Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones
Abstract
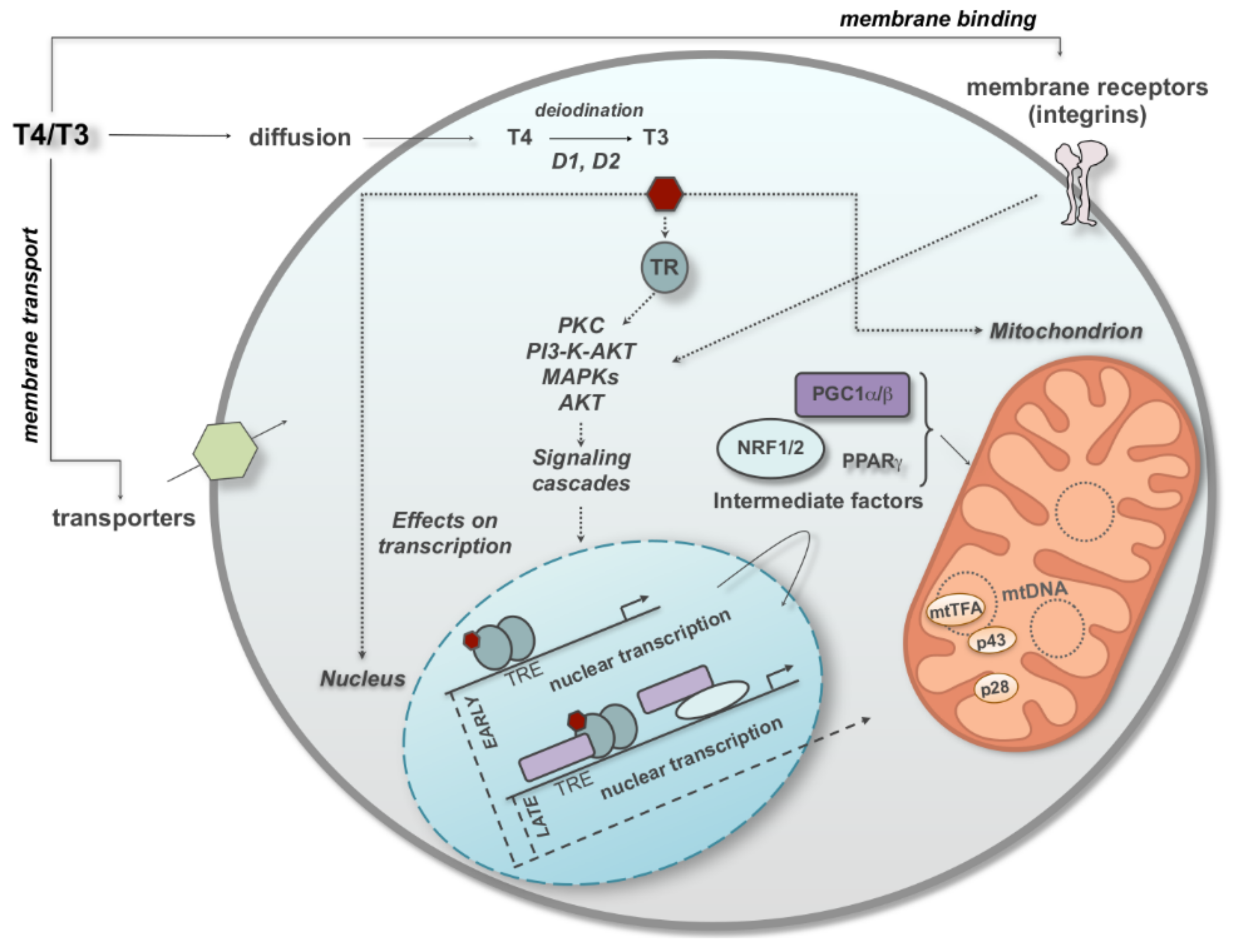
1. Introduction
1.1. Respiratory Chain, Oxidative Phosphorylation
1.2. Thyroid Hormones, General Concepts
2. Thyroid Hormones and the OXPHOS
Thyroid Hormones and the Mitochondrial Efficiency
3. Nongenomic Regulation of Mitochondrial Respiratory Chain by TH with a Look at the Effects of T2
4. Iodothyronines and Respiratory Supercomplexes
5. Iodothyronines, Mitochondrial Dynamics and Mitophagy
6. Thyromimetics and Mitochondria
7. Conclusions
A Final Speculation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Letts, J.A.; Sazanov, L.A. Clarifying the supercomplex: The higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol. 2017, 24, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Trombetti, F.; Pagliarani, A.; Ventrella, V.; Algieri, C.; Tioli, G.; Lenaz, G. Molecular and Supramolecular Structure of the Mitochondrial Oxidative Phosphorylation System: Implications for Pathology. Life 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Galemou Yoga, E.; Angerer, H.; Parey, K.; Zickermann, V. Respiratory complex I—Mechanistic insights and advances in structure determination. Biochim. Biophys. Acta Bioenergy 2020, 1861, 148153. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.S.; D’Imprima, E.; Vonck, J. Mitochondrial Respiratory Chain Complexes. Subcell. Biochem. 2018, 87, 167–227. [Google Scholar] [CrossRef]
- Xia, D.; Esser, L.; Tang, W.K.; Zhou, F.; Zhou, Y.; Yu, L.; Yu, C.A. Structural analysis of cytochrome bc1 complexes: Implications to the mechanism of function. Biochim. Biophys. Acta 2013, 1827, 1278–1294. [Google Scholar] [CrossRef]
- Zong, S.; Wu, M.; Gu, J.; Liu, T.; Guo, R.; Yang, M. Structure of the Intact 14-Subunit Human Cytochrome c Oxidase. Cell Res. 2018, 28, 1026–1034. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial Complex II: At the Crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Orr, A.L.; Brand, M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Structure and Mechanisms of F-Type ATP Synthases. Annu. Rev. Biochem. 2019, 88, 515–549. [Google Scholar] [CrossRef]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenergy 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial proton leaks and uncoupling proteins. Biochim. Biophys. Acta Bioenergy 2021, 1862, 148428. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Chouchani, E.T.; Kazak, L.; Angelin, A.; Fedorenko, A.; Long, J.Z.; Vidoni, S.; Garrity, R.; Cho, J.; Terada, N.; et al. H+ transport is an integral function of the mitochondrial ADP/ATP carrier. Nature 2019, 571, 515–520. [Google Scholar] [CrossRef]
- Brand, M.D.; Brindle, K.M.; Buckingham, J.A.; Harper, J.A.; Rolfe, D.F.; Stuart, J.A. The significance and mechanism of mitochondrial proton conductance. Int. J. Obes. Relat. Metab. Disord. 1999, 23, S4–S11. [Google Scholar] [CrossRef]
- Busiello, R.A.; Savarese, S.; Lombardi, A. Mitochondrial uncoupling proteins and energy metabolism. Front. Physiol. 2015, 6, 36. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 1955, 217, 409–427. [Google Scholar] [CrossRef]
- Moreno, M.; de Lange, P.; Lombardi, A.; Silvestri, E.; Lanni, A.; Goglia, F. Metabolic effects of thyroid hormone derivatives. Thyroid 2008, 18, 239–253. [Google Scholar] [CrossRef]
- Orozco, A.; Navarrete-Ramírez, P.; Olvera, A.; García-G, C. 3,5-Diiodothyronine (T2) is on a role. A new hormone in search of recognition. Gen. Comp. Endocrinol. 2014, 203, 174–180. [Google Scholar] [CrossRef]
- Senese, R.; Cioffi, F.; de Lange, P.; Goglia, F.; Lanni, A. Thyroid: Biological actions of ‘nonclassical’ thyroid hormones. J. Endocrinol. 2014, 221, R1–R12. [Google Scholar] [CrossRef]
- Zucchi, R.; Accorroni, A.; Chiellini, G. Update on 3-iodothyronamine and its neurological and metabolic actions. Front. Physiol. 2014, 5, 402. [Google Scholar] [CrossRef]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B.M.L.C. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [Google Scholar] [CrossRef]
- Russo, S.C.; Salas-Lucia, F.; Bianco, A.C. Deiodinases and the Metabolic Code for Thyroid Hormone Action. Endocrinology 2021, 162, bqab059. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.; Hernandez, A. ABCD of Thyroid Hormone Action: After and Before Cloning of Deiodinase Genes. Endocrinology 2021, 162, bqab151. [Google Scholar] [CrossRef] [PubMed]
- Siegrist-Kaiser, C.; Burger, A.G. Modification of the side chain of thyroid hormones. In Thyroid Hormone Metabolism; Wu, S.Y., Visser, T.J., Eds.; CRC: Boca Raton, FL, USA, 1994; pp. 175–198. [Google Scholar]
- Singh, B.K.; Sinha, R.A.; Yen, P.M. Novel Transcriptional Mechanisms for Regulating Metabolism by Thyroid Hormone. Int. J. Mol. Sci. 2018, 19, 3284. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Leonard, J.L.; Lin, H.Y.; Leinung, M.; Mousa, S.A. Molecular Basis of Nongenomic Actions of Thyroid Hormone. Vitam. Horm. 2018, 106, 67–96. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Goglia, F. Mitochondrial Actions of Thyroid Hormone. Compr. Physiol. 2016, 6, 1591–1607. [Google Scholar] [CrossRef]
- Harper, M.E.; Brand, M.D. Use of top-down elasticity analysis to identify sites of thyroid hormone-induced thermogenesis. Proc. Soc. Exp. Biol. Med. 1995, 208, 228–237. [Google Scholar] [CrossRef]
- Rolfe, D.F.; Hulbert, A.J.; Brand, M.D. Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen consuming tissues of the rat. Biochem. Biophys. Acta 1994, 1118, 405–416. [Google Scholar] [CrossRef]
- Hafner, R.P.; Brown, G.C.; Brand, M.D. Thyroid-hormone control of state-3 respiration in isolated rat liver mitochondria. Biochem. J. 1990, 265, 731–734. [Google Scholar] [CrossRef]
- Hafner, R.P.; Nobes, C.D.; McGown, A.D.; Brand, M.D. Altered relationship between proton motive force and respiration rate in non-phosphorylating liver mitochondria isolated from rats of different thyroid hormone status. Eur. J. Biochem. 1988, 178, 511–518. [Google Scholar] [CrossRef]
