Abstract
Aging is the greatest risk factor for nearly all major chronic diseases, including cardiovascular diseases, cancer, Alzheimer’s and other neurodegenerative diseases of aging. Age-related impairment of immune function (immunosenescence) is one important cause of age-related morbidity and mortality, which may extend beyond its role in infectious disease. One aspect of immunosenescence that has received less attention is age-related natural killer (NK) cell dysfunction, characterized by reduced cytokine secretion and decreased target cell cytotoxicity, accompanied by and despite an increase in NK cell numbers with age. Moreover, recent studies have revealed that NK cells are the central actors in the immunosurveillance of senescent cells, whose age-related accumulation is itself a probable contributor to the chronic sterile low-grade inflammation developed with aging (“inflammaging”). NK cell dysfunction is therefore implicated in the increasing burden of infection, malignancy, inflammatory disorders, and senescent cells with age. This review will focus on recent advances and open questions in understanding the interplay between systemic inflammation, senescence burden, and NK cell dysfunction in the context of aging. Understanding the factors driving and enforcing NK cell aging may potentially lead to therapies countering age-related diseases and underlying drivers of the biological aging process itself.
1. Introduction
Aging is the most significant cause of morbidity and mortality in the developed world, and increasingly in developing countries as well [1,2]. While the decline in smoking prevalence and advancements in medical science have resulted in an increased lifespan, there is an increasing population prevalence of the diseases and debilities of aging associated with the not-as-rapid increase in health span [2,3]. Immunosenescence, the age-related decline in immune function, is a critical cause of age-related ill-health, driving dramatic increases in vulnerability to infectious disease with age (including the dramatic age-related rise in morbidity and mortality from COVID-19) [4,5]. In addition to immunosenescence, chronic sterile low-grade inflammation associated with aging (“inflammaging”) is likely contributing to the pathogenesis of multiple chronic diseases of aging [6,7].
NK cells are large granular innate immune cells that belong to the family of group 1 innate lymphocytes (ILC1) [8]. They play a vital role as immunological first responders, rapidly eliminating cells infected with viruses or other intracellular pathogens as well as pre-malignant cells [9], and have recently been identified as the principal actors in immune surveillance of senescent cells [10,11,12], which accumulate with age and are implicated in inflammaging, several age-related diseases of aging, and the underlying biological aging process itself [13,14]. Yet, comparatively little is known about the effects of aging on NK cells as compared to the cells in the adaptive immune system [15]. In this review, we discuss what is known about the phenotype and drivers of age-related NK cell dysfunction, areas of uncertainty, potential sources of new insight, and approaches to NK cell immune rejuvenation and fortification. Understanding and remediating the age-related decline in NK cell function could be an important means of ameliorating critical proximate causes of age-related ill-health and death and opposing one of the underlying drivers of aging.
2. NK Cells in Immunity: An Overview
NK cells eliminate target cells through direct cell-to-cell contact-based NK cell cytotoxicity (NKCC) and by secreting cytokines, such as interferon gamma (INF-γ) or tumor necrosis factor alpha (TNF-α) [16,17], which recruit additional immune cells to the site of infection or inflammation. NK cells represent ≈5–10% of peripheral blood lymphocytes (PBMC), with approximately 2 billion NK cells circulating in adult humans [18]. NK cells reside in different tissues, including bone marrow, lymph nodes, liver, skin, lung, and, to a lesser extent, secondary lymphoid organs [19]; however, this has not been extensively studied.
The human NK cell population can be subdivided based on the expression of the surface markers CD56 and CD16, with CD56dim NK cells being largely cytotoxic and CD56bright NK cells primarily responsible for the secretion of cytokines, such as IFN-γ and TNF-α [20,21]. However, in absolute numbers, CD56bright NK cells, located predominantly in peripheral and lymphoid tissues [22,23], outnumber CD56dim NK cells. The CD56dim NK cell subpopulation represent at least 90% of peripheral blood NK cells, whereas the CD56bright NK cells constitute the remaining [24]. NK cell phenotypes and their changes with age are described in greater depth in the subsequent sections.
The process of NK cell differentiation and maturation has been extensively reviewed elsewhere and is beyond the scope of this review [25]. In a brief summary, human NK cells develop from common lymphoid progenitor (CLP) cells derived from CD34+ hematopoietic stem cells (HSCs), the common precursors of NK, T, B, and other lymphoid cells [26,27]. CLP cells express different markers, including CD38, CD7, CD10, CD127, and CD122. The expression of CD122 (IL-2Rβ/IL-15R) marks the irreversible fate of CLPs starting to differentiate into NK lineage [28,29,30]. NK cells can act as a bridge between innate and adaptive immunity, as they can interact with T and B cells via their costimulatory ligands, such as CD40L and OX40L, which further promote NK cell differentiation [31,32].
NK cells interact with target cells via activating and inhibitory receptors, which bind to the corresponding ligands on the target cell’s surface. Depending on the balance of these ligand–receptor interactions, the NK cell determines whether or not to eliminate the target cell [33]. This review focuses on the alteration of the function of human NK cells with age; the NK cell receptors in humans and rodents have been extensively reviewed elsewhere [9,34]; we will therefore only provide a brief summary of this topic, as a background for what follows.
NKG2D, Ly49, and natural cytotoxicity receptors (NCRs), such as NKp46, are some of the main activating receptors for NK cells [16,35]. When the NKG2D or Ly49 interact with target cell ligands, such as MICA/MICB or ULBPs [36], their signal is mediated intracellularly through the YINM and immunoreceptor tyrosine-based activation motif (ITAM), respectively [36,37,38]. The phosphorylation of YINM or ITAM by tyrosine kinases leads to a cascade that results in the release of perforins and granzymes from NK cells to induce apoptosis in target cells [37,38]. In addition, NK cells also secrete cytokines and chemokines, which mediate their effector function directly on the target cells (cytotoxic signal) or through the recruitment of other immune cells, such as macrophages and neutrophils, to the site of inflammation [39,40].
The killer cell immunoglobulin-like receptors (KIR) and NKG2A are the major families of inhibitory receptors expressed on NK cells [41]. KIR bind to the HLA-A/B/C ligand, whereas NKG2A recognizes the inhibitory HLA-E [42,43]. These ligands bind to their respective receptors via immunoreceptor tyrosine-based inhibitory motifs (ITIM), leading to the inactivation of NK cells [44]. In addition, KIR and NK2GA are also known to coordinate NK cell tolerance [45]. Major histocompatibility complex class I (MHC-I) present non-self antigens for target engagement by CD8+ T-cells, but, conversely, are essential ligands for inhibitory receptors on NK cells for both mice and humans [46]. NK cells can recognize MHC- I directly (class Ia) or indirectly (class Ib). Reduced expression of MHC-I in cancer cells or cells infected with certain viruses lowers the inhibitory bar for attack by NK cells, enabling these cells’ elimination [47].
Mice and humans differ in the mechanism by which MHC class Ia is recognized: human NK cells use KIR to recognize MHC Ia, while mice use lectin-like receptors of the Ly49 family [48,49,50]. However, mice and humans have similar mechanisms for recognizing MHC class Ib, which involve NKG2D in both species [48,49,50]. Despite the differences in the ligand recognition mechanisms, the intracellular signaling mechanism involved in NK cell activation is conserved in these two species [48].
3. Changes in NK Cell Numbers and Subpopulations with Age
Several studies have characterized changes in the absolute number of circulating NK cells as well as the distribution of NK cell subsets with increasing age. Age does not appear to influence NK progenitor numbers in the peripheral blood or in the bone marrow [51]. Nonetheless, although there is some disagreement in the field, most studies report an increase in NK cell number with age (11 of 13 reported studies) (Table 1).

Table 1.
Changes in natural killer cell phenotype, subset, and cytotoxicity throughout the aging process. Summary of age-related changes in natural killer cell phenotype, subset distribution, and cytotoxicity. Age-related changes in natural killer cell phenotype, subset distribution, and cytotoxicity against cancer cell lines.
The markers used to label NK cells, or the gating strategy used to count them, may at least partially explain the discrepancy in the studies. The earliest studies relied on CD16 to distinguish NK cells from other lymphocytes, which may have resulted in an incomplete picture of total NK cell number. For example, Zang et al. used CD3 and CD16 expression to distinguish NK cells and reported no differences in peripheral NK cell numbers with age [69]. However, CD56 is now regarded as a more reliable and general marker of human NK cells than CD16. While CD16 is often expressed on the more mature subset, CD56dim NK chambers, the CD56bright NK cell subset sometimes lacks or expresses very low levels of CD16. It is possible that these earlier studies reported changes in CD56dim NK cell populations. Later studies, which included CD56 to label NK cells, reported that absolute cell numbers increased with age (Table 1). With regards to changes in NK cell subpopulations, the studies listed in Table 1 almost unanimously report that there is a significant decrease in the percentage of CD56bright cells (Table 1: six of six studies) [62,64,65,66,67,68] and a significant increase in CD56dim cells (five of five studies) [62,65,66,67,68].
Unbiased approaches, such as single-cell RNA expression analysis, offer a possible approach to resolving some of the conflicting data on the effect of aging on NK cells. Transcriptional clustering has revealed two distinct signatures of splenic NK cells, which have a more active gene expression signature than NK cells present in the blood [70]. RNA-sequencing has confirmed the prevalence of several distinct subpopulations of NK cells in the human bone marrow and blood based on the expression of several conserved markers [71]. These findings expand upon the classical NK cell developmental model (CD56bright → CD56dimCD57− → CD56dimCD57+) and identify two subpopulations in the CD57+ population, distinguished by the expression of CX3CR1 and HAVCR2 to differentiate the terminally differentiated NK cell population from the matured NK population [71]. In addition, single-cell RNA expression analysis of NK cells isolated from peripheral blood from healthy subjects reveals even more heterogeneity among NK cells, with up to ten subsets of NK cells identified by unsupervised clustering, with conserved makers per cluster independent of individual variation or stimulation [72]. Further, Smith et al. identified three novel NK cell populations; CD56neg type I interferon-responding NK cells have increased granzyme B mRNA in response to IL2 and a small population with low ribosomal expression [72]. Similar methods were employed to analyze specific gene expression variances of NK cells in the context of aging, which demonstrated an increase in expanded late low-cytotoxic subsets with enriched apoptotic signaling pathways and decreased virus defense responses, as compared to the younger group [73].
An important limitation to our insight into the effect of aging on NK cell phenotype is that the great majority of studies, especially in humans, have only investigated age-related changes in circulating NK. Dogra et al. have substantially advanced our understanding by examining NK cells from multiple anatomic sites and compartments of 60 autopsies of subjects across a wide range of ages (5–92 years) and ethnic backgrounds [74]. Notably, across donors, the dominance of CD56brightCD16− vs. CD56dimCD16+ was tissue-specific, whether pooled or at the individual donor level. CD56dimCD16+ NK cells dominated in blood and in tissues rich in it, such as the bone marrow, spleen, and lungs. Moreover, CD56dimCD16+ NK cells were substantially more likely to express CD57 (indicative of terminal differentiation) in tissues where this subset is dominant [74]
In contrast with previous reports (Table 1: [62,65,66,67,68]), Dogra et al. found no relationship between the ratio between CD56brightCD16− and CD56dimCD16+ NK cell subsets and age in blood or any other tissue. It will be important to understand why this one impressive study came to such a different result on this front.
The frequency of terminally differentiated CD56dimCD16+CD57+ NK cells nominally rose with age in blood, bone marrow, spleen, and lung, but the trend was only statistically significant in the bone marrow [74]. The lack of a clear age-related change in this subset in blood is broadly consistent with limited and inconsistent prior reports [64,66]. Age was also unrelated to the level of granzyme B expression by CD56dimCD16+ cells, nor the tissue-specific NK cell expression of the signaling adaptor FcεRIγ, an indicator of antibody-dependent cellular cytotoxicity (ADCC) potential [74]. Unlike its effects on T-cell differentiation and polyclonality (which clearly interfaces with aging, despite its uncertain effects on functional immune senescence [15]), cytomegalovirus serostatus did not impact NK cell frequency or tissue distribution [74].
