Myogenic Precursor Cells Show Faster Activation and Enhanced Differentiation in a Male Mouse Model Selected for Advanced Endurance Exercise Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Line Establishment
2.2. Animals and Study Design
2.3. Satellite Cell Isolation and Culture
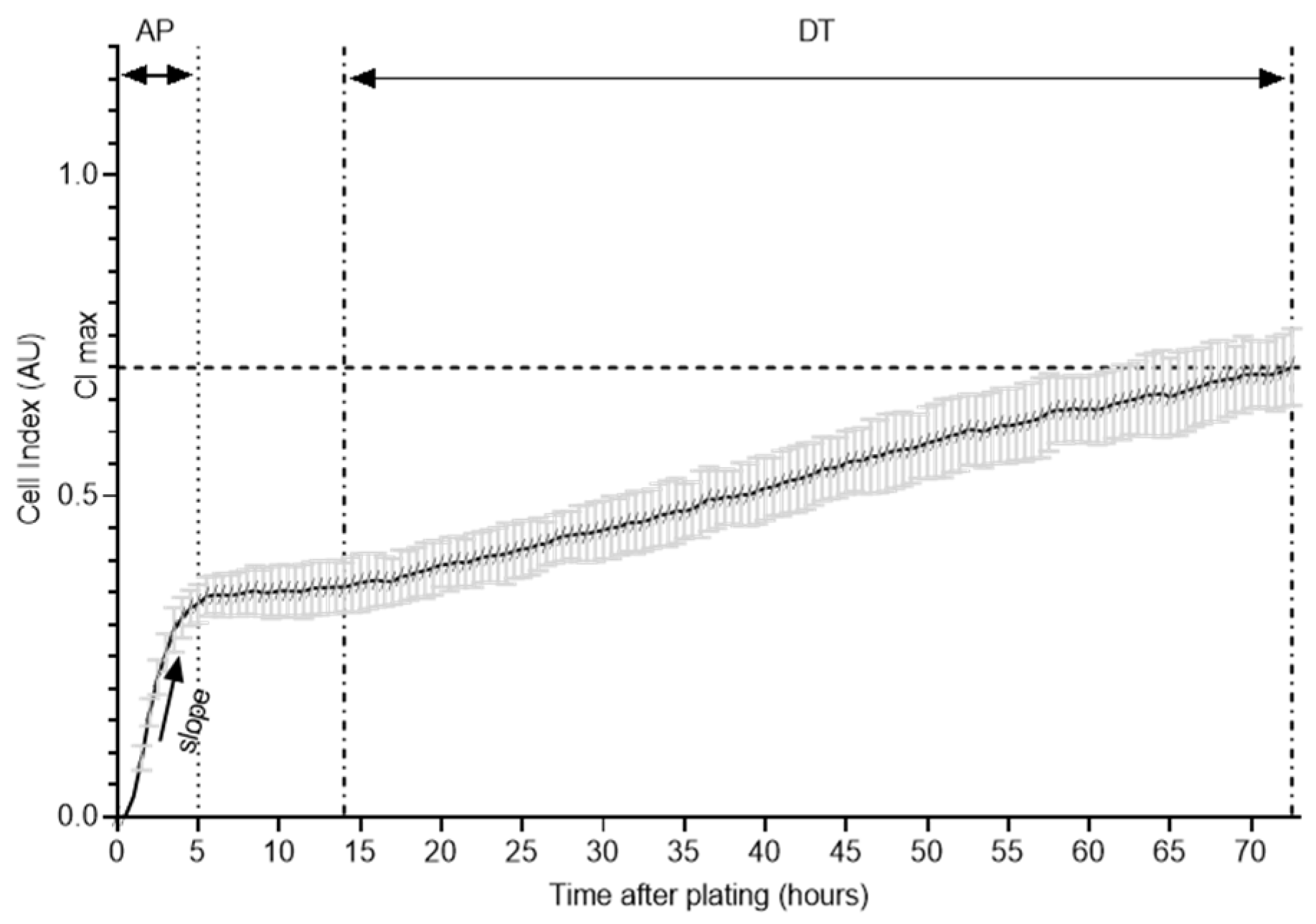
2.4. xCELLigence
2.5. Immunofluorescence Staining for Myogenic Markers Pax7 and MyoD
2.6. Differentiation of SATC
2.7. Immunofluorescence for Myosin Heavy Chain and Determination of the Fusion Index
2.8. Creatine Kinase (CK) Measurement
2.9. Statistical Analyses
3. Results
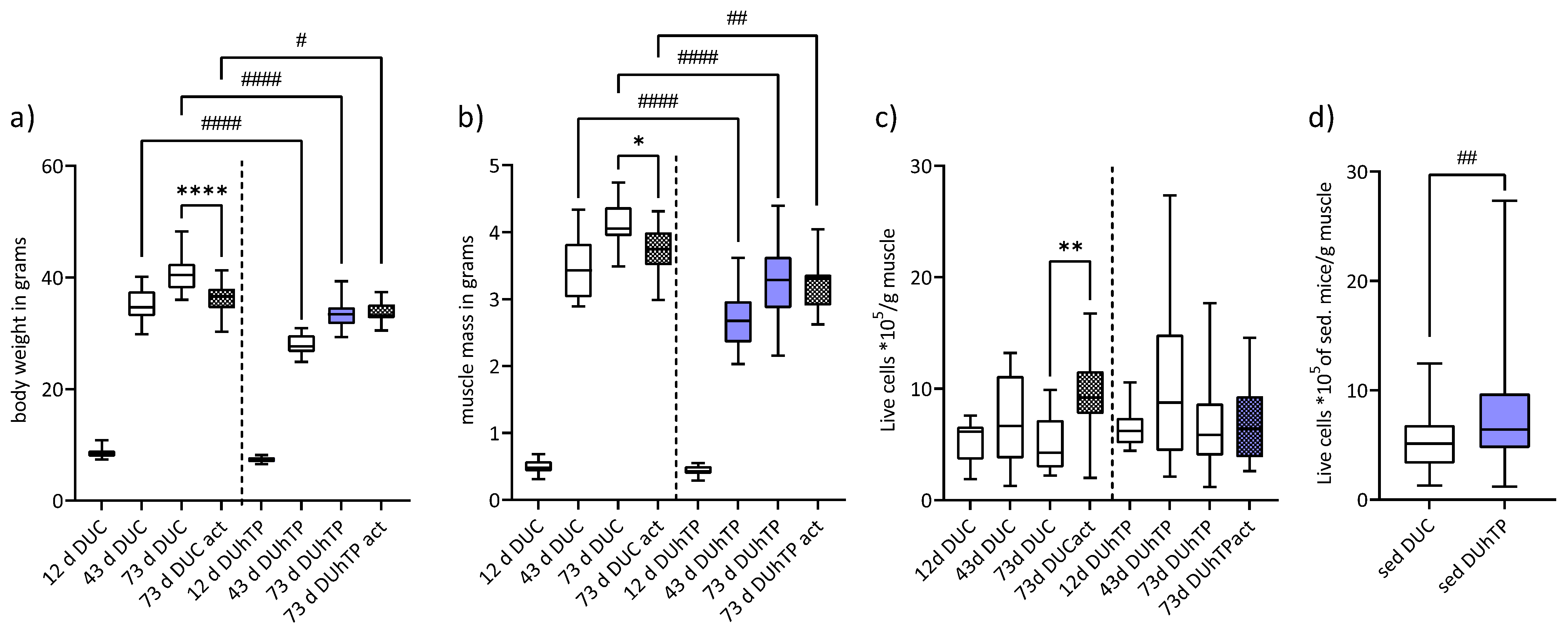
3.1. Voluntary Running Performance, Body and Muscle Weight, and Satellite Cell Yield
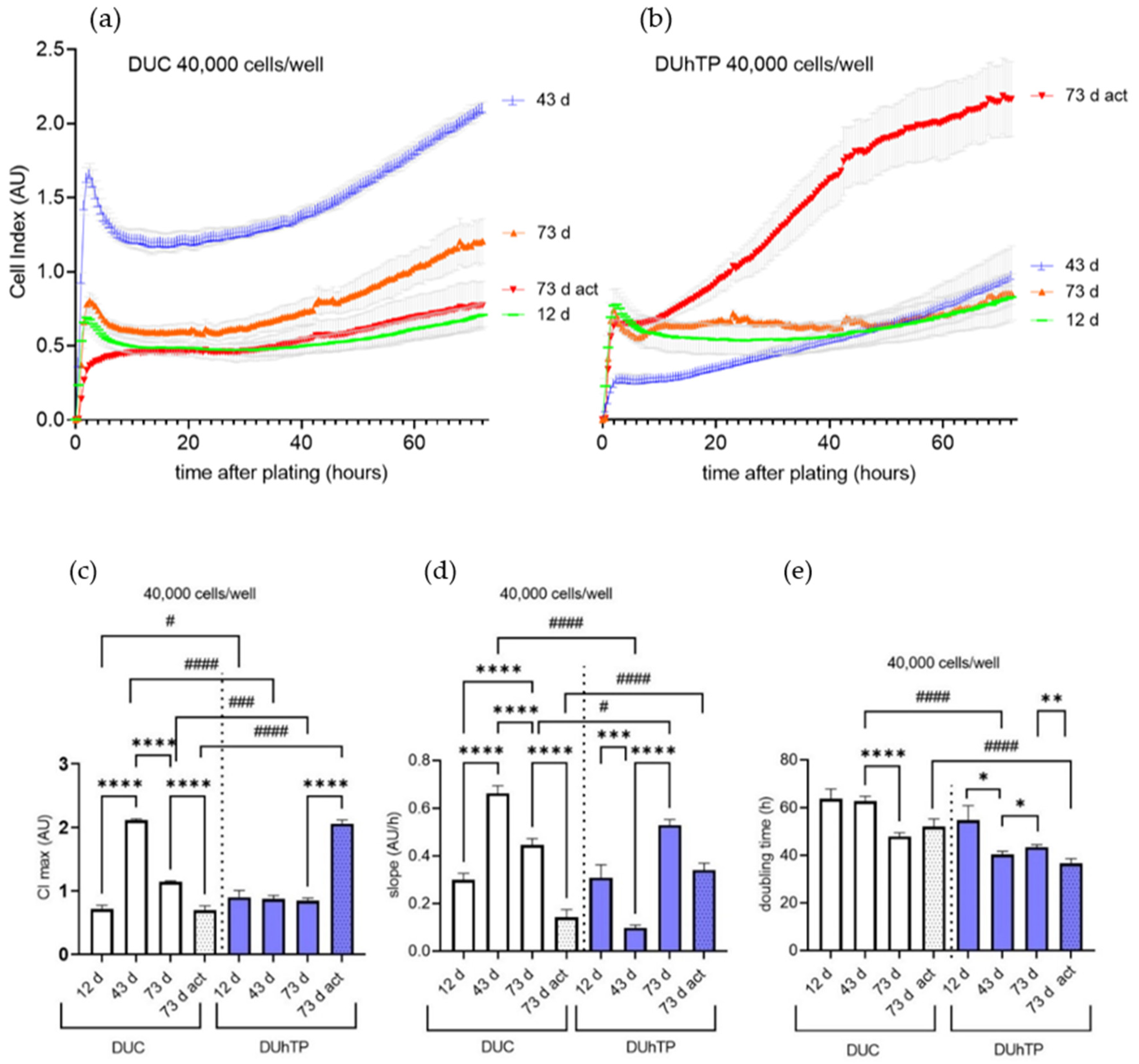
3.2. Growth Kinetics of Isolated Satellite Cells
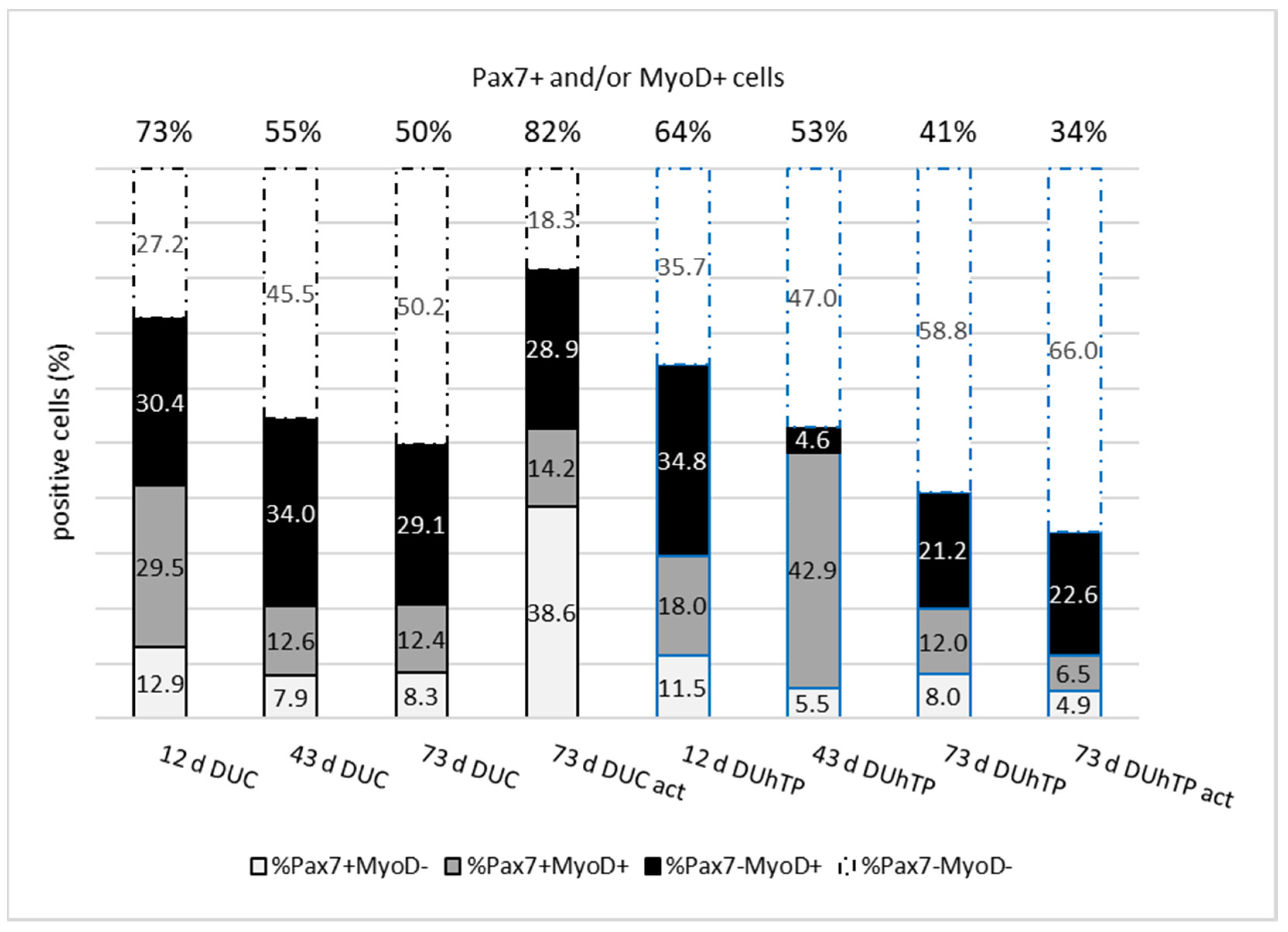
3.3. Pax7 and MyoD Expression in MPC/SATC Cultures from Male DUC and DUhTP Mice
3.4. MPC/SATC of the DUhTP Line Showed a Higher Ability to Fuse and to Differentiate
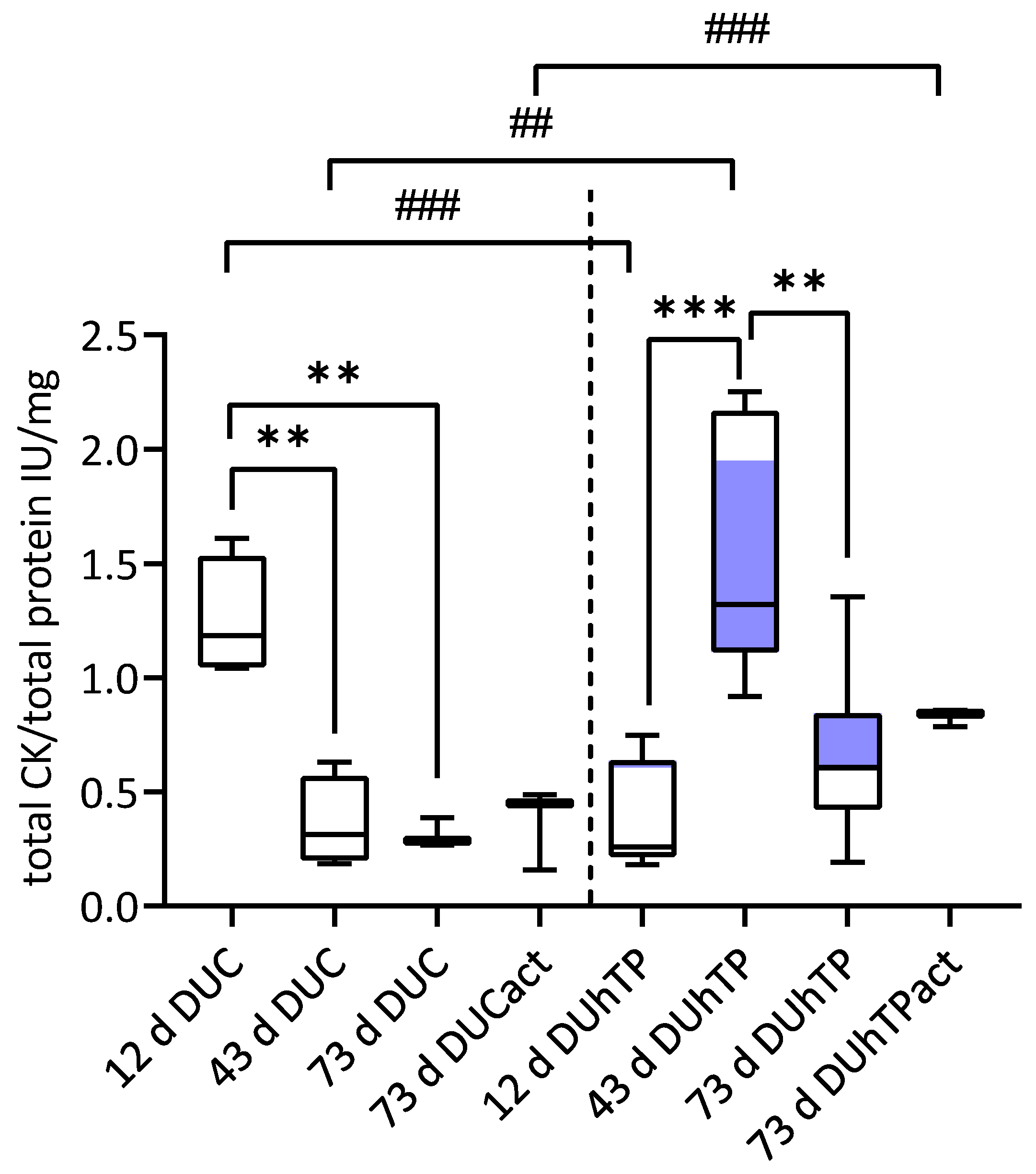
3.5. Cellular Creatine Kinase (CK) Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, R.B.; Biérinx, A.-S.; Gnocchi, V.F.; Zammit, P.S. Dynamics of Muscle Fibre Growth During Postnatal Mouse Development. BMC Dev. Biol. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Gokhin, D.S.; Ward, S.R.; Bremner, S.N.; Lieber, R.L. Quantitative Analysis of Neonatal Skeletal Muscle Functional Improvement in the Mouse. J. Exp. Biol. 2008, 211, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S. Skeletal Muscle Stem Cells in Developmental Versus Regenerative Myogenesis. J. Intern. Med. 2009, 266, 372–389. [Google Scholar] [CrossRef]
- Keefe, A.C.; Lawson, J.A.; Flygare, S.D.; Fox, Z.D.; Colasanto, M.P.; Mathew, S.; Yandell, M.; Kardon, G. Muscle Stem Cells Contribute to Myofibres in Sedentary Adult Mice. Nat. Commun. 2015, 6, 7087. [Google Scholar] [CrossRef]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, J.; Christensen, J.; Xiang, W.; Tierney, M.T.; Boscolo, F.S.; Sacco, A.; Brack, A.S. Early Forming Label-Retaining Muscle Stem Cells Require P27kip1 for Maintenance of the Primitive State. Development 2014, 141, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Schultz, E. Satellite Cell Proliferative Compartments in Growing Skeletal Muscles. Dev. Biol. 1996, 175, 84–94. [Google Scholar] [CrossRef]
