Pilot Study of the Effects of Chronic Intracerebroventricular Infusion of Human Anti-IgLON5 Disease Antibodies in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibody Purification from Patients with Anti-IgLON5 Disease and Control Subjects
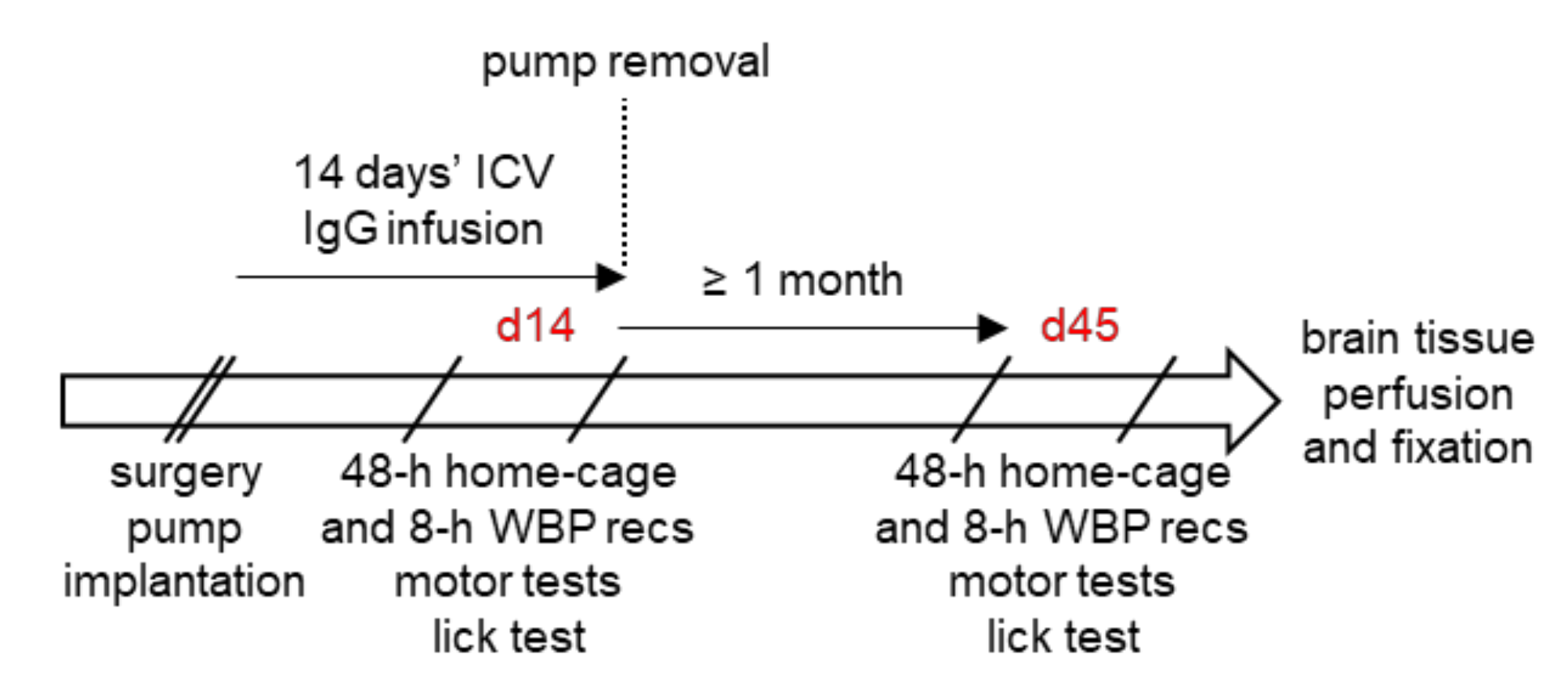
2.3. Overview of the Experimental Protocol
2.4. Surgery and ICV Antibody Infusion
2.5. Neuropathological Examination
2.6. Assessment of Daily EMG Activity Profiles
2.7. Assessment of Sleep Phenotype
2.8. Assessment of Sleep-Related Breathing Phenotype
2.9. Assessment of Ataxia and Gait Instability
2.10. Assessment of Licking Behavior
2.11. Statistical Analysis
3. Results
3.1. Specificity of the Purified Antibodies
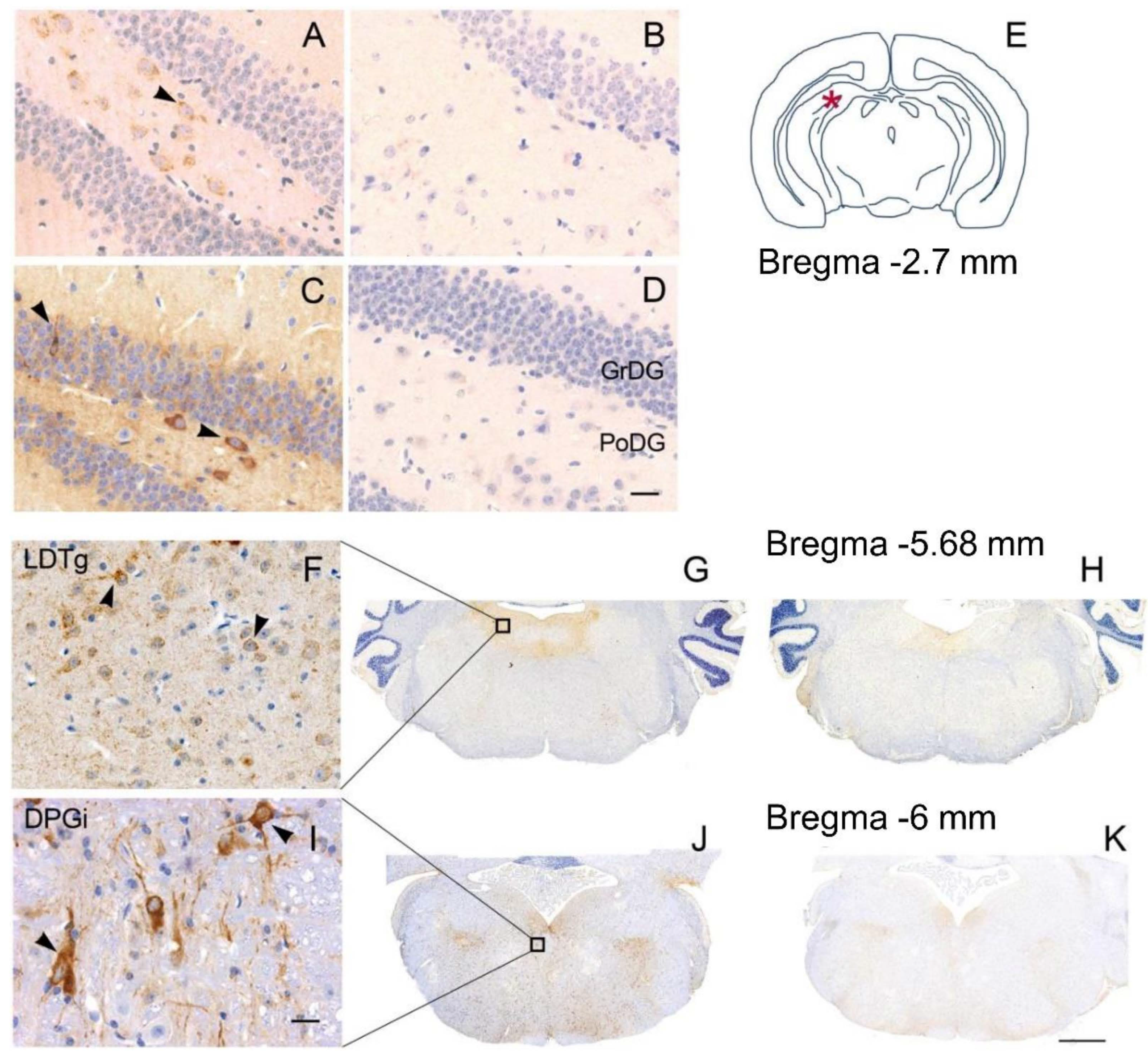
3.2. Neuropathology
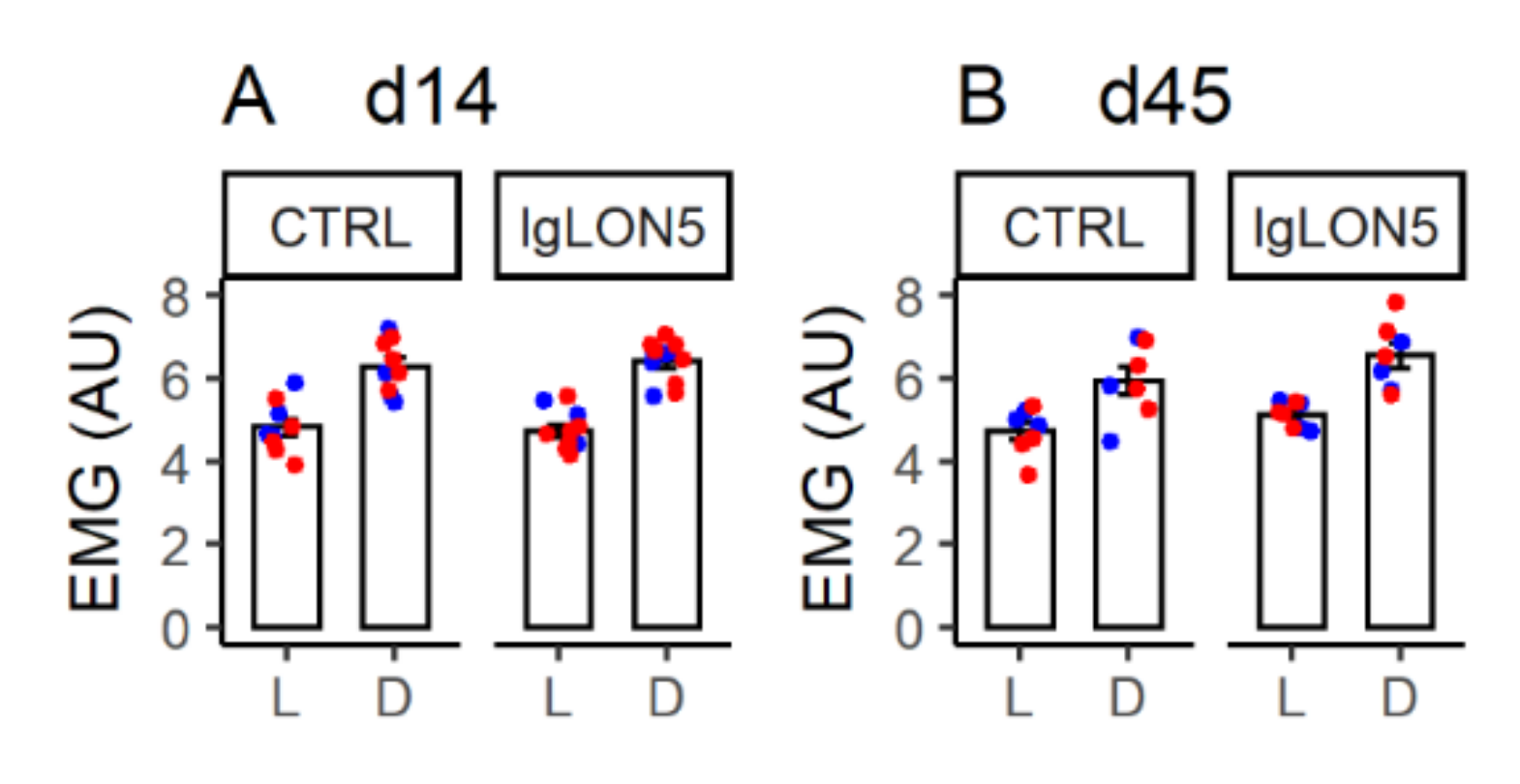
3.3. Daily EMG Activity Profiles
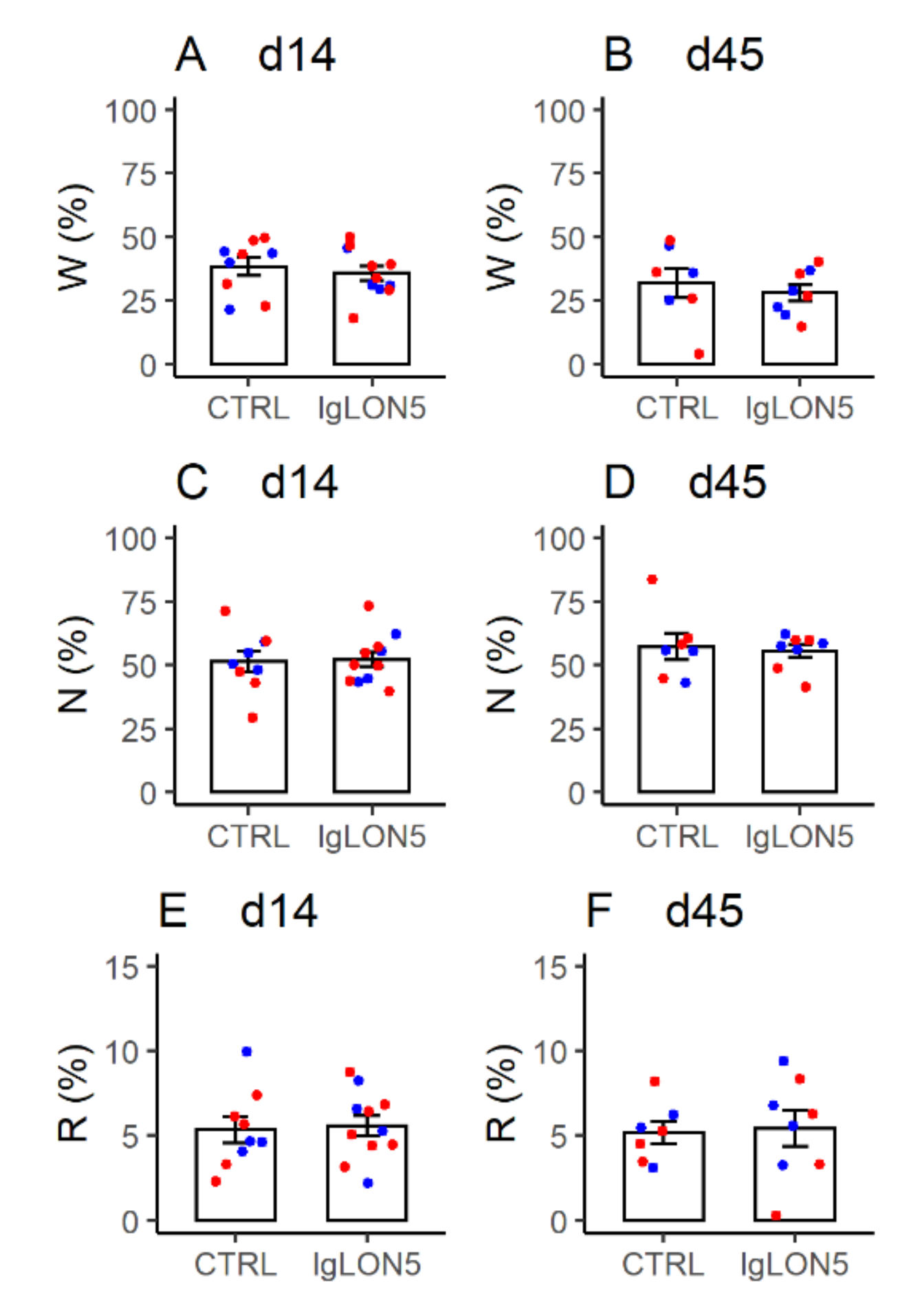
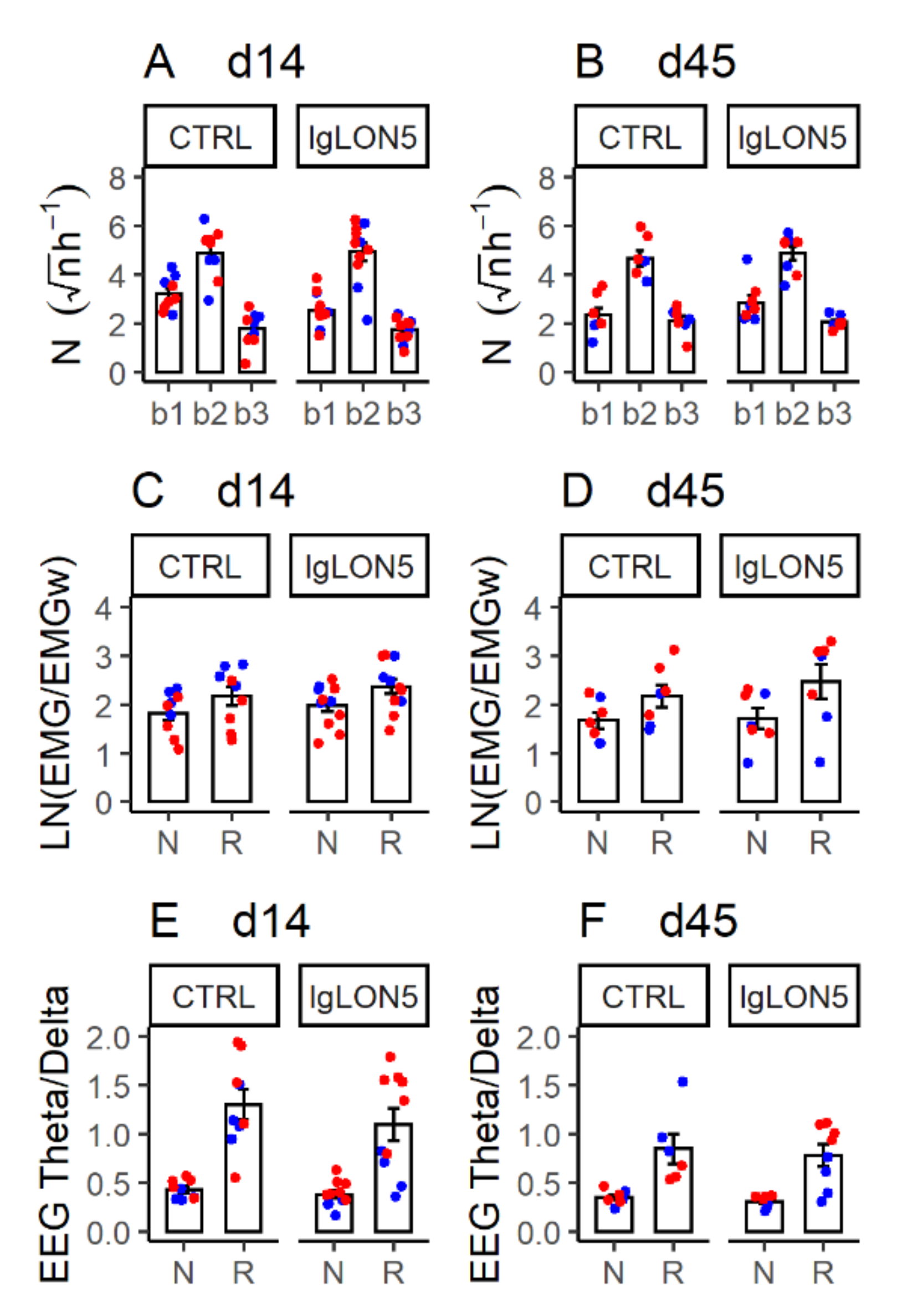
3.4. Sleep Phenotype
3.5. Sleep-Related Breathing Phenotype
3.6. Ataxia and Gait Instability
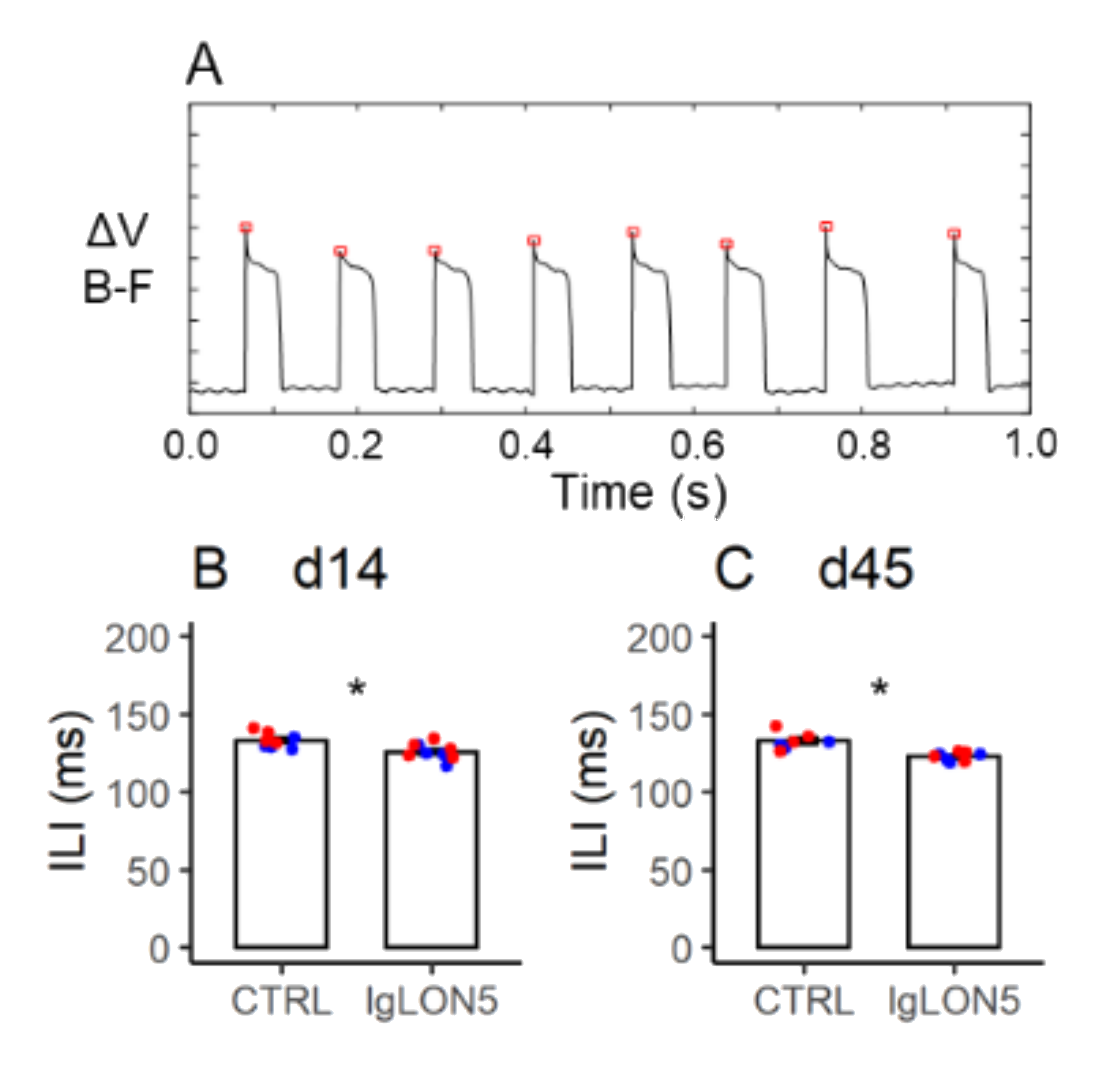
3.7. Licking Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ♀ hTau and WT d14 and d45 Total N = 15 | ♀ hTau and WT d14 Total N = 20 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.192 | 0.902 | 0.854 |
| Mouse Strain (hTau vs. WT) | 0.855 | 0.988 | N/A |
| Ab x Strain | 0.945 | 0.911 | N/A |
| LD period (light vs. dark) | <0.001 * | <0.001 * | <0.001 * |
| Ab x LD | 0.161 | 0.558 | 0.783 |
| Strain x LD | 0.174 | 0.107 | N/A |
| Ab x Strain x LD | 0.352 | 0.557 | N/A |
| Time (d14 vs. d45) | 0.308 | N/A | 0.428 |
| Ab x Time | 0.076 | N/A | 0.608 |
| Strain x Time | 0.502 | N/A | N/A |
| Ab x Strain x Time | 0.572 | N/A | N/A |
| Time x LD | 0.735 | N/A | 0.533 |
| Ab x Time x LD | 0.682 | N/A | 0.391 |
| Time x LD x Strain | 0.419 | N/A | N/A |
| Ab x Time x LD x Strain | 0.990 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 15 | ♀ hTau and WT d14 Total N = 20 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.434 | 0.571 | 0.281 |
| Mouse Strain (hTau vs. WT) | 0.756 | 0.669 | N/A |
| Ab x Strain | 0.235 | 0.979 | N/A |
| Time (d14 vs. d45) | 0.052 | N/A | 0.849 |
| Ab x Time | 0.838 | N/A | 0.855 |
| Strain x Time | 0.794 | N/A | N/A |
| Ab x Strain x Time | 0.912 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 15 | ♀ hTau and WT d14 Total N = 20 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.808 | 0.933 | 0.363 |
| Mouse Strain (hTau vs. WT) | 0.915 | 0.867 | N/A |
| Ab x Strain | 0.330 | 0.684 | N/A |