- Dummler, K.; Muller, S.; Seitz, H.J. Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem. J. 1996, 317, 913–918. [Google Scholar] [CrossRef]
- Seitz, H.J.; Muller, M.J.; Soboll, S. Rapid thyroid-hormone effect on mitochondrial and cytosolic ATP/ADP ratios in the intact liver cell. Biochem. J. 1985, 227, 149–153. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wiêckowski, M.R.; Wojtczak, L. Thyroid hormone-induced expression of the ADP/ATP carrier and its effect on fatty acid-induced uncoupling of oxidative phosphorylation. FEBS Lett. 1997, 416, 19–22. [Google Scholar] [CrossRef]
- Denton, R.L.; McCormac, J.G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am. J. Physiol. 1985, 249, E543–E554. [Google Scholar] [CrossRef]
- Weitzel, J.M.; Radtke, C.; Seitz, H.J. Two thyroid hormone-mediated gene expression patterns in vivo identified by cDNA expression arrays in rat. Nucleic Acids Res. 2001, 29, 5148–5155. [Google Scholar] [CrossRef]
- Wulf, A.; Harneit, A.; Kröger, M.; Kebenko, M.; Wetzel, M.G.; Weitzel, J.M. T3-mediated expression of PGC-1alpha via a far upstream located thyroid hormone response element. Mol. Cell. Endocrinol. 2008, 287, 90–95. [Google Scholar] [CrossRef]
- Weitzel, J.M.; Iwen, K.A. Coordination of mitochondrial biogenesis by thyroid hormone. Mol. Cell. Endocrinol. 2011, 342, 1–7. [Google Scholar] [CrossRef]
- Venditti, P.; Napolitano, G.; Fasciolo, G.; Di Meo, S. Thyroid state affects H2O2 removal by rat heart mitochondria. Arch. Biochem. Biophys. 2019, 662, 61–67. [Google Scholar] [CrossRef]
- Yehuda-Shnaidman, E.; Kalderon, B.; Bar-Tana, J. Thyroid hormone, thyromimetics, and metabolic efficiency. Endocr. Rev. 2014, 35, 35–58. [Google Scholar] [CrossRef]
- Vaitkus, J.A.; Farrar, J.S.; Celi, F.S. Thyroid Hormone Mediated Modulation of Energy Expenditure. Int. J. Mol. Sci. 2015, 16, 16158–16175. [Google Scholar] [CrossRef]
- Yau, W.W.; Yen, P.M. Thermogenesis in Adipose Tissue Activated by Thyroid Hormone. Int. J. Mol. Sci. 2020, 21, 3020. [Google Scholar] [CrossRef]
- Magnus-Levy, A. Uber den respiratorischen Gaswechsel unter dem Ein fluss der Thyroidea sowie unter verschiedenen pathologischen Zustanden. Berl. Klin. Wochenschr. 1895, 34, 650–654. [Google Scholar]
- Hess, B.; Martius, C. The mode of action of thyroxin. Arch. Biochem. Biophys. 1951, 33, 486–487. [Google Scholar]
- Lardy, H.A.; Feldcott, G. Metabolic effects of thyroxine in vitro. Ann. N. Y. Acad. Sci. 1951, 54, 636–648. [Google Scholar] [CrossRef]
- Brookes, P.S.; Hulbert, A.J.; Brand, M.D. The proton permeability of liposomes made from mitochondrial inner membrane phospholipids: No effect of fatty acid composition. Biochim. Biophys. Acta 1997, 1330, 157–164. [Google Scholar] [CrossRef][Green Version]
- Bertholet, A.M.; Kazak, L.; Chouchani, E.T.; Bogaczynska, M.G.; Paranjpe, I.; Wainwright, G.L.; Bétourné, A.; Kajimura, S.; Spiegelman, B.M.; Kirichok, Y. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab. 2017, 25, 811–822.e4. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef]
- Bround, M.J.; Bers, D.M.; Molkentin, J.D. A 20/20 view of ANT function in mitochondrial biology and necrotic cell death. J. Mol. Cell. Cardiol. 2020, 144, A3–A13. [Google Scholar] [CrossRef]
- Ribeiro, M.O.; Bianco, S.D.; Kaneshige, M.; Schultz, J.J.; Cheng, S.Y.; Bianco, A.C.; Brent, G.A. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-beta isoform specific and required for adaptive thermogenesis. Endocrinology 2010, 151, 432–440. [Google Scholar] [CrossRef]
- Herz, C.T.; Kiefer, F.W. Adipose tissue browning in mice and humans. J. Endocrinol. 2019, 241, R97–R109. [Google Scholar] [CrossRef]
- Krause, K. Novel Aspects of White Adipose Tissue Browning by Thyroid Hormones. Exp. Clin. Endocrinol. Diabetes 2020, 128, 446–449. [Google Scholar] [CrossRef]
- Larkin, S.; Mull, E.; Miao, W.; Pittner, R.; Albrandt, K.; Moore, C.; Young, A.; Denaro, M.; Beaumont, K. Regulation of the third member of the uncoupling protein family, UCP3, by cold and thyroid hormone. Biochem. Biophys. Res. Commun. 1997, 240, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Yoshimatsu, H.; Kakuma, T.; Hidaka, S.; Kurokawa, M.; Sakata, T. Enhanced expression of uncoupling protein 2 gene in rat white adipose tissue and skeletal muscle following chronic treatment with thyroid hormone. FEBS Lett. 1997, 418, 323–326. [Google Scholar] [CrossRef]
- Cioffi, F.; Senese, R.; de Lange, P.; Goglia, F.; Lanni, A.; Lombardi, A. Uncoupling proteins: A complex journey to function discovery. Biofactors 2009, 35, 417–428. [Google Scholar] [CrossRef] [PubMed]
- De Lange, P.; Lanni, A.; Beneduce, L.; Moreno, M.; Lombardi, A.; Silvestri, E.; Goglia, F. Uncoupling protein-3 is a molecular determinant for the regulation of restingmetabolic rate by thyroid hormone. Endocrinology 2001, 142, 3414–3420. [Google Scholar] [CrossRef] [PubMed]
- Lanni, A.; Beneduce, L.; Lombardi, A.; Moreno, M.; Boss, O.; Muzzin, P.; Giacobino, J.P.; Goglia, F. Expression of uncoupling protein-3 and mitochondrial activity in the transition from hypothyroid to hyperthyroid state in rat skeletal muscle. FEBS Lett. 1999, 444, 250–254. [Google Scholar] [CrossRef]
- Lanni, A.; De Felice, M.; Lombardi, A.; Moreno, M.; Fleury, C.; Ricquier, D.; Goglia, F. Induction of UCP2 mRNA by thyroid hormones in rat heart. FEBS Lett. 1997, 418, 171–174. [Google Scholar] [CrossRef]
- Brand, M.D.; Esteves, T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef]
- Flandin, P.; Lehr, L.; Asensio, C.; Giacobino, J.P.; Rohner-Jeanrenaud, F.; Muzzin, P.; Jimenez, M. Uncoupling protein-3 as a molecular determinant of the action of 3,5,3′-triiodothyronine on energy metabolism. Endocrine 2009, 36, 246–254. [Google Scholar] [CrossRef][Green Version]
- Lombardi, A.; Busiello, R.A.; Napolitano, L.; Cioffi, F.; Moreno, M.; de Lange, P.; Silvestri, E.; Lanni, A.; Goglia, F. UCP3 translocates lipid hydroperoxide and mediates lipid hydroperoxide-dependent mitochondrial uncoupling. J. Biol. Chem. 2010, 285, 16599–16605. [Google Scholar] [CrossRef]