- Schultz, E. A Quantitative Study of the Satellite Cell Population in Postnatal Mouse Lumbrical Muscle. Anat. Rec. 1974, 180, 589–595. [Google Scholar] [CrossRef]
- Ontell, M.; Feng, K.C.; Klueber, K.; Dunn, R.F.; Taylor, F. Myosatellite Cells, Growth, and Regeneration in Murine Dystrophic Muscle: A Quantitative Study. Anat. Rec. 1984, 208, 159–174. [Google Scholar] [CrossRef]
- Allbrook, D.B.; Han, M.F.; Hellmuth, A.E. Population of Muscle Satellite Cells in Relation to Age and Mitotic Activity. Pathology 1971, 3, 223–243. [Google Scholar] [CrossRef]
- Shinin, V.; Gayraud-Morel, B.; Gomès, D.; Tajbakhsh, S. Asymmetric Division and Cosegregation of Template DNA Strands in Adult Muscle Satellite Cells. Nat. Cell Biol. 2006, 8, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Pallafacchina, G.; François, S.; Regnault, B.; Czarny, B.; Dive, V.; Cumano, A.; Montarras, D.; Buckingham, M. An Adult Tissue-Specific Stem Cell in Its Niche: A Gene Profiling Analysis of in Vivo Quiescent and Activated Muscle Satellite Cells. Stem Cell Res. 2010, 4, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Friday, B.B.; Horsley, V.; Pavlath, G.K. Calcineurin Activity Is Required for the Initiation of Skeletal Muscle Differentiation. J. Cell Biol. 2000, 149, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Oustanina, S.; Hause, G.; Braun, T. Pax7 Directs Postnatal Renewal and Propagation of Myogenic Satellite Cells but Not Their Specification. Embo. J. 2004, 23, 3430–3439. [Google Scholar] [CrossRef] [PubMed]
- Relaix, F.; Montarras, D.; Zaffran, S.; Gayraud-Morel, B.; Rocancourt, D.; Tajbakhsh, S.; Mansouri, A.; Cumano, A.; Buckingham, M. Pax3 and Pax7 Have Distinct and Overlapping Functions in Adult Muscle Progenitor Cells. J. Cell Biol. 2006, 172, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lepper, C.; Conway, S.J.; Fan, C.-M. Adult Satellite Cells and Embryonic Muscle Progenitors Have Distinct Genetic Requirements. Nature 2009, 460, 627–631. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 up-Regulation Inhibits Myogenesis and Cell Cycle Progression in Satellite Cells: A Potential Mechanism for Self-Renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef]
- Liu, W.; Wen, Y.; Bi, P.; Lai, X.; Liu, X.S.; Kuang, S. Hypoxia Promotes Satellite Cell Self-Renewal and Enhances the Efficiency of Myoblast Transplantation. Development 2012, 139, 2857–2865. [Google Scholar] [CrossRef]