| Time (d14 vs. d45) | 0.070 | N/A | 0.999 |
| Ab x Time | 0.946 | N/A | 0.871 |
| Strain x Time | 0.573 | N/A | N/A |
| Ab x Strain x Time | 0.238 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 15 | ♀ hTau and WT d14 Total N = 20 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.690 | 0.861 | 0.995 |
| Mouse Strain (hTau vs. WT) | 0.614 | 0.683 | N/A |
| Ab x Strain | 0.412 | 0.683 | N/A |
| Time (d14 vs. d45) | 0.536 | N/A | 0.065 |
| Ab x Time | 0.851 | N/A | 0.446 |
| Strain x Time | 0.854 | N/A | N/A |
| Ab x Strain x Time | 0.743 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 15 | ♀ hTau and WT d14 Total N = 20 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.704 | 0.180 | 0.553 |
| Mouse Strain (hTau vs. WT) | 0.043 * | 0.57 | N/A |
| Ab x Strain | 0.756 | 0.133 | N/A |
| Dur (4–8 s vs. 12–120 s vs. > 120 s) | <0.001 * | <0.001 * | <0.001 * |
| Ab x Dur | 0.430 | 0.300 | 0.556 |
| Strain x Dur | 0.078 | 0.168 | N/A |
| Ab x Strain x Dur | 0.816 | 0.649 | N/A |
| Time (d14 vs. d45) | 0.977 | N/A | 0.871 |
| Ab x Time | 0.278 | N/A | 0.746 |
| Strain x Time | 0.939 | N/A | N/A |
| Ab x Strain x Time | 0.140 | N/A | N/A |
| Time x Dur | 0.215 | N/A | 0.486 |
| Ab x Time x Dur | 0.417 | N/A | 0.596 |
| Time x Dur x Strain | 0.264 | N/A | N/A |
| Ab x Time x Dur x Strain | 0.322 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 15 | ♀ hTau and WT d14 Total N = 20 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.848 | 0.378 | 0.458 |
| Mouse Strain (hTau vs. WT) | 0.415 | 0.024 * | N/A |
| Ab x Strain | 0.341 | 0.344 | N/A |
| SS (NREM sleep vs. REM sleep) | <0.001 * | <0.001 * | 0.005 * |
| Ab x SS | 0.326 | 0.841 | 0.682 |
| Strain x SS | 0.029 * | 0.179 | N/A |
| Ab x Strain x SS | 0.145 | 0.023 * | N/A |
| Time (d14 vs. d45) | 0.133 | N/A | 0.205 |
| Ab x Time | 0.552 | N/A | 0.589 |
| Strain x Time | 0.007 * | N/A | N/A |
| Ab x Strain x Time | 0.663 | N/A | N/A |
| Time x SS | 0.019 * | N/A | 0.169 |
| Ab x Time x SS | 0.352 | N/A | 0.186 |
| Time x SS x Strain | 0.001 * | N/A | N/A |
| Ab x Time x SS x Strain | 0.903 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 15 | ♀ hTau and WT d14 Total N = 20 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.293 | 0.194 | 0.934 |
| Mouse Strain (hTau vs. WT) | 0.046 * | 0.004 * | N/A |
| Ab x Strain | 0.132 | 0.098 | N/A |
| SS (NREM sleep vs. REM sleep) | <0.001 * | <0.001 * | 0.001 * |
| Ab x SS | 0.330 | 0.331 | 0.947 |
| Strain x SS | 0.137 | 0.015 * | N/A |
| Ab x Strain x SS | 0.142 | 0.062 | N/A |