- Costford, S.R.; Chaudhry, S.N.; Salkhordeh, M.; Harper, M.E. Effects of the presence, absence, and overexpression of uncoupling protein-3 on adiposity and fuel metabolism in congenic mice. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1304–E1312. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Samec, S. Uncoupling proteins: Their roles in adaptive thermogenesis and substrate metabolism reconsidered. Br. J. Nutr. 2001, 86, 123–139. [Google Scholar] [CrossRef]
- Goglia, F.; Skulachev, V.P. A function for novel uncoupling proteins: Antioxidant defense of mitochondrial matrix by translocating fatty acid peroxides from the inner to the outer membrane leaflet. FASEB J. 2003, 17, 1585–1591. [Google Scholar] [CrossRef]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef]
- Kinnally, K.W.; Peixoto, P.M.; Ryu, S.Y.; Dejean, L.M. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta 2011, 1813, 616–622. [Google Scholar] [CrossRef]
- Miura, T.; Tanno, M. The mPTP and its regulatory proteins: Final common targets of signalling pathways for protection against necrosis. Cardiovasc. Res. 2012, 94, 181–189. [Google Scholar] [CrossRef]
- Azzolin, L.; von Stockum, S.; Basso, E.; Petronilli, V.; Forte, M.A.; Bernardi, P. The mitochondrial permeability transition from yeast to mammals. FEBS Lett. 2010, 584, 2504–2509. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Yaniv, Y.; Nuss, H.B.; Wang, S.; Sollott, S.J. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc. Res. 2009, 83, 213–225. [Google Scholar] [CrossRef]
- Haworth, R.A.; Hunter, D.R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979, 195, 460–467. [Google Scholar] [CrossRef]
- Raaflaub, J. Swelling of isolated mitochondria of the liver and their susceptibility to physicochemical influences. Helv. Physiol. Pharmacol. Acta 1953, 11, 142–156. [Google Scholar]
- Tapley, D.F. The effect of thyroxine and other substances on the swelling of isolated rat liver mitochondria. J. Biol. Chem. 1956, 222, 325–339. [Google Scholar] [CrossRef]
- Lehninger, A.L. Thyroxine and the swelling and contraction cycle in mitochondria. Ann. N. Y. Acad. Sci. 1960, 86, 484–493. [Google Scholar] [CrossRef]
- Kalderon, B.; Hermesh, O.; Bar-Tana, J. Mitochondrial permeability transition is induced by in vivo thyroid hormone treatment. Endocrinology 1995, 136, 3552–3556. [Google Scholar] [CrossRef]
- Castilho, R.F.; Kowaltowski, A.J.; Vercesi, A.E. 3,5,3′-triiodothyronine induces mitochondrial permeability transition mediated by reactive oxygen species and membrane protein thiol oxidation. Arch. Biochem. Biophys. 1998, 354, 151–157. [Google Scholar] [CrossRef]
- Venditti, P.; De Rosa, R.; Di Meo, S. Effect of thyroid state on susceptibility to oxidants and swelling of mitochondria from rat tissues. Free Radic. Biol. Med. 2003, 35, 485–494. [Google Scholar] [CrossRef]
- Yehuda-Shnaidman, E.; Kalderon, B.; Azazmeh, N.; Bar-Tana, J. Gating of the mitochondrial permeability transition pore by thyroid hormone. FASEB J. 2010, 24, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Endlicher, R.; Drahota, Z.; Červinková, Z. In vitro and in vivo activation of mitochondrial membrane permeability transition pore using triiodothyronine. Physiol. Res. 2016, 65, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Casolo, V.; Petrussa, E.; Peresson, C.; Patui, S.; Bertolini, A.; Passamonti, S.; Braidot, E.; Zancani, M. The mitochondrial permeability transition pore (PTP)—An example of multiple molecular exaptation? Biochim. Biophys. Acta 2012, 1817, 2072–2086. [Google Scholar] [CrossRef]
- Karch, J.; Molkentin, J.D. Identifying the components of the elusive mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA 2014, 111, 10396–10397. [Google Scholar] [CrossRef]
- Alavian, K.N.; Beutner, G.; Lazrove, E.; Sacchetti, S.; Park, H.A.; Licznerski, P.; Li, H.; Nabili, P.; Hockensmith, K.; Graham, M.; et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA 2014, 111, 10580–10585. [Google Scholar] [CrossRef]
- Kokoszka, J.E.; Waymire, K.G.; Levy, S.E.; Sligh, J.E.; Cai, J.; Jones, D.P.; MacGregor, G.R.; Wallace, D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004, 427, 461–465. [Google Scholar] [CrossRef]
- Gutiérrez-Aguilar, M.; Douglas, D.L.; Gibson, A.K.; Domeier, T.L.; Molkentin, J.D.; Baines, C.P. Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J. Mol. Cell. Cardiol. 2014, 72, 316–325. [Google Scholar] [CrossRef]
- Drahota, Z.; Endlicher, R.; Staňková, P.; Rychtrmoc, D.; Milerová, M.; Cervinková, Z. Characterization of calcium, phosphate and peroxide interactions in activation of mitochondrial swelling using derivative of the swelling curves. J. Bioenergy Biomembr. 2012, 44, 309–315. [Google Scholar] [CrossRef]
- Di Lisa, F.; Bernardi, P. Modulation of Mitochondrial Permeability Transition in Ischemia-Reperfusion Injury of the Heart. Advantages and Limitations. Curr. Med. Chem. 2015, 22, 2480–2487. [Google Scholar] [CrossRef]
- Bonora, M.; Wieckowski, M.R.; Chinopoulos, C.; Kepp, O.; Kroemer, G.; Galluzzi, L.; Pinton, P. Molecular mechanisms of cell death: Central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015, 34, 1475–1486. [Google Scholar] [CrossRef]
- Traba, J.; Del Arco, A.; Duchen, M.R.; Szabadkai, G.; Satrústegui, J. SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca2+ buffering. Cell Death Differ. 2012, 19, 650–660. [Google Scholar] [CrossRef]
- Moro, L.; Marra, E.; Capuano, F.; Greco, M. Thyroid hormone treatment of hypothyroid rats restores the regenerative capacity and the mitochondrial membrane permeability properties of the liver after partial hepatectomy. Endocrinology 2004, 145, 5121–5128. [Google Scholar] [CrossRef]
- Del Viscovo, A.; Secondo, A.; Esposito, A.; Goglia, F.; Moreno, M.; Canzoniero, L.M. Intracellular and plasma membrane-initiated pathways involved in the [Ca2C]i elevations induced by iodothyronines (T3 and T2) in pituitary GH3 cells. Am. J. Physiol. 2012, 302, E1419–E1430. [Google Scholar]
