Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer
Abstract
:1. Background
2. Methods
2.1. Study Design and Population
2.2. HLA-Typing
2.3. Viral Assays
2.4. Flow Cytometric Analysis
2.5. Intracellular Cytokine Staining Assays
2.6. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Early Two-Fold Decrease of CA-HIV-DNA following Anti-PD-1
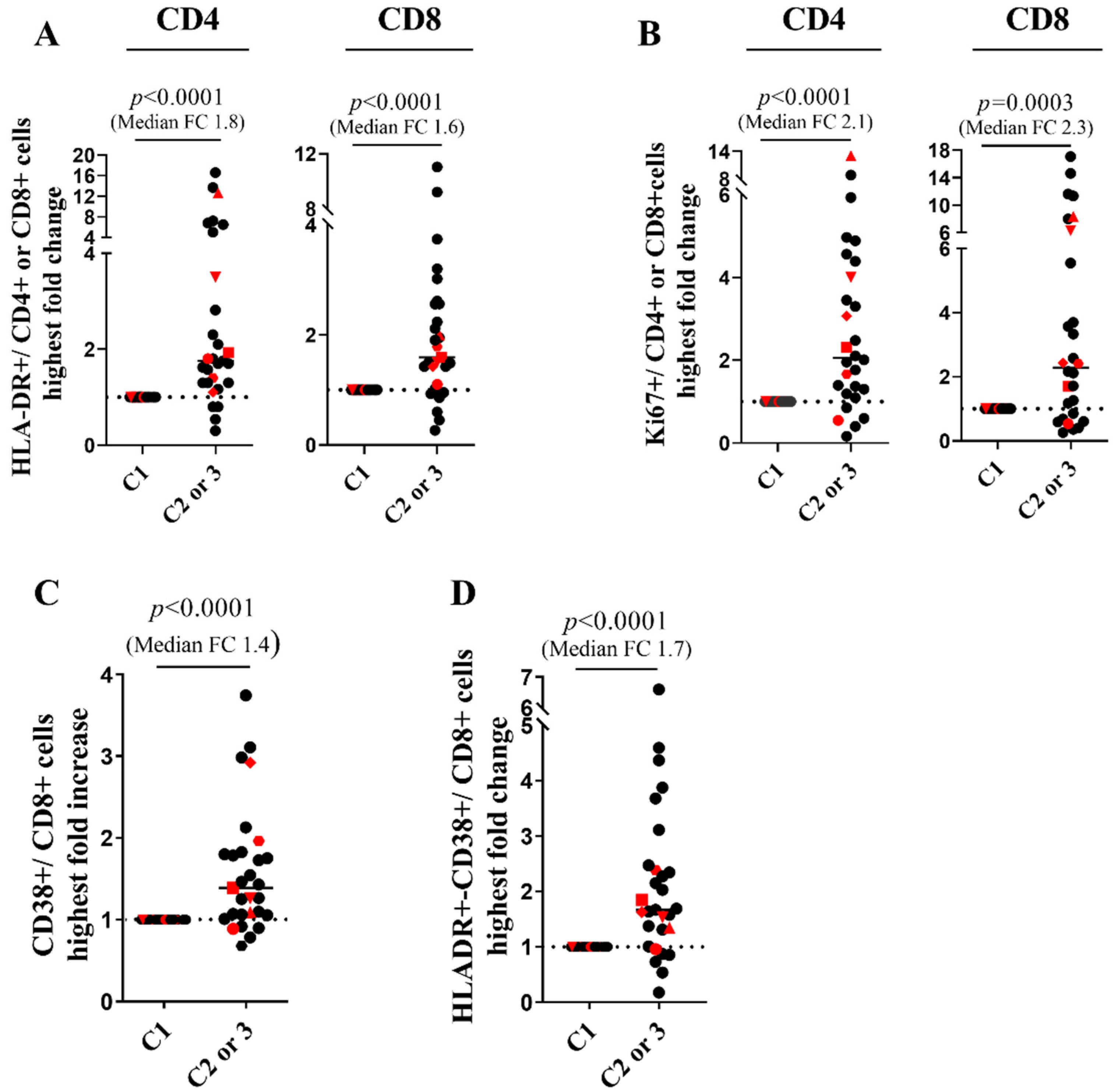
3.3. CD4 Stability and Early T Cell Activation
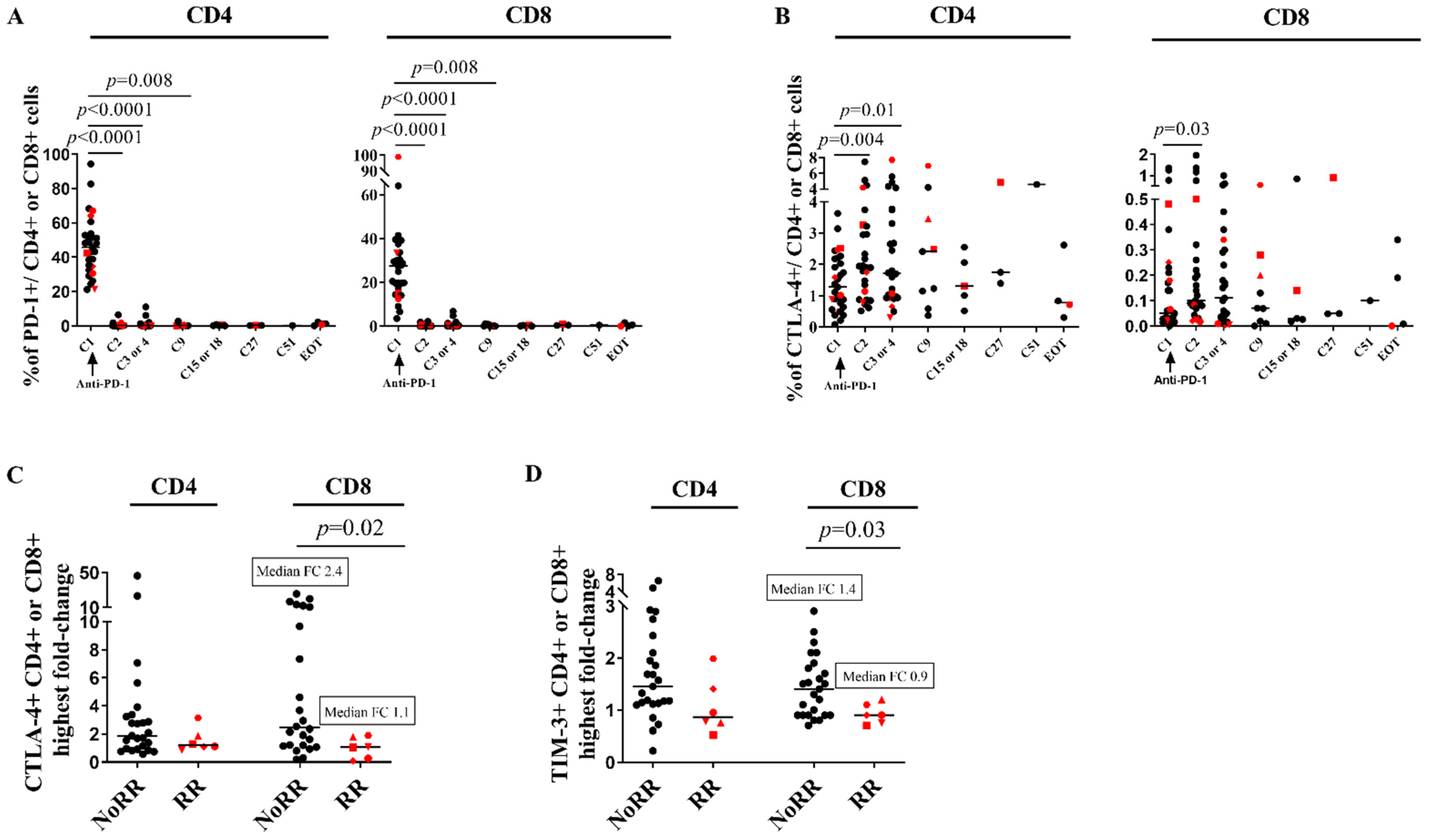
3.4. CTLA-4 Is Upregulated on CD4 Cells and ICP Compensatory Mechanisms Are Less Pronounced in Patients RR
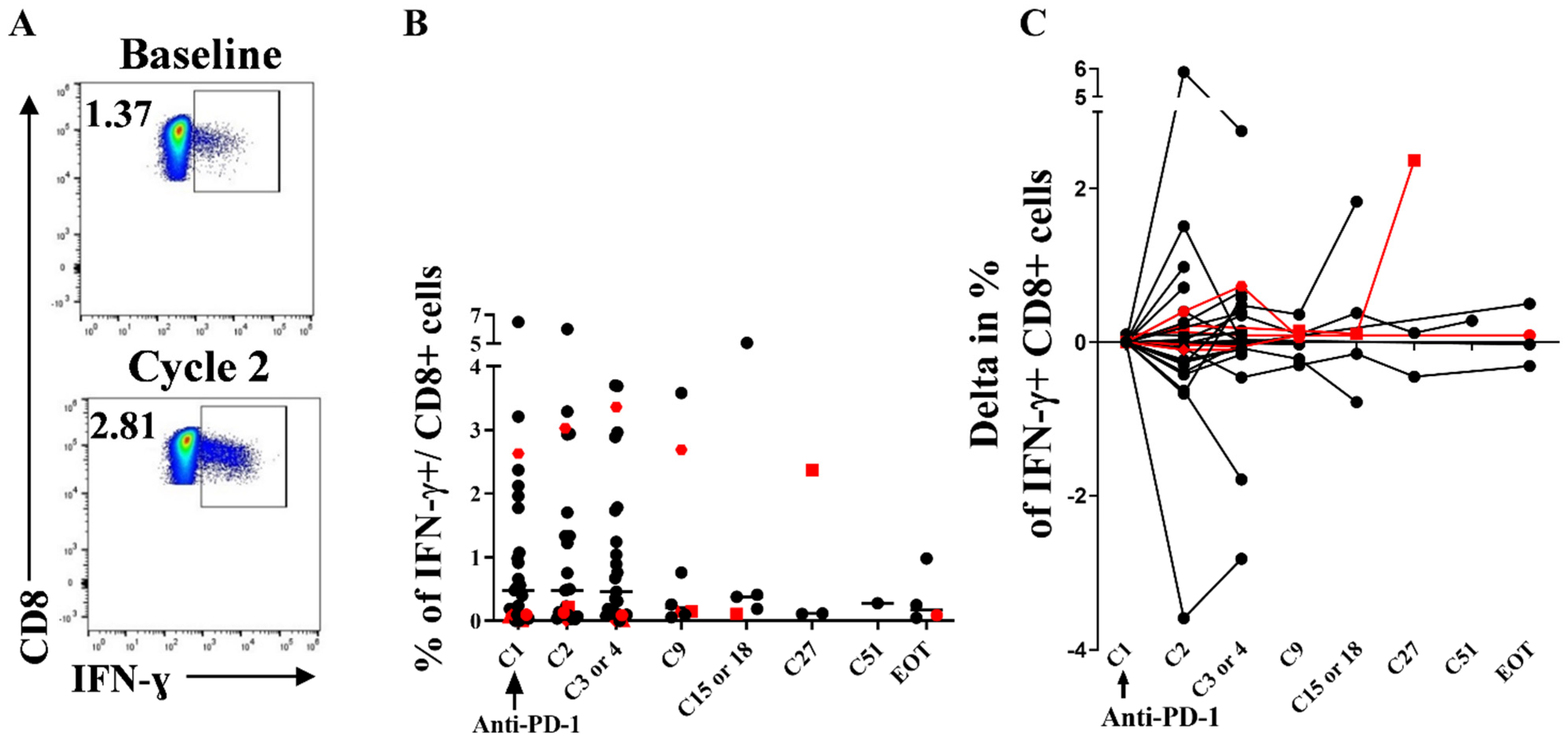
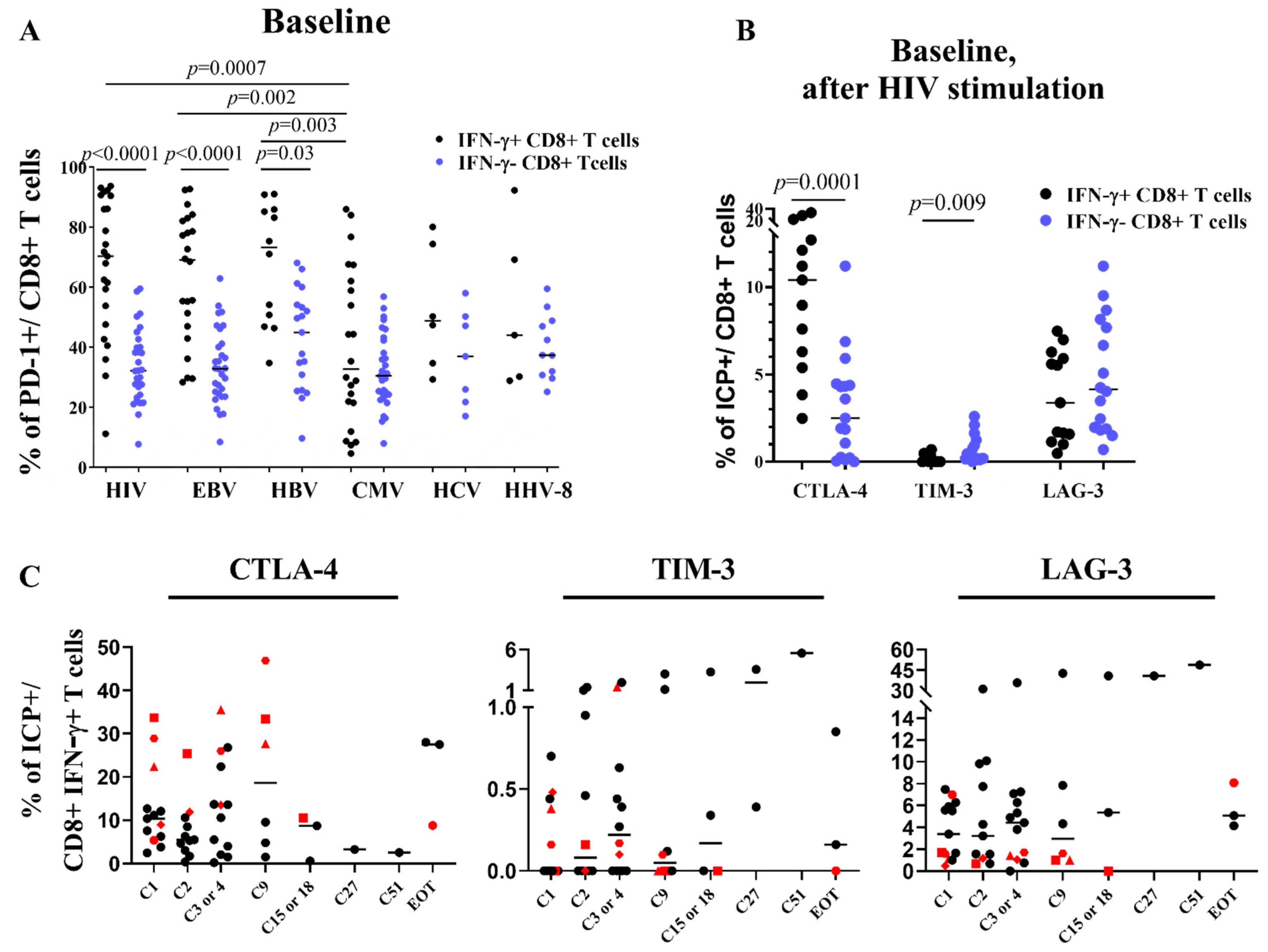
3.5. Stability of Peripheral HIV-Specific T Cells Despite PD-1 Overexpression at Baseline
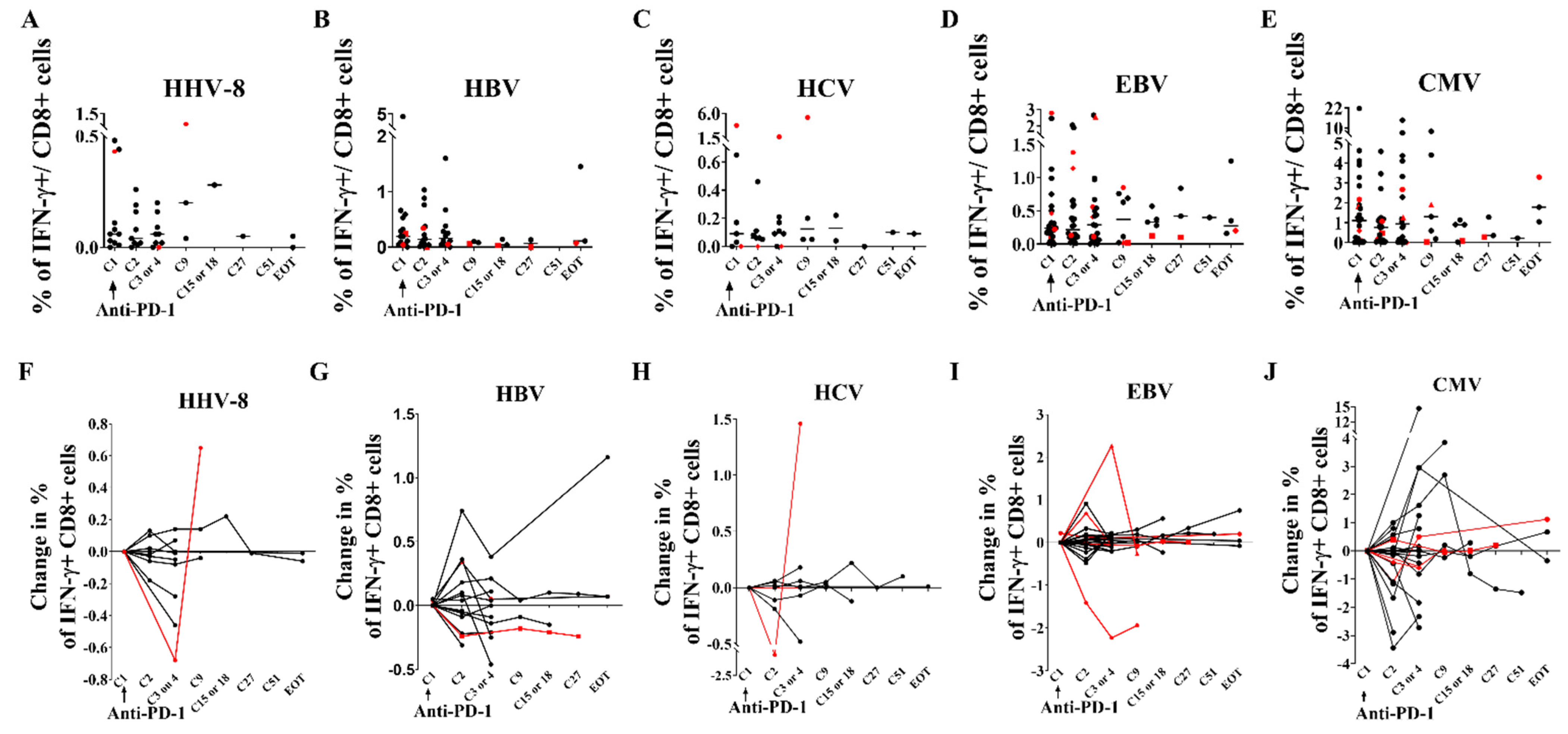
3.6. Immunological and Virological Parameters of Other Viruses Are Not Modified
3.7. Immuno-Virological Profiling of RRs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Members of the ANRS: Oncovirim, Study Group
Members of the French Cooperative Thoracic Intergroup (IFCT) CHIVA-2 Investigators
References
- Wherry, E.J. T Cell Exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Fromentin, R.; DaFonseca, S.; Costiniuk, C.T.; El-Far, M.; Procopio, F.A.; Hecht, F.M.; Hoh, R.; Deeks, S.G.; Hazuda, D.J.; Lewin, S.R.; et al. PD-1 Blockade Potentiates HIV Latency Reversal Ex Vivo in CD4+ T Cells from ART-Suppressed Individuals. Nat. Commun. 2019, 10, 814. [Google Scholar] [CrossRef]
- Fromentin, R.; Bakeman, W.; Lawani, M.B.; Khoury, G.; Hartogensis, W.; DaFonseca, S.; Killian, M.; Epling, L.; Hoh, R.; Sinclair, E.; et al. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLOS Pathog. 2016, 12, e1005761. [Google Scholar] [CrossRef] [PubMed]
- Evans, V.A.; van der Sluis, R.M.; Solomon, A.; Dantanarayana, A.; McNeil, C.; Garsia, R.; Palmer, S.; Fromentin, R.; Chomont, N.; Sékaly, R.-P.; et al. Programmed Cell Death-1 Contributes to the Establishment and Maintenance of HIV-1 Latency. AIDS 2018, 32, 1491–1497. [Google Scholar] [CrossRef]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 Expression on HIV-Specific T Cells Is Associated with T-Cell Exhaustion and Disease Progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Fontenot, A.P.; Mack, D.G.; Lozupone, C.; Dillon, S.; Meditz, A.; Wilson, C.C.; Connick, E.; Palmer, B.E. Programmed Death 1 Expression on HIV-Specific CD4+ T Cells Is Driven by Viral Replication and Associated with T Cell Dysfunction. J. Immunol. 