| Time (d14 vs. d45) | 0.036 * | N/A | 0.018 * |
| Ab x Time | 0.595 | N/A | 0.753 |
| Strain x Time | 0.104 | N/A | N/A |
| Ab x Strain x Time | 0.506 | N/A | N/A |
| Time x SS | 0.117 | N/A | 0.063 |
| Ab x Time x SS | 0.535 | N/A | 0.460 |
| Time x SS x Strain | 0.249 | N/A | N/A |
| Ab x Time x SS x Strain | 0.642 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 13 | ♀ hTau and WT d14 Total N = 19 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.665 | 0.337 | 0.307 |
| Mouse Strain (hTau vs. WT) | 0.169 | 0.019 * | N/A |
| Ab x Strain | 0.345 | 0.412 | N/A |
| SS (NREM sleep vs. REM sleep) | 0.431 | 0.065 | 0.002 * |
| Ab x SS | 0.384 | 0.542 | 0.354 |
| Strain x SS | 0.551 | 0.678 | N/A |
| Ab x Strain x SS | 0.798 | 0.382 | N/A |
| Time (d14 vs. d45) | 0.693 | N/A | 0.946 |
| Ab x Time | 0.633 | N/A | 0.844 |
| Strain x Time | 0.032 * | N/A | N/A |
| Ab x Strain x Time | 0.342 | N/A | N/A |
| Time x SS | 0.095 | N/A | 0.002 * |
| Ab x Time x SS | 0.146 | N/A | 0.506 |
| Time x SS x Strain | 0.289 | N/A | N/A |
| Ab x Time x SS x Strain | 0.189 | N/A | N/A |
| ♀ hTau and WT d14 and d45 Total N = 13 | ♀ hTau and WT d14 Total N = 19 | ♂ hTau d14 and d45 Total N = 7 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.011 * | 0.237 | 0.395 |
| Mouse Strain (hTau vs. WT) | 0.832 | 0.998 | N/A |
| Ab x Strain | 0.797 | 0.910 | N/A |
| SS (NREM sleep vs. REM sleep) | 0.075 | 0.183 | 0.198 |
| Ab x SS | 0.255 | 0.868 | 0.516 |
| Strain x SS | 0.050 | 0.334 | N/A |
| Ab x Strain x SS | 0.358 | 0.671 | N/A |
| Time (d14 vs. d45) | 0.209 | N/A | 0.469 |
| Ab x Time | 0.971 | N/A | 0.396 |
| Strain x Time | 0.557 | N/A | N/A |
| Ab x Strain x Time | 0.683 | N/A | N/A |
| Time x SS | 0.532 | N/A | 0.421 |
| Ab x Time x SS | 0.893 | N/A | 0.434 |
| Time x SS x Strain | 0.517 | N/A | N/A |
| Ab x Time x SS x Strain | 0.956 | N/A | N/A |
| ♀ hTau and WT | ♂ hTau | |||
|---|---|---|---|---|
| d14 Total N = 20 | d45 Total N = 16 | d14 Total N = 8 | d45 Total N = 7 | |
| Mann-Whitney U P value | 0.331 | 0.721 | 0.786 | 0.057 |
| ♀ hTau and WT d14 and d45 Total N = 13 | ♀ hTau and WT d14 Total N = 17 | ♂ hTau d14 and d45 Total N = 6 | |
|---|---|---|---|
| ANOVA factor or interaction | p value | p value | p value |
| Ab (Pt-IgG vs. Ctrl-IgG) | 0.015 * | 0.101 | 0.948 |
| Mouse Strain (hTau vs. WT) | 0.136 | 0.104 | N/A |
| Ab x Strain | 0.209 | 0.183 | N/A |
| Time (d14 vs. d45) | 0.206 | N/A | 0.527 |
| Ab x Time | 0.282 | N/A | 0.544 |
| Strain x Time | 0.787 | N/A | N/A |
| Ab x Strain x Time | 0.509 | N/A | N/A |