- Neal, A.; Boldrin, L.; Morgan, J.E. The Satellite Cell in Male and Female, Developing and Adult Mouse Muscle: Distinct Stem Cells for Growth and Regeneration. PLoS ONE 2012, 7, e37950. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar]
- Yoshida, N.; Yoshida, S.; Koishi, K.; Masuda, K.; Nabeshima, Y. Cell Heterogeneity Upon Myogenic Differentiation: Down-Regulation of Myod and Myf-5 Generates’ Reserve Cells’. J. Cell Sci. 1998, 111, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Biressi, S.; Molinaro, M.; Cossu, G. Cellular Heterogeneity During Vertebrate Skeletal Muscle Development. Dev. Biol. 2007, 308, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Andrés, V.; Walsh, K. Myogenin Expression, Cell Cycle Withdrawal, and Phenotypic Differentiation Are Temporally Separable Events That Precede Cell Fusion Upon Myogenesis. J. Cell Biol. 1996, 132, 657–666. [Google Scholar] [CrossRef]
- Lehka, L.; Rędowicz, M.J. Mechanisms Regulating Myoblast Fusion: A Multilevel Interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef]
- Fukada, S.I. The Roles of Muscle Stem Cells in Muscle Injury, Atrophy and Hypertrophy. J. Biochem. 2018, 163, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Egner, I.M.; Bruusgaard, J.C.; Gundersen, K. Satellite Cell Depletion Prevents Fiber Hypertrophy in Skeletal Muscle. Development 2016, 143, 2898–2906. [Google Scholar] [CrossRef] [PubMed]
- Goh, Q.; Millay, D.P. Requirement of Myomaker-Mediated Stem Cell Fusion for Skeletal Muscle Hypertrophy. Elife 2017, 6, e20007. [Google Scholar] [CrossRef]
- Murach, K.A.; White, S.H.; Wen, Y.; Ho, A.; Dupont-Versteegden, E.E.; McCarthy, J.J.; Peterson, C.A. Differential Requirement for Satellite Cells During Overload-Induced Muscle Hypertrophy in Growing Versus Mature Mice. Skelet. Muscle 2017, 7, 14. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Mula, J.; Miyazaki, M.; Erfani, R.; Garrison, K.; Farooqui, A.B.; Srikuea, R.; Lawson, B.A.; Grimes, B.; Keller, C.; et al. Effective Fiber Hypertrophy in Satellite Cell-Depleted Skeletal Muscle. Development 2011, 138, 3657–3666. [Google Scholar] [CrossRef]
- Koulmann, N.; Bigard, A.X. Interaction between Signalling Pathways Involved in Skeletal Muscle Responses to Endurance Exercise. Pflug. Arch. 2006, 452, 125–139. [Google Scholar] [CrossRef]
- SShamim, B.; Hawley, J.A.; Camera, D.M. Protein Availability and Satellite Cell Dynamics in Skeletal Muscle. Sports Med. 2018, 48, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, R.; Heintz, C. Enhancement of Skeletal Muscle Regeneration. Dev. Dyn. 1994, 201, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, M.; Naito, H.; Ogura, Y.; Kojima, A.; Goto, K.; Katamoto, S. Effects of Voluntary Wheel Running on Satellite Cells in the Rat Plantaris Muscle. J. Sports Sci. Med. 2009, 8, 51–57. [Google Scholar] [PubMed]