- Mangiullo, R.; Gnoni, A.; Damiano, F.; Siculella, L.; Zanotti, F.; Papa, S.; Gnoni, G.V. 3,5-diiodo-L-thyronine upregulates rat-liver mitochondrial FoF1-ATP synthase by GA-binding protein/nuclear respiratory factor-2. Biochim. Biophys. Acta 2010, 1797, 233–240. [Google Scholar] [CrossRef]
- Silvestri, E.; Lombardi, A.; Coppola, M.; Gentile, A.; Cioffi, F.; Senese, R.; Goglia, F.; Lanni, A.; Moreno, M.; de Lange, P. Differential Effects of 3,5-Diiodo-L-Thyronine and 3,5,3′-Triiodo-L-Thyronine on Mitochondrial Respiratory Pathways in Liver from Hypothyroid Rats. Cell. Physiol. Biochem. 2018, 47, 2471–2483. [Google Scholar] [CrossRef]
- Palacios-Romero, R.; Mowbray, J. Evidence for the rapid direct control both in vivo and in vitro of the efficiency of oxidative phosphorylation by 3,5,3-Tri-iodo-thyronine in rats. Biochem. J. 1979, 184, 527–538. [Google Scholar] [CrossRef]
- Sterling, K.; Brenner, M.A.; Sakurada, T. Rapid effect of triiodothyronine on the mitochondrial pathway in rat liver in vivo. Science 1980, 210, 340–343. [Google Scholar] [CrossRef]
- Sterling, K.; Brenner, M.A. Thyroid hormone action: Effect of triiodothyronine on mitochondrial adenine nucleotide translocase in vivo and in vitro. Metabolism 1995, 34, 193–199. [Google Scholar] [CrossRef]
- Sterling, K.; Milch, P.O.; Brenner, M.A.; Lazarus, J.H. Thyroid hormone action: The mitochondrial pathway. Science 1977, 197, 996–999. [Google Scholar] [CrossRef]
- Thomas, W.E.; Crespo-Armas, A.; Mowbray, J. The influence of nanomolar calcium ions and physiological levels of thyroid hormone on oxidative phosphorylation in rat liver mitochondria. A possible signal amplification control mechanism. Biochem. J. 1987, 247, 315–320. [Google Scholar] [CrossRef]
- Goglia, F.; Torresani, J.; Bugli, P.; Barletta, A.; Liverini, G. In vitro binding of triiodothyronine to rat liver mitochondria. Pflügers Archiv. 1981, 390, 120–124. [Google Scholar] [CrossRef]
- Hashizume, K.; Ichikawa, K. Localization of 3,5,3′-l-triiodothyronine receptor in rat mitochondrial membrane. Biochem. Biophys. Res. Commun. 1982, 106, 920–926. [Google Scholar] [CrossRef]
- Sterling, K.; Milch, P.O. Thyroid hormone binding by a component of mitochondrial membrane. Proc. Natl. Acad. Sci. USA 1975, 72, 3225–3229. [Google Scholar]
- Senese, R.; Lasala, P.; Leanza, C.; de Lange, P. New avenues for regulation of lipid metabolism by thyroid hormones and analogs. Front. Physiol. 2014, 5, 475. [Google Scholar] [CrossRef]
- Goglia, F. The effects of 3,5-diiodothyronine on energy balance. Front. Physiol. 2015, 5, 528. [Google Scholar] [CrossRef]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef]
- Pessemesse, L.; Lepourry, L.; Bouton, K.; Levin, J.; Cabello, G.; Wrutniak-Cabello, C.; Casas, F. p28, a truncated form of TRa1 regulates mitochondrial physiology. FEBS Lett. 2014, 588, 4037–4043. [Google Scholar] [CrossRef] [PubMed]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Mitochondrial T3 receptor and targets. Mol. Cell. Endocrinol. 2017, 458, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wrutniak-C, C.; Cassar-Malek, I.; Marchal, S.; Rascle, A.; Heusser, S.; Keller, J.M.; Flèchon, J.; Dauça, M.; Samarut, J.; Ghysdael, J.; et al. A 43-kDa protein related to c-Erb A alpha 1 is located in the mitochondrial matrix of rat liver. J. Biol. Chem. 1995, 270, 16347–16354. [Google Scholar] [CrossRef]
- Wrutniak-Cabello, C.; Carazo, A.; Casas, F.; Cabello, G. Triiodothyronine mitochondrial receptors: Import and molecular mechanisms. J. Soc. Biol. 2008, 202, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Thyroid hormone action in mitochondria. J. Mol. Endocrinol. 2001, 26, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Chocron, E.S.; Sayre, N.L.; Holstein, D.; Saelim, N.; Ibdah, J.A.; Dong, L.Q.; Zhu, X.; Cheng, S.Y.; Lechleiter, J.D. The trifunctional protein mediates thyroid hormone receptor-dependent stimulation of mitochondria metabolism. Mol. Endocrinol. 2012, 26, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Rochard, P.; Rodier, A.; Casas, F.; Cassar-Malek, I.; Marchal-Victorion, S.; Daury, L.; Wrutniak, C.; Cabello, G. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J. Biol. Chem. 2000, 275, 2733–2744. [Google Scholar] [CrossRef]
- Seyer, P.; Grandemange, S.; Busson, M.; Carazo, A.; Gamalèri, F.; Pessemesse, L.; Casas, F.; Cabello, G.; Wrutniak-Cabello, C. Mitochondrial activity regulates myoblast differentiation by control of c-Myc expression. J. Cell. Physiol. 2006, 207, 75–86. [Google Scholar] [CrossRef]
- Seyer, P.; Grandemange, S.; Rochard, P.; Busson, M.; Pessemesse, L.; Casas, F.; Cabello, G.; Wrutniak-Cabello, C. P43-dependent mitochondrial activity regulates myoblast differentiation and slow myosin isoform expression by control of Calcineurin expression. Exp. Cell Res. 2011, 317, 2059–2071. [Google Scholar] [CrossRef]
- Blanchet, E.; Pessemesse, L.; Feillet-Coudray, C.; Coudray, C.; Cabello, C.; Bertrand-Gaday, C.; Casas, F. p43, a Truncated Form of Thyroid Hormone Receptor α, Regulates Maturation of Pancreatic β Cells. Int. J. Mol. Sci. 2021, 22, 2489. [Google Scholar] [CrossRef]
- Horst, C.; Rokos, H.; Seitz, H.J. Rapid stimulation of hepatic oxygen consumption by 3,5-di-iodo-L-thyronine. Biochem. J. 1989, 261, 945–950. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Cioffi, M.; Goglia, F. Effect of 3,3′-diiodothyronine and 3,5-diiodothyronine on rat liver oxidative capacity. Mol. Cell. Endocrinol. 1992, 86, 143–148. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Cioffi, M.; Goglia, F. Effect of 3,3′-di-iodothyronine and 3,5-di-iodothyronine on rat liver mitochondria. J. Endocrinol. 1993, 136, 59–64. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; Goglia, F. Rapid stimulation in vitro of rat liver cytochrome oxidase activity by 3,5-diiodo-L-thyronine and by 3,3′-diiodo-L-thyronine. Mol. Cell. Endocrinol. 1994, 99, 89–94. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; Goglia, F. Calorigenic effect of diiodothyronines in the rat. J. Physiol. 1996, 494, 831–837. [Google Scholar] [CrossRef]
- Goglia, F. Biological effects of 3,5-diiodothyronine (T2). Biochemistry 2005, 70, 164–172. [Google Scholar] [CrossRef]