References
- Gaig, C.; Compta, Y.; Heidbreder, A.; Marti, M.J.; Titulaer, M.J.; Crijnen, Y.; Högl, B.; Lewerenz, J.; Erro, M.E.; Garcia-Monco, J.C.; et al. Frequency and Characterization of Movement Disorders in Anti-IgLON5 Disease. Neurology 2021, 97, e1367–e1381. [Google Scholar] [CrossRef] [PubMed]
- Sabater, L.; Gaig, C.; Gelpi, E.; Bataller, L.; Lewerenz, J.; Torres-Vega, E.; Contreras, A.; Giometto, B.; Compta, Y.; Embid, C.; et al. A Novel Non-Rapid-Eye Movement and Rapid-Eye-Movement Parasomnia with Sleep Breathing Disorder Associated with Antibodies to IgLON5: A Case Series, Characterisation of the Antigen, and Post-Mortem Study. Lancet Neurol. 2014, 13, 575–586. [Google Scholar] [CrossRef]
- Ranaivoson, F.M.; Turk, L.S.; Ozgul, S.; Kakehi, S.; von Daake, S.; Lopez, N.; Trobiani, L.; De Jaco, A.; Denissova, N.; Demeler, B.; et al. A Proteomic Screen of Neuronal Cell-Surface Molecules Reveals IgLONs as Structurally Conserved Interaction Modules at the Synapse. Structure 2019, 27, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Vanaveski, T.; Singh, K.; Narvik, J.; Eskla, K.-L.; Visnapuu, T.; Heinla, I.; Jayaram, M.; Innos, J.; Lilleväli, K.; Philips, M.-A.; et al. Promoter-Specific Expression and Genomic Structure of IgLON Family Genes in Mouse. Front. Neurosci. 2017, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Gaig, C.; Iranzo, A.; Cajochen, C.; Vilaseca, I.; Embid, C.; Dalmau, J.; Graus, F.; Santamaria, J. Characterization of the Sleep Disorder of Anti-IgLON5 Disease. Sleep 2019, 42, zsz133. [Google Scholar] [CrossRef]
- Gaig, C.; Compta, Y. Neurological Profiles beyond the Sleep Disorder in Patients with Anti-IgLON5 Disease. Curr. Opin. Neurol. 2019, 32, 493–499. [Google Scholar] [CrossRef]
- Honorat, J.A.; Komorowski, L.; Josephs, K.A.; Fechner, K.; St Louis, E.K.; Hinson, S.R.; Lederer, S.; Kumar, N.; Gadoth, A.; Lennon, V.A.; et al. IgLON5 Antibody: Neurological Accompaniments and Outcomes in 20 Patients. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e385. [Google Scholar] [CrossRef]
- Ni, Y.; Shen, D.; Zhang, Y.; Song, Y.; Gao, Y.; Zhou, Q.; He, L.; Yin, D.; Wang, Y.; Song, F.; et al. Expanding the Clinical Spectrum of Anti-IgLON5 Disease: A Multicenter Retrospective Study. Eur. J. Neurol. 2021, 29, 267–276. [Google Scholar] [CrossRef]
- Gelpi, E.; Höftberger, R.; Graus, F.; Ling, H.; Holton, J.L.; Dawson, T.; Popovic, M.; Pretnar-Oblak, J.; Högl, B.; Schmutzhard, E.; et al. Neuropathological Criteria of Anti-IgLON5-Related Tauopathy. Acta Neuropathol. 2016, 132, 531–543. [Google Scholar] [CrossRef]