2007, 179, 1979–1987. [Google Scholar] [CrossRef]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.-R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 Expression on HIV-Specific CD8+ T Cells Leads to Reversible Immune Dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef]
- Velu, V.; Titanji, K.; Zhu, B.; Husain, S.; Pladevega, A.; Lai, L.; Vanderford, T.H.; Chennareddi, L.; Silvestri, G.; Freeman, G.J.; et al. Enhancing SIV-Specific Immunity in Vivo by PD-1 Blockade. Nature 2009, 458, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.L.; Bosch, R.J.; Ritz, J.; Hataye, J.M.; Aga, E.; Tressler, R.L.; Mason, S.W.; Hwang, C.K.; Grasela, D.M.; Ray, N.; et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 1725–1733. [Google Scholar] [CrossRef]
- Abbar, B.; Baron, M.; Katlama, C.; Marcelin, A.-G.; Veyri, M.; Autran, B.; Guihot, A.; Spano, J.-P. Immune Checkpoint Inhibitors in People Living with HIV: What about Anti-HIV Effects? AIDS Lond. Engl. 2019, 34, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Guihot, A.; Marcelin, A.-G.; Massiani, M.-A.; Samri, A.; Soulié, C.; Autran, B.; Spano, J.-P. Drastic Decrease of the HIV Reservoir in a Patient Treated with Nivolumab for Lung Cancer. Ann. Oncol. 2018, 29, 517–518. [Google Scholar] [CrossRef]
- Wightman, F.; Solomon, A.; Kumar, S.S.; Urriola, N.; Gallagher, K.; Hiener, B.; Palmer, S.; Mcneil, C.; Garsia, R.; Lewin, S.R. Effect of Ipilimumab on the HIV Reservoir in an HIV-Infected Individual with Metastatic Melanoma. AIDS Lond. Engl. 2015, 29, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.A.; Rajdev, L.; Rhodes, A.; Dantanarayana, A.; Tennakoon, S.; Chea, S.; Spelman, T.; Lensing, S.; Rutishauser, R.; Bakkour, S.; et al. Impact of Anti-PD-1 and Anti-CTLA-4 on the HIV Reservoir in People Living with HIV with Cancer on Antiretroviral Therapy: The AIDS Malignancy Consortium-095 Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e1973–e1981. [Google Scholar] [CrossRef] [PubMed]