Further investigation of age-related changes in the heterogeneity amongst NK cell populations may be vital in understanding the mechanism behind the loss of their functions with age.
4. Effect of Age on NK Cell Cytokine Production and Secretion
Decreased NK cell cytotoxic subtypes and function are associated with an increased risk of viral infections and cancers [75,76,77]. Dysfunctional NK cells are typically identified by decreased NK effector functions, such as impaired NKCC, as well as reduced IFN-γ secretion and expression of perforin and granzyme [78]. Release of cytokines, such as IFN-γ and TNF-α, activated the NK cells help coordinate a broader immune response, including the recruitment of macrophages [79] and neutrophils [80] at the site of cancer [81], infection [82], or senescent cells [83].
One potential cause of NK cell dysfunction with age may be changes in the systemic milieu [84,85]. Particularly notable is the well-established age-related decline in interleukin-2 (IL-2) production in humans [86]; IL-2 is a potent enhancer of both NKCC and NK cytokine secretion, such as TNF-α [87]. One study reported that TNF-α secretion is sustained in IL-2-stimulated NK cells from otherwise-healthy aging donors, despite their reduced IL-2 production [62]. Other studies have found that NK cells from older donors increase production of IFN-γ and/or TNF-α following IL-2 treatment [64,88], but to a lesser degree than that which occurs with younger donors [59,89]. Similar to IL-2, interleukin-12 (IL-12) enhances the cytotoxicity of NK cells and promotes IFN-γ secretion by NK cells [90,91]. Studies have reported a reduction in cytokines released by NK cells from older donors, despite activation with IL-2 [92,93,94], and chemokine production in older subjects, despite stimulation with IL-2 or IL-12 [95,96]. One caveat to these findings is that a reduction in cytokine or chemokine production per cell with age may possibly be attributable to the age-related shift from CD56bright to CD56dim NK cells, as the former are responsible for producing these mediators. Further investigation of age-related changes in the cytokine and chemokine production and secretion amongst NK cell populations may be vital in understanding the mechanism behind the decrease in NK cell activation and recruitment.
5. Changes in NK Cell Cytotoxic Activity with Age
A decline in NKCC with age has been well documented and has been linked to increased incidence of infectious diseases [73,97,98]. Age-related NKCC impairment has also been implicated in enabling the emergence of multiple noncommunicable diseases of aging [99]. For example, adult cancer incidence and mortality for all-site invasive cancers and for many individual cancer types rise progressively with age [100,101] and the decline in NK cell function with age is implicated in this increased risk [102]. NK cell surveillance provides a critical early defense against precancerous and cancerous cells [103,104]. Depressed NK cell activity in animals results in elevated tumor incidence [105] or increased metastasis [106,107]. Relatives of patients with familial melanoma [108] and individuals with a high family incidence of cancer [109] exhibit low NK cell activity compared to healthy controls; similarly, liver cirrhosis patients with reduced NKCC are prospectively at an elevated risk of progressing to liver cancer [110], and one study reported low NKCC to be longitudinally associated with a risk of cancer at all sites in the general Japanese population aged 40 and over [111]. A longitudinal study demonstrated a declining efficacy of NK cell cytotoxicity, coinciding with elevated risk of cancer in both males and females with age [84].
These epidemiological findings are supported by laboratory data. More than half of the studies (6 of 11 studies—Table 1) report a decrease with age in NK cell cytotoxicity against K562 cancer cells. The non-unanimous findings can be attributed to the lack in consistent NK cell markers, leading to potential contamination; in addition, some of the reports used IL-2, which augments the innate NKCC ability. One study [67] reported an age-related rise in expression of the inhibitory receptor KLRG1, particularly in CD56dim NK cells; CD56dim NK cells expressing high levels of KLRG1 had impaired cytotoxicity against the MCF-10A cancer line, which expresses high levels of KLRG1’s ligand E-cadherin, and silencing RNA against KLRG1 enhanced these cells’ NKCC against this target cell type. Notably, however, one study reported an increase in cytotoxic activity with age, associated with a shift from the CD56+57− to the CD56+57+ phenotype [54]. The impact of aging on NK cell toxicity against senescent cells remains to be investigated. Identifying the cause(s) for the age-related decline in NKCC is a necessary step toward identifying interventions to retard or reverse this decline, and thus potentially mitigate its contribution to age-related diseases.
NK cell killing of target cells is dependent on the NK cell receptors necessary for target cell recognition. Age-related declines in the percentage of NK cells expressing the activating receptors, NKp30 or NKp46 [66,112,113], have been reported. One study reported an age-related increase in NK cells expressing the inhibitory receptor KLRG1, particularly but not only in the CD56dim subset [67]; contrarily, another group reported a decline in both NKG2A and KLRG1 [64]. The percentage of NK cells expressing inhibitory KIRs has been reported to increase with age [54]. Taken together, these results point towards an alteration in the ability to receive pro-activation signaling from the target cells, possibly indicating an overall shift towards a more inhibitory phenotype.
As a possible indication of the functional significance of such changes, researchers have investigated diversity and frequency of KIR genotypes in otherwise-healthy aging persons [114] and centenarians [115] as compared to younger controls. The first study did not reveal a clear enrichment of either KIR diversity or particular KIR genes in older compared to younger Irish subjects, suggesting that neither confers a late-life survivorship advantage [114]. The analysis of the HLA-DRB1 and KIR receptors/HLA ligand frequencies in centenarians and controls from Sicily also demonstrated no significant difference in KIR receptors [115].
A reduction in NK cell perforin expression has been reported to be the mechanism behind age-dependent decline in NK cell cytotoxic function [116]. However, one study found no significant differences in the perforin content, distribution, and utilization in the lytic assays in NK cells from young (≤35-year-old) and old (≥61-year-old) groups, but evidence of impaired perforin release through degranulation upon coculture with target cells [113]. Certain compounds, such as ionomycin and phorbol myristate acetate (PMA), are used in vitro to activate NK cells, bypassing the need for target cell surface receptors to induce NK cell degranulation [99]. NK cells from older donors maintain their sensitivity to this assay, suggesting that the age-related decline in NKCC may be due to loss of these discussed receptors and/or to cell signaling, and not the ability to produce cytotoxic granules when activated [88,89,99].
These results collectively indicate that NK cells from older subjects have undergone biological changes, which impact their ability to interact with other immune cells and their target cells. One hypothesis to explain the increase in the numbers of cytotoxic NK cells with age may be that it is an attempt to compensate for the age-related loss in effector function at the individual NK cell level.
6. Effect of Age on NK Cell Signal Transduction
While it has become clear that NK cell function declines with age, few studies provide clues about the underlying causes. The process of NK cell cytotoxicity is a complex and tightly coordinated series of events, which ultimately result in the release of lytic granules into the immunological synapse (IS) between the NK cell and a bound target cell (Figure 1) [117]. Several key checkpoints in this pathway involve the turnover of phosphoinositide molecules by phospholipase C (PLC), calcium mobilization and influx, polarization of secretory lysosomes to the IS, and fusion of these vesicles with the NK cell membrane [117,118,119,120]. Ca2+ release is also required for critical degranulation checkpoints, such as migration of the mitochondria to the IS (which controls subsequent Ca2+ influx) and fusion of secretory lysosomes with the NK cell membrane [121,122].
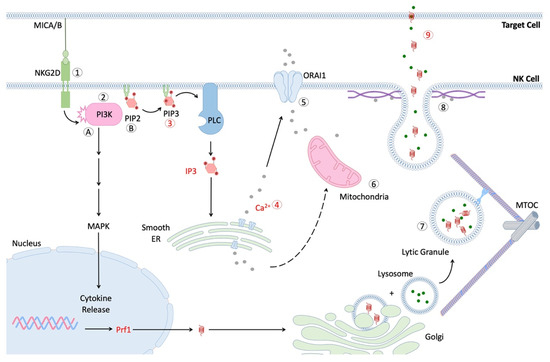
Figure 1.
Scheme of NK cell signaling after formation of immunological synapse (IS). Interaction of activating receptor with cognate ligand leads to phosphorylation of cytoplasmic tail and recruitment of PI3K [61,120]. Pathway (A) activation of PI3K and PLC lead to downstream signaling via MAPK and NFκB pathways, respectively, thus facilitating the production and secretion of cytokines, such as IFN-γ. Activation of MAPK signaling also contributes to MTOC migration and the polarization of lytic granules. Pathway (B). Conversion of phosphatidylinositol-4, 5-bisphosphate (PIP2) to phosphatidylinositol-3, 4, 5-triphosphate (PIP3) allows for subsequent generation of the secondary messenger inositol trisphosphate (IP3) via phospholipase C (PLC) [117]. IP3 contributes to the release of internal calcium stores from the smooth endoplasmic reticulum [123], which in turn facilitates mitochondrial migration to IS and influx of extracellular calcium via the ORAI1 transporter [118,119]. Additionally, the microtubule organization center (MTOC) migrates towards the IS and allows for efficient transport of lytic granules (secretory lysosomes) to travel to the IS [124,125]. Lastly, lytic granules fuse with the NK cell membrane in a calcium-dependent manner and release perforin and granzymes into the IS [126]. These proteins form holes in the target cell membrane and induce apoptosis.
The second messenger, phosphoinositol phosphate 3 (IP3), is critical to this process, as it stimulates the release of internal Ca2+ stores, which leads to downstream activation events. The IP3 production, following exposure to K562 cancer cells, was significantly reduced in NK cells from older subjects [61]. As noted previously, one study reported that the Ca2+ mobilization in NK cells from older donors is reduced, relative to the level observed in NK cells from younger donors in response to IL-2 [62].The decline in the mobilization of Ca2+ resulting from the impaired production of IP3 might therefore contribute to the impaired cytotoxicity of the NK observed in older subjects [118].
In addition to the decreased Ca2+ mobilization, NK cells from older individuals exhibit diminished perforin expression and release, which likely contributes to the age-dependent decline in NK cell toxicity [113,116]. One study measured the abundance of intracellular perforin in unstimulated CD56+CD16+ NK cells from various age groups and demonstrated an age-dependent decline in the percentage of perforin-positive NK cells in individuals that are 70 years and older, which was only partially remedied by treatment with IL-2 [116]. In another study with a much larger sample size, no decline in the expression of perforin with age was observed nor were changes in the fusion of secretory lysosomes with the NK cell membrane [113]. However, NK cells from older donors in this study did exhibit diminished perforin binding to the surface of K562 cancer cells [113]. As noted previously, the decline in IL-2 production with aging is well-known in humans [86]; thus, it is possible that in vitro experiments using activated NK cells could hide any difference in resting perforin expression between young and old NK cells [127].
7. NK Cell Exhaustion
The phenomenon of cell exhaustion is well known and characterized in T cells; however, it is not known whether NK cells undergo a similar state of exhaustion [128,129]. The impairment of the cytotoxic activity of NK cells in cancer patients is well known. For instance, a significant reduction in the numbers of NK cells expressing NKG2D, NKp30, NKp46, and perforin was observed in patients suffering from pancreatic, gastric, and colorectal cancers [130]. A similar loss in cytolytic function of NK cells is also observed in patients suffering from chronic viral infection [131]. An investigation of the molecular signaling in these NK cells may shed new light on any potential exhaustion phenotype in NK cells and help to distinguish them from senescent NK cells.
While NK cells themselves are short lived (around two weeks), human NK cells exhibit telomere shortening and a decrease in telomerase activity with age [132,133]. Cellular differentiation has a role in telomere shortening, leading to the more mature CD56bright NK cells having shorter relative telomere length than the immature CD56dim subset [132,134], but all subsets of NK cells have decreased telomere length with age, with CD56bright cells demonstrating the greatest decrease [132]. Interestingly, NK cells expressing the activation markers, NKG2D and LFA-1, have shorter telomeres as compared to those without, while those expressing inhibitory markers, such as KIR or NKG2A, had an extremely heterogenous telomere length with no real pattern [134]. Taken together, these studies point to telomere attrition as a potential factor for diminished NK cell function with age, but more research would need to be conducted in order to determine any actual functional effects on cytotoxicity.