- Cagnin, A.; Mariotto, S.; Fiorini, M.; Gaule, M.; Bonetto, N.; Tagliapietra, M.; Buratti, E.; Zanusso, G.; Ferrari, S.; Monaco, S. Microglial and Neuronal TDP-43 Pathology in Anti-IgLON5-Related Tauopathy. J. Alzheimers Dis. 2017, 59, 13–20. [Google Scholar] [CrossRef]
- Erro, M.E.; Sabater, L.; Martínez, L.; Herrera, M.; Ostolaza, A.; García de Gurtubay, I.; Tuñón, T.; Graus, F.; Gelpi, E. Anti-IGLON5 Disease: A New Case without Neuropathologic Evidence of Brainstem Tauopathy. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e651. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.C.; Ramanathan, S. Cell Surface Antibody-Associated Neurodegeneration: The Case of Anti-IgLON5 Antibodies. Neurology 2017, 88, 1688–1690. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol. Rev. 2017, 97, 839–887. [Google Scholar] [CrossRef]
- Bivona, G.; Gambino, C.M.; Lo Sasso, B.; Scazzone, C.; Giglio, R.V.; Agnello, L.; Ciaccio, M. Serum Vitamin D as a Biomarker in Autoimmune, Psychiatric and Neurodegenerative Diseases. Diagnostics 2022, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Hunt, C.; Macedo E Cordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of Immune Activation on the Kynurenine Pathway and Depression Symptoms—A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Cabezudo-García, P.; Mena-Vázquez, N.; Estivill Torrús, G.; Serrano-Castro, P. Response to Immunotherapy in Anti-IgLON5 Disease: A Systematic Review. Acta Neurol. Scand. 2020, 141, 263–270. [Google Scholar] [CrossRef]
- Rose, N.R.; Bona, C. Defining Criteria for Autoimmune Diseases (Witebsky’s Postulates Revisited). Immunol. Today 1993, 14, 426–430. [Google Scholar] [CrossRef]
- Sabater, L.; Planagumà, J.; Dalmau, J.; Graus, F. Cellular Investigations with Human Antibodies Associated with the Anti-IgLON5 Syndrome. J. Neuroinflamm. 2016, 13, 226. [Google Scholar] [CrossRef]
- Landa, J.; Gaig, C.; Plagumà, J.; Saiz, A.; Antonell, A.; Sanchez-Valle, R.; Dalmau, J.; Graus, F.; Sabater, L. Effects of IgLON5 Antibodies on Neuronal Cytoskeleton: A Link between Autoimmunity and Neurodegeneration. Ann. Neurol. 2020, 88, 1023–1027. [Google Scholar] [CrossRef]
- Ryding, M.; Gamre, M.; Nissen, M.S.; Nilsson, A.C.; Okarmus, J.; Poulsen, A.A.E.; Meyer, M.; Blaabjerg, M. Neurodegeneration Induced by Anti-IgLON5 Antibodies Studied in Induced Pluripotent Stem Cell-Derived Human Neurons. Cells 2021, 10, 837. [Google Scholar] [CrossRef] [PubMed]
