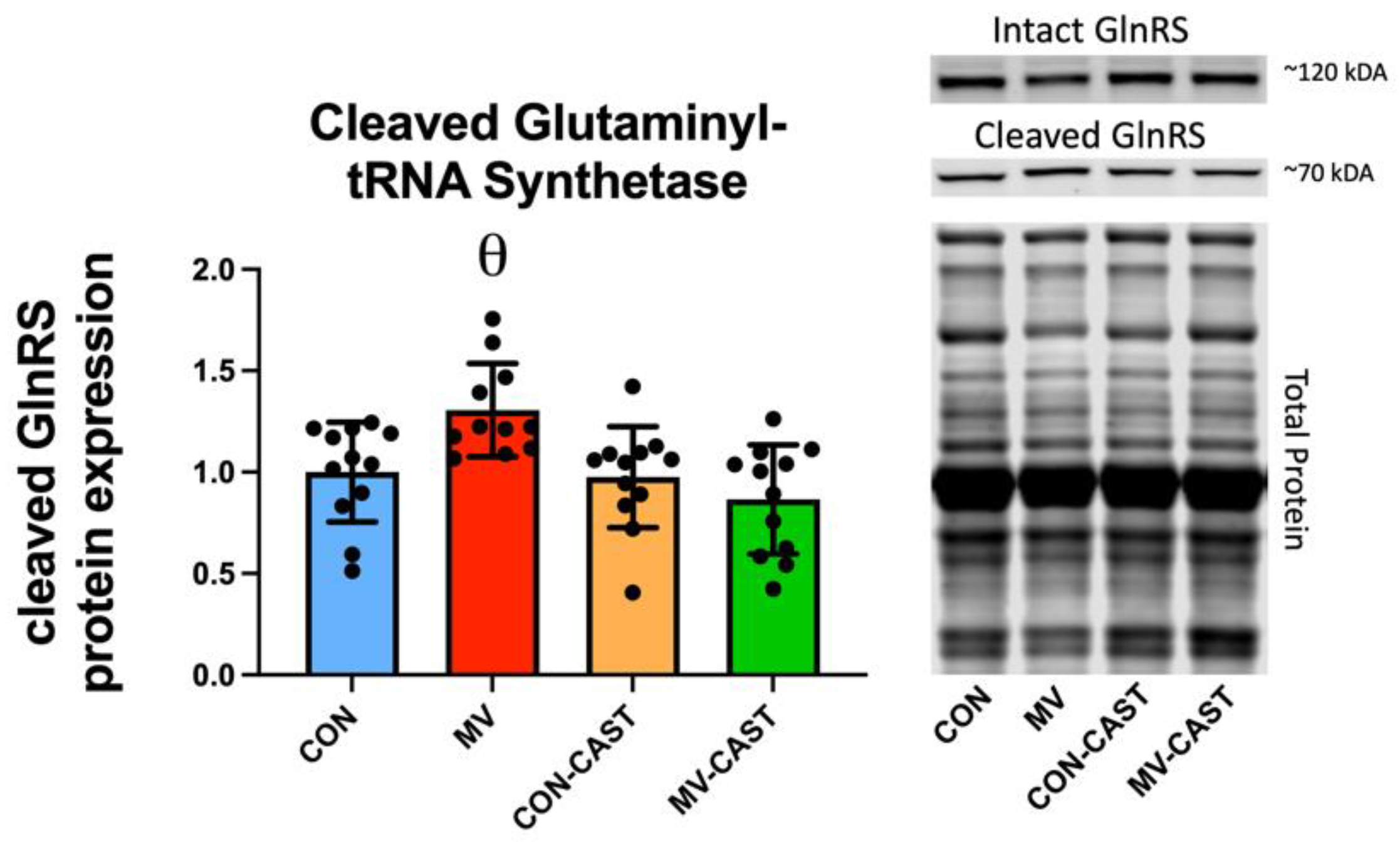
3.3. Prevention of MV-Induced Calpain Activation in the Diaphragm Does Not Prevent the Down-Regulation of Anabolic Signaling
4. Discussion
4.1. Summary of Experimental Results
4.2. Critique of Experimental Approach
4.3. Calpains Play an Essential Role in MV-Induced Depression of Protein Synthesis in Diaphragm Muscle
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vassilakopoulos, T.; Petrof, B.J. Ventilator-induced Diaphragmatic Dysfunction. Am. J. Respir. Crit. Care Med. 2004, 169, 336–341. [Google Scholar] [CrossRef]
- Dres, M.; Demoule, A. Diaphragm dysfunction during weaning from mechanical ventilation: An underestimated phenomenon with clinical implications. Crit. Care 2018, 22, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hermans, G.; Agten, A.; Testelmans, D.; Decramer, M.; Gayan-Ramirez, G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: A prospective observational study. Crit. Care 2010, 14, R127. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.Y.; Suh, H.J.; Hong, S.-B.; Koh, Y.; Lim, C.-M. Diaphragm dysfunction assessed by ultrasonography: Influence on weaning from mechanical ventilation*. Crit. Care Med. 2011, 39, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Funk, G.-C.; Anders, S.; Breyer, M.-K.; Burghuber, O.C.; Edelmann, G.; Heindl, W.; Hinterholzer, G.; Kohansal, R.; Schuster, R.; Schwarzmaier-D’Assie, A.; et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur. Respir. J. 2009, 35, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnelier, A.; Tonnelier, J.M.; Nowak, E.; Gut-Gobert, C.; Prat, G.; Renault, A.; Boles, J.M.; L’Her, E. Clinical relevance of classification according to weaning difficulty. Respir Care 2011, 56, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Béduneau, G.; Pham, T.; Schortgen, F.; Piquilloud, L.; Zogheib, E.; Jonas, M.; Grelon, F.; Runge, I.; Terzi, N.; Grangé, S.; et al. Epidemiology of Weaning Outcome according to a New Definition. The WIND Study. Am. J. Respir. Crit. Care Med. 2017, 195, 772–783. [Google Scholar] [CrossRef]
- Powers, S.K.; Wiggs, M.P.; Sollanek, K.J.; Smuder, A.J. Ventilator-induced diaphragm dysfunction: Cause and effect. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R464–R477. [Google Scholar] [CrossRef] [Green Version]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid Disuse Atrophy of Diaphragm Fibers in Mechanically Ventilated Humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Nelson, W.B.; Smuder, A.J.; Hudson, M.; Talbert, E.; Powers, S.K. Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit. Care Med. 2012, 40, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Smuder, P.A.J.; Nelson, P.W.B.; Hudson, P.M.B.; Kavazis, P.A.N.; Powers, P.S.K. Inhibition of the Ubiquitin–Proteasome Pathway Does Not Protect against Ventilator-induced Accelerated Proteolysis or Atrophy in the Diaphragm. Anesthesiology 2014, 121, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smuder, A.J.; Sollanek, K.J.; Nelson, W.B.; Min, K.; Talbert, E.E.; Kavazis, A.N.; Hudson, M.B.; Sandri, M.; Szeto, H.H.; Powers, S.K. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free. Radic. Biol. Med. 2018, 115, 179–190. [Google Scholar] [CrossRef]