- Goglia, F.; Lanni, A.; Barth, J.; Kadenbach, B. Interaction of diiodothyronines with isolated cytochrome c oxidase. FEBS Lett. 1994, 346, 295–298. [Google Scholar] [CrossRef]
- Arnold, S.; Goglia, F.; Kadenbach, B. 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur. J. Biochem. 1998, 252, 325–330. [Google Scholar] [CrossRef]
- Kadenbach, B.; Hüttemann, M.; Arnold, S.; Lee, I.; Bender, E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic. Biol. Med. 2000, 29, 211–221. [Google Scholar] [CrossRef]
- Qin, L.; Mills, D.A.; Buhrow, L.; Hiser, C.; Ferguson-Miller, S. A conserved steroid binding site in cytochrome C oxidase. Biochemistry 2008, 47, 9931–9933. [Google Scholar] [CrossRef][Green Version]
- Oleynikov, I.P.; Azarkina, N.V.; Vygodina, T.V.; Konstantinov, A.A. Interaction of Cytochrome C Oxidase with Steroid Hormones. Cells 2020, 9, 2211. [Google Scholar] [CrossRef] [PubMed]
- Oleynikov, I.P.; Azarkina, N.V.; Vygodina, T.V. Direct Interaction of Mitochondrial Cytochrome c Oxidase with Thyroid Hormones: Evidence for Two Binding Sites. Cells 2022, 11, 908. [Google Scholar] [CrossRef]
- Lombardi, A.; Lanni, A.; Moreno, M.; Brand, M.D.; Goglia, F. Effect of 3,5-di-iodo-L-thyronine on the mitochondrial energy-transduction apparatus. Biochem. J. 1998, 330, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Priore, P.; Gnoni, G.V.; Papa, S.; Zanotti, F.; Gnoni, A. 3,5-Diiodo-L-thyronine administration to hypothyroid rats rapidly enhances fatty acid oxidation rate and bioenergetic parameters in liver cells. PLoS ONE 2013, 8, e52328. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Lanni, A.; de Lange, P.; Silvestri, E.; Grasso, P.; Senese, R.; Goglia, F.; Moreno, M. Acute administration of 3,5-diiodo-L-thyronine to hypothyroid rats affects bioenergetic parameters in rat skeletal muscle mitochondria. FEBS Lett. 2007, 581, 5911–5916. [Google Scholar] [CrossRef] [PubMed]
- De Lange, P.; Cioffi, F.; Senese, R.; Moreno, M.; Lombardi, A.; Silvestri, E.; De Matteis, R.; Lionetti, L.; Mollica, M.P.; Goglia, F.; et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes 2011, 60, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, E.; Cioffi, F.; Glinni, D.; Ceccarelli, M.; Lombardi, A.; de Lange, P.; Chambery, A.; Severino, V.; Lanni, A.; Goglia, F.; et al. Pathways affected by 3,5-diiodo-l-thyronine in liver of high fat-fed rats: Evidence from two-dimensional electrophoresis, blue-native PAGE, and mass spectrometry. Mol. Biosyst. 2010, 6, 2256–2271. [Google Scholar] [CrossRef]
- Silvestri, E.; Coppola, M.; Cioffi, F.; Goglia, F. Proteomic approaches for the study of tissue specific effects of 3,5,3′-triiodo-L-thyronine and 3,5-diiodo-L-thyronine in conditions of altered energy metabolism. Front. Physiol. 2014, 5, 491. [Google Scholar] [CrossRef][Green Version]
- Lombardi, A.; de Lange, P.; Silvestri, E.; Busiello, R.A.; Lanni, A.; Goglia, F.; Moreno, M. 3,5-Diiodo-L-thyronine rapidly enhances mitochondrial fatty acid oxidation rate and thermogenesis in rat skeletal muscle: AMP-activated protein kinase involvement. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E497–E502. [Google Scholar] [CrossRef]
- Moreno, M.; Silvestri, E.; Coppola, M.; Goldberg, I.J.; Huang, L.S.; Salzano, A.M.; D’Angelo, F.; Ehrenkranz, J.R.; Goglia, F. 3,5,3′-Triiodo-L-Thyronine- and 3,5-Diiodo-L-Thyronine- Affected Metabolic Pathways in Liver of LDL Receptor Deficient Mice. Front. Physiol. 2016, 7, 545. [Google Scholar] [CrossRef]
- Da Silva Teixeira, S.; Filgueira, C.; Sieglaff, D.H.; Benod, C.; Villagomez, R.; Minze, L.J.; Zhang, A.; Webb, P.; Nunes, M.T. 3,5-diiodothyronine (3,5-T2) reduces blood glucose independently of insulin sensitization in obese mice. Acta Physiol. 2017, 220, 238–250. [Google Scholar] [CrossRef]
- Lietzow, J.; Golchert, J.; Pietzner, M.; Völker, U.; Poutanen, M.; Ohlsson, C.; Homuth, G.; Köhrle, J. Comparative Analysis of the Effects of Long-Term 3,5-diiodothyronine Treatment on the Murine Hepatic Proteome and Transcriptome Under Conditions of Normal Diet and High-Fat Diet. Thyroid 2021, 31, 1135–1146. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Canesi, L.; De Matteis, R.; Goglia, F.; Cioffi, F.; Fugassa, E.; Gallo, G.; Vergani, L. Direct effects of iodothyronines on excess fat storage in rat hepatocytes. J. Hepatol. 2011, 54, 1230–1236. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Canesi, L.; Goglia, F.; Ravera, S.; Panfoli, I.; Gallo, G.; Vergani, L. Non-receptor-mediated actions are responsible for the lipid-lowering effects of iodothyronines in FaO rat hepatoma cells. J. Endocrinol. 2011, 210, 59–69. [Google Scholar] [CrossRef]
- Scanlan, T.S.; Suchland, K.L.; Hart, M.E.; Chiellini, G.; Huang, Y.; Kruzich, P.J.; Frascarelli, S.; Crossley, D.A.; Bunzow, J.R.; Ronca-Testoni, S.; et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. 2004, 10, 638–642. [Google Scholar] [CrossRef]
- Rutigliano, G.; Bandini, L.; Sestito, S.; Chiellini, G. 3-Iodothyronamine and Derivatives: New Allies against Metabolic Syndrome? Int. J. Mol. Sci. 2020, 21, 2005. [Google Scholar] [CrossRef]
- Cumero, S.; Fogolari, F.; Domenis, R.; Zucchi, R.; Mavelli, I.; Contessi, S. Mitochondrial F0F1-ATP synthase is a molecular target of 3-iodothyronamine, an endogenous metabolite of thyroid hormone. Br. J. Pharmacol. 2012, 166, 2331–2347. [Google Scholar] [CrossRef]
- Venditti, P.; Napolitano, G.; Di Stefano, L.; Chiellini, G.; Zucchi, R.; Scanlan, T.S.; Di Meo, S. Effects of the thyroid hormone derivatives 3-iodothyronamine and thyronamine on rat liver oxidative capacity. Mol. Cell. Endocrinol. 2011, 341, 55–62. [Google Scholar] [CrossRef]
- Lehmphul, I.; Hoefig, C.S.; Köhrle, J. 3-Iodothyronamine reduces insulin secretion in vitro via a mitochondrial mechanism. Mol. Cell. Endocrinol. 2018, 460, 219–228. [Google Scholar] [CrossRef]
- Ball, S.G.; Sokolov, J.; Chin, W.W. 3,5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J. Mol. Endocrinol. 1997, 19, 137–147. [Google Scholar] [CrossRef]
