Cerebral Organoids Maintain the Expression of Neural Stem Cell-Associated Glycoepitopes and Extracellular Matrix
Abstract
:1. Introduction
2. Materials and Methods
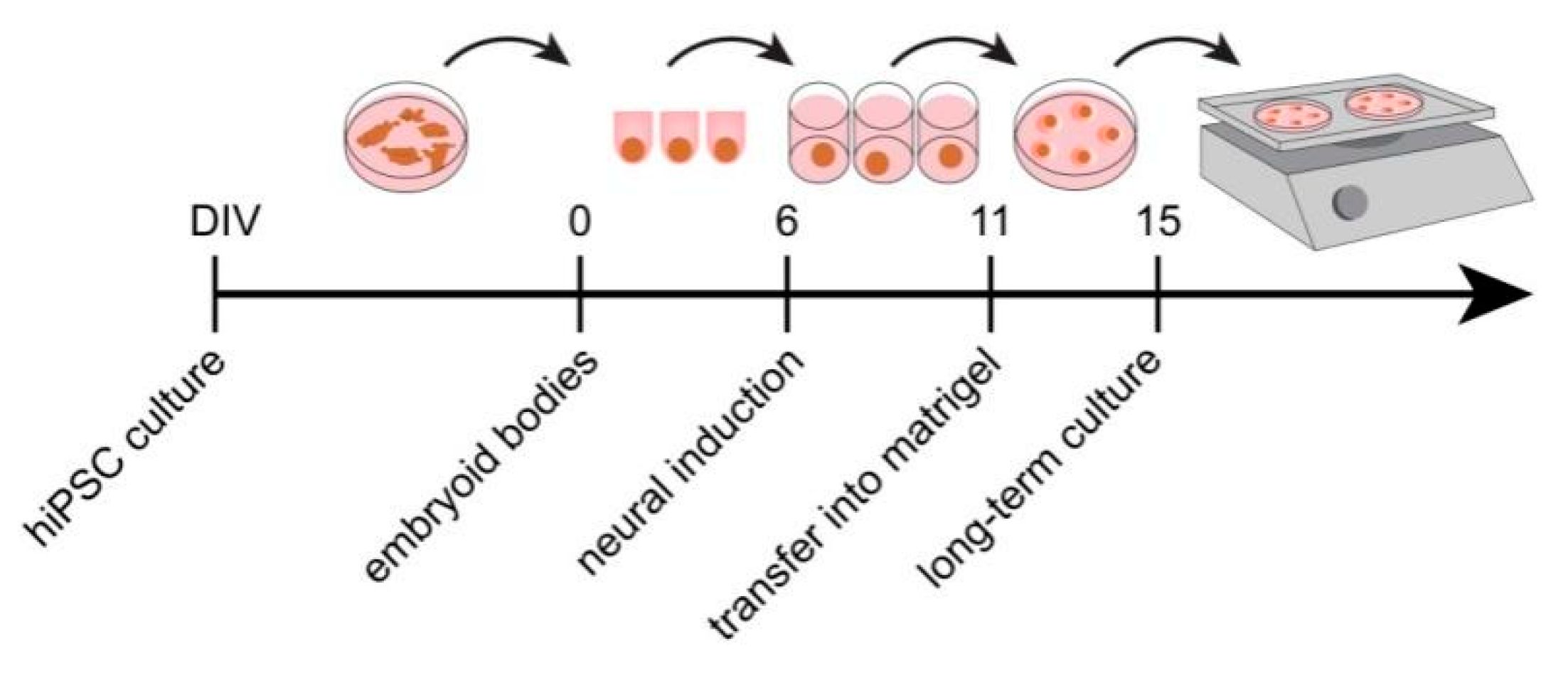
2.1. Cell Culture of Human Induced Pluripotent Stem Cells (hiPSCs)
2.1.1. Generation of Cells with Neural Identity
2.1.2. hEB Formation (0–6 DIV)
2.1.3. Neural Induction (6–11 DIV)
2.1.4. Transfer of Neuroepithelial Tissue into Matrigel (11 DIV)
2.1.5. Static Organoid Culture (11–15 DIV)
2.1.6. Dynamic Organoid Culture (15 DIV and Later)
2.1.7. Preparation of Cerebral Organoids for Cryosectioning
2.2. Immunohistochemistry and Fluorescence Microscopy
3. Results
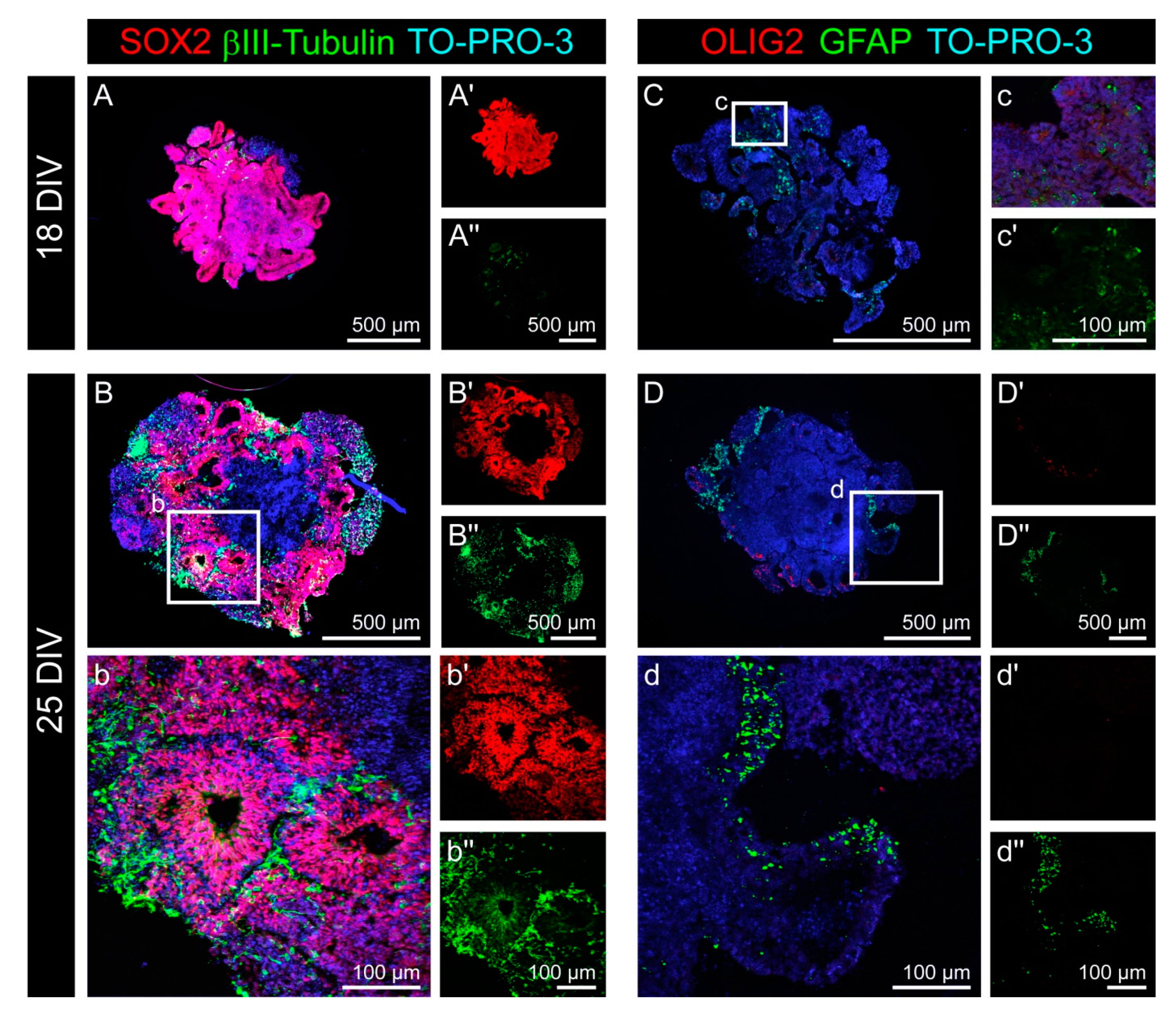
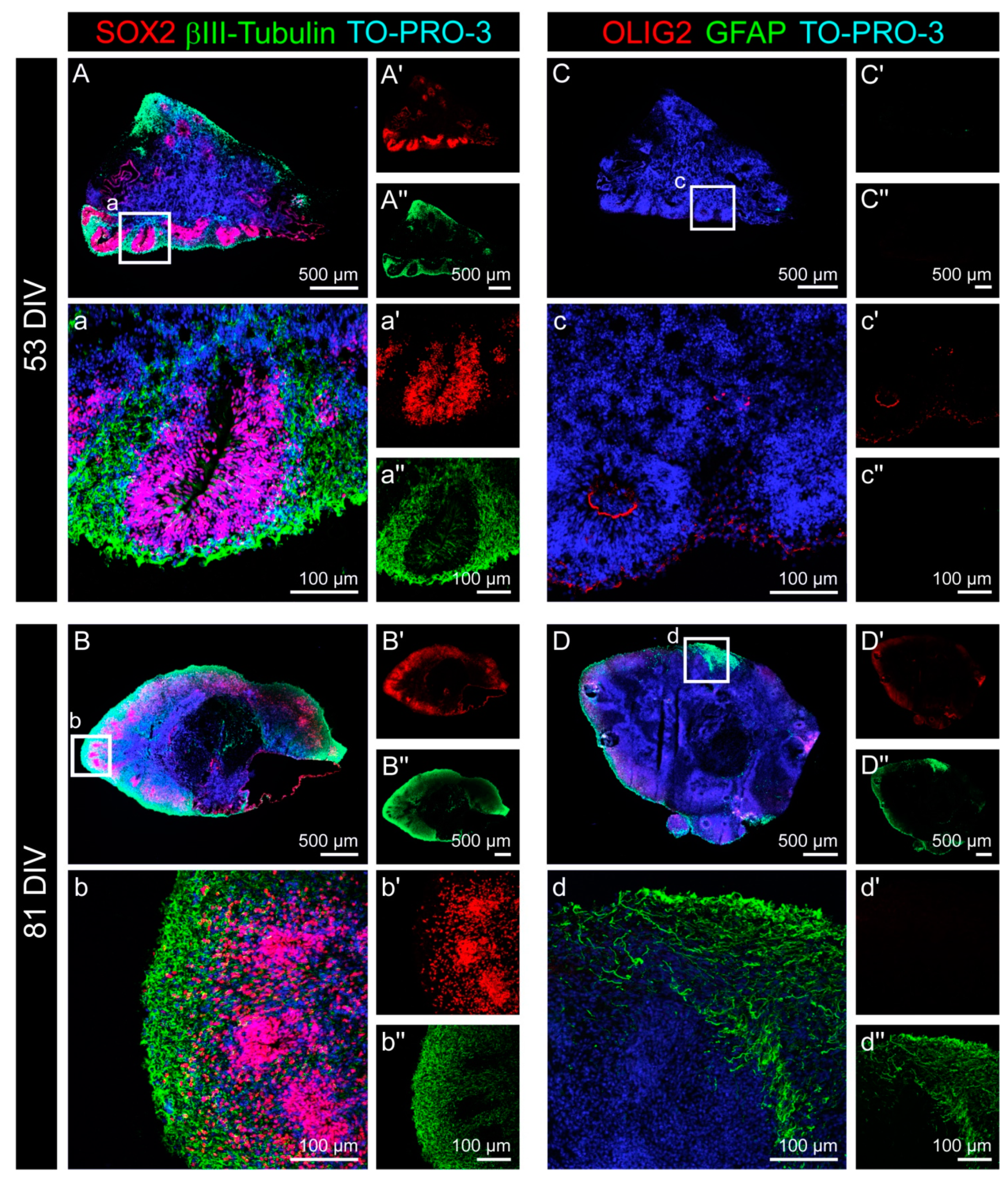
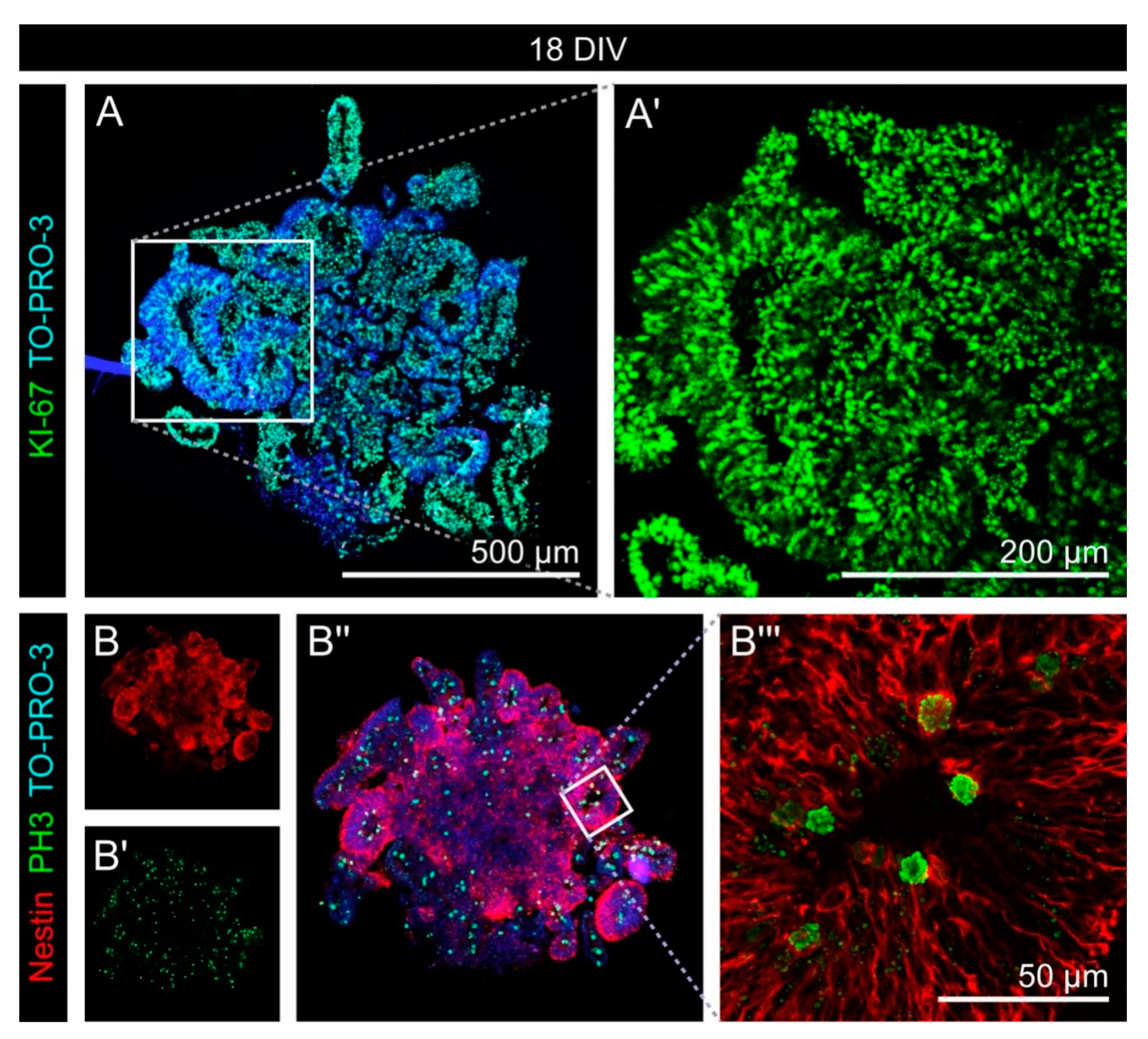
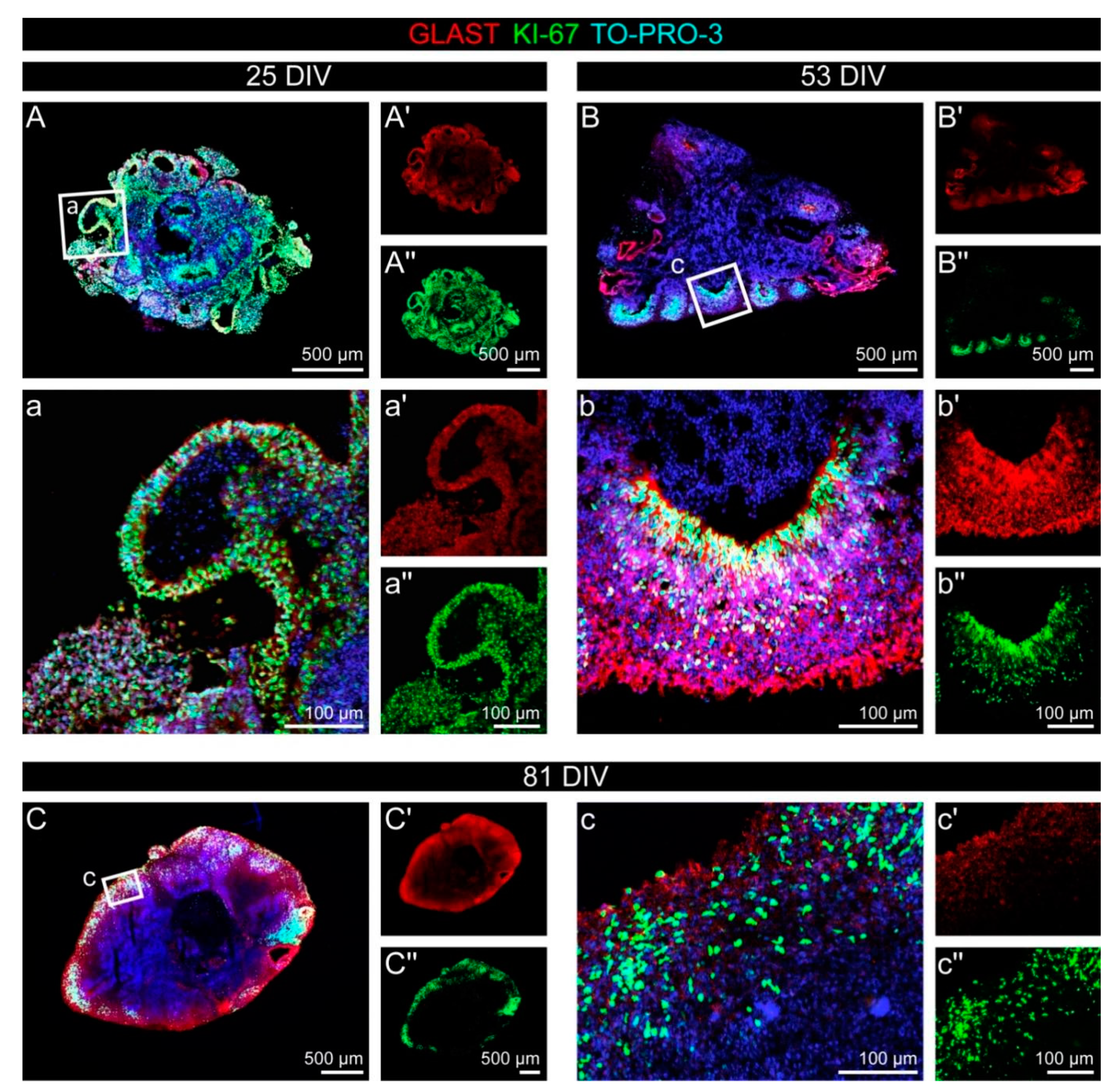
3.1. Cell Fate and Proliferation in Human Cerebral Organoids
3.2. Cell Types in the Cerebral Organoids
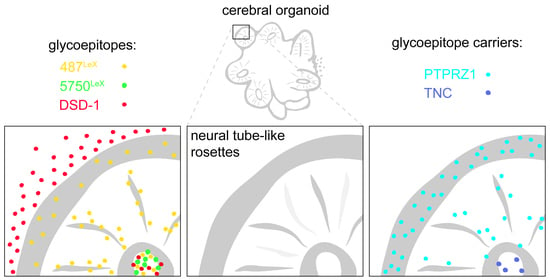
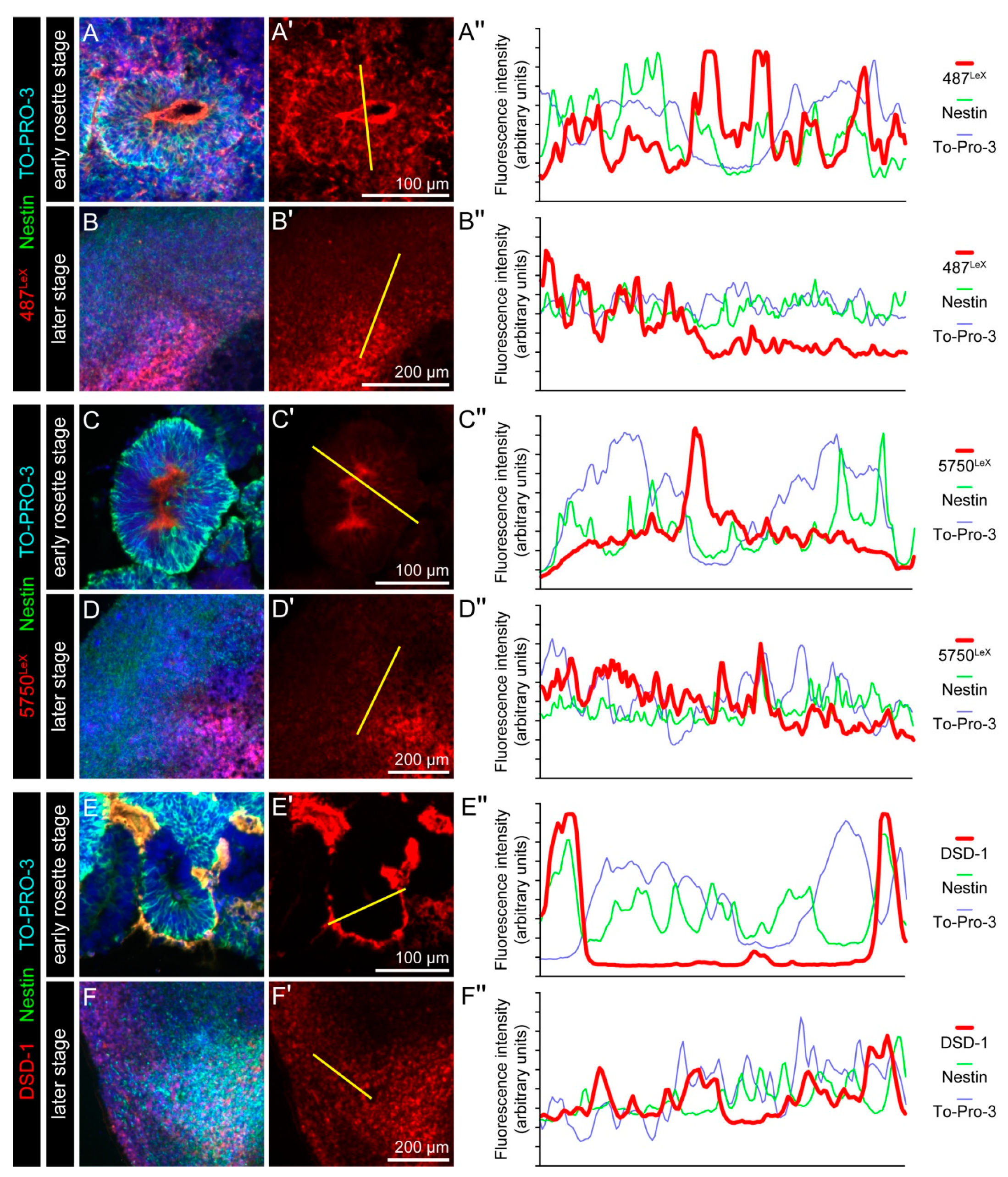
3.3. Expression of LewisX (LeX) Motifs and Potential Carriers in Cerebral Organoids
3.4. Expression of the DSD-1 Epitope in Cerebral Organoids
3.5. Overview: Neural Stem Cell-Related Carbohydrates in Human Cerebral Organoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Cell Culture of Human Induced Pluripotent Stem Cells (hiPSCs)
Appendix A.1.1. Generation of Cells with Neural Identity
Appendix A.1.2. hEB Formation (0–6 DIV)
Appendix A.1.3. Neural Induction (6–11 DIV)
Appendix A.1.4. Transfer of Neuroepithelial Tissue into Matrigel (11 DIV)
Appendix A.1.5. Static Organoid Culture (11–15 DIV)
Appendix A.1.6. Dynamic Organoid Culture (15 DIV and Later)
Appendix A.1.7. Preparation of Cerebral Organoids for Cryosectioning
Appendix A.2. Immunohistochemistry and Fluorescence Microscopy
Appendix B
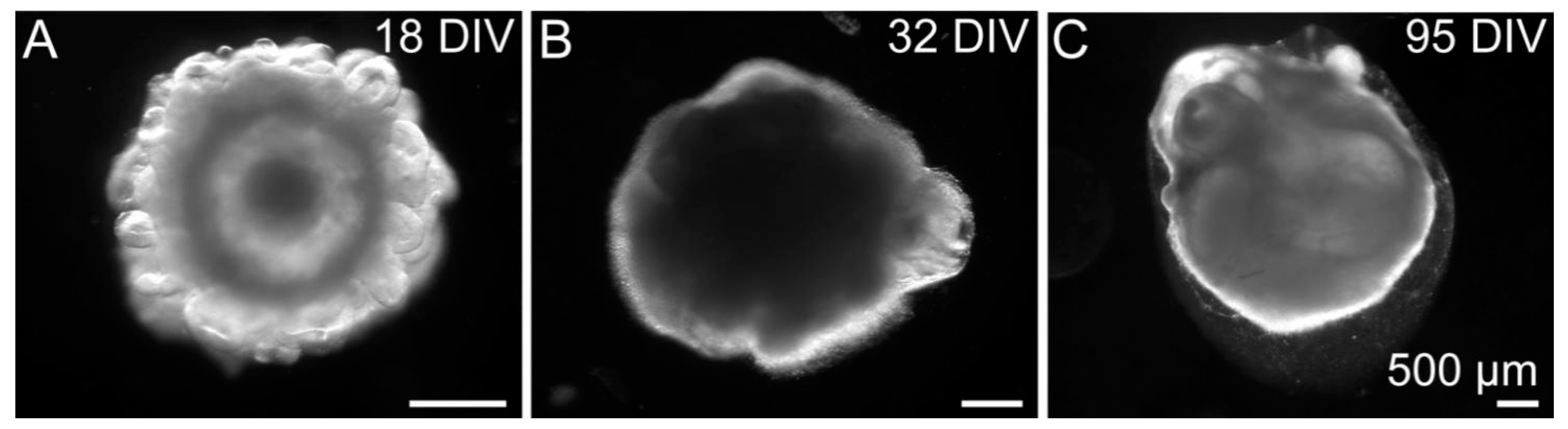
Appendix C
| Medium | Source/Components |
|---|---|
| mTeSR™1 medium | Stemcell Technologies (see below) |
| Neural Induction Medium | DMEM/F12 + 15 mM (3574.5 μg/mL) HEPES 1% (v/v) N2 1% (v/v) GlutaMAX 1 μg/mL Heparin 1% (v/v) MEM-NEAA |
| Organoid Differentiation Medium | DMEM/F12 and Neurobasal (50:50) 0.5% (v/v) N2 1% GlutaMAX 0.025% Insulin (v/v) 0.5% MEM-NEAA 1% Penicillin-streptomycin 0.035% 2-Mercaptoethanol in DMEM/F12 (1:100) 1% B27 (B27 w/o vitamin A) |
| Medium/Supplement | Source | Cat# | Lot |
|---|---|---|---|