- Macedo, C.; Webber, S.A.; Donnenberg, A.D.; Popescu, I.; Hua, Y.; Green, M.; Rowe, D.; Smith, L.; Brooks, M.M.; Metes, D. EBV-Specific CD8+ T Cells from Asymptomatic Pediatric Thoracic Transplant Patients Carrying Chronic High EBV Loads Display Contrasting Features: Activated Phenotype and Exhausted Function. J. Immunol. Baltim. Md 1950 2011, 186, 5854–5862. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Bord, E.; Broge, T.A.; Glotzbecker, B.; Mills, H.; Gheuens, S.; Rosenblatt, J.; Avigan, D.; Koralnik, I.J. Increased Program Cell Death-1 (PD-1) Expression on T Lymphocytes of Patients with Progressive Multifocal Leukoencephalopathy (PML). J. Acquir. Immune Defic. Syndr. 2012, 60, 244–248. [Google Scholar] [CrossRef]
- Wykes, M.N.; Lewin, S.R. Immune Checkpoint Blockade in Infectious Diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 Blockade with Nivolumab with and without Therapeutic Vaccination for Virally Suppressed Chronic Hepatitis B: A Pilot Study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Gardiner, D.; Lalezari, J.; Lawitz, E.; DiMicco, M.; Ghalib, R.; Reddy, K.R.; Chang, K.-M.; Sulkowski, M.; Marro, S.O.; Anderson, J.; et al. A Randomized, Double-Blind, Placebo-Controlled Assessment of BMS-936558, a Fully Human Monoclonal Antibody to Programmed Death-1 (PD-1), in Patients with Chronic Hepatitis C Virus Infection. PLoS ONE 2013, 8, e63818. [Google Scholar] [CrossRef]
- Roos-Weil, D.; Weiss, N.; Guihot, A.; Uzunov, M.; Bellanger, A.; Eymard, B.; Saadoun, D.; Houillier, C.; Idbaih, A.; Demeret, S.; et al. Immune Checkpoint Inhibitors for Progressive Multifocal Leukoencephalopathy: A New Gold Standard? J. Neurol. 2021, 268, 2458–2465. [Google Scholar] [CrossRef]
- Chen, J.; Del Valle, L.; Lin, H.-Y.; Plaisance-Bonstaff, K.; Forrest, J.C.; Post, S.R.; Qin, Z. Expression of PD-1 and PD-Ls in Kaposi’s Sarcoma and Regulation by Oncogenic Herpesvirus Lytic Reactivation. Virology 2019, 536, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Delyon, J.; Bizot, A.; Battistella, M.; Madelaine, I.; Vercellino, L.; Lebbé, C. PD-1 Blockade with Nivolumab in Endemic Kaposi Sarcoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.R.; Schulz, T.F.; Whitby, D.; Cook, P.M.; Boshoff, C.; Rainbow, L.; Howard, M.R.; Gao, S.J.; Bohenzky, R.A.; Simmonds, P.; et al. Prevalence of Kaposi’s Sarcoma Associated Herpesvirus Infection Measured by Antibodies to Recombinant Capsid Protein and Latent Immunofluorescence Antigen. Lancet Lond. Engl. 1996, 348, 1133–1138. [Google Scholar] [CrossRef]