8. Impact of the Aging Systemic Milieu on NK Cells
To this point we have largely focused on predominantly cell-autonomous changes in NK cells with age, but changes in the local microenvironment during NK cell maturation or locally at the site of abnormal cells during aging may also skew NK cell behavior. Pro-inflammatory and anti-inflammatory cytokines work together to mediate proper immune responses and are vital to the healthy aging of an individual [135]. Chronic sterile systemic inflammation is often considered emblematic of dysfunction associated with aging, and is a powerful risk factor for morbidity and mortality in age-related diseases, such as sarcopenia [136], obesity [137], and Alzheimer’s [138]. Following transplantation into old mice, NK cells isolated from young mice exhibit significant impairment in NK cell function in ex vivo assays, whereas the transfer of NK cells from old mice to young mice seems to fully restore their cytotoxic potential [139]. These findings suggest a strong influence of host tissue microenvironment on NK cell function in aging and are consistent with in vitro stimulation experiments of human NK cells [64,88]. However, the interpretation of studies in aging mice must be taken with caution, as mouse NK cells are functionally quite different from those of humans at the protein level (mouse have much lower perforin and granzyme levels) and in terms of localization patterns [140].
The aged environment might lack the stimulating factors necessary to achieve maximal NK function. With age, the levels of IL-2 [86] and IL-15 [141], vital cytokines for NK cell development and survival, decline, which may contribute to the decline in NK cell surveillance with age. In parallel, the levels of inflammatory cytokines, such as IL-6 [142] and GDF15 [143,144], increase with age, contributing to the aged inflammatory microenvironment. Treatment of NK cells in culture with interleukins 2, 12, or 15 (IL-2, IL-12, IL-15) and interferon-α (IFN-α) can increase their cytotoxicity towards cancer cells and even toward cancer lines that are generally resistant to NK cell killing [145,146].
Studies are mixed on whether stimulation of aged NK cells with IL-2 ex vivo is sufficient to restore NKCC and related activities. One study reported full recovery of NKCC following IL-2 stimulation of in NK cells from old (60–80 years old) and even very old (80–100) vs. middle-aged (18–60) subjects [89]. However, others report that although NK cell activation by cytokine treatment does improve cytotoxicity in old-derived NK cells, it never reaches the level observed in NK cells derived from young donors, indicating a disruption in cell-level activation signaling with age [64,147]. One study demonstrated a decreased sensitivity of NK cells to IL-2 when derived from older donors [62], which may at least in part be explained by the use of the SENIEUR patient selection criteria in this study, which attempted to avoid confounding factors that elicit strong immunological responses, such as current infection, cancer, recent myocardial infarction or stroke, lymphoproliferative disorders, or laboratory findings outside the reference values for the subjects’ age [148].
The evidence is similarly mixed on whether IL-2 stimulation of aged NK cells is sufficient to normalize production and secretion of NKCC effectors. One study reported an age-dependent decline in the percentage of perforin-positive NK cells derived from individuals that are 70 years and older, which was only partially remedied by treatment with IL-2 [116]. However, in another study with a much larger sample size, no decline in the expression of perforin with age was observed, nor were changes in the fusion of secretory lysosomes with the NK cell membrane; however, they did demonstrate diminished perforin binding to the surface of K562 cancer cells [113]. As the decline in IL-2 production with aging is well known in humans [86], it is possible that in vitro experiments using activated NK cells could hide any difference in resting perforin expression between young and old NK cells [127].
Epigenetics makers are known to be heavily influenced as organisms age. While there not many studies on the role of epigenetic modifications on the differentiation and function of NK cells, especially not in relation to age, it is very clear that the activation state of NK cells is epigenetically regulated [149]. NK cell activity is modulated by the complex interaction of activating and inhibiting receptors, and how much they can be activated seem to be modulated by epigenetics [150]. The exposure to cytokines, such as IL-6 or INF-γ, can influence NK cell activation; however, the strength of the response is modified by prior exposure to other stimuli, which alter the epigenetic state of the promotor for their receptors [151]. Exposure to factors that are known to influence systemic aging—such as CMV infection [152], exercise [153], and emotional stress [154], have also been demonstrated to change the epigenetic status of NK cells. More broadly speaking, long-term exposures to a variety of stresses lead to epigenetic modifications [149,152]
Interestingly, it seems that the differentiation of CD56dim NK cells affects epigenetic clocks, which use the level and pattern of DNA methylation in cells either in blood or other tissues to predict biological age and mortality [155]. Further, Jonkman et al. reported that cytotoxic NK cells as well as T-cells seem to be the important drivers of these clocks, and that blood CD56dim and CD57bright NK cells from the same donor are assessed by several such clocks to be biologically much older than CD56bright cells [155]. Certainly, our understanding of the role of the interaction of aged NK cells with the systemic milieu is incomplete, suggesting that additional cell-autonomous or microenvironmental factors remain to be identified.
9. Neuroendocrine Signaling and NK Cell Aging
Neuroendocrine factors affect NK cells, and many studies have demonstrated the influence of stress [156] and the nervous system on NK cell activity [157,158,159]. Some of these factors are known to change with age: for instance, glucocorticoid (GC) levels increase with age in human serum [160], even in relatively healthy aging people, as assessed by the SENIEUR criteria [161], and GCs were demonstrated to have an inhibitory effect on NK cell functions [162]. This effect is due to decreased gene expression for essential genes, such as perforin and granzyme B, leading to an overall reduction in cytotoxicity [116] and IFN-γ production [163].
The serotonin receptor agonist, quipazine, enhanced NK cell function, whereas various dopamine/serotonin antagonists inhibited CD16-mediated NK cell function [112]. In addition, NK cells express very high levels of dopamine receptors, and their activation seems to have an inhibitory function on human NK cells [164]. Similarly, epinephrine and norepinephrine seem to primarily inhibit NK cell cytotoxicity and cytokine production [165,166].
Neuropeptides present in the peripheral blood bind to and can modulate NK cell activity [167]. Galanin modulates IFN-γ secretion and sensitizes NK cells towards specific cytokines [168], while neuropeptide substance P increases the cytotoxicity of NK cells and helps with NK cell migration [168,169]. Both galanin and neuropeptide substance P decrease expression with age [170,171], suggesting that the observed decline with age may contribute to the age-related impairment in NK function.
Insulin-like growth factor 1 (IGF-1) is an important growth factor that promotes NK cell development from CD34+ cells and increases NK cell cytotoxicity [172]. IGF-1 levels decline with age [173,174]. Additionally, the secretome of senescent cells contains several pro-inflammatory factors, including TNF-α [175], which has been demonstrated to induce a state of IGF resistance [176]. Combined, the reduction in circulating IGF-1 and tissue resistance to IGF-1 signaling may contribute to the age-related changes to NK cells and the ensuing decrease in senescent cell clearing.
Other growth factors, such as platelet-derived growth factors D and DD (PDGF-D and PDGF-DD), are able to interact with the surface of NK cells to induce NK cell survival [177] and cytokine secretion [178]. There are conflicting results on the age-induced changes to levels of PDGF, with reports of decreased [179], unchanged [180], and increased [181] PDGF levels with age. These studies focus on PDGF in general rather than the PDGF-D and PDGF-DD specifically, and thus these levels would need to be examined in the context of age to further understand their role in NK cell aging.
In contrast to IGF-1 and PDGF, transforming growth factor beta (TGF-β) has been demonstrated to inhibit NK cell development and function [182,183]. TGF-β has been demonstrated to block IL-15-induced metabolic activity and proliferation of NK cells by inhibiting the mechanistic target of rapamycin (mTOR) activity [184] and the expression of NKp30 [185] and NKG2D receptors [186]. Senescent fibroblasts secrete TGF- β [187], and plasma TGF- β levels have been demonstrated to increase with age [188].
10. Reducing the Impacts of Secondary Aging on NK Cell Function
Ewald W. Busse is credited with introducing the conceptual distinction between primary aging, denoting age changes driven by intrinsic metabolic processes, and secondary aging, driven by supernumerary cellular and molecular damage inflicted through lifestyle and environmental exposures [189,190]. Recent years have observed rapid advances in the biology of aging, leading to the discovery, development, and translation from animal models of candidate interventions targeting primary aging processes, but drivers of secondary aging are already amenable to intervention today [191,192].
10.1. Micronutrient Deficiency
One recognized source of secondary aging in older persons is micronutrient deficiencies, resulting from some combination of malabsorption, low intake, and an increase in the aging body’s requirements [193,194,195,196,197]. A dysregulation of essential nutrients, such as vitamin B6, vitamin B12, folic acid, and zinc, which are essential for cell-mediated immunity [198,199,200], are linked to various age-related pathologies and diseases, including inflammation [201,202], bone aging [203] and osteoporosis [204], and Alzheimer’s disease [205,206], in addition to a decrease in NK cell count and/or cytotoxicity [197,207,208,209].
Nutrient supplementation studies demonstrate significant promise in mitigating these secondary aging effects. Studies have demonstrated that the age-related decrease in zinc levels correlate with a reduction in NK cell cytotoxicity [210]. Zinc supplementation in NK cell cultures improves the age-related decline in proliferation/differentiation of CD34+ cells, in part by restoring the transcription factor Gata-3 [51]. NK cell cytotoxicity in healthy elderly subjects is reported to be partially restored through supplementation with zinc [210,211].
Similarly, vitamin B12 and folate (vitamin B9) dysregulation have been correlated with lower NK cell counts and activity, and can be improved through supplementation, which increases NK cell counts and activity in aging rats [200,209]. Again, a nutritional formula containing 120 IU vitamin E, 3.8 µg vitamin B12, 400 µg folic acid, and other nutrients increased the NK activity relative to the placebo in healthy subjects aged 70 years and older [212].
To our knowledge, the effect of vitamin D supplementation on NK cell function has not yet been tested in studies in aging humans, but the mechanisms of action of vitamin D in immunity are thought to principally involve the innate immune system [213]. A meta-analysis of individual participant data (IPD) from randomized controlled trials of vitamin D supplementation in subjects from birth up to 95 years of age reported a reduced risk of acute respiratory tract infections, particularly in trials involving daily or weekly vitamin D and in subjects with baseline deficient 25-hydroxyvitamin D levels [213]. Inconclusive data suggest that vitamin D deficiency may increase risk of infection with SARS-CoV-2 and/or adverse outcomes in COVID-19 [214], and evidence supports a potential role for vitamin D repletion in the prevention and amelioration of acute respiratory distress syndrome [215,216]. NK cell numbers and/or cytolytic activity were positively associated with serum vitamin D levels in a cross-sectional study in otherwise-healthy nonagenarians and centenarians [217].
In addition, the effect of supplementation with other nutrients whose deficiency is implicated in age-related immune dysfunction, such as vitamin E [218,219] on NK cell activity in aging subjects, remains to be investigated.
10.2. Stress Reduction
As noted above, GC levels rise with age [160] and are further elevated in association with elevated psychological stress [161] and impaired NK cell functions [162]. Consistent with this, extensive evidence has demonstrated that subjective stress levels and stressful life events predict impaired immunity [220], including increased susceptibility to induced rhinovirus infection [221] and less durable antibody titers after vaccination [222]. The increase in influenza-specific IFN-γ production following influenza vaccination and following four weeks of aerobic exercise appeared to be partially mediated by psychosocial factors [223]. Again, we are unaware of human challenge studies testing the effects of induced or self-reported stress on NK activity, but a study in mice subjected to selective REM sleep deprivation found that the ensuing impairment in NK cell numbers and activity was mediated by an upregulation in NK cell β–adrenergic receptors, possibly caused by an increase in GC levels, which was itself caused by the sleep restriction protocol [224].
A potential approach to reducing secondary aging of the immune system (including possibly NK function) in aging is therefore intervention against subjective stress and the associated elevation in GC levels. Modest evidence suggests that such modalities, as cognitive, psychological, or exercise interventions to lower chronic stress, may improve immune function [225]. Magnesium supplementation in older subjects has also been reported to lower nighttime GC levels [226,227].