| 2-Mercaptoethanol | Sigma-Aldrich, Steinheim, Germany | M7522 | BCBD8822V |
| B-27™ Supplement (50X), minus vitamin A | Gibco/Thermo, Darmstadt, Germany | 12587010 | 1920047 |
| B-27™ Supplement (50X), serum free | Gibco/Thermo, Darmstadt, Germany | 17504044 | 1855270 |
| DMEM/F-12 | Gibco/Thermo, Darmstadt, Germany | 1132074 | 1944326 |
| GlutaMAX™ Supplement | Gibco/Thermo, Darmstadt, Germany | 35050061 | 1919085 |
| Heparin sodium salt from porcine intestinal mucosa | Sigma-Aldrich, Steinheim, Germany | 3149 | SLBC9940V |
| HEPES solution | Sigma-Aldrich, Steinheim, Germany | H0887 | RNBG5446 |
| Insulin solution, human | Sigma-Aldrich, Steinheim, Germany | I9278 | SLBV1793 |
| MEM non-essential amino acid solution (MEM-NEAA) (100×) | Sigma-Aldrich, Steinheim, Germany | M7145 | RNBG2199 |
| mTeSR™1; 5X Supplement | Stemcell Technologies, Cologne, Germany | 85852 | SLBV4129, SLBW3665 |
| mTeSR™1; Basal Medium | Stemcell Technologies, Cologne, Germany | 85851 | AC10750263, AC11627263 |
| N-2 Supplement (100X) | Gibco/Thermo, Darmstadt, Germany | 17502048 | 1852441, 1861414 |
| Neurobasal™ medium | Gibco/Thermo, Darmstadt, Germany | 21103049 | 1944312 |
| Penicillin-streptomycin | Sigma-Aldrich, Steinheim, Germany | P4333 | 066M4791V |
| ROCK inhibitor (Y-27632) | Merck, Darmstadt, Germany | SCM075 | 2905652 |
| Buffer/Solution | Components |
|---|---|
| Gelatin/sucrose solution | PBS 7.5% (w/v) gelatin 10.0% (w/v) sucrose |
| Blocking buffer | PBS 10% (v/v) bovine serum albumin (BSA) 1% (v/v) goat serum 0.5% Triton X 100 (v/v) |
| Paraformaldehyde solution | PBS 4% paraformaldehyde, pH 7.4 |
| Phosphate-buffered saline (PBS) | H20 137 mM NaCl 3.0 mM KCL 6.5 mM Na2HPO4 · 2H2O 1.5 mM KH2PO4 pH 7.3 |
| Nuclear Dye | Source | Cat# | Lot | Dilution (IHC) |
|---|---|---|---|---|
| TO-PRO®-3 | Thermo Scientific, Waltham, MA, USA | T3605 | 1622799 | 1:500 |
| Enzyme/Reagent | Source | Cat# | Lot |
|---|---|---|---|
| Accutase® solution | Sigma-Aldrich, Steinheim, Germany | A1110501 | 1819345 |
| Matrigel® Basement Membrane Matrix | Corning, Wiesbaden, Germany | 354234 | 8015325 |
| Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix | Corning, Wiesbaden, Germany | 354230 | 5341006 7065004 |
| Normal serum (goat) unconjugated | Dianova, Hamburg, Germany | 005-000-121 | 137206 |
| Material | Source | Cat# | Lot |
|---|---|---|---|
| Cell culture dishes, Nunclon™ Delta (Ø 60 mm) | Nunc/Thermo, Roskilde, Denmark | 150288 | 119704 |