- Lallemand, F.; Desire, N.; Rozenbaum, W.; Nicolas, J.C.; Marechal, V. Quantitative Analysis of Human Herpesvirus 8 Viral Load Using a Real-Time PCR Assay. J. Clin. Microbiol. 2000, 38, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Avettand-Fènoël, V.; Chaix, M.-L.; Blanche, S.; Burgard, M.; Floch, C.; Toure, K.; Allemon, M.-C.; Warszawski, J.; Rouzioux, C.; French Pediatric Cohort Study ANRS-CO 01 Group. LTR Real-Time PCR for HIV-1 DNA Quantitation in Blood Cells for Early Diagnosis in Infants Born to Seropositive Mothers Treated in HAART Area (ANRS CO 01). J. Med. Virol. 2009, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Nakid-Cordero, C.; Arzouk, N.; Gauthier, N.; Tarantino, N.; Larsen, M.; Choquet, S.; Burrel, S.; Autran, B.; Vieillard, V.; Guihot, A. Skewed T Cell Responses to Epstein-Barr Virus in Long-Term Asymptomatic Kidney Transplant Recipients. PLoS ONE 2019, 14, e0224211. [Google Scholar] [CrossRef]
- Guihot, A.; Oksenhendler, E.; Galicier, L.; Marcelin, A.-G.; Papagno, L.; Bedin, A.-S.; Agbalika, F.; Dupin, N.; Cadranel, J.; Autran, B.; et al. Multicentric Castleman Disease Is Associated with Polyfunctional Effector Memory HHV-8-Specific CD8+ T Cells. Blood 2008, 111, 1387–1395. [Google Scholar] [CrossRef]
- Achenbach, C.J.; Assoumou, L.; Deeks, S.G.; Wilkin, T.J.; Berzins, B.; Casazza, J.P.; Lambert-Niclot, S.; Koup, R.A.; Costagliola, D.; Calvez, V.; et al. Effect of Therapeutic Intensification Followed by HIV DNA Prime and RAd5 Boost Vaccination on HIV-Specific Immunity and HIV Reservoir (EraMune 02): A Multicentre Randomised Clinical Trial. Lancet HIV 2015, 2, e82–e91. [Google Scholar] [CrossRef]
- Calin, R.; Hamimi, C.; Lambert-Niclot, S.; Carcelain, G.; Bellet, J.; Assoumou, L.; Tubiana, R.; Calvez, V.; Dudoit, Y.; Costagliola, D.; et al. Treatment Interruption in Chronically HIV-Infected Patients with an Ultralow HIV Reservoir. AIDS Lond. Engl. 2016, 30, 761–769. [Google Scholar] [CrossRef]
- Michot, J.-M.; Mouraud, S.; Adam, J.; Lazarovici, J.; Bigenwald, C.; Rigaud, C.; Tselikas, L.; Dartigues, P.; Danu, A.; Bigorgne, A.; et al. CD8+ T Lymphocytes Immune Depletion and LAG-3 Overexpression in Hodgkin Lymphoma Tumor Microenvironment Exposed to Anti-PD-1 Immunotherapy. Cancers 2021, 13, 5487. [Google Scholar] [CrossRef] [PubMed]