10.3. Sleep Health and Circadian Alignment
The quality and quantity of sleep and the amplitude of circadian rhythms decline with age [228], and low objectively measured sleep quantity and/or quality are linked to neurodegenerative [229] and other diseases of aging [230,231], as well as total mortality [232,233,234]. Insufficient or circadian-misaligned sleep are known to cause impaired immune function [235,236]. A single night of imposed total [237] or even partial [238,239] sleep restriction, or nights of shorter vs. longer self-reported sleep [240,241] are followed by lower NK cell number and/or functions in humans the following day. Conversely, a night of recovery sleep allows NK activity to normalize [238,239], although IL-2 levels remained suppressed [238].
Consistent with this, animal studies demonstrate that chronic partial sleep restriction accelerates the growth and metastasis of experimental cancer associated with a decrease in tumor dendritic, NK, and T-cells [242,243], while better sleep is associated with longer survival in human cancer patients [244,245].
Therefore, the means to improve sleep quality or quantity, or to entrain circadian rhythms, may be a potential means to ameliorate secondary immunological aging and age impairments in NK function. In addition to cognitive-behavioral therapy and therapeutic sleep restriction for insomnia and sleep hygiene for more general poor sleep [246], some specific interventions demonstrated to improve sleep in older persons include magnesium supplementation [226,227] and creating a peripheral temperature gradient via wearing socks in bed [247,248] or through pre-bed hot baths [249].
Tobacco smoking and obesity also suppress NK cell activity, which reverses upon smoking cessation or weight loss, respectively, and should be pursued if the patient can be engaged [250].
11. Harnessing NK Cells as Immunosenolytics
Senescent cells are cells that have undergone an irreversible cell-cycle arrest in response to a range of stressors or as part of physiological processes, and they are known to secrete a pleiotropy of inflammatory, growth, and matrix-degrading factors, collectively termed the senescence-associated phenotype (SASP) [13,251]. By restricting tissue renewal with age and through promotion of inflammation, senescent cells have been implicated as drivers of the degenerative aging processes and contribute to specific diseases of aging [14].
Senolytics (drugs that selectively remove senescent cells from the body) have been demonstrated to increase the health span in aging laboratory animals and are advancing into clinical trials for human translation [10,252]. However, they bear a significant potential limitation. Such drugs rely on the differential activity of essential cell metabolic pathways, including anti-apoptotic, pro-survival, mitochondrial metabolic, kinase [83,253], and glutaminolytic pathways [254]. Such mechanisms of action necessarily perturb the healthy function of non-senescent cells, raising the specter of important side-effects, despite the overall positive effects on the health span and lifespan observed in animal models of aging and its diseases. A more physiological strategy that exploits the intrinsic immunosurveillance of senescent cells might offer greater potential to favorably impact aging and diseases of aging.
Recently, the discovery of NK cell-mediated immune surveillance of senescent cells has led to an interest in harnessing this effector function as an approach to combating aging and age-related diseases [10,11,12]. As noted previously, NK cells are the chief lymphocytes responsible for the clearance of senescent cells during wound resolution, development, and other physiological processes, and also from aging or diseased tissues. Thus, understanding the causes of the NK cell functional decline with age may allow us to develop therapeutic strategies to rejuvenate or fortify NK-mediated immunosurveillance of senescent cells.
One potential approach would be to reverse age-related deficits in the intrinsic capacities of this process. As we have discussed, NK function declines with age, and although the impact of aging on NK cell toxicity against senescent cells has not yet been elucidated, the known mechanisms driving and enforcing the decline in other NK cell functions may present targets for restoring the immunosurveillance of cancerous, virally infected, and senescent cells.
A second potential immunosurveillance-enhancing approach would be to target mechanisms whereby senescent cells evade immunological clearance. Such mechanisms include the shedding of NK binding ligands from the senescent cell surface [255] by matrix metalloproteinases (which are part of the SASP) [256] and upregulation of the inhibitory receptor HLA-E [257]. Although chronic inhibition of these mechanisms may be expected to lead to excessive or off-target removal of senescent or other cells, a more transient approach, similar to that which has been employed in most preclinical studies of senolytic drugs, may open a favorable therapeutic window.
A third approach would be to fortify the endogenous armamentarium of the immune system via transfer therapy of expanded and potentially engineered NK cells. Due to their significant involvement in the immune surveillance of cancer cells, NK cells have recently become a promising tool in cancer immunotherapy [258]. Several advancements have been made to enhance the efficacy of NK cells in response to the tumor microenvironment inhibition of NK cell activity [259], including the chimeric antigen receptor (CAR)-NK cell production and ex vivo activation of NK cells by cytokines [259,260].
CAR-T cells targeting a candidate senescent cell surface marker were reported to extend survival in a murine model of post-chemotherapy senescent cell accumulation, and to partially normalize liver fibrosis resulting from either treatment with CCl4 or from diet-induced nonalcoholic steatohepatitis (NASH) [261]. Similar strategies could be exploited to engineer NK cells to target senescent cells for elimination from tissues. NK cells, rather than T-cells, are the principal actors in the immune surveillance of senescent cells specifically, but even in the cancer context, CAR-NK cells offer a number of potential advantages over CAR-T therapy [262,263]. First, the profile of cytokines released by NK cells is thought to be substantially less likely to lead to a systemic inflammatory response; such a “cytokine release syndrome” is a critical side-effect of CAR-T therapy. The risk is further reduced by less expansion of CAR-NK cells after the infusion into patients. Additionally, CAR-NK cells do not persist as long in the body as CAR-T cells after mounting an attack on tumor cells in vivo, which lowers the risk of graft vs. host disease and malignant transformation. While CAR-T cells lose their cytotoxic capacity if expression of the CAR system is lost or its target antigen is not present on subpopulations of target cells, CAR-NK, regardless, would retain their native receptors as a backup system for targeting aberrant cells.
12. Perspective and Conclusions
NK cells play critical roles in combating cancer, disease, senescence, and infection, yet their function appears to decline with age. As part of immunosenescence, decline in NK cell cytotoxicity is likely a major cause of the increased susceptibility to viral infection, increased overall disease burden, and accumulation of senescent and cancer cells observed in the elderly [264]. Considering this, a growing number of studies have sought to probe specific changes that occur in NK cell biology and functionality with aging. Uncovering changes with age in gene expression, marker expression/distribution, degranulation, and internal or systemic signaling can lead to a better understanding of how NK cells age and the impact of their local environment. These insights can aid in the development of tailored rejuvenation and intervention strategies, which may restore the functionality of native NK cells or enable the augmentation of their forces with allogenic or autologus cells.
A recent study using mass cytometry (also known as or cytometry by time-of-flight, or CyTOF) has uncovered between 6000 and 30,000 distinct NK cell phenotypes within a given individual based on unique combinations of 35 cell surface antigens, whose expression is determined by both by genetics and the environment [265]. A further characterization of surface antigens and phenotypic expression changes with age will be important to developing better therapeutic interventions for improving NK cell functions for age-related diseases.
The transplantation of allogenic NK cells has demonstrated great promise in cancer treatment and as such, demonstrate promise for the transplantation of young allogenic or cord-blood-derived NK cells for treatment of various age-related diseases and aging in general [266,267]. Furthermore, CAR-T cells have proven to be powerful tools for immunotherapy for the treatment of cancer [268,269] and, recently, CAR-NK cell have emerged as an attractive alternative [263,268]. In recent clinical trials, CAR-NK cell immunotherapy has not only been found to be effective, it has also significantly improved affordability (by using off-the-shelf NK cells), reduced sensitivity to immune checkpoints, and lowered the probability of nonspecific neurotoxicity, as compared to CAR-T therapy [270,271].
In sum, much is known about the changes with age in NK cell function, but much less about the factors driving it. New tools and technologies will increasingly enable the understanding and therapeutic intervention of NK cell aging, allowing people of increasingly advanced ages to live lives more free of infection, cancer, senescence, and ultimately advance the widely emerging revolution in longevity therapeutics.
Author Contributions
Conceptualization, A.S. (Amit Sharma) and A.S. (Alexandra Stolzing); writing—original draft preparation, A.B. and E.F.; writing—review and editing, A.B., G.Z., M.R. and T.D.A.; analysis, A.S. (Amit Sharma), A.S. (Alexandra Stolzing) and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the SENS Research Foundation (SRF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Acknowledgments
Graphical abstract was created using BioRender.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sierra, F. Geroscience and the challenges of aging societies. Aging Med. 2019, 2, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Rae, M.J.; Butler, R.N.; Campisi, J.; de Grey, A.D.; Finch, C.E.; Gough, M.; Martin, G.M.; Vijg, J.; Perrott, K.M.; Logan, B.J. The demographic and biomedical case for late-life interventions in aging. Sci. Transl. Med. 2010, 2, 40cm21. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N. The Geroscience Hypothesis: Is It Possible to Change the Rate of Aging? In Advances in Geroscience; Sierra, F., Kohanski, R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–36. [Google Scholar]
- Akbar, A.N.; Gilroy, D.W. Aging immunity may exacerbate COVID-19. Science 2020, 369, 256–257. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Barbé-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The interplay between immunosenescence and age-related diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, B. The Development and Diversity of ILCs, NK Cells and Their Relevance in Health and Diseases. Adv. Exp. Med. Biol. 2017, 1024, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; An, J.; Zou, M.H. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Passos, J.F.; Khosla, S.; Tchkonia, T.; Kirkland, J.L. Reducing Senescent Cell Burden in Aging and Disease. Trends Mol. Med. 2020, 26, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Žugich, J. The twilight of immunity: Emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.S.; Pabst, R. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol. Lett. 2007, 108, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Ferlazzo, G. Natural killer cell distribution and trafficking in human tissues. Front. Immunol. 2012, 3, 347. [Google Scholar] [CrossRef]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56bright NK Cells: An Update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef]