| Cell culture dishes (Ø 90 mm) | VWR, Darmstadt, Germany | 734-2320 | 100729073 |
| Costar® Ultra-Low Attachment multiple well plate (24 wells) | Corning, Wiesbaden, Germany | 3473 | 32017029 |
| Costar® Ultra-Low Attachment multiple well plate (96 wells) Round bottom | Corning, Wiesbaden, Germany | CLS7007 | 10217014 |
| ImmuMount™ | Thermo Scientific, Waltham, MA, USA | 9990402 | 421566 |
| Nunc™ 4-well Cell culture multidishes | Nunc/Thermo, Roskilde, Denmark | 176740 | 152255 |
| Nunc™ 6-well Cell culture multidishes | Nunc/Thermo, Roskilde, Denmark | 140675 | 147735 |
| SuperFrost Plus™ adhesion slides | Menzel/Thermo, Braunschweig, Germany | J1800AMNZ | 032018 040416 |
| Parafilm® PM996 | Bemis, Neenah, WI, USA | PM996 | N/A |
| Roti®-Liquid barrier marker | Carl Roth, Karlsruhe, Germany | AN92.1 | 268273002 |
| Tissue freezing medium | Leica, Wetzlar, Germany | 14020108926 | 03808615 |
| Software | Source | RRID |
|---|---|---|
| Adobe Illustrator CS6 | Adobe Systems, San Jose, CA, USA | RRID:SCR_010279 |
| Adobe Photoshop CS6 | Adobe Systems, San Jose, CA, USA | RRID:SCR_014199 |
| FIJI/ImageJ V 1.51h | National Institutes of Health, Bethesda, MD, USA [68,69] | RRID:SCR_002285 |
| Inkscape V 0.92.1 | Inkscape Community, N/A | RRID:SCR_014479 |
| Zen black edition V 2.3 SP1 | Carl Zeiss, Jena, Germany | RRID:SCR_018163 |
| Zen blue edition V 2.3 | Carl Zeiss, Jena, Germany | RRID:SCR_013672 |
References
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.; Copp, A.J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, K.R.; Huttner, W.B. How the extracellular matrix shapes neural development. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Nishiwaki, T.; Shintani, T.; Hamanaka, H.; Noda, M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM). J. Biol. Chem. 1996, 271, 21446–21452. [Google Scholar] [CrossRef] [Green Version]
- Hennen, E.; Faissner, A. LewisX: A neural stem cell specific glycan? Int. J. Biochem. Cell Biol. 2012, 44, 830–833. [Google Scholar] [CrossRef]
- Capela, A.; Temple, S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 2002, 35, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Hennen, E.; Czopka, T.; Faissner, A. Structurally distinct LewisX glycans distinguish subpopulations of neural stem/progenitor cells. J. Biol. Chem. 2011, 286, 16321–16331. [Google Scholar] [CrossRef] [Green Version]