- McGary, C.S.; Deleage, C.; Harper, J.; Micci, L.; Ribeiro, S.P.; Paganini, S.; Kuri-Cervantes, L.; Benne, C.; Ryan, E.S.; Balderas, R.; et al. CTLA-4+PD-1− Memory CD4+ T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity 2017, 47, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, R.M.; Kumar, N.A.; Pascoe, R.D.; Zerbato, J.M.; Evans, V.A.; Dantanarayana, A.I.; Anderson, J.L.; Sékaly, R.P.; Fromentin, R.; Chomont, N.; et al. Combination Immune Checkpoint Blockade to Reverse HIV Latency. J. Immunol. 2020, 204, ji1901191. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Toor, S.M.; Khalaf, S.; Elkord, E. Breast Cancer Cells and PD-1/PD-L1 Blockade Upregulate the Expression of PD-1, CTLA-4, TIM-3 and LAG-3 Immune Checkpoints in CD4+ T Cells. Vaccines 2019, 7, 149. [Google Scholar] [CrossRef]
- Osa, A.; Uenami, T.; Koyama, S.; Fujimoto, K.; Okuzaki, D.; Takimoto, T.; Hirata, H.; Yano, Y.; Yokota, S.; Kinehara, Y.; et al. Clinical Implications of Monitoring Nivolumab Immunokinetics in Non–Small Cell Lung Cancer Patients. JCI Insight 2018, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Samri, A.; Durier, C.; Urrutia, A.; Sanchez, I.; Gahery-Segard, H.; Imbart, S.; Sinet, M.; Tartour, E.; Aboulker, J.P.; Autran, B.; et al. Evaluation of the interlaboratory concordance in quantification of human immunodeficiency virus-specific T cells with a gamma interferon enzyme-linked immunospot assay. Clin. Vaccine. Immunol. CVI 2006, 13, 684–697. [Google Scholar] [CrossRef]
- Sacre, K.; Carcelain, G.; Cassoux, N.; Fillet, A.-M.; Costagliola, D.; Vittecoq, D.; Salmon, D.; Amoura, Z.; Katlama, C.; Autran, B. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J. Exp. Med. 2005, 201, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.; Bertoletti, A. Quantity and quality of virus-specific CD8 cell response: Relevance to the design of a therapeutic vaccine for chronic HBV infection. Mol. Immunol. 2001, 38, 467–473. [Google Scholar] [CrossRef]
- Webster, G.J.; Reignat, S.; Brown, D.; Ogg, G.S.; Jones, L.; Seneviratne, S.L.; Williams, R.; Dusheiko, G.; Bertoletti, A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: Implications for immunotherapy. J. Virol. 2004, 78, 5707–5719. [Google Scholar] [CrossRef]