- Mendoza, A.; Navarrete-Ramírez, P.; Hernández-Puga, G.; Villalobos, P.; Holzer, G.; Renaud, J.P.; Laudet, V.; Orozco, A. 3,5-T2 is an alternative ligand for the thyroid hormone receptor beta1. Endocrinology 2013, 154, 2948–2958. [Google Scholar] [CrossRef]
- Höchli, M.; Hackenbrock, C.R. Fluidity in mitochondrial membranes: Thermotropic lateral translational motion of intramembrane particles. Proc. Natl. Acad. Sci. USA 1976, 73, 1636–1640. [Google Scholar] [CrossRef]
- Guo, R.; Zong, S.; Wu, M.; Gu, J.; Yang, M. Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell 2017, 170, 1247–1257.e12. [Google Scholar] [CrossRef]
- Schägger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef]
- Javadov, S.; Jang, S.; Chapa-Dubocq, X.R.; Khuchua, Z.; Camara, A.K. Mitochondrial respiratory supercomplexes in mammalian cells: Structural versus functional role. J. Mol. Med. 2021, 99, 57–73. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Eubel, H.; Keegstra, W.; Boekema, E.J.; Braun, H.P. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc. Natl. Acad. Sci. USA 2005, 102, 3225–3229. [Google Scholar] [CrossRef]
- Heinemeyer, J.; Braun, H.P.; Boekema, E.J.; Kouril, R. A structural model of the cytochrome C reductase/oxidase supercomplex from yeast mitochondria. J. Biol. Chem. 2007, 282, 12240–12248. [Google Scholar] [CrossRef]
- Schäfer, E.; Seelert, H.; Reifschneider, N.H.; Krause, F.; Dencher, N.A.; Vonck, J. Architecture of active mammalian respiratory chain supercomplexes. J. Biol. Chem. 2006, 281, 15370–15375. [Google Scholar] [CrossRef]
- Caruana, N.J.; Stroud, D.A. The road to the structure of the mitochondrial respiratory chain supercomplex. Biochem. Soc. Trans. 2020, 48, 621–629. [Google Scholar] [CrossRef]
- Davies, K.M.; Blum, T.B.; Kühlbrandt, W. Conserved in situ arrangement of complex I and III(2) in mitochondrial respiratory chain supercomplexes of mammals, yeast, and plants. Proc. Natl. Acad. Sci. USA 2018, 115, 3024–3029. [Google Scholar] [CrossRef]
- Brzezinski, P. New Structures Reveal Interaction Dynamics in Respiratory Supercomplexes. Trends Biochem. Sci. 2020, 45, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002, 277, 43553–43556. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, K.; Gohil, V.; Stuart, R.A.; Hunte, C.; Brandt, U.; Greenberg, M.L.; Schägger, H. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 2003, 278, 52873–52880. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Mileykovskaya, E.; Dowhan, W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem. Phys. Lipids 2014, 179, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Arnarez, C.; Marrink, S.J.; Periole, X. Molecular mechanism of cardiolipin-mediated assembly of respiratory chain supercomplexes. Chem. Sci. 2016, 7, 4435–4443. [Google Scholar] [CrossRef]
- Basu Ball, W.; Neff, J.K.; Gohil, V.M. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018, 592, 1273–1290. [Google Scholar] [CrossRef]
- Lu, Y.W.; Acoba, M.G.; Selvaraju, K.; Huang, T.C.; Nirujogi, R.S.; Sathe, G.; Pandey, A.; Claypool, S.M. Human adenine nucleotide translocases physically and functionally interact with respirasomes. Mol. Biol. Cell 2017, 28, 1489–1506. [Google Scholar] [CrossRef]
- Parodi-Rullán, R.M.; Chapa-Dubocq, X.; Guzmán-Hernández, R.; Jang, S.; A Torres-Ramos, C.; Ayala-Peña, S.; Javadov, S. The Role of Adenine Nucleotide Translocase in the Assembly of Respiratory Supercomplexes in Cardiac Cells. Cells 2019, 8, 1247. [Google Scholar] [CrossRef]
- Silvestri, E.; Senese, R.; De Matteis, R.; Cioffi, F.; Moreno, M.; Lanni, A.; Gentile, A.; Busiello, R.A.; Salzano, A.M.; Scaloni, A.; et al. Absence of uncoupling protein 3 at thermoneutrality influences brown adipose tissue mitochondrial functionality in mice. FASEB J. 2020, 34, 15146–15163. [Google Scholar] [CrossRef]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodríguez-Hernández, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- Maranzana, E.; Barbero, G.; Falasca, A.I.; Lenaz, G.; Genova, M.L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal. 2013, 19, 1469–1480. [Google Scholar] [CrossRef]
- Moreno-Lastres, D.; Fontanesi, F.; García-Consuegra, I.; Martín, M.A.; Arenas, J.; Barrientos, A.; Ugalde, C. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012, 15, 324–335. [Google Scholar] [CrossRef]
- Moe, A.; Kovalova, T.; Król, S.; Yanofsky, D.J.; Bott, M.; Sjöstrand, D.; Rubinstein, J.L.; Högbom, M.; Brzezinski, P. The respiratory supercomplex from C. glutamicum. Structure 2021, 30, 338–349.e3. [Google Scholar] [CrossRef]
- Enríquez, J.A. Supramolecular Organization of Respiratory Complexes. Annu. Rev. Physiol. 2016, 78, 533–561. [Google Scholar] [CrossRef]
- Genova, M.L.; Lenaz, G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta 2014, 1837, 427–443. [Google Scholar] [CrossRef]
- Hirst, J. Mitochondrial complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Blaza, J.N.; Serreli, R.; Jones, A.J.; Mohammed, K.; Hirst, J. Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc. Natl. Acad. Sci. USA 2014, 111, 15735–15740. [Google Scholar] [CrossRef]
- Milenkovic, D.; Blaza, J.N.; Larsson, N.G.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef]
- Mejia, E.M.; Nguyen, H.; Hatch, G.M. Mammalian cardiolipin biosynthesis. Chem. Phys. Lipids 2014, 179, 11–16. [Google Scholar] [CrossRef]
- Cavallo, A.; Gnoni, A.; Conte, E.; Siculella, L.; Zanotti, F.; Papa, S.; Gnoni, G.V. 3,5-diiodo-L-thyronine increases FoF1-ATP synthase activity and cardiolipin level in liver mitochondria of hypothyroid rats. J. Bioenergy Biomembr. 2011, 43, 349–357. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Demori, I.; Vecchione, G.; Compalati, A.D.; Gallo, G.; Goglia, F.; De Matteis, R.; Silvestri, E.; Vergani, L. Triglyceride Mobilization from Lipid Droplets Sustains the Anti-Steatotic Action of Iodothyronines in Cultured Rat Hepatocytes. Front. Physiol. 2016, 6, 418. [Google Scholar] [CrossRef]