- Clement, A.M.; Nadanaka, S.; Masayama, K.; Mandl, C.; Sugahara, K.; Faissner, A. The DSD-1 carbohydrate epitope depends on sulfation, correlates with chondroitin sulfate D motifs, and is sufficient to promote neurite outgrowth. J. Biol. Chem. 1998, 273, 28444–28453. [Google Scholar] [CrossRef] [Green Version]
- von Holst, A.; Sirko, S.; Faissner, A. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J. Neurosci. 2006, 26, 4082–4094. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, E.; Theocharidis, U.; May, M.; Lessmann, K.; Schroeder, T.; Faissner, A. Sulfation of Glycosaminoglycans Modulates the Cell Cycle of Embryonic Mouse Spinal Cord Neural Stem Cells. Front. Cell Dev. Biol. 2021, 9, 643060. [Google Scholar] [CrossRef] [PubMed]
- Karus, M.; Samtleben, S.; Busse, C.; Tsai, T.; Dietzel, I.D.; Faissner, A.; Wiese, S. Normal sulfation levels regulate spinal cord neural precursor cell proliferation and differentiation. Neural Dev. 2012, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garwood, J.; Rigato, F.; Heck, N.; Faissner, A. Tenascin glycoproteins and the complementary ligand DSD-1-PG/phosphacan– structuring the neural extracellular matrix during development and repair. Restor. Neurol. Neurosci. 2001, 19, 51–64. [Google Scholar]
- Grumet, M.; Milev, P.; Sakurai, T.; Karthikeyan, L.; Bourdon, M.; Margolis, R.K.; Margolis, R.U. Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J. Biol. Chem. 1994, 269, 12142–12146. [Google Scholar] [CrossRef]
- Faissner, A.; Roll, L.; Theocharidis, U. Tenascin-C in the matrisome of neural stem and progenitor cells. Mol. Cell. Neurosci. 2017, 81, 22–31. [Google Scholar] [CrossRef]
- Gates, M.A.; Thomas, L.B.; Howard, E.M.; Laywell, E.D.; Sajin, B.; Faissner, A.; Götz, B.; Silver, J.; Steindler, D.A. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J. Comp. Neurol. 1995, 361, 249–266. [Google Scholar] [CrossRef]
- Kazanis, I.; Ffrench-Constant, C. Extracellular matrix and the neural stem cell niche. Dev. Neurobiol. 2011, 71, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Klausmeyer, A.; Garwood, J.; Faissner, A. Differential expression of phosphacan/RPTPbeta isoforms in the developing mouse visual system. J. Comp. Neurol. 2007, 504, 659–679. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Miller, J.A.; Horvath, S.; Geschwind, D.H. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 12698–12703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Pan, L.; Pembroke, W.G.; Rexach, J.E.; Godoy, M.I.; Condro, M.C.; Alvarado, A.G.; Harteni, M.; Chen, Y.W.; Stiles, L.; et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat. Commun. 2021, 12, 3958. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, P.; Opitz, T.; Steinbeck, J.A.; Ladewig, J.; Brüstle, O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA 2009, 106, 3225–3230. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, M.; Roll, L.; Langenstroth, D.; Brüstle, O.; Faissner, A. Glycoconjugates reveal diversity of human neural stem cells (hNSCs) derived from human induced pluripotent stem cells (hiPSCs). Cell Tissue Res. 2017, 368, 531–549. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Gehart, H.; Artegiani, B.; Löpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.A.; Gogola, E.; Sachs, N.; Barazas, M.; Annunziato, S.; De Ruiter, J.R.; Velds, A.; Blatter, S.; Houthuijzen, J.M.; van de Ven, M.; et al. BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat. Methods 2018, 15, 134–140. [Google Scholar] [CrossRef]
- Bell, C.C.; Dankers, A.C.A.; Lauschke, V.M.; Sison-Young, R.; Jenkins, R.; Rowe, C.; Goldring, C.E.; Park, K.; Regan, S.L.; Walker, T.; et al. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicol. Sci. 2018, 162, 655–666. [Google Scholar] [CrossRef] [Green Version]
- Nyga, A.; Cheema, U.; Loizidou, M. 3D tumour models: Novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 2011, 5, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerakis, Y.; Hetz, C. Brain organoids: A next step for humanized Alzheimer’s disease models? Mol. Psychiatry 2019, 24, 474–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariappan, A.; Goranci-Buzhala, G.; Ricci-Vitiani, L.; Pallini, R.; Gopalakrishnan, J. Trends and challenges in modeling glioma using 3D human brain organoids. Cell Death Differ. 2021, 28, 15–23. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Faissner, A.; Clement, A.; Lochter, A.; Streit, A.; Mandl, C.; Schachner, M. Isolation of a neural chondroitin sulfate proteoglycan with neurite outgrowth promoting properties. J. Cell. Biol. 1994, 126, 783–799. [Google Scholar] [CrossRef]
- Streit, A.; Faissner, A.; Gehrig, B.; Schachner, M. Isolation and biochemical characterization of a neural proteoglycan expressing the L5 carbohydrate epitope. J. Neurochem. 1990, 55, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Faissner, A.; Kruse, J. J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron 1990, 5, 627–637. [Google Scholar] [CrossRef]