- Depla, E.; Van der Aa, A.; Livingston, B.D.; Crimi, C.; Allosery, K.; De Brabandere, V.; Krakover, J.; Murthy, S.; Huang, M.; Power, S.; et al. Rational Design of a Multiepitope Vaccine Encoding T-Lymphocyte Epitopes for Treatment of Chronic Hepatitis B Virus Infections. J. Virol. 2008, 82, 435–450. [Google Scholar] [CrossRef]
- Malmassari, S.; Lone, Y.C.; Zhang, M.; Transy, C.; Michel, M.L. In vivo hierarchy of immunodominant and subdominant HLA-A*0201-restricted T-cell epitopes of HBx antigen of hepatitis B virus. Microbes Infect. 2005, 7, 626–634. [Google Scholar] [CrossRef]
- Tsai, S.L.; Lee, T.H.; Chien, R.N.; Liao, S.K.; Lin, C.L.; Kuo, G.C.; Liaw, Y.F. A method to increase tetramer staining efficiency of CD8+ T cells with MHC–peptide complexes: Therapeutic applications in monitoring cytotoxic T lymphocyte activity during hepatitis B and C treatment. J. Immunol. Methods 2004, 285, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Schnuriger, A.; Dominguez, S.; Guiguet, M.; Harfouch, S.; Samri, A.; Ouazene, Z.; Slama, L.; Simon, A.; Valantin, M.A.; Thibault, V.; et al. Acute hepatitis C in HIV-infected patients: Rare spontaneous clearance correlates with weak memory CD4 T-cell responses to hepatitis C virus. AIDS 2009, 23, 2079–2089. [Google Scholar] [CrossRef] [PubMed]


| Pt | Cohort | Age | Sex | Type of Cancers | Pv Line | ICB Type | ART | CD4 Count (/mm3) | CD4/CD8 Ratio | HIV VL (cp/mL) | HIV-DNA(cp/10 cells) | HLA Typing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CHIVA-2 | 71 | M | NSCLC | 2 | Nivo | Rilpivirine, Dolutegravir | 183 | 0.5 | 28 | <40 | A*01:03 B*37:49 |
| 2 | CHIVA-2 | 68 | M | NSCLC | 2 | Nivo | Abacavir, Nevirapine | NA | NA | <1 | 227 | A*03:11 B*07:27 |
| 3 | CHIVA-2 | 56 | M | NSCLC | 1 | Nivo | Emtricitabine tenofovir disoproxil, Maraviroc | 831 | 0.4 | 193 | 818 | A*24:29 B*38:44 |
| 4 | CHIVA-2 | 59 | M | NSCLC | 1 | Nivo | Emtricitabine, Rilpivirine, Tenofovir alafenamide | 596 | 0.9 | <1 | <40 | A*02:29 B*49:58 |
| 5 | CHIVA-2 | 65 | M | NSCLC | 2 | Nivo | Tenofovir, Emtricitabine, Bictegravir | 451 | 0.9 * | 352 | 904 | A*02:29 B*07:15 |
| 6 | CHIVA-2 | 55 | F | NSCLC | 1 | Nivo | Dolutegravir, Abacavir, Lamivudine | 499 | 0.6 | 138 | 1287 | A*29:30 B*37:44 |