- Penack, O.; Gentilini, C.; Fischer, L.; Asemissen, A.M.; Scheibenbogen, C.; Thiel, E.; Uharek, L. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia 2005, 19, 835840. [Google Scholar] [CrossRef]
- Carrega, P.; Bonaccorsi, I.; Di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 2014, 192, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Lugthart, G.; Melsen, J.E.; Vervat, C.; van Ostaijen-Ten Dam, M.M.; Corver, W.E.; Roelen, D.L.; van Bergen, J.; van Tol, M.J.; Lankester, A.C.; Schilham, M.W. Human Lymphoid Tissues Harbor a Distinct CD69+CXCR6+ NK Cell Population. J. Immunol. 2016, 197, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Freud, A.G.; Caligiuri, M.A. Location and cellular stages of natural killer cell development. Trends Immunol. 2013, 34, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Klein Wolterink, R.G.; Garcia-Ojeda, M.E.; Vosshenrich, C.A.; Hendriks, R.W.; Di Santo, J.P. The intrathymic crossroads of T and NK cell differentiation. Immunol. Rev. 2010, 238, 126137. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Grzywacz, B.; Miller, J.S. Human NK Cell Development: One Road or Many? Front. Immunol. 2019, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Luetke-Eversloh, M.; Killig, M.; Romagnani, C. Signatures of human NK cell development and terminal differentiation. Front. Immunol. 2013, 4, 499. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, H.; Pricop, L.; Herberman, R.B.; Whiteside, T.L. Expression and function of CD7 molecule on human natural killer cells. J. Immunol. 1994, 152, 517–526. [Google Scholar] [PubMed]
- Higuchi, Y.; Zeng, H.; Ogawa, M. CD38 expression by hematopoietic stem cells of newborn and juvenile mice. Leukemia 2003, 17, 171–174. [Google Scholar] [CrossRef]
- Zingoni, A.; Sornasse, T.; Cocks, B.G.; Tanaka, Y.; Santoni, A.; Lanier, L.L. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J. Immunol. 2004, 173, 3716–3724. [Google Scholar] [CrossRef]
- Blanca, I.R.; Bere, E.W.; Young, H.A.; Ortaldo, J.R. Human B cell activation by autologous NK cells is regulated by CD40-CD40 ligand interaction: Role of memory B cells and CD5+ B cells. J. Immunol. 2001, 167, 6132–6139. [Google Scholar] [CrossRef] [PubMed]
- Moretta, L.; Montaldo, E.; Vacca, P.; Del Zotto, G.; Moretta, F.; Merli, P.; Locatelli, F.; Mingari, M.C. Human natural killer cells: Origin, receptors, function, and clinical applications. Int. Arch. Allergy Immunol. 2014, 164, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Wu, J.; Bakker, A.B.; Phillips, J.H.; Lanier, L.L. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J. Immunol. 1998, 161, 7–10. [Google Scholar] [PubMed]
- Cosman, D.; Mullberg, J.; Sutherland, C.L.; Chin, W.; Armitage, R.; Fanslow, W.; Kubin, M.; Chalupny, N.J. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Jamieson, A.M.; Diefenbach, A.; McMahon, C.W.; Xiong, N.; Carlyle, J.R.; Raulet, D.H. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 2002, 17, 19–29. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef]
- Gayoso, I.; Sanchez-Correa, B.; Campos, C.; Alonso, C.; Pera, A.; Casado, J.G.; Morgado, S.; Tarazona, R.; Solana, R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011, 3, 337–343. [Google Scholar] [CrossRef]
- Narni-Mancinelli, E.; Ugolini, S.; Vivier, E. Tuning the threshold of natural killer cell responses. Curr. Opin. Immunol. 2013, 25, 53–58. [Google Scholar] [CrossRef]
- Canossi, A.; Aureli, A.; Del Beato, T.; Rossi, P.; Franceschilli, L.; De Sanctis, F.; Sileri, P.; di Lorenzo, N.; Buonomo, O.; Lauro, D.; et al. Role of KIR and CD16A genotypes in colorectal carcinoma genetic risk and clinical stage. J. Transl. Med. 2016, 14, 239. [Google Scholar] [CrossRef]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; Lopez-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Latour, S.; Davidson, D. Negative regulation of immunoreceptor signaling. Annu. Rev. Immunol. 2002, 20, 669–707. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, W.M.; Kim, S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol. Rev. 2006, 214, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kadri, N.; Thanh, T.L.; Höglund, P. Selection, tuning, and adaptation in mouse NK cell education. Immunol. Rev. 2015, 267, 167–177. [Google Scholar] [CrossRef]
- Pahl, J.; Cerwenka, A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology 2017, 222, 11–20. [Google Scholar] [CrossRef]
- Moesta, A.K.; Parham, P. Diverse functionality among human NK cell receptors for the C1 epitope of HLA-C: KIR2DS2, KIR2DL2, and KIR2DL3. Front. Immunol. 2012, 3, 336. [Google Scholar] [CrossRef]
- Makrigiannis, A.P.; Anderson, S.K. Ly49 gene expression in different inbred mouse strains. Immunol. Res. 2000, 21, 39–47. [Google Scholar] [CrossRef]
- Sternberg-Simon, M.; Brodin, P.; Pickman, Y.; Onfelt, B.; Karre, K.; Malmberg, K.J.; Hoglund, P.; Mehr, R. Natural killer cell inhibitory receptor expression in humans and mice: A closer look. Front. Immunol. 2013, 4, 65. [Google Scholar] [CrossRef]
- Muzzioli, M.; Stecconi, R.; Moresi, R.; Provinciali, M. Zinc improves the development of human CD34+ cell progenitors towards NK cells and increases the expression of GATA-3 transcription factor in young and old ages. Biogerontology 2009, 10, 593–604. [Google Scholar] [CrossRef]
- Ligthart, G.J.; van Vlokhoven, P.C.; Schuit, H.R.; Hijmans, W. The expanded null cell compartment in ageing: Increase in the number of natural killer cells and changes in T-cell and NK-cell subsets in human blood. Immunology 1986, 59, 353–357. [Google Scholar] [PubMed]
- Facchini, A.; Mariani, E.; Mariani, A.R.; Papa, S.; Vitale, M.; Manzoli, F.A. Increased number of circulating Leu 11+ (CD 16) large granular lymphocytes and decreased NK activity during human ageing. Clin. Exp. Immunol. 1987, 68, 340–347. [Google Scholar] [PubMed]
- Krishnaraj, R.; Svanborg, A. Preferential accumulation of mature NK cells during human immunosenescence. J. Cell. Biochem. 1992, 50, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Zamai, L.; Neri, L.M.; Galanzi, A.; Facchini, A.; Rana, R.; Cataldi, A.; Papa, S. The impairment of natural killer function in the healthy aged is due to a postbinding deficient mechanism. Cell. Immunol. 1992, 145, 1–10. [Google Scholar] [CrossRef]
- Sansoni, P.; Cossarizza, A.; Brianti, V.; Fagnoni, F.; Snelli, G.; Monti, D.; Marcato, A.; Passeri, G.; Ortolani, C.; Forti, E.; et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood 1993, 82, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Monaco, M.C.; Cattini, L.; Sinoppi, M.; Facchini, A. Distribution and lytic activity of NK cell subsets in the elderly. Mech. Ageing Dev. 1994, 76, 177–187. [Google Scholar] [CrossRef]
- Kutza, J.; Murasko, D.M. Effects of aging on natural killer cell activity and activation by interleukin-2 and IFN-α. Cell. Immunol. 1994, 155, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kutza, J.; Murasko, D.M. Age-associated decline in IL-2 and IL-12 induction of LAK cell activity of human PBMC samples. Mech. Ageing Dev. 1996, 90, 209–222. [Google Scholar] [CrossRef]
- Mariani, E.; Sgobbi, S.; Meneghetti, A.; Tadolini, M.; Tarozzi, A.; Sinoppi, M.; Cattini, L.; Facchini, A. Perforins in human cytolytic cells: The effect of age. Mech. Ageing Dev. 1996, 92, 195–209. [Google Scholar] [CrossRef]
- Mariani, E.; Mariani, A.R.; Meneghetti, A.; Tarozzi, A.; Cocco, L.; Facchini, A. Age-dependent decreases of NK cell phosphoinositide turnover during spontaneous but not Fc-mediated cytolytic activity. Int. Immunol. 1998, 10, 981–989. [Google Scholar] [CrossRef][Green Version]
- Borrego, F.; Alonso, M.C.; Galiani, M.D.; Carracedo, J.; Ramirez, R.; Ostos, B.; Pena, J.; Solana, R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp. Gerontol. 1999, 34, 253–265. [Google Scholar] [CrossRef]
- Lutz, C.T.; Moore, M.B.; Bradley, S.; Shelton, B.J.; Lutgendorf, S.K. Reciprocal age related change in natural killer cell receptors for MHC class I. Mech. Ageing Dev. 2005, 126, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Le Garff-Tavernier, M.; Beziat, V.; Decocq, J.; Siguret, V.; Gandjbakhch, F.; Pautas, E.; Debre, P.; Merle-Beral, H.; Vieillard, V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 2010, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Hayhoe, R.P.; Henson, S.M.; Akbar, A.N.; Palmer, D.B. Variation of human natural killer cell phenotypes with age: Identification of a unique KLRG1-negative subset. Hum. Immunol. 2010, 71, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Oliveira, A.; Smith-Carvalho, M.; Porto, L.C.; Cardoso-Oliveira, J.; Ribeiro Ados, S.; Falcao, R.R.; Abdelhay, E.; Bouzas, L.F.; Thuler, L.C.; Ornellas, M.H.; et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum. Immunol. 2011, 72, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Muller-Durovic, B.; Lanna, A.; Covre, L.P.; Mills, R.S.; Henson, S.M.; Akbar, A.N. Killer Cell Lectin-like Receptor G1 Inhibits NK Cell Function through Activation of Adenosine 5’-Monophosphate-Activated Protein Kinase. J. Immunol. 2016, 197, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Gounder, S.S.; Abdullah, B.J.J.; Radzuanb, N.; Zain, F.; Sait, N.B.M.; Chua, C.; Subramani, B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal. Cell. Pathol. 2018, 2018, 7871814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wallace, D.L.; de Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.; et al. In vivo kinetics of human natural killer cells: The effects of ageing and acute and chronic viral infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Crinier, A.; Milpied, P.; Escaliere, B.; Piperoglou, C.; Galluso, J.; Balsamo, A.; Spinelli, L.; Cervera-Marzal, I.; Ebbo, M.; Girard-Madoux, M.; et al. High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity 2018, 49, 971–986.e5. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Siebert, J.R.; Burns, R.; Gerbec, Z.J.; Bonacci, B.; Rymaszewski, A.; Rau, M.; Riese, M.J.; Rao, S.; Carlson, K.S.; et al. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nat. Commun. 2019, 10, 3931. [Google Scholar] [CrossRef]
- Smith, S.L.; Kennedy, P.R.; Stacey, K.B.; Worboys, J.D.; Yarwood, A.; Seo, S.; Solloa, E.H.; Mistretta, B.; Chatterjee, S.S.; Gunaratne, P.; et al. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv. 2020, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, X.; Le, W.; Xie, L.; Li, H.; Wen, W.; Wang, S.; Ma, S.; Huang, Z.; Ye, J.; et al. A human circulating immune cell landscape in aging and COVID-19. Protein Cell 2020, 11, 740–770. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 2020, 180, 749–763.e13. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; An, E.; Shioi, Y.; Nakamura, K.; Luo, S.; Yokose, N.; Minami, S.; Dan, K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 2001, 124, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Yokose, N.; Tamura, H.; An, E.; Nakamura, K.; Dan, K.; Nomura, T. Natural killer cells in the late decades of human life. Clin. Immunol. Immunopathol. 1997, 84, 269–275. [Google Scholar] [CrossRef]
- Pierson, B.; Miller, J. CD56+bright and CD56+dim natural killer cells in patients with chronic myelogenous leukemia progressively decrease in number, respond less to stimuli that recruit clonogenic natural killer cells, and exhibit decreased proliferation on a per cell basis. Blood 1996, 88, 2279–2287. [Google Scholar] [CrossRef]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell. Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Vieira, S.M.; Lemos, H.P.; Grespan, R.; Napimoga, M.H.; Dal-Secco, D.; Freitas, A.; Cunha, T.M.; Verri, W.A., Jr.; Souza-Junior, D.A.; Jamur, M.C.; et al. A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br. J. Pharm. 2009, 158, 779–789. [Google Scholar] [CrossRef]