- Götz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Murciano, A.; Zamora, J.; López-Sánchez, J.; Frade, J.M. Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol. Cell. Neurosci. 2002, 21, 285–300. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Madhavan, M.; Nevin, Z.S.; Shick, H.E.; Garrison, E.; Clarkson-Paredes, C.; Karl, M.; Clayton, B.L.L.; Factor, D.C.; Allan, K.C.; Barbar, L.; et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 2018, 15, 700–706. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Min Yang, S.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Elkabetz, Y.; Panagiotakos, G.; Al Shamy, G.; Socci, N.D.; Tabar, V.; Studer, L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008, 22, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Karus, M.; Hennen, E.; Safina, D.; Klausmeyer, A.; Wiese, S.; Faissner, A. Differential expression of micro-heterogeneous LewisX-type glycans in the stem cell compartment of the developing mouse spinal cord. Neurochem. Res. 2013, 38, 1285–1294. [Google Scholar] [CrossRef]
- Pollen, A.A.; Nowakowski, T.J.; Chen, J.; Retallack, H.; Sandoval-Espinosa, C.; Nicholas, C.R.; Shuga, J.; Liu, S.J.; Oldham, M.C.; Diaz, A.; et al. Molecular identity of human outer radial glia during cortical development. Cell 2015, 163, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Garwood, J.; Schnädelbach, O.; Clement, A.; Schütte, K.; Bach, A.; Faissner, A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J. Neurosci. 1999, 19, 3888–3899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milev, P.; Chiba, A.; Häring, M.; Rauvala, H.; Schachner, M.; Ranscht, B.; Margolis, R.K.; Margolis, R.U. High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-ζ/β with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J. Biol. Chem. 1998, 273, 6998–7005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, N.X.; Saito, H. A human transmembrane protein-tyrosine-phosphatase, PTP zeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc. Natl. Acad. Sci. USA 1992, 89, 7417–7421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, N.; He, J.; Yajima, Y.; Mikami, T.; Sugahara, K.; Yabe, T. Heterogeneity of the chondroitin sulfate portion of phosphacan/6B4 proteoglycan regulates its binding affinity for pleiotrophin/heparin binding growth-associated molecule. J. Biol. Chem. 2003, 278, 35805–35811. [Google Scholar] [CrossRef] [Green Version]