- Smith, H.K.; Merry, T.L. Voluntary Resistance Wheel Exercise During Post-Natal Growth in Rats Enhances Skeletal Muscle Satellite Cell and Myonuclear Content at Adulthood. Acta Physiol. 2012, 204, 393–402. [Google Scholar] [CrossRef]
- Li, P.; Akimoto, T.; Zhang, M.; Williams, R.S.; Yan, Z. Resident Stem Cells Are Not Required for Exercise-Induced Fiber-Type Switching and Angiogenesis but Are Necessary for Activity-Dependent Muscle Growth. Am. J. Physiol. Cell Physiol. 2006, 290, C1461–C1468. [Google Scholar] [CrossRef]
- Masschelein, E.; D’Hulst, G.; Zvick, J.; Hinte, L.; Soro-Arnaiz, I.; Gorski, T.; Von Meyenn, F.; Bar-Nur, O.; De Bock, K. Exercise Promotes Satellite Cell Contribution to Myofibers in a Load-Dependent Manner. Skelet. Muscle 2020, 10, 21. [Google Scholar] [CrossRef]
- Shefer, G.; Rauner, G.; Yablonka-Reuveni, Z.; Benayahu, D. Reduced Satellite Cell Numbers and Myogenic Capacity in Aging Can Be Alleviated by Endurance Exercise. PLoS ONE 2010, 5, e13307. [Google Scholar] [CrossRef]
- Falkenberg, H.; Langhammer, M.; Renne, U. Comparison of Biochemical Blood Traits after Long-Term Selection on High or Low Locomotory Activity in Mice. Arch. Anim. Breed. 2000, 43, 513–522. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Walz, C.; Spitschak, M.; Wirthgen, E.; Walz, M.; Langhammer, M.; Tuchscherer, A.; Naumann, R.; Hoeflich, A. Partial Phenotype Conversion and Differential Trait Response to Conditions of Husbandry in Mice. J. Comp. Physiol. B 2018, 188, 527–539. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Ohde, D.; Walz, C.; Langhammer, M.; Schultz, J.; Hoeflich, A. Analysis of Activity-Dependent Energy Metabolism in Mice Reveals Regulation of Mitochondrial Fission and Fusion mRNA by Voluntary Physical Exercise in Subcutaneous Fat from Male Marathon Mice (Duhtp). Cells 2020, 9, 2697. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin Is Elevated in Skeletal Muscle and Serum of Mice Immediately after Acute Exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Brenmoehl, J.; Ohde, D.; Walz, C.; Schultz, J.; Tuchscherer, A.; Rieder, F.; Renne, U.; Hoeflich, A. Dynamics of Fat Mass in Duhtp Mice Selected for Running Performance—Fat Mobilization in a Walk. Obes. Facts 2015, 8, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Brenmoehl, J.; Ohde, D.; Albrecht, E.; Walz, C.; Tuchscherer, A.; Hoeflich, A. Browning of Subcutaneous Fat and Higher Surface Temperature in Response to Phenotype Selection for Advanced Endurance Exercise Performance in Male Duhtp Mice. J. Comp. Physiol. B 2017, 187, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Sonia, A.-M.; Rochat, A.; Mademtzoglou, D.; Morais, J.; de Reyniès, A.; Auradé, F.; Chang, T.H.-T.; Zammit, P.S.; Relaix, F. Gene Expression Profiling of Muscle Stem Cells Identifies Novel Regulators of Postnatal Myogenesis. Front. Cell Dev. Biol. 2016, 4, 58. [Google Scholar]
- Dietl, G.; Langhammer, M.; Renne, U. Model Simulations for Genetic Random Drift in the Outbred Strain Fzt:Du. Arch. Tierz. 2004, 47, 595–604. [Google Scholar] [CrossRef]
- Atienza, J.M.; Zhu, J.; Wang, X.; Xu, X.; Abassi, Y. Dynamic Monitoring of Cell Adhesion and Spreading on Microelectronic Sensor Arrays. J. Biomol. Screen 2005, 10, 795–805. [Google Scholar] [CrossRef]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The Xcelligence System for Real-Time and Label-Free Monitoring of Cell Viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar]
- Miersch, C.; Stange, K.; Röntgen, M. Separation of Functionally Divergent Muscle Precursor Cell Populations from Porcine Juvenile Muscles by Discontinuous Percoll Density Gradient Centrifugation. BMC Cell Biol. 2018, 19, 2. [Google Scholar] [CrossRef]
- Kirstein, S.L.; Atienza, J.M.; Xi, B.; Zhu, J.; Yu, N.; Wang, X.; Xu, X.; Abassi, Y.A. Live Cell Quality Control and Utility of Real-Time Cell Electronic Sensing for Assay Development. Assay Drug Dev. Technol. 2006, 4, 545–553. [Google Scholar] [CrossRef]
- Weintraub, H.; Dwarki, V.J.; Verma, I.; Davis, R.; Hollenberg, S.; Snider, L.; Lassar, A.; Tapscott, S.J. Muscle-Specific Transcriptional Activation by Myod. Genes Dev. 1991, 5, 1377–1386. [Google Scholar] [CrossRef]
- Manzanares, G.; Brito-Da-Silva, G.; Gandra, P. Voluntary Wheel Running: Patterns and Physiological Effects in Mice. Braz. J. Med. Biol. Res. 2018, 52, e7830. [Google Scholar] [CrossRef] [PubMed]
- Oydanich, M.; Babici, D.; Zhang, J.; Rynecki, N.; Vatner, D.E.; Vatner, S.F. Mechanisms of Sex Differences in Exercise Capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R832–R838. [Google Scholar] [CrossRef] [PubMed]