- Billiau, A. Interferon-gamma: Biology and role in pathogenesis. Adv. Immunol. 1996, 62, 61–130. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef] [PubMed]
- Soto-Gamez, A.; Quax, W.J.; Demaria, M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J. Mol. Biol. 2019, 431, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Carson, W.E.; Fehniger, T.A.; Haldar, S.; Eckhert, K.; Lindemann, M.J.; Lai, C.F.; Croce, C.M.; Baumann, H.; Caligiuri, M.A. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Investig. 1997, 99, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Pahlavani, M.A.; Richardson, A. The effect of age on the expression of interleukin-2. Mech. Ageing Dev. 1996, 89, 125–154. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, Z.; Wei, H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front. Immunol. 2017, 8, 930. [Google Scholar] [CrossRef] [PubMed]
- Kaszubowska, L.; Foerster, J.; Schetz, D.; Kmiec, Z. CD56bright cells respond to stimulation until very advanced age revealing increased expression of cellular protective proteins SIRT1, HSP70 and SOD2. Immun. Ageing 2018, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Kaszubowska, L.; Foerster, J.; Kaczor, J.J.; Schetz, D.; Slebioda, T.J.; Kmiec, Z. NK cells of the oldest seniors represent constant and resistant to stimulation high expression of cellular protective proteins SIRT1 and HSP70. Immun. Ageing 2018, 15, 12. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Niu, J.; Zhou, Z.; Zhang, J.; Tian, Z. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Hum. Immunol. 2008, 69, 490–500. [Google Scholar] [CrossRef]
- Gately, M.K.; Renzetti, L.M.; Magram, J.; Stern, A.S.; Adorini, L.; Gubler, U.; Presky, D.H. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998, 16, 495–521. [Google Scholar] [CrossRef]
- Krishnaraj, R.; Bhooma, T. Cytokine sensitivity of human NK cells during immunosenescence. 2. IL2-induced interferon gamma secretion. Immunol. Lett. 1996, 50, 59–63. [Google Scholar] [CrossRef]
- Krishnaraj, R. Senescence and cytokines modulate the NK cell expression. Mech. Ageing Dev. 1997, 96, 89–101. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L.; Neri, S.; Dolzani, P.; Meneghetti, A.; Silvestri, T.; Ravaglia, G.; Forti, P.; Cattini, L.; Facchini, A. RANTES and MIP-1alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp. Gerontol. 2002, 37, 219–226. [Google Scholar] [CrossRef]
- Mariani, E.; Meneghetti, A.; Neri, S.; Ravaglia, G.; Forti, P.; Cattini, L.; Facchini, A. Chemokine production by natural killer cells from nonagenarians. Eur. J. Immunol. 2002, 32, 1524–1529. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L.; Meneghetti, A.; Dolzani, P.; Mazzetti, I.; Neri, S.; Ravaglia, G.; Forti, P.; Facchini, A. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech. Ageing Dev. 2001, 122, 1383–1395. [Google Scholar] [CrossRef]
- Orange, J.S.; Ballas, Z.K. Natural killer cells in human health and disease. Clin. Immunol. 2006, 118, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.; Altfeld, M. Control of human viral infections by natural killer cells. Annu. Rev. Immunol. 2013, 31, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, J.; Lord, J.M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res. Rev. 2013, 12, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Henley, S.J.; Singh, S.D.; King, J.; Wilson, R.J.; O’Neil, M.E.; Ryerson, A.B. Invasive Cancer Incidence and Survival-United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; Ershler, W.B. Cancer and ageing: A nexus at several levels. Nat. Rev. Cancer 2005, 5, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.Y.; Powis, S.J. Does Natural Killer Cell Deficiency (NKD) Increase the Risk of Cancer? NKD May Increase the Risk of Some Virus Induced Cancer. Front. Immunol. 2019, 10, 1703. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Sunwoo, J.B.; Bui, J.D. Cancer Immunosurveillance and Immunoediting by Natural Killer Cells. Cancer J. 2013, 19, 483–489. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Meyers, K.M.; Prieur, D.J.; Starkey, J.R. Role of NK cells in tumour growth and metastasis in beige mice. Nature 1980, 284, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P.; Edwards, A.; Honeyman, M.; McCarthy, W.H. Low natural-killer-cell activity in familial melanoma patients and their relatives. Br. J. Cancer 1979, 40, 113–122. [Google Scholar] [CrossRef]
- Strayer, D.R.; Carter, W.A.; Mayberry, S.D.; Pequignot, E.; Brodsky, I. Low natural cytotoxicity of peripheral blood mononuclear cells in individuals with high familial incidences of cancer. Cancer Res. 1984, 44, 370–374. [Google Scholar]
- Nakajima, T.; Mizushima, N.; Kanai, K. Relationship between natural killer activity and development of hepatocellular carcinoma in patients with cirrhosis of the liver. Jpn. J. Clin. Oncol. 1987, 17, 327–332. [Google Scholar]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Tarazona, R.; DelaRosa, O.; Alonso, C.; Ostos, B.; Espejo, J.; Pena, J.; Solana, R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech. Ageing Dev. 2000, 121, 77–88. [Google Scholar] [CrossRef]
- Hazeldine, J.; Hampson, P.; Lord, J.M. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell 2012, 11, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.D.; Ross, O.A.; Curran, M.D.; Rea, I.M.; Middleton, D. Investigation of KIR diversity in immunosenescence and longevity within the Irish population. Exp. Gerontol. 2004, 39, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Listi, F.; Caruso, C.; Colonna-Romano, G.; Lio, D.; Nuzzo, D.; Candore, G. HLA and KIR frequencies in Sicilian Centenarians. Rejuvenation Res. 2010, 13, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, D.; Laskarin, G.; Rubesa, G.; Strbo, N.; Bedenicki, I.; Manestar, D.; Glavas, M.; Christmas, S.E.; Podack, E.R. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood 1998, 92, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L.; Long, E.O. Cytotoxic immunological synapses. Immunol. Rev. 2010, 235, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Maul-Pavicic, A.; Chiang, S.C.; Rensing-Ehl, A.; Jessen, B.; Fauriat, C.; Wood, S.M.; Sjoqvist, S.; Hufnagel, M.; Schulze, I.; Bass, T.; et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc. Natl. Acad. Sci. USA 2011, 108, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- McCarl, C.A.; Khalil, S.; Ma, J.; Oh-hora, M.; Yamashita, M.; Roether, J.; Kawasaki, T.; Jairaman, A.; Sasaki, Y.; Prakriya, M.; et al. Store-operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J. Immunol. 2010, 185, 5845–5858. [Google Scholar] [CrossRef] [PubMed]
- Zweifach, A. Target-cell contact activates a highly selective capacitative calcium entry pathway in cytotoxic T lymphocytes. J. Cell Biol. 2000, 148, 603–614. [Google Scholar] [CrossRef]
- Schwindling, C.; Quintana, A.; Krause, E.; Hoth, M. Mitochondria positioning controls local calcium influx in T cells. J. Immunol. 2010, 184, 184–190. [Google Scholar] [CrossRef]
- Pores-Fernando, A.T.; Zweifach, A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol. Rev. 2009, 231, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Korkotian, E. Endoplasmic reticulum calcium stores in dendritic spines. Front. Neuroanat. 2014, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Kopf, A.; Kiermaier, E. Dynamic Microtubule Arrays in Leukocytes and Their Role in Cell Migration and Immune Synapse Formation. Front. Cell Dev. Biol. 2021, 9, 635511. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.P.; Pandey, R.; Zheng, R.; Suhoski, M.M.; Monaco-Shawver, L.; Orange, J.S. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J. Exp. Med. 2007, 204, 2305–2320. [Google Scholar] [CrossRef] [PubMed]
- Krzewski, K.; Coligan, J.E. Human NK cell lytic granules and regulation of their exocytosis. Front. Immunol. 2012, 3, 335. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, H.; Goses, Y.; Reshef, T.; Klajman, A. Interleukin-2 production and activity in aged humans. Mech. Ageing Dev. 1985, 32, 213–226. [Google Scholar] [CrossRef]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Peng, Y.P.; Zhu, Y.; Zhang, J.J.; Xu, Z.K.; Qian, Z.Y.; Dai, C.C.; Jiang, K.R.; Wu, J.L.; Gao, W.T.; Li, Q.; et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J. Transl. Med. 2013, 11, 262. [Google Scholar] [CrossRef]
- Sun, C.; Sun, H.Y.; Xiao, W.H.; Zhang, C.; Tian, Z.G. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharm. Sin. 2015, 36, 1191–1199. [Google Scholar] [CrossRef]
- Fali, T.; Papagno, L.; Bayard, C.; Mouloud, Y.; Boddaert, J.; Sauce, D.; Appay, V. New Insights into Lymphocyte Differentiation and Aging from Telomere Length and Telomerase Activity Measurements. J. Immunol. 2019, 202, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Meneghetti, A.; Formentini, I.; Neri, S.; Cattini, L.; Ravaglia, G.; Forti, P.; Facchini, A. Telomere length and telomerase activity: Effect of ageing on human NK cells. Mech. Ageing Dev. 2003, 124, 403–408. [Google Scholar] [CrossRef]
- Ouyang, Q.; Baerlocher, G.; Vulto, I.; Lansdorp, P.M. Telomere length in human natural killer cell subsets. Ann. N. Y. Acad. Sci. 2007, 1106, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Alemán, H.; Esparza, J.; Ramirez, F.A.; Astiazaran, H.; Payette, H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Aging 2011, 40, 469–475. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Swardfager, W.; Lanctôt, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A Meta-Analysis of Cytokines in Alzheimer’s Disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef]
- Shehata, H.M.; Hoebe, K.; Chougnet, C.A. The aged nonhematopoietic environment impairs natural killer cell maturation and function. Aging Cell 2015, 14, 191–199. [Google Scholar] [CrossRef]
- Sungur, C.M.; Murphy, W.J. Utilization of mouse models to decipher natural killer cell biology and potential clinical applications. Hematology 2013, 2013, 227–233. [Google Scholar] [CrossRef]
- Quinn, L.S.; Anderson, B.G.; Strait-Bodey, L.; Wolden-Hanson, T. Serum and muscle interleukin-15 levels decrease in aging mice: Correlation with declines in soluble interleukin-15 receptor alpha expression. Exp. Gerontol. 2010, 45, 106–112. [Google Scholar] [CrossRef]
- Jergovic, M.; Thompson-Ryder, H.L.; Asghar, A.; Nikolich-Zugich, J. The role of Interleukin-6 in age-related frailty syndrome. J. Immunol. 2020, 204, 59.17. [Google Scholar]
- Liu, H.; Huang, Y.; Lyu, Y.; Dai, W.; Tong, Y.; Li, Y. GDF15 as a biomarker of ageing. Exp. Gerontol. 2021, 146, 111228. [Google Scholar] [CrossRef] [PubMed]
- Tavenier, J.; Rasmussen, L.J.H.; Andersen, A.L.; Houlind, M.B.; Langkilde, A.; Andersen, O.; Petersen, J.; Nehlin, J.O. Association of GDF15 with Inflammation and Physical Function during Aging and Recovery after Acute Hospitalization: A Longitudinal Study of Older Patients and Age-Matched Controls. J. Gerontol. Ser. A 2021, 76, 964–974. [Google Scholar] [CrossRef]
- Tomescu, C.; Chehimi, J.; Maino, V.C.; Montaner, L.J. Retention of viability, cytotoxicity, and response to IL-2, IL-15, or IFN-alpha by human NK cells after CD107a degranulation. J. Leukoc. Biol. 2009, 85, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Jasaputra, D.K.; Sumitro, S.B.; Widodo, M.A.; Mozef, T.; Rizal, R.; Kusuma, H.S.; Laksmitawati, D.R.; Murti, H.; Bachtiar, I.; et al. Effect of interleukins (IL-2, IL-15, IL-18) on receptors activation and cytotoxic activity of natural killer cells in breast cancer cell. Afr. Health Sci. 2020, 20, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, J.; Holm, J.; Schersten, T. Incidence of bacteria after skin wash of surgical patients in hospitals. Lakartidningen 1979, 76, 969–970. [Google Scholar]
- Ligthart, G.J.; Corberand, J.X.; Fournier, C.; Galanaud, P.; Hijmans, W.; Kennes, B.; Müller-Hermelink, H.K.; Steinmann, G.G. Admission criteria for immunogerontological studies in man: The SENIEUR protocol. Mech. Ageing Dev. 1984, 28, 47–55. [Google Scholar] [CrossRef]