- Whidden, M.A.; Smuder, A.J.; Wu, M.; Hudson, M.; Nelson, W.B.; Powers, S.K. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J. Appl. Physiol. 2010, 108, 1376–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyatt, H.W.; Ozdemir, M.; Yoshihara, T.; Nguyen, B.L.; Deminice, R.; Powers, S.K. Calpains play an essential role in mechanical ventilation-induced diaphragmatic weakness and mitochondrial dysfunction. Redox Biol. 2020, 38, 101802. [Google Scholar] [CrossRef] [PubMed]
- Maes, K.; Testelmans, D.; Powers, S.; Decramer, M.; Gayan-Ramirez, G. Leupeptin Inhibits Ventilator-induced Diaphragm Dysfunction in Rats. Am. J. Respir. Crit. Care Med. 2007, 175, 1134–1138. [Google Scholar] [CrossRef]
- Smith, I.J.; Dodd, S.L. Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signalling pathway in rat diaphragm muscle. Exp. Physiol. 2007, 92, 561–573. [Google Scholar] [CrossRef]
- Smith, I.J.; Lecker, S.H.; Hasselgren, P.-O. Calpain activity and muscle wasting in sepsis. Am. J. Physiol. Metab. 2008, 295, E762–E771. [Google Scholar] [CrossRef] [Green Version]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The Calpain System. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Smuder, A.J.; Falk, D.J.; Sollanek, K.J.; Nelson, W.B.; Powers, S.K. Delivery of Recombinant Adeno-Associated Virus Vectors to Rat Diaphragm Muscle via Direct Intramuscular Injection. Hum. Gene Ther. Methods 2013, 24, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.A.; Hornberger, T.A. Measuring protein synthesis with SUnSET: A valid alternative to traditional techniques? Exerc Sport Sci Rev. 2013, 41, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-X.; Jeromin, A. Spectrin Breakdown Products (SBDPs) as Potential Biomarkers for Neurodegenerative Diseases. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2012, 1, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.-S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, H.-Y.; Zhou, X.-L.; Ruan, Z.-R.; Sun, W.-C.; Eriani, G.; Wang, E.-D. Calpain Cleaves Most Components in the Multiple Aminoacyl-tRNA Synthetase Complex and Affects Their Functions. J. Biol. Chem. 2015, 290, 26314–26327. [Google Scholar] [CrossRef] [Green Version]
- Kwon, N.H.; Fox, P.L.; Kim, S. Aminoacyl-tRNA synthetases as therapeutic targets. Nat. Rev. Drug Discov. 2019, 18, 629–650. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Zergeroglu, A.M.; Lennon, S.L.; Sugiura, T.; Yimlamai, T.; Enns, D.; Belcastro, A.; Powers, S.K. Mechanical Ventilation–induced Diaphragmatic Atrophy Is Associated with Oxidative Injury and Increased Proteolytic Activity. Am. J. Respir. Crit. Care Med. 2002, 166, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Agten, A.; Maes, K.; Smuder, A.; Powers, S.K.; Decramer, M.; Gayan-Ramirez, G. N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation*. Crit. Care Med. 2011, 39, 777–782. [Google Scholar] [CrossRef]