| 7 | CHIVA-2 | 68 | M | NSCLC | 1 | Nivo | Abacavir, Efavirenz | 241 | 0.7 | <20 | 187 | A*02:11 B*39:40 |
| 8 | CHIVA-2 | 53 | F | NSCLC | 1 | Nivo | Efavirenz, Emtricitabine, Tenofovir | 291 | 0.4 | <20 | 66 | A*02:66 B*49:52 |
| 9 | CHIVA-2 | 58 | M | NSCLC | 1 | Nivo | Dolutegravir, Abacavir, Lamivudine | 249 | 0.2 | <20 | 851 | A*01:03 B*08:51 |
| 10 | CHIVA-2 | 59 | M | NSCLC | 1 | Nivo | Lamivudine, Dolutegravir, Abacavir | 583 | 0.4 | <1 | 231 | A*02:03 B*18 27 |
| 11 | Onco VIHAC | 62 | M | Melanoma | 0 | Nivo | Elvitegravir, Emtricitabine, Tenofovir | 455 | 0.8 | <1 | 166 | A*02:26 B* 07:08 |
| 12 | Onco VIHAC | 69 | M | NSCLC | 0 | Pembro | Emtricitabine, Rilpivirine, Tenofovir | 273 | 0.4 | <1 | <40 | A*02:24 B*35:57 |
| 13 | Onco VIHAC | 75 | M | NSCLC | 1 | Nivo | Lamivudine, Dolutegravir | 217 | 1 | 42 | 218 | A*25:31 B*40:51 |
| 14 | Onco VIHAC | 60 | F | NSCLC | 0 | Pembro | Darunavir, Norvir, Raltegravir | 888 | 2.1 | 21 | 1749 | A*31:68 B*07:07 |
| 15 | Onco VIHAC | 63 | M | NSCLC | 1 | Nivo | Dolutegravir, Abacavir, Lamivudine | 238 | 1.8 | <1 | <40 | A*03:23 B*44:53 |
| 16 | Onco VIHAC | 53 | M | HL | 3 | Nivo | Dolutegravir, Lamivudine | 373 | 0.3 | <20 | 173 | A*33:68 B*14:44 |
| 17 | Onco VIHAC | 53 | M | NSCLC | 2 | Nivo | Abacavir, Lamivudine | 405 | 0.6 | <1 | 620 | A*02:29 B*40:49 |
| 18 | Onco VIHAC | 64 | M | Bladder | 2 | Pembro | Abacavir, Lamivudine, Nivérapine | 449 | 1.1 | <1 | 213 | A*02:31 B*07:40 |
| 19 | Onco VIHAC | 62 | M | Oropharynx | 2 | Nivo | Darunavir, Ritonavir | 162 | 0.5 | <20 | 80 | A*02:11 B*15:40 |
| 20 | Onco VIHAC | 58 | M | Kaposi Sarcoma | 4 | Nivo | Dolutegravir, Abacavir, Lamivudine | 728 | 2.1 | 47 | 409 | A*33:68 B*14:44 |
| 21 | Onco VIHAC | 62 | M | Anal | 2 | Nivo | Dolutegravir, Lamivudine | 209 | 1.2 | <20 | 231 | A*02:24 B*35:44 |
| 22 | Onco VIHAC | 52 | M | Head and neck | 1 | Nivo | Darunavir, Norvir, Raltegravir | 369 | 0.8 | <20 | 191 | A*30:33 B*07:15 |
| 23 | Onco VIHAC | 71 | F | Head and neck | 1 | Nivo | Dolutegravir | 333 | 1.1 | <1 | <40 | A*02:24 B*44:50 |
| 24 | Onco VIHAC | 63 | M | Eye | 2 | Cemi | Bictegravir, Emtricitabne, Tenofovir | 45 | 0.2 | 29 | <40 | A*02:33 B*14:53 |
| 25 | Onco VIHAC | 70 | M | Melanoma | 0 | Pembro | Emtricitabine, Tenofovir, Névirapine | 434 | 0.5 | <1 | 181 | A*01:03 B*07:51 |