- Wiencke, J.K.; Butler, R.; Hsuang, G.; Eliot, M.; Kim, S.; Sepulveda, M.A.; Siegel, D.; Houseman, E.A.; Kelsey, K.T. The DNA methylation profile of activated human natural killer cells. Epigenetics 2016, 11, 363–380. [Google Scholar] [CrossRef]
- Schenk, A.; Bloch, W.; Zimmer, P. Natural Killer Cells—An Epigenetic Perspective of Development and Regulation. Int. J. Mol. Sci. 2016, 17, 326. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Miller, J.; Anderson, S.; Bryceson, Y. Epigenetic regulation of NK cell differentiation and effector functions. Front. Immunol. 2013, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Merino, A.; Zhang, B.; Dougherty, P.; Luo, X.; Wang, J.; Blazar, B.R.; Miller, J.S.; Cichocki, F. Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J. Clin. Investig. 2019, 129, 3770–3785. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Bloch, W.; Schenk, A.; Zopf, E.M.; Hildebrandt, U.; Streckmann, F.; Beulertz, J.; Koliamitra, C.; Schollmayer, F.; Baumann, F. Exercise-induced Natural Killer Cell Activation is Driven by Epigenetic Modifications. Int. J. Sports Med. 2015, 36, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Misale, M.S.; Witek Janusek, L.; Tell, D.; Mathews, H.L. Chromatin organization as an indicator of glucocorticoid induced natural killer cell dysfunction. Brain Behav. Immun. 2018, 67, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, T.H.; Dekkers, K.F.; Slieker, R.C.; Grant, C.D.; Ikram, M.A.; van Greevenbroek, M.M.J.; Franke, L.; Veldink, J.H.; Boomsma, D.I.; Slagboom, P.E.; et al. Functional genomics analysis identifies T and NK cell activation as a driver of epigenetic clock progression. Genome Biol. 2022, 23, 24. [Google Scholar] [CrossRef]
- Wyman, P.A.; Moynihan, J.; Eberly, S.; Cox, C.; Cross, W.; Jin, X.; Caserta, M.T. Association of Family Stress with Natural Killer Cell Activity and the Frequency of Illnesses in Children. Arch. Pediatrics Adolesc. Med. 2007, 161, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, N.J.; Cox, W.I.; Horner, H.C. Direct suppression of natural killer activity in human peripheral blood leukocyte cultures by glucocorticoids and its modulation by interferon. Cancer Res. 1983, 43, 4019–4025. [Google Scholar] [PubMed]
- Nair, M.P.; Schwartz, S.A. Immunomodulatory effects of corticosteroids on natural killer and antibody-dependent cellular cytotoxic activities of human lymphocytes. J. Immunol. 1984, 132, 2876–2882. [Google Scholar]
- Parrillo, J.E.; Fauci, A.S. Comparison of the Effector Cells in Human Spontaneous Cellular Cytotoxicity and Antibody-Dependent Cellular Cytotoxicity: Differential Sensitivity of Effector Cells to In Vivo and In Vitro Corticosteroids. Scand. J. Immunol. 1978, 8, 99–107. [Google Scholar] [CrossRef]
- Yiallouris, A.; Tsioutis, C.; Agapidaki, E.; Zafeiri, M.; Agouridis, A.P.; Ntourakis, D.; Johnson, E.O. Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Front. Endocrinol. 2019, 10, 54. [Google Scholar] [CrossRef]
- Luz, C.; Dornelles, F.; Preissler, T.; Collaziol, D.; da Cruz, I.M.; Bauer, M.E. Impact of psychological and endocrine factors on cytokine production of healthy elderly people. Mech. Ageing Dev. 2003, 124, 887–895. [Google Scholar] [CrossRef]
- Gatti, G.; Cavallo, R.; Sartori, M.L.; del Ponte, D.; Masera, R.; Salvadori, A.; Carignola, R.; Angeli, A. Inhibition by cortisol of human natural killer (NK) cell activity. J. Steroid Biochem. 1987, 26, 49–58. [Google Scholar] [CrossRef]
- Eddy, J.L.; Krukowski, K.; Janusek, L.; Mathews, H.L. Glucocorticoids regulate natural killer cell function epigenetically. Cell. Immunol. 2014, 290, 120–130. [Google Scholar] [CrossRef]
- Duan, X.; Lu, J.; Wang, H.; Liu, X.; Wang, J.; Zhou, K.; Jiang, W.; Wang, Y.; Fang, M. Bidirectional factors impact the migration of NK cells to draining lymph node in aged mice during influenza virus infection. Exp. Gerontol. 2017, 96, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, K.; Hermodsson, S.; Strannegård, O. Evidence for a beta-adrenoceptor-mediated regulation of human natural killer cells. J. Immunol. 1985, 134, 4095–4099. [Google Scholar] [PubMed]
- Sun, Z.; Hou, D.; Liu, S.; Fu, W.; Wang, J.; Liang, Z. Norepinephrine inhibits the cytotoxicity of NK92-MI cells via the β2-adrenoceptor/cAMP/PKA/p-CREB signaling pathway. Mol. Med. Rep. 2018, 17, 8530–8535. [Google Scholar] [CrossRef] [PubMed]
- Stepien, H.; Zelazowski, P.; Döhler, K.D.; Pawlikowski, M. Neuropeptides and Natural Killer Cell Activity. In Progress in Neuropeptide Research; Birkhäuser: Basel, Switzerland, 1988; pp. 25–31. [Google Scholar] [CrossRef]
- Koller, A.; Bianchini, R.; Schlager, S.; Münz, C.; Kofler, B.; Wiesmayr, S. The neuropeptide galanin modulates natural killer cell function. Neuropeptides 2017, 64, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Feistritzer, C.; Clausen, J.; Sturn, D.H.; Djanani, A.; Gunsilius, E.; Wiedermann, C.J.; Kähler, C.M. Natural killer cell functions mediated by the neuropeptide substance P. Regul. Pept. 2003, 116, 119–126. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Marconi, G.D.; Zara, S.; Cataldi, A.; Porzionato, A.; Di Giulio, C. In the carotid body, galanin is a signal for neurogenesis in young, and for neurodegeneration in the old and in drug-addicted subjects. Front. Physiol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Barbariga, M.; Rabiolo, A.; Fonteyne, P.; Bignami, F.; Rama, P.; Ferrari, G. The Effect of Aging on Nerve Morphology and Substance P Expression in Mouse and Human Corneas. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5329–5335. [Google Scholar] [CrossRef]
- Ni, F.; Sun, R.; Fu, B.; Wang, F.; Guo, C.; Tian, Z.; Wei, H. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat. Commun. 2013, 4, 1479. [Google Scholar] [CrossRef]
- Bidlingmaier, M.; Friedrich, N.; Emeny, R.T.; Spranger, J.; Wolthers, O.D.; Roswall, J.; Körner, A.; Obermayer-Pietsch, B.; Hübener, C.; Dahlgren, J.; et al. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: Results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J. Clin. Endocrinol. Metab. 2014, 99, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Ramsey, M.; Carter, C.S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 2005, 4, 195–212. [Google Scholar] [CrossRef]
- Salminen, A. Feed-forward regulation between cellular senescence and immunosuppression promotes the aging process and age-related diseases. Ageing Res. Rev. 2021, 67, 101280. [Google Scholar] [CrossRef]
- O’Connor, J.C.; McCusker, R.H.; Strle, K.; Johnson, R.W.; Dantzer, R.; Kelley, K.W. Regulation of IGF-I function by proinflammatory cytokines: At the interface of immunology and endocrinology. Cell. Immunol. 2008, 252, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, T.; Wu, X.; Mansour, A.G.; Lu, T.; Zhang, J.; Wang, L.S.; Caligiuri, M.A.; Yu, J. PDGF-D-PDGFRβ signaling enhances IL-15-mediated human natural killer cell survival. Proc. Natl. Acad. Sci. USA 2022, 119, e2114134119. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Edeling, M.A.; Trifonov, V.; Luo, J.; Goyal, P.; Bohl, B.; Bando, J.K.; Kim, A.H.; Walker, J.; Andahazy, M.; et al. Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell 2018, 172, 534–548.e19. [Google Scholar] [CrossRef] [PubMed]
- Drubaix, I.; Giakoumakis, A.; Robert, L.; Robert, A.M. Preliminary data on the age-dependent decrease in basic fibroblast growth factor and platelet-derived growth factor in the human vein wall and in their influence on cell proliferation. Gerontology 1998, 44, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Benatti, B.B.; Silvério, K.G.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Influence of aging on biological properties of periodontal ligament cells. Connect. Tissue Res. 2008, 49, 401–408. [Google Scholar] [CrossRef]
- Zhou, L.; Dong, J.; Yu, M.; Yin, H.; She, M. Age-dependent increase of NF-κB translocation and PDGF-B expression in aortic endothelial cells of hypercholesterolemic rats. Exp. Gerontol. 2003, 38, 1161–1168. [Google Scholar] [CrossRef]
- Slattery, K.; Gardiner, C.M. NK Cell Metabolism and TGF–Implications for Immunotherapy. Front. Immunol. 2019, 10, 2915. [Google Scholar] [CrossRef]
- Wilson, E.B.; El-Jawhari, J.J.; Neilson, A.L.; Hall, G.D.; Melcher, A.A.; Meade, J.L.; Cook, G.P. Human tumour immune evasion via TGF-β blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS ONE 2011, 6, e22842. [Google Scholar] [CrossRef] [PubMed]
- Viel, S.; Marçais, A.; Guimaraes, F.S.-F.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016, 9, ra19. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.; Steinle, A. Impairment of NKG2D-Mediated Tumor Immunity by TGF-β. Front. Immunol. 2019, 10, 2689. [Google Scholar] [CrossRef] [PubMed]
- Frippiat, C.; Chen, Q.M.; Zdanov, S.; Magalhaes, J.P.; Remacle, J.; Toussaint, O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J. Biol. Chem. 2001, 276, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Forsey, R.J.; Thompson, J.M.; Ernerudh, J.; Hurst, T.L.; Strindhall, J.; Johansson, B.; Nilsson, B.O.; Wikby, A. Plasma cytokine profiles in elderly humans. Mech. Ageing Dev. 2003, 124, 487–493. [Google Scholar] [CrossRef]
- Anstey, K.; Stankov, L.; Lord, S. Primary aging, secondary aging, and intelligence. Psychol. Aging 1993, 8, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Busse, E.W.; Pfeiffer, E. Behavior and Adaptation in Late Life; Little, Brown: Boston, MA, USA, 1977. [Google Scholar]
- Zhimulev, I.F.; Belyaeva, E.S.; Bgatov, A.V.; Baricheva, E.M.; Vlassova, I.E. Cytogenetic and molecular aspects of position effect variegation in Drosophila melanogaster. II. Peculiarities of morphology and genetic activity of the 2B region in the T(1;2)dorvar7 chromosome in males. Chromosoma 1988, 96, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zealley, B.; de Grey, A.D. Strategies for engineered negligible senescence. Gerontology 2013, 59, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, Á.J.R. Zinc, aging, and immunosenescence: An overview. Pathobiol. Aging Age-Relat. Dis. 2015, 5, 25592. [Google Scholar] [CrossRef]
- Prasad, A.S.; Fitzgerald, J.T.; Hess, J.W.; Kaplan, J.; Pelen, F.; Dardenne, M. Zinc deficiency in elderly patients. Nutrition 1993, 9, 218–224. [Google Scholar] [PubMed]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ströhle, A.; Hahn, A. Age-associated changes in the metabolism of vitamin B(12) and folic acid: Prevalence, aetiopathogenesis and pathophysiological consequences. Z. Gerontol. Geriatr. 2004, 37, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Mocchegiani, E.; Rink, L. Correlation between zinc status and immune function in the elderly. Biogerontology 2006, 7, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Elmadfa, I.; Meyer, A.L. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Apostolopoulos, V. Vitamin B12, Folic Acid, and the Immune System. In Nutrition and Immunity; Springer: Cham, Switzerland, 2019; pp. 103–114. [Google Scholar] [CrossRef]
- Nour Zahi Gammoh, L.R. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Mertens, K.; Lowes, D.A.; Webster, N.R.; Talib, J.; Hall, L.; Davies, M.J.; Beattie, J.H.; Galley, H.F. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br. J. Anasthesia 2015, 114, 990–999. [Google Scholar] [CrossRef]
- Busse, B.; Bale, H.A.; Zimmermann, E.A.; Panganiban, B.; Barth, H.D.; Carriero, A.; Vettorazzi, E.; Zustin, J.; Hahn, M.; Ager, J.W., 3rd; et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci. Transl. Med. 2013, 5, 193ra188. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Lee, J.; Terasawa, T.; Lau, J.; Trikalinos, T.A. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: An updated meta-analysis for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011, 155, 827–838. [Google Scholar] [CrossRef]