- Ono, Y.; Saido, T.C.; Sorimachi, H. Calpain research for drug discovery: Challenges and potential. Nat. Rev. Drug Discov. 2016, 15, 854–876. [Google Scholar] [CrossRef]
- Wendt, A.; Thompson, V.; Goll, D. Interaction of calpastatin with calpain: A review. Biol. Chem. 2004, 385, 465–472. [Google Scholar] [CrossRef]
- Antonellis, A.; Green, E.D. The Role of Aminoacyl-tRNA Synthetases in Genetic Diseases. Annu. Rev. Genom. Hum. Genet. 2008, 9, 87–107. [Google Scholar] [CrossRef]
- Furuno, K.; Goldberg, A.L.; Hasselgren, P.O.; Zamir, O.; James, J.H.; Fischer, J.E.; Hall-Angerås, M.; Angerås, U.; Benson, D.; Ballard, F.J.; et al. The activation of protein degradation in muscle by Ca2+ or muscle injury does not involve a lysosomal mechanism. Biochem. J. 1986, 237, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Hyatt, H.W.; Powers, S.K. The Role of Calpains in Skeletal Muscle Remodeling with Exercise and Inactivity-induced Atrophy. Endoscopy 2020, 41, 994–1008. [Google Scholar] [CrossRef]
- Hyatt, H.; Deminice, R.; Yoshihara, T.; Powers, S.K. Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: A review of the causes and effects. Arch. Biochem. Biophys. 2018, 662, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Shenton, D.; Smirnova, J.B.; Selley, J.N.; Carroll, K.; Hubbard, S.J.; Pavitt, G.D.; Ashe, M.P.; Grant, C.M. Global Translational Responses to Oxidative Stress Impact upon Multiple Levels of Protein Synthesis. J. Biol. Chem. 2006, 281, 29011–29021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyatt, H.W.; Powers, S.K. Disturbances in Calcium Homeostasis Promotes Skeletal Muscle Atrophy: Lessons from Ventilator-Induced Diaphragm Wasting. Front. Physiol. 2020, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Kakinuma, K.; Torii, F.; Irie, A.; Nakagawa, K.; Labeit, S.; Abe, K.; Suzuki, K.; Sorimachi, H. Possible Regulation of the Conventional Calpain System by Skeletal Muscle-specific Calpain, p94/Calpain 3. J. Biol. Chem. 2004, 279, 2761–2771. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyatt, H.W.; Ozdemir, M.; Bomkamp, M.P.; Powers, S.K. Activation of Calpain Contributes to Mechanical Ventilation-Induced Depression of Protein Synthesis in Diaphragm Muscle. Cells 2022, 11, 1028. https://doi.org/10.3390/cells11061028
Hyatt HW, Ozdemir M, Bomkamp MP, Powers SK. Activation of Calpain Contributes to Mechanical Ventilation-Induced Depression of Protein Synthesis in Diaphragm Muscle. Cells. 2022; 11(6):1028. https://doi.org/10.3390/cells11061028
Chicago/Turabian StyleHyatt, Hayden W., Mustafa Ozdemir, Matthew P. Bomkamp, and Scott K. Powers. 2022. "Activation of Calpain Contributes to Mechanical Ventilation-Induced Depression of Protein Synthesis in Diaphragm Muscle" Cells 11, no. 6: 1028. https://doi.org/10.3390/cells11061028
APA StyleHyatt, H. W., Ozdemir, M., Bomkamp, M. P., & Powers, S. K. (2022). Activation of Calpain Contributes to Mechanical Ventilation-Induced Depression of Protein Synthesis in Diaphragm Muscle. Cells, 11(6), 1028. https://doi.org/10.3390/cells11061028