- Bartling, B.; Al-Robaiy, S.; Lehnich, H.; Binder, L.; Hiebl, B.; Simm, A. Sex-Related Differences in the Wheel-Running Activity of Mice Decline with Increasing Age. Exp. Gerontol. 2017, 87, 139–147. [Google Scholar] [CrossRef] [PubMed]
- McGillivray, D.G.; Garland, T.; Dlugosz, E.M.; Chappell, M.A.; Syme, D.A. Changes in Efficiency and Myosin Expression in the Small-Muscle Phenotype of Mice Selectively Bred for High Voluntary Running Activity. J. Exp. Biol. 2009, 212, 977–985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wozniak, A.C.; Pilipowicz, O.; Yablonka-Reuveni, Z.; Greenway, S.; Craven, S.; Scott, E.; Anderson, J.E. C-Met Expression and Mechanical Activation of Satellite Cells on Cultured Muscle Fibers. J. Histochem. Cytochem. 2003, 51, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; King, K.Y.; Brett, J.O.; Cromie, M.J.; Charville, G.W.; Maguire, K.K.; Brunson, C.; Mastey, N.; Liu, L.; Tsai, C.R.; et al. Mtorc1 Controls the Adaptive Transition of Quiescent Stem Cells from G0 to G(Alert). Nature 2014, 510, 393–396. [Google Scholar] [CrossRef]
- Joseph, R.T.; Schroeder, M.D.; Ma, C.; Rando, T.A. Hgfa Is an Injury-Regulated Systemic Factor That Induces the Transition of Stem Cells into GAlert. Cell Rep. 2017, 19, 479–486. [Google Scholar]
- Sellathurai, J.; Cheedipudi, S.; Dhawan, J.; Schrøder, H.D. A Novel in Vitro Model for Studying Quiescence and Activation of Primary Isolated Human Myoblasts. PLoS ONE 2013, 8, e64067. [Google Scholar] [CrossRef]
- Rouger, K.; Brault, M.; Daval, N.; Leroux, I.; Guigand, L.; Lesoeur, J.; Fernandez, B.; Cherel, Y. Muscle Satellite Cell Heterogeneity: In Vitro and in Vivo Evidences for Populations That Fuse Differently. Cell Tissue Res. 2004, 317, 319–326. [Google Scholar] [CrossRef]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle Satellite Cells Adopt Divergent Fates: A Mechanism for Self-Renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Kubori, T.; Kohara, N.; Iizuka, T.; Kimura, J. Muscle Afferent Block for the Treatment of Oromandibular Dystonia. Mov. Disord. 1998, 13, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Halevy, O.; Piestun, Y.; Allouh, M.; Rosser, B.W.; Rinkevich, Y.; Reshef, R.; Rozenboim, I.; Wleklinski-Lee, M.; Yablonka-Reuveni, Z. Pattern of Pax7 Expression During Myogenesis in the Posthatch Chicken Establishes a Model for Satellite Cell Differentiation and Renewal. Dev. Dyn. 2004, 231, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Sreenivas, P.; Cheedipudi, S.; Reddy, V.R.; Shashidhara, L.S.; Chilukoti, R.K.; Mylavarapu, M.; Dhawan, J. Distinct Transcriptional Networks in Quiescent Myoblasts: A Role for Wnt Signaling in Reversible vs. Irreversible Arrest. PLoS ONE 2014, 8, e65097. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem Cell Function, Self-Renewal, and Behavioral Heterogeneity of Cells from the Adult Muscle Satellite Cell Niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, V.F.; White, R.B.; Ono, Y.; Ellis, J.A.; Zammit, P.S. Further Characterisation of the Molecular Signature of Quiescent and Activated Mouse Muscle Satellite Cells. PLoS ONE 2009, 4, e5205. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Murase, T.; Kondo, S.; Okuda, A.; Inagawa-Ogashiwa, M. Muscle Reconstitution by Muscle Satellite Cell Descendants with Stem Cell-Like Properties. Development 2004, 131, 5481–5490. [Google Scholar] [CrossRef][Green Version]
- Miersch, C.; Stange, K.; Hering, S.; Kolisek, M.; Viergutz, T.; Röntgen, M. Molecular and Functional Heterogeneity of Early Postnatal Porcine Satellite Cell Populations Is Associated with Bioenergetic Profile. Sci. Rep. 2017, 7, 45052. [Google Scholar] [CrossRef]
- Nadia, C.; Kadi, F.; Féasson, L.; Denis, C. Satellite Cell Frequency in Skeletal Muscle of Old Men: Effects of Endurance Training. Muscle Nerve 2003, 28, 87–92. [Google Scholar]
- John, L.; Bischoff, R. Differentiation of Creatine Phosphokinase During Myogenesis: Quantitative Fractionation of Isozymes. Dev. Biol. 1977, 57, 330–344. [Google Scholar]
- Kuang, S.; Chargé, S.B.; Seale, P.; Huh, M.; Rudnicki, M.A. Distinct Roles for Pax7 and Pax3 in Adult Regenerative Myogenesis. J. Cell Biol. 2006, 172, 103–113. [Google Scholar] [CrossRef]
- Appell, H.-J.; Forsberg, S.; Hollmann, W. Satellite Cell Activation in Human Skeletal Muscle after Training: Evidence for Muscle Fiber Neoformation. Int. J. Sports Med. 1988, 9, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Yutzey, K.E.; Rhodes, S.J.; Konieczny, S.F. Differential Trans Activation Associated with the Muscle Regulatory Factors Myod1, Myogenin, and Mrf4. Mol. Cell. Biol. 1990, 10, 3934–3944. [Google Scholar] [PubMed]