- Giacco, A.; Delli Paoli, G.; Senese, R.; Cioffi, F.; Silvestri, E.; Moreno, M.; Ruoppolo, M.; Caterino, M.; Costanzo, M.; Lombardi, A.; et al. The saturation degree of fatty acids and their derived acylcarnitines determines the direct effect of metabolically active thyroid hormones on insulin sensitivity in skeletal muscle cells. FASEB J. 2019, 33, 1811–1823. [Google Scholar] [CrossRef]
- Nakada, K.; Inoue, K.; Hayashi, J. Interaction theory of mammalian mitochondria. Biochem. Biophys. Res. Commun. 2001, 288, 743–746. [Google Scholar] [CrossRef]
- Arimura, S.; Yamamoto, J.; Aida, G.P.; Nakazono, M.; Tsutsumi, N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc. Natl. Acad. Sci. USA 2004, 101, 7805–7808. [Google Scholar] [CrossRef]
- Skulachev, V.P. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem. Sci. 2001, 26, 23–29. [Google Scholar] [CrossRef]
- Molina, A.J.; Shirihai, O.S. Monitoring mitochondrial dynamics with photoactivatable [corrected] green fluorescent protein. Methods Enzymol. 2009, 457, 289–304. [Google Scholar] [CrossRef]
- Malka, F.; Lombès, A.; Rojo, M. Organization, dynamics and transmission of mitochondrial DNA: Focus on vertebrate nucleoids. Biochim. Biophys. Acta 2006, 1763, 463–472. [Google Scholar] [CrossRef][Green Version]
- Legros, F.; Lombès, A.; Frachon, P.; Rojo, M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell 2002, 13, 4343–4354. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Critical dependence of neurons on mitochondrial dynamics. Curr. Opin. Cell Biol. 2006, 18, 453–459. [Google Scholar] [CrossRef]
- Meeusen, S.L.; Nunnari, J. Mitochondrial fusion in vitro. Methods Mol. Biol. 2007, 372, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; van der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 2001, 12, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Miyata, N.; Kuge, O.; Mihara, K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J. Cell Biol. 2016, 212, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Stotland, A.; Gottlieb, R.A. Mitochondrial quality control: Easy come, easy go. Biochim. Biophys. Acta 2015, 1853, 2802–2811. [Google Scholar] [CrossRef]
- Cogliati, S.; Frezza, C.; Soriano, M.E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef]
- Gilkerson, R.W.; Selker, J.M.; Capaldi, R.A. The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett. 2003, 546, 355–358. [Google Scholar] [CrossRef]
- Plecitá-Hlavatá, L.; Ježek, P. Integration of superoxide formation and cristae morphology for mitochondrial redox signaling. Int. J. Biochem. Cell Biol. 2016, 80, 31–50. [Google Scholar] [CrossRef]
- Van der Laan, M.; Horvath, S.E.; Pfanner, N. Mitochondrial contact site and cristae organizing system. Curr. Opin. Cell Biol. 2016, 41, 33–42. [Google Scholar] [CrossRef]
- Zick, M.; Rabl, R.; Reichert, A.S. Cristae formation—Linking ultrastructure and function of mitochondria. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 5–19. [Google Scholar] [CrossRef]
- Hackenbrock, C.R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. Reversible Ultrastructural Changes with Change in Metabolic Steady State in Isolated Liver Mitochondria. J. Cell Biol. 1966, 30, 269–297. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Wilkens, V.; Kohl, W.; Busch, K. Restricted diffusion of OXPHOS complexes in dynamic mitochondria delays their exchange between cristae and engenders a transitory mosaic distribution. J. Cell Sci. 2013, 126, 103–116. [Google Scholar] [CrossRef]
- Wolf, D.M.; Segawa, M.; Kondadi, A.K.; Anand, R.; Bailey, S.T.; Reichert, A.S.; van der Bliek, A.M.; Shackelford, D.B.; Liesa, M.; Shirihai, O.S. Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J. 2019, 38, e101056. [Google Scholar] [CrossRef]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Martins de Brito, O.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef]
- Hoppins, S.; Collins, S.R.; Cassidy-Stone, A.; Hummel, E.; Devay, R.M.; Lackner, L.L.; Westermann, B.; Schuldiner, M.; Weissman, J.S.; Nunnari, J. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell Biol. 2011, 195, 323–340. [Google Scholar] [CrossRef]
- Jiko, C.; Davies, K.M.; Shinzawa-Itoh, K.; Tani, K.; Maeda, S.; Mills, D.J.; Tsukihara, T.; Fujiyoshi, Y.; Kühlbrandt, W.; Gerle, C. Bovine F1Fo ATP synthase monomers bend the lipid bilayer in 2D membrane crystals. Elife 2015, 4, e06119. [Google Scholar] [CrossRef]
- Blum, T.B.; Hahn, A.; Meier, T.; Davies, K.M.; Kühlbrandt, W. Dimers of Mitochondrial ATP Synthase Induce Membrane Curvature and Self-Assemble into Rows. Proc. Natl. Acad. Sci. USA 2019, 116, 4250–4255. [Google Scholar] [CrossRef]
- Chen, H.; Vermulst, M.; Wang, Y.E.; Chomyn, A.; Prolla, T.A.; McCaffery, J.M.; Chan, D.C. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 2010, 141, 280–289. [Google Scholar] [CrossRef]
- Tondera, D.; Grandemange, S.; Jourdain, A.; Karbowski, M.; Mattenberger, Y.; Herzig, S.; Da Cruz, S.; Clerc, P.; Raschke, I.; Merkwirth, C.; et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009, 28, 1589–1600. [Google Scholar] [CrossRef]
- Wang, C.W.; Klionsky, D.J. The molecular mechanism of autophagy. Mol. Med. 2003, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I. Proteasomal and Autophagic Degradation Systems. Annu. Rev. Biochem. 2017, 86, 193–224. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, R.; Tata, J.R.; Lindberg, O.; Ernster, L. The relationship between the structure and activity of rat skeletal muscle mitochondria after thyroidectomy and thyroid hormone treatment. J. Cell Biol. 1965, 26, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Venediktova, N.I.; Pavlik, L.L.; Belosludtseva, N.V.; Khmil, N.V.; Murzaeva, S.V.; Mironova, G.D. Formation of lamellar bodies in rat liver mitochondria in hyperthyroidism. J. Bioenergy Biomembr. 2018, 50, 289–295. [Google Scholar] [CrossRef]
- Jakovcic, S.; Swift, H.H.; Gross, N.J.; Rabinowitz, M. Biochemical and stereological analysis of rat liver mitochondria in different thyroid states. J. Cell Biol. 1978, 77, 887–901. [Google Scholar] [CrossRef]