- Andorfer, C.; Kress, Y.; Espinoza, M.; de Silva, R.; Tucker, K.L.; Barde, Y.-A.; Duff, K.; Davies, P. Hyperphosphorylation and Aggregation of Tau in Mice Expressing Normal Human Tau Isoforms. J. Neurochem. 2003, 86, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Andorfer, C.; Acker, C.M.; Kress, Y.; Hof, P.R.; Duff, K.; Davies, P. Cell-Cycle Reentry and Cell Death in Transgenic Mice Expressing Nonmutant Human Tau Isoforms. J. Neurosci. 2005, 25, 5446–5454. [Google Scholar] [CrossRef] [PubMed]
- Dengler-Crish, C.M.; Smith, M.A.; Wilson, G.N. Early Evidence of Low Bone Density and Decreased Serotonergic Synthesis in the Dorsal Raphe of a Tauopathy Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Davidowitz, E.J.; Krishnamurthy, P.K.; Lopez, P.; Jimenez, H.; Adrien, L.; Davies, P.; Moe, J.G. In Vivo Validation of a Small Molecule Inhibitor of Tau Self-Association in Htau Mice. J. Alzheimers Dis. 2020, 73, 147–161. [Google Scholar] [CrossRef]
- Neddens, J.; Daurer, M.; Loeffler, T.; Alzola Aldamizetxebarria, S.; Flunkert, S.; Hutter-Paier, B. Constant Levels of Tau Phosphorylation in the Brain of Htau Mice. Front. Mol. Neurosci. 2020, 13, 136. [Google Scholar] [CrossRef]
- Planagumà, J.; Leypoldt, F.; Mannara, F.; Gutiérrez-Cuesta, J.; Martín-García, E.; Aguilar, E.; Titulaer, M.J.; Petit-Pedrol, M.; Jain, A.; Balice-Gordon, R.; et al. Human N-Methyl D-Aspartate Receptor Antibodies Alter Memory and Behaviour in Mice. Brain 2015, 138, 94–109. [Google Scholar] [CrossRef]
- Maphis, N.; Xu, G.; Kokiko-Cochran, O.N.; Jiang, S.; Cardona, A.; Ransohoff, R.M.; Lamb, B.T.; Bhaskar, K. Reactive Microglia Drive Tau Pathology and Contribute to the Spreading of Pathological Tau in the Brain. Brain 2015, 138, 1738–1755. [Google Scholar] [CrossRef]
- Oshio, K.; Watanabe, H.; Song, Y.; Verkman, A.S.; Manley, G.T. Reduced Cerebrospinal Fluid Production and Intracranial Pressure in Mice Lacking Choroid Plexus Water Channel Aquaporin-1. FASEB J. 2005, 19, 76–78. [Google Scholar] [CrossRef]
- Silvani, A.; Bastianini, S.; Berteotti, C.; Franzini, C.; Lenzi, P.; Lo Martire, V.; Zoccoli, G. Sleep Modulates Hypertension in Leptin-Deficient Obese Mice. Hypertension 2009, 53, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Bastianini, S.; Silvani, A.; Berteotti, C.; Elghozi, J.