- Lassar, A.B.; Buskin, J.N.; Lockshon, D.; Davis, R.L.; Apone, S.; Hauschka, S.D.; Weintraub, H. Myod Is a Sequence-Specific DNA Binding Protein Requiring a Region of Myc Homology to Bind to the Muscle Creatine Kinase Enhancer. Cell 1989, 58, 823–831. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.; Wu, J.; Estall, J.; Irving, B.; et al. A Pgc-1alpha Isoform Induced by Resistance Training Regulates Skeletal Muscle Hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Kadi, F.; Charifi, N.; Denis, C.; Lexell, J.; Andersen, J.L.; Schjerling, P.; Olsen, S.; Kjaer, M. The Behaviour of Satellite Cells in Response to Exercise: What Have We Learned from Human Studies? Pflug. Arch. 2005, 451, 319–327. [Google Scholar] [CrossRef]
- Brooks, M.J.; Hajira, A.; Mohamed, J.S.; Alway, S.E. Voluntary Wheel Running Increases Satellite Cell Abundance and Improves Recovery from Disuse in Gastrocnemius Muscles from Mice. J. Appl. Physiol. 2018, 124, 1616–1628. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ogawa, R.; Watanabe, Y.; Uezumi, A.; Miyagoe-Suzuki, Y.; Tsujikawa, K.; Yamamoto, H.; Takeda, S.; Fukada, S.-I. Calcitonin Receptor and Odz4 Are Differently Expressed in Pax7-Positive Cells During Skeletal Muscle Regeneration. J. Mol. Histol. 2012, 43, 581–587. [Google Scholar] [CrossRef]
- Ishii, K.; Suzuki, N.; Mabuchi, Y.; Ito, N.; Kikura, N.; Fukada, S.-I.; Okano, H.; Takeda, S.; Akazawa, C. Muscle Satellite Cell Protein Teneurin-4 Regulates Differentiation During Muscle Regeneration. Stem Cells 2015, 33, 3017–3027. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkov, S.; Brenmoehl, J.; Langhammer, M.; Hoeflich, A.; Röntgen, M. Myogenic Precursor Cells Show Faster Activation and Enhanced Differentiation in a Male Mouse Model Selected for Advanced Endurance Exercise Performance. Cells 2022, 11, 1001. https://doi.org/10.3390/cells11061001
Petkov S, Brenmoehl J, Langhammer M, Hoeflich A, Röntgen M. Myogenic Precursor Cells Show Faster Activation and Enhanced Differentiation in a Male Mouse Model Selected for Advanced Endurance Exercise Performance. Cells. 2022; 11(6):1001. https://doi.org/10.3390/cells11061001
Chicago/Turabian StylePetkov, Stefan, Julia Brenmoehl, Martina Langhammer, Andreas Hoeflich, and Monika Röntgen. 2022. "Myogenic Precursor Cells Show Faster Activation and Enhanced Differentiation in a Male Mouse Model Selected for Advanced Endurance Exercise Performance" Cells 11, no. 6: 1001. https://doi.org/10.3390/cells11061001
APA StylePetkov, S., Brenmoehl, J., Langhammer, M., Hoeflich, A., & Röntgen, M. (2022). Myogenic Precursor Cells Show Faster Activation and Enhanced Differentiation in a Male Mouse Model Selected for Advanced Endurance Exercise Performance. Cells, 11(6), 1001. https://doi.org/10.3390/cells11061001