- Meng, K.; Rodriguez-Peña, A.; Dimitrov, T.; Chen, W.; Yamin, M.; Noda, M.; Deuel, T.F. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc. Natl. Acad. Sci. USA 2000, 97, 2603–2608. [Google Scholar] [CrossRef] [Green Version]
- Pariser, H.; Ezquerra, L.; Herradon, G.; Perez-Pinera, P.; Deuel, T.F. Fyn is a downstream target of the pleiotrophin/receptor protein tyrosine phosphatase β/ζ-signaling pathway: Regulation of tyrosine phosphorylation of Fyn by pleiotrophin. Biochem. Biophys. Res. Commun. 2005, 332, 664–669. [Google Scholar] [CrossRef]
- Van Camp, J.K.; Beckers, S.; Zegers, D.; Van Hul, W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. Rep. 2014, 10, 207–229. [Google Scholar] [CrossRef]
- Matrone, C.; Petrillo, F.; Nasso, R.; Ferretti, G. Fyn Tyrosine Kinase as Harmonizing Factor in Neuronal Functions and Dysfunctions. Int. J. Mol. Sci. 2020, 21, 4444. [Google Scholar] [CrossRef]
- Milev, P.; Monnerie, H.; Popp, S.; Margolis, R.K.; Margolis, R.U. The core protein of the chondroitin sulfate proteoglycan phosphacan is a high-affinity ligand of fibroblast growth factor-2 and potentiates its mitogenic activity. J. Biol. Chem. 1998, 273, 21439–21442. [Google Scholar] [CrossRef] [Green Version]
- Akita, K.; von Holst, A.; Furukawa, Y.; Mikami, T.; Sugahara, K.; Faissner, A. Expression of multiple chondroitin/dermatan sulfotransferases in the neurogenic regions of the embryonic and adult central nervous system implies that complex chondroitin sulfates have a role in neural stem cell maintenance. Stem Cells 2008, 26, 798–809. [Google Scholar] [CrossRef]
- Bjornsson, C.S.; Apostolopoulou, M.; Tian, Y.; Temple, S. It takes a village: Constructing the neurogenic niche. Dev. Cell 2015, 32, 435–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormel, P.R.; Vieira de Sá, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A.; et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.; Barry, G. Building a Human Brain for Research. Front. Mol. Neurosci. 2020, 13, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paşca, S.P. Assembling human brain organoids. Science 2019, 363, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.A.; Reumann, D.; Bian, S.; Lévi-Strauss, J.; Knoblich, J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 2017, 14, 743–751. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