-L.; Franzini, C.; Lenzi, P.; Lo Martire, V.; Zoccoli, G. Sleep Related Changes in Blood Pressure in Hypocretin-Deficient Narcoleptic Mice. Sleep 2011, 34, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Ferri, R.; Lo Martire, V.; Bastianini, S.; Berteotti, C.; Salvadè, A.; Plazzi, G.; Zucconi, M.; Ferini-Strambi, L.; Bassetti, C.L.; et al. Muscle Activity During Sleep in Human Subjects, Rats, and Mice: Towards Translational Models of REM Sleep Without Atonia. Sleep 2017, 40, zsx029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silvani, A.; Berteotti, C.; Bastianini, S.; Cohen, G.; Lo Martire, V.; Mazza, R.; Pagotto, U.; Quarta, C.; Zoccoli, G. Cardiorespiratory Anomalies in Mice Lacking CB1 Cannabinoid Receptors. PLoS ONE 2014, 9, e100536. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, M.L.; Berteotti, C.; Alvente, S.; Bastianini, S.; Guidi, S.; Lo Martire, V.; Matteoli, G.; Silvani, A.; Stagni, F.; Bosi, M.; et al. Obstructive Sleep Apneas Naturally Occur in Mice during REM Sleep and Are Highly Prevalent in a Mouse Model of Down Syndrome. Neurobiol. Dis. 2021, 159, 105508. [Google Scholar] [CrossRef] [PubMed]
- Bastianini, S.; Alvente, S.; Berteotti, C.; Lo Martire, V.; Silvani, A.; Swoap, S.J.; Valli, A.; Zoccoli, G.; Cohen, G. Accurate Discrimination of the Wake-Sleep States of Mice Using Non-Invasive Whole-Body Plethysmography. Sci. Rep. 2017, 7, 41698. [Google Scholar] [CrossRef] [PubMed]
- Bastianini, S.; Alvente, S.; Berteotti, C.; Bosi, M.; Lo Martire, V.; Silvani, A.; Valli, A.; Zoccoli, G. Post-Sigh Sleep Apneas in Mice: Systematic Review and Data-Driven Definition. J. Sleep Res. 2019, 28, e12845. [Google Scholar] [CrossRef]
- Guyenet, S.J.; Furrer, S.A.; Damian, V.M.; Baughan, T.D.; La Spada, A.R.; Garden, G.A. A Simple Composite Phenotype Scoring System for Evaluating Mouse Models of Cerebellar Ataxia. J. Vis. Exp. 2010, 39, 1787. [Google Scholar] [CrossRef]
- Lo Martire, V.; Alvente, S.; Bastianini, S.; Berteotti, C.; Bombardi, C.; Calandra-Buonaura, G.; Capellari, S.; Cohen, G.; Cortelli, P.; Gasparini, L.; et al. Mice Overexpressing Lamin B1 in Oligodendrocytes Recapitulate the Age-Dependent Motor Signs, but Not the Early Autonomic Cardiovascular Dysfunction of Autosomal-Dominant Leukodystrophy (ADLD). Exp. Neurol. 2018, 301, 1–12. [Google Scholar] [CrossRef]
- Hayar, A.; Bryant, J.L.; Boughter, J.D.; Heck, D.H. A Low-Cost Solution to Measure Mouse Licking in an Electrophysiological Setup with a Standard Analog-to-Digital Converter. J. Neurosci. Methods 2006, 153, 203–207. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Kenny, A.; de Leo, G.; Beamer, E.H.; Engel, T. Tau Phosphorylation in a Mouse Model of Temporal Lobe Epilepsy. Front. Aging Neurosci. 2019, 11, 308. [Google Scholar] [CrossRef]