- Kruman, I.I.; Kumaravel, T.S.; Lohani, A.; Pedersen, W.A.; Cutler, R.G.; Kruman, Y.; Haughey, N.; Lee, J.; Evans, M.; Mattson, M.P. Folic Acid Deficiency and Homocysteine Impair DNA Repair in Hippocampal Neurons and Sensitize Them to Amyloid Toxicity in Experimental Models of Alzheimer’s Disease. J. Neurosci. 2002, 22, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Partearroyo, T.; Úbeda, N.; Montero, A.; Achón, M.; Varela-Moreiras, G. Vitamin B12 and Folic Acid Imbalance Modifies NK Cytotoxicity, Lymphocytes B and Lymphoprolipheration in Aged Rats. Nutrients 2013, 5, 4836–4848. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Jahaj, E.; Pratikaki, M.; Keskinidou, C.; Detsika, M.; Grigoriou, E.; Psarra, K.; Orfanos, S.E.; Tsirogianni, A.; Dimopoulou, I.; et al. Vitamin D deficiency correlates with a reduced number of natural killer cells in intensive care unit (ICU) and non-ICU patients with COVID-19 pneumonia. Hell. J. Cardiol. 2021, 62, 381. [Google Scholar] [CrossRef] [PubMed]
- Tamura, J.; Kubota, K.; Murakami, H.; Sawamura, M.; Matsushima, T.; Tamura, T.; Saitoh, T.; Kurabayshi, H.; Naruse, T. Immunomodulation by vitamin B12: Augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 2001, 116, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Neri, S.; Cattini, L.; Mocchegiani, E.; Malavolta, M.; Dedoussis, G.V.; Kanoni, S.; Rink, L.; Jajte, J.; Facchini, A. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: Interactive influence of +647 MT1a and −174 IL-6 polymorphic alleles. Exp. Gerontol. 2008, 43, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J. Clin. Immunol. 2001, 21, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Bunout, D.; Barrera, G.; Hirsch, S.; Gattas, V.; de la Maza, M.P.; Haschke, F.; Steenhout, P.; Klassen, P.; Hager, C.; Avendaño, M.; et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. JPEN J. Parenter. Enter. Nutr. 2004, 28, 348–354. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mei, K.; Xie, L.; Yuan, P.; Ma, J.; Yu, P.; Zhu, W.; Zheng, C.; Liu, X. Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: A meta-analysis and GRADE assessment of cohort studies and RCTs. Nutr. J. 2021, 20, 89. [Google Scholar] [CrossRef]
- Dancer, R.C.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M.; Rowiesha, M.; Elmashad, A.; Elrifaey, S.M.; Elhorany, H.; Koura, H.G. Vitamin D status in preterm neonates and the effects of its supplementation on respirat.tory distress syndrome. Pediatr. Pulmonol. 2020, 55, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Ravaglia, G.; Forti, P.; Meneghetti, A.; Tarozzi, A.; Maioli, F.; Boschi, F.; Pratelli, L.; Pizzoferrato, A.; Piras, F.; et al. Vitamin D, thyroid hormones and muscle mass influence natural killer (NK) innate immunity in healthy nonagenarians and centenarians. Clin. Exp. Immunol. 1999, 116, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Meydani, S.N. Age-associated changes in immune function: Impact of vitamin E intervention and the underlying mechanisms. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 283–289. [Google Scholar] [CrossRef]
- Madison, A.A.; Shrout, M.R.; Renna, M.E.; Kiecolt-Glaser, J.K. Psychological and Behavioral Predictors of Vaccine Efficacy: Considerations for COVID-19. Perspect. Psychol. Sci. 2021, 16, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Tyrrell, D.A.; Smith, A.P. Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 1991, 325, 606–612. [Google Scholar] [CrossRef]
- Glaser, R.; Sheridan, J.; Malarkey, W.B.; MacCallum, R.C.; Kiecolt-Glaser, J.K. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom. Med. 2000, 62, 804–807. [Google Scholar] [CrossRef]
- Kohut, M.L.; Lee, W.; Martin, A.; Arnston, B.; Russell, D.W.; Ekkekakis, P.; Yoon, K.J.; Bishop, A.; Cunnick, J.E. The exercise-induced enhancement of influenza immunity is mediated in part by improvements in psychosocial factors in older adults. Brain Behav. Immun. 2005, 19, 357–366. [Google Scholar] [CrossRef]
- De Lorenzo, B.H.; de Oliveira Marchioro, L.; Greco, C.R.; Suchecki, D. Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology 2015, 57, 134–143. [Google Scholar] [CrossRef]
- Vedhara, K.; Ayling, K.; Sunger, K.; Caldwell, D.M.; Halliday, V.; Fairclough, L.; Avery, A.; Robles, L.; Garibaldi, J.; Welton, N.J.; et al. Psychological interventions as vaccine adjuvants: A systematic review. Vaccine 2019, 37, 3255–3266. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Antonijevic, I.A.; Künzel, H.; Uhr, M.; Wetter, T.C.; Golly, I.C.; Steiger, A.; Murck, H. Oral Mg2+ supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry 2002, 35, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Kimiagar, M.; Sadeghniiat, K.; Shirazi, M.M.; Hedayati, M.; Rashidkhani, B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J. Res. Med. Sci. 2012, 17, 1161–1169. [Google Scholar] [PubMed]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.M.; Videnovic, A. Chronic sleep disturbance and neural injury: Links to neurodegenerative disease. Nat. Sci. Sleep 2016, 8, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Mariani, S.; Redline, S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2020, 75, 991–999. [Google Scholar] [CrossRef]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef] [PubMed]
- Guida, J.L.; Alfini, A.J.; Gallicchio, L.; Spira, A.P.; Caporaso, N.E.; Green, P.A. Association of objectively measured sleep with frailty and 5-year mortality in community-dwelling older adults. Sleep 2021, 44, zsab003. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef]
- Kripke, D.F.; Langer, R.D.; Elliott, J.A.; Klauber, M.R.; Rex, K.M. Mortality related to actigraphic long and short sleep. Sleep Med. 2011, 12, 28–33. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflug. Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Mishima, K.; Satoh, K.; Tozawa, T.; Mishima, Y.; Shimizu, T.; Hishikawa, Y. Total sleep deprivation induces an acute and transient increase in NK cell activity in healthy young volunteers. Sleep 2001, 24, 804–809. [Google Scholar] [PubMed]
- Irwin, M.; McClintick, J.; Costlow, C.; Fortner, M.; White, J.; Gillin, J.C. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996, 10, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.; Mascovich, A.; Gillin, J.C.; Willoughby, R.; Pike, J.; Smith, T.L. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom. Med. 1994, 56, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Fondell, E.; Axelsson, J.; Franck, K.; Ploner, A.; Lekander, M.; Bälter, K.; Gaines, H. Short natural sleep is associated with higher T cell and lower NK cell activities. Brain Behav. Immun. 2011, 25, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Shakhar, K.; Valdimarsdottir, H.B.; Guevarra, J.S.; Bovbjerg, D.H. Sleep, fatigue, and NK cell activity in healthy volunteers: Significant relationships revealed by within subject analyses. Brain Behav. Immun. 2007, 21, 180–184. [Google Scholar] [CrossRef]
- De Lorenzo, B.H.P.; Novaes, E.B.R.R.; Paslar Leal, T.; Piqueira Garcia, N.; Martins Dos Santos, R.M.; Alvares-Saraiva, A.M.; Perez Hurtado, E.C.; Braga Dos Reis, T.C.; Duarte Palma, B. Chronic Sleep Restriction Impairs the Antitumor Immune Response in Mice. Neuroimmunomodulation 2018, 25, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Song, P.; Hang, K.; Chen, Z.; Zhu, Z.; Zhang, Y.; Xu, J.; Qin, J.; Wang, B.; Qu, W.; et al. Sleep Deprivation Disturbs Immune Surveillance and Promotes the Progression of Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 727959. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.P.; Geller, D.A.; Antoni, M.; Donnell, D.M.; Tsung, A.; Marsh, J.W.; Burke, L.; Penedo, F.; Terhorst, L.; Kamarck, T.W.; et al. Sleep duration is associated with survival in advanced cancer patients. Sleep Med. 2017, 32, 208–212. [Google Scholar] [CrossRef]
- Palesh, O.; Aldridge-Gerry, A.; Zeitzer, J.M.; Koopman, C.; Neri, E.; Giese-Davis, J.; Jo, B.; Kraemer, H.; Nouriani, B.; Spiegel, D. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep 2014, 37, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Raymann, R.J.; Swaab, D.F.; Van Someren, E.J. Skin temperature and sleep-onset latency: Changes with age and insomnia. Physiol. Behav. 2007, 90, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Lee, J.Y. Effects of feet warming using bed socks on sleep quality and thermoregulatory responses in a cool environment. J. Physiol. Anthr. 2018, 37, 13. [Google Scholar] [CrossRef] [PubMed]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R.; Castriotta, R.J. Before-bedtime passive body heating by warm shower or bath to improve sleep: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 46, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Terunuma, H.; Nieda, M. Immunosurveillance of Cancer and Viral Infections with Regard to Alterations of Human NK Cells Originating from Lifestyle and Aging. Biomedicines 2021, 9, 557. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Clinical strategies and animal models for developing senolytic agents. Exp. Gerontol. 2015, 68, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Johmura, Y.; Yamanaka, T.; Omori, S.; Wang, T.W.; Sugiura, Y.; Matsumoto, M.; Suzuki, N.; Kumamoto, S.; Yamaguchi, K.; Hatakeyama, S.; et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 2021, 371, 265–270. [Google Scholar] [CrossRef]
- Muñoz, D.P.; Yannone, S.M.; Daemen, A.; Sun, Y.; Vakar-Lopez, F.; Kawahara, M.; Freund, A.M.; Rodier, F.; Wu, J.D.; Desprez, P.Y.; et al. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight 2019, 5, e124716. [Google Scholar] [CrossRef] [PubMed]
- Zingoni, A.; Cecere, F.; Vulpis, E.; Fionda, C.; Molfetta, R.; Soriani, A.; Petrucci, M.T.; Ricciardi, M.R.; Fuerst, D.; Amendola, M.G.; et al. Genotoxic Stress Induces Senescence-Associated ADAM10-Dependent Release of NKG2D MIC Ligands in Multiple Myeloma Cells. J. Immunol. 2015, 195, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Valipour, B.; Velaei, K.; Abedelahi, A.; Karimipour, M.; Darabi, M.; Charoudeh, H.N. NK cells: An attractive candidate for cancer therapy. J. Cell. Physiol. 2019, 234, 19352–19365. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Colonna, M. Tailoring Natural Killer cell immunotherapy to the tumour microenvironment. Semin. Immunol. 2017, 31, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Miller, J.S. NK cells in therapy of cancer. Crit. Rev. Oncog. 2014, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Pupovac, A.; Evtimov, V.; Boyd, N.; Shu, R.; Boyd, R.; Trounson, A. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells 2021, 10, 1058. [Google Scholar] [CrossRef]
- Wang, L.; Dou, M.; Ma, Q.; Yao, R.; Liu, J. Chimeric antigen receptor (CAR)-modified NK cells against cancer: Opportunities and challenges. Int. Immunopharmacol. 2019, 74, 105695. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.O.; Mitchell, W.A.; Lapenna, A.; Pitts, D.; Aspinall, R. Immunological pathogenesis of main age-related diseases and frailty: Role of immunosenescence. Eur. Geriatr. Med. 2010, 1, 112–121. [Google Scholar] [CrossRef]
- Horowitz, A.; Strauss-Albee, D.M.; Leipold, M.; Kubo, J.; Nemat-Gorgani, N.; Dogan, O.C.; Dekker, C.L.; Mackey, S.; Maecker, H.; Swan, G.E.; et al. Genetic and Environmental Determinants of Human NK Cell Diversity Revealed by Mass Cytometry. Sci. Transl. Med. 2013, 5, 208ra145. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.E.; Gill, S.; Negrin, R.S. Biology and clinical effects of natural killer cells in allogeneic transplantation. Curr. Opin. Oncol. 2010, 22, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Lupo, K.B.; Matosevic, S. Natural Killer Cells as Allogeneic Effectors in Adoptive Cancer Immunotherapy. Cancers 2019, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Labanieh, L.; Majzner, R.G.; Mackall, C.L. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2018, 2, 377–391. [Google Scholar] [CrossRef]
- Pfefferle, A.; Huntington, N.D. You Have Got a Fast CAR: Chimeric Antigen Receptor NK Cells in Cancer Therapy. Cancers 2020, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).