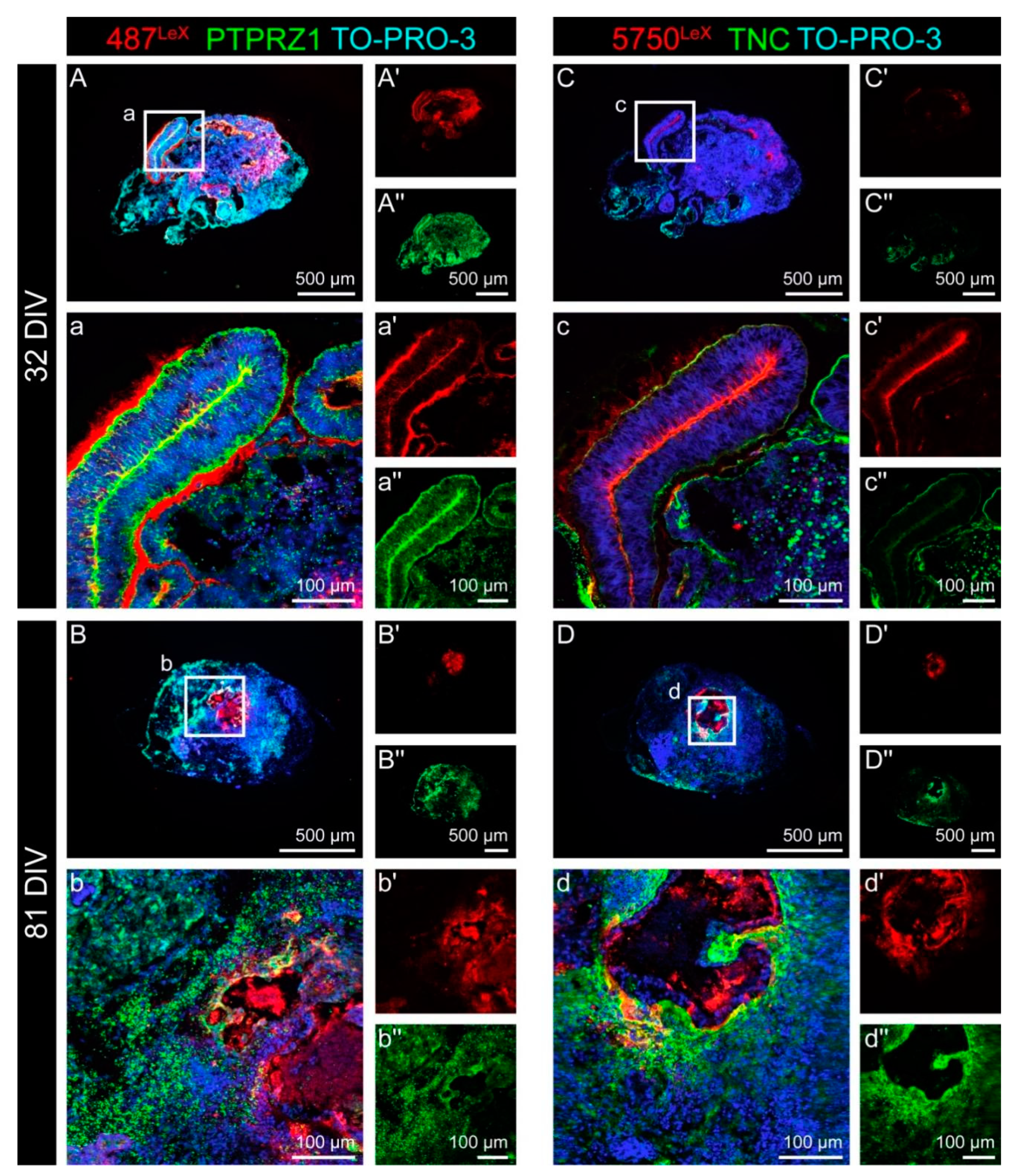

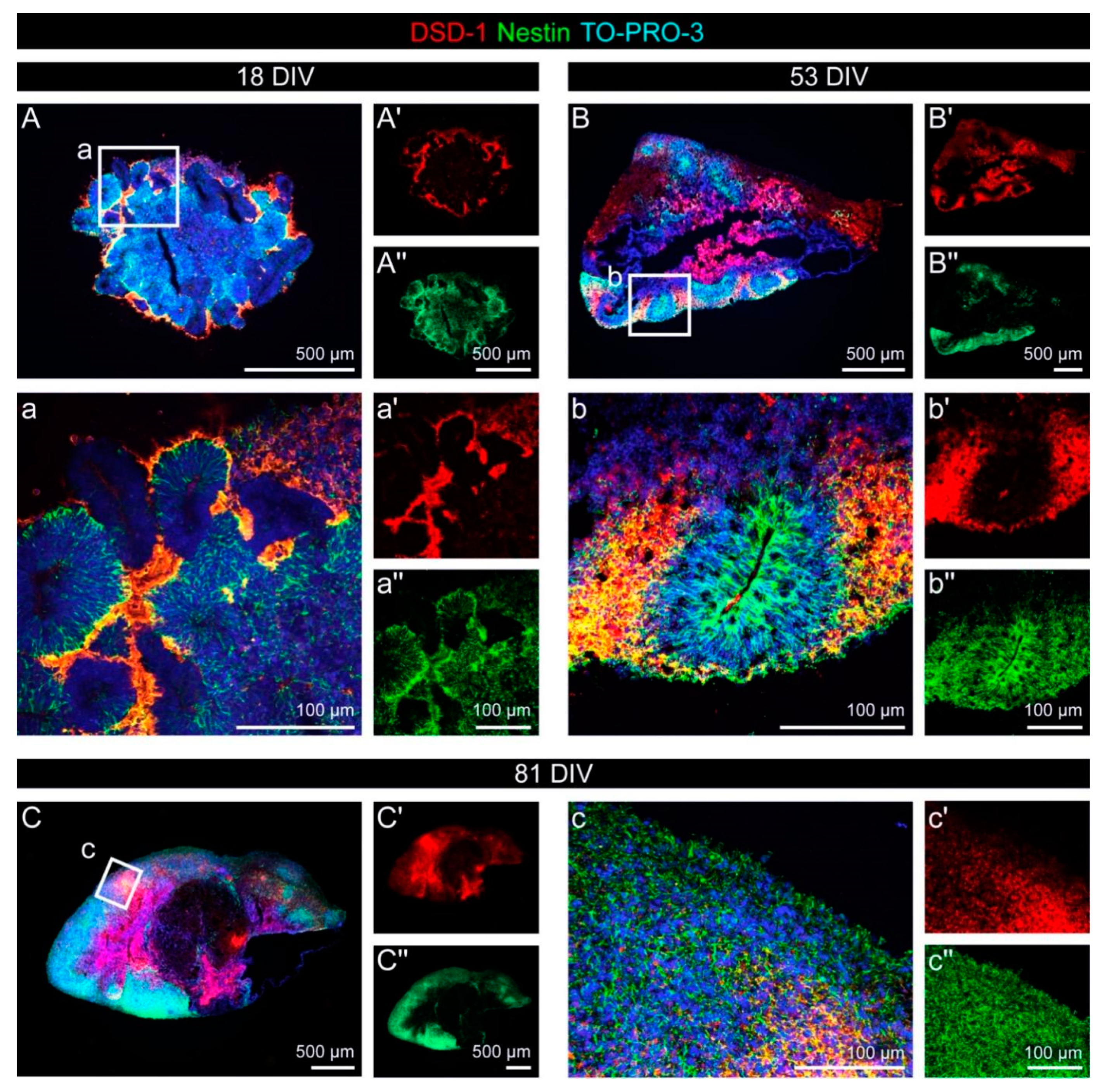

| Antibody | Species | Source | Cat# | Lot | Dilution (IHC) | RRID/Reference |
|---|---|---|---|---|---|---|
| 473HD (DSD-1 epitope) | Rat IgM | Faissner et al. (1994) | N/A | 12/06 | 1:350 | [40] |
| 487LeX (LewisX epitope) | Rat IgM | Streit et al. (1990) | N/A | 9/08 | 1:350 | [41] |
| 5750LeX (LewisX epitope) | Rat IgM | Hennen et al. (2011) | N/A | 2/08 | 1:50 | [9] |
| GFAP | Mouse IgG | Sigma-Aldrich | G3893 | 038M4864V | 1:300 | RRID:AB_477010 |
| GLAST (EAAT1) | Rabbit IgG | Cell Signaling Technology | 5684 | 0001 | 1:100 | RRID:AB_10695722 |
| KAF12 (Tenascin C) | Rabbit polyclonal | Faissner and Kruse (1990) | N/A | 7/96 | 1:300 | [42] |
| KAF13 (PTPRZ1) | Rabbit polyclonal | Faissner et al. (1994) | N/A | N/A | 1:300 | [40] |
| KI-67 | Mouse IgM | Sigma-Aldrich | P6834 | 034144776 | 1:50 | RRID:AB_261141 |
| Nestin | Mouse IgG | Santa Cruz Biotechnology | sc-23927 | A2914 | 1:250 | RRID:AB_627994 |
| OLIG2 | Rabbit polyclonal | Millipore | AB9610 | 3071572 | 1:400 | RRID:AB_570666 |
| Phospho-Histone H3 | Rabbit polyclonal | Millipore | 06-570 | 2825969 | 1:300 | RRID:AB_310177 |
| SOX2 | Rabbit polyclonal | Millipore | AB5603 | 3108482 | 1:100 | RRID:AB_2286686 |
| βIII-Tubulin | Mouse IgG | Sigma-Aldrich | T8660 | 097M4835V | 1:250 | RRID:AB_477590 |
| Antibody; Conjugation | Species | Cat# | Lot | Dilution (IHC) | Source/RRID |
|---|---|---|---|---|---|
| Anti-rabbit IgG (H + L); Alexa Fluor® 488 | Goat | 111-545-045 | 135978 | 1:300 | Jackson ImmunoResearch, Ely, UK, RRID:AB_2338049 |
| Anti-rabbit IgG (H + L); Cy™3 | Goat | 111-165-045 | 125365 | 1:300 | Jackson ImmunoResearch, Ely, UK, RRID:AB_2338003 |
| Anti-mouse IgG + IgM (H + L); Alexa Fluor® 488 | Goat | 115-545-044 | 130731 | 1:300 | Jackson ImmunoResearch, Ely, UK, RRID:AB_2338844 |
| Anti-mouse IgG + IgM (H + L); Cy™3 | Goat | 115-165-068 | 136057 | 1:300 | Jackson ImmunoResearch, Ely, UK, RRID:AB_2338686 |
| Anti-rat IgM (µ-chain); Cy™3 | Goat | 112-165-075 | 128380 | 1:300 | Jackson ImmunoResearch, Ely, UK, RRID:AB_2338249 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roll, L.; Lessmann, K.; Brüstle, O.; Faissner, A. Cerebral Organoids Maintain the Expression of Neural Stem Cell-Associated Glycoepitopes and Extracellular Matrix. Cells 2022, 11, 760. https://doi.org/10.3390/cells11050760
Roll L, Lessmann K, Brüstle O, Faissner A. Cerebral