| 26 | Onco VIHAC | 56 | M | NSCLC | 2 | Pembro | Emtricitabine, Tenofovir, Darunavir, Ritonavir | 424 | 0.8 | <20 | 166 | A*11:11 B*15:27 |
| 27 | Onco VIHAC | 62 | M | Bladder | 1 | Pembro | Efavirenz, Emtricitabine, Tenofovir | 915 | 1,4 | <20 | 99 | A*24:24 B*44:44 |
| 28 | Onco VIHAC | 58 | M | NSCLC | 0 | Pembro | Darunavir, Doletugravir, Ritonavir, Tenofovir | 192 | 0,4 | <1 | 73 | A*02:32 B*44:51 |
| 29 | Onco VIHAC | 62 | M | NSCLC | 1 | Nivo | Raltegravir, Emtricitabine, Tenofovir | 534 | 0.3 | <1 | 99 | NA |
| 30 | Onco VIHAC | 60 | M | Bladder | 2 | Pembro | Bictegravir, Emtricitabne, Tenofovir | 969 | 0.6 | <1 | 363 | NA |
| 31 | Onco VIHAC | 59 | M | NSCLC | 1 | Nivo | Darunavir, Ritonavir | 699 | 1 | <1 | 251 | NA |
| 32 | Onco VIHAC | 60 | M | NSCLC | 0 | Pembro | Bictegravir, Emtricitabne, Tenofovir | 169 | 0.4 | <1 | 59 | NA |
| all | OncoVIHAC 69% | 61 | M 88% | NSCLC 63% Bladder 9% | 1 | Nivo 69% Pembro 28% | 369 | 1 | 20 | 184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baron, M.; Soulié, C.; Lavolé, A.; Assoumou, L.; Abbar, B.; Fouquet, B.; Rousseau, A.; Veyri, M.; Samri, A.; Makinson, A.; et al. Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer. Cells 2022, 11, 1015. https://doi.org/10.3390/cells11061015
Baron M, Soulié C, Lavolé A, Assoumou L, Abbar B, Fouquet B, Rousseau A, Veyri M, Samri A, Makinson A, et al. Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer. Cells. 2022; 11(6):1015. https://doi.org/10.3390/cells11061015
Chicago/Turabian StyleBaron, Marine, Cathia Soulié, Armelle Lavolé, Lambert Assoumou, Baptiste Abbar, Baptiste Fouquet, Alice Rousseau, Marianne Veyri, Assia Samri, Alain Makinson, and et al. 2022. "Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer" Cells 11, no. 6: 1015. https://doi.org/10.3390/cells11061015
APA StyleBaron, M., Soulié, C., Lavolé, A., Assoumou, L., Abbar, B., Fouquet, B., Rousseau, A., Veyri, M., Samri, A., Makinson, A., Choquet, S., Mazières, J., Brosseau, S., Autran, B., Costagliola, D., Katlama, C., Cadranel, J., Marcelin, A.-G., Lambotte, O., ... The ANRS Co 24 OncoVIHAC Study Group. (2022). Impact of Anti PD-1 Immunotherapy on HIV Reservoir and Anti-Viral Immune Responses in People Living with HIV and Cancer. Cells, 11(6), 1015. https://doi.org/10.3390/cells11061015