- Vega-Nunez, E.; Alvarez, A.M.; Menendez-Hurtado, A.; Santos, A.; Perez-Castillo, A. Neuronal mitochondrial morphology and transmembrane potential are severely altered by hypothyroidism during rat brain development. Endocrinology 1997, 138, 3771–3778. [Google Scholar] [CrossRef][Green Version]
- Silvestri, E.; Cioffi, F.; De Matteis, R.; Senese, R.; de Lange, P.; Coppola, M.; Salzano, A.M.; Scaloni, A.; Ceccarelli, M.; Goglia, F.; et al. 3,5-Diiodo-L-Thyronine Affects Structural and Metabolic Features of Skeletal Muscle Mitochondria in High-Fat-Diet Fed Rats Producing a Co-adaptation to the Glycolytic Fiber Phenotype. Front. Physiol. 2018, 9, 194. [Google Scholar] [CrossRef]
- Lino, C.A.; Barreto-Chaves, M.L.M. Modulation of the ubiquitin proteasome system (UPS) in thyroid hormone-induced cardiac hypertrophy. FASEB J. 2012, 26, 1139-6. [Google Scholar] [CrossRef]
- Sinha, R.A.; You, S.H.; Zhou, J.; Siddique, M.M.; Bay, B.H.; Zhu, X.; Privalsky, M.L.; Cheng, S.Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, S.L.; Lin, S.L.; Tsai, C.Y.; Chuang, W.Y.; Lin, Y.H.; Huang, Y.H.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene 2017, 36, 5274–5284. [Google Scholar] [CrossRef]
- Iannucci, L.F.; Cioffi, F.; Senese, R.; Goglia, F.; Lanni, A.; Yen, P.M.; Sinha, R.A. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci. Rep. 2017, 7, 2023. [Google Scholar] [CrossRef]
- Lesmana, R.; Sinha, R.A.; Singh, B.K.; Zhou, J.; Ohba, K.; Wu, Y.; Yau, W.W.; Bay, B.H.; Yen, P.M. Thyroid Hormone Stimulation of Autophagy Is Essential for Mitochondrial Biogenesis and Activity in Skeletal Muscle. Endocrinology 2016, 157, 23–38. [Google Scholar] [CrossRef]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar] [CrossRef]
- Venditti, P.; Chiellini, G.; Di Stefano, L.; Napolitano, G.; Zucchi, R.; Columbano, A.; Scanlan, T.S.; Di Meo, S. The TRbeta-selective agonist, GC-1, stimulates mitochondrial oxidative processes to a lesser extent than triiodothyronine. J. Endocrinol. 2010, 205, 279–289. [Google Scholar] [CrossRef]
- Saponaro, F.; Sestito, S.; Runfola, M.; Rapposelli, S.; Chiellini, G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Front. Med. 2020, 7, 331. [Google Scholar] [CrossRef]
- Cable, E.E.; Finn, P.D.; Stebbins, J.W.; Hou, J.; Ito, B.R.; van Poelje, P.D.; Linemeyer, D.L.; Erion, M.D. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 2009, 49, 407–417. [Google Scholar] [CrossRef]
- Zhou, J.; Waskowicz, L.R.; Lim, A.; Liao, X.H.; Lian, B.; Masamune, H.; Refetoff, S.; Tran, B.; Koeberl, D.D.; Yen, P.M. A Liver-Specific Thyromimetic, VK2809, Decreases Hepatosteatosis in Glycogen Storage Disease Type Ia. Thyroid 2019, 29, 1158–1167. [Google Scholar] [CrossRef]
- Mousa, S.A.; O’Connor, L.J.; Bergh, J.J.; Davis, F.B.; Scanlan, T.S.; Davis, P.J. The proangiogenic action of thyroid hormone analogue GC-1 is initiated at an integrin. J. Cardiovasc. Pharmacol. 2005, 46, 356–360. [Google Scholar] [CrossRef]
- Cioffi, F.; Zambad, S.P.; Chhipa, L.; Senese, R.; Busiello, R.A.; Tuli, D.; Munshi, S.; Moreno, M.; Lombardi, A.; Gupta, R.C.; et al. TRC150094, a novel functional analog of iodothyronines, reduces adiposity by increasing energy expenditure and fatty acid oxidation in rats receiving a high-fat diet. FASEB J. 2010, 24, 3451–3461. [Google Scholar] [CrossRef]
- Joshi, D.; Jamadarkhana, P.; Kumbhare, S.; Singh, A.; Kotecha, J.; Bunger, D.; Shiwalkar, A.; Mohanan, A.; Dutt, C. Safety, Tolerability, and Pharmacokinetics of a Novel Mitochondrial Modulator, TRC150094, in Overweight and Obese Subjects: A Randomized Phase-I Clinical Trial. Front. Pharmacol. 2021, 12, 729424. [Google Scholar] [CrossRef] [PubMed]
- Goglia, F.; Moreno, M.; Lanni, A. Action of thyroid hormones at the cellular level: The mitochondrial target. FEBS Lett. 1999, 452, 115–120. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; Goglia, F. Biochemical and functional differences in rat liver mitochondrial subpopulations obtained at different gravitational forces. Int. J. Biochem. Cell Biol. 1996, 28, 337–343. [Google Scholar] [CrossRef]
- Solanes, G.; Pedraza, N.; Calvo, V.; Vidal-Puig, A.; Lowell, B.B.; Villarroya, F. Thyroid hormones directly activate the expression of the human and mouse uncoupling protein-3 genes through a thyroid response element in the proximal promoter region. Biochem. J. 2005, 386, 505–513. [Google Scholar] [CrossRef]

| Parameters | Reported Changes | TH | References |
|---|---|---|---|
| Activity of electron transport chain components | increase | T3, T4 | [29,30] |
| P/O ratio | no change or decrease | T4 | [43,44] |
| Redox slipping | increase | T3 | [26] (and references within) |
| Proton leak | increase | T3, T2 | [26,41] (and references within) |
| Activity of uncoupling proteins (ANT, UCPs) | increase | T3 | [31,32,33,52,53,54,55,56,57] |
| PTP opening | increase | T3 | [39,73,74,75,76,77] |
| Activity of K-glycerophosphate dehydrogenase, succinic dehydrogenase, NADH dehydrogenase and calcium uptake | increase | T2, T3, T4 | [34,88] |
| Cardiolipin-synthase activity | increase | T3 | [26] (and references within) |
| Reduced cytochromes | increase | T3 | [26,38] (and references within) |
| Transcription of nuclear and mitochondrial respiratory genes | increase | T3, T2 | [35,36,37,89,90] |
| Supercomplex aggregation and activity | increase | T3, T2 | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioffi, F.; Giacco, A.; Goglia, F.; Silvestri, E. Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones. Cells 2022, 11, 997. https://doi.org/10.3390/cells11060997
Cioffi F, Giacco A, Goglia F, Silvestri E. Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones. Cells. 2022; 11(6):997. https://doi.org/10.3390/cells11060997
Chicago/Turabian StyleCioffi, Federica, Antonia Giacco, Fernando Goglia, and Elena Silvestri. 2022. "Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones" Cells 11, no. 6: 997. https://doi.org/10.3390/cells11060997
APA StyleCioffi, F., Giacco, A., Goglia, F., & Silvestri, E. (2022). Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones. Cells, 11(6), 997. https://doi.org/10.3390/cells11060997