- El Khoury, N.B.; Gratuze, M.; Petry, F.; Papon, M.-A.; Julien, C.; Marcouiller, F.; Morin, F.; Nicholls, S.B.; Calon, F.; Hébert, S.S.; et al. Hypothermia Mediates Age-Dependent Increase of Tau Phosphorylation in Db/Db Mice. Neurobiol. Dis. 2016, 88, 55–65. [Google Scholar] [CrossRef]
- Ren, Q.-G.; Gong, W.-G.; Wang, Y.-J.; Zhou, Q.-D.; Zhang, Z.-J. Citalopram Attenuates Tau Hyperphosphorylation and Spatial Memory Deficit Induced by Social Isolation Rearing in Middle-Aged Rats. J. Mol. Neurosci. 2015, 56, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hesser, C.M.; Lind, F.; Linnarsson, D. Significance of Airway Resistance for the Pattern of Breathing and Lung Volumes in Exercising Humans. J. Appl. Physiol. 1990, 68, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Vajnerová, O.; Bielavská, E.; Jiruska, P.; Brozek, G. Level of Vigilance Influences Licking Frequency in Rats. Physiol. Res. 2003, 52, 243–249. [Google Scholar] [PubMed]
- Geiszler, P.C.; Barron, M.R.; Pardon, M.-C. Impaired Burrowing Is the Most Prominent Behavioral Deficit of Aging Htau Mice. Neuroscience 2016, 329, 98–111. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvente, S.; Matteoli, G.; Molina-Porcel, L.; Landa, J.; Alba, M.; Bastianini, S.; Berteotti, C.; Graus, F.; Lo Martire, V.; Sabater, L.; et al. Pilot Study of the Effects of Chronic Intracerebroventricular Infusion of Human Anti-IgLON5 Disease Antibodies in Mice. Cells 2022, 11, 1024. https://doi.org/10.3390/cells11061024
Alvente S, Matteoli G, Molina-Porcel L, Landa J, Alba M, Bastianini S, Berteotti C, Graus F, Lo Martire V, Sabater L, et al. Pilot Study of the Effects of Chronic Intracerebroventricular Infusion of Human Anti-IgLON5 Disease Antibodies in Mice. Cells. 2022; 11(6):1024. https://doi.org/10.3390/cells11061024
Chicago/Turabian StyleAlvente, Sara, Gabriele Matteoli, Laura Molina-Porcel, Jon Landa, Mercedes Alba, Stefano Bastianini, Chiara Berteotti, Francesc Graus, Viviana Lo Martire, Lidia Sabater, and et al. 2022. "Pilot Study of the Effects of Chronic Intracerebroventricular Infusion of Human Anti-IgLON5 Disease Antibodies in Mice" Cells 11, no. 6: 1024. https://doi.org/10.3390/cells11061024
APA StyleAlvente, S., Matteoli, G., Molina-Porcel, L., Landa, J., Alba, M., Bastianini, S., Berteotti, C., Graus, F., Lo Martire, V., Sabater, L., Zoccoli, G., & Silvani, A. (2022). Pilot Study of the Effects of Chronic Intracerebroventricular Infusion of Human Anti-IgLON5 Disease Antibodies in Mice. Cells, 11(6), 1024. https://doi.org/10.3390/cells11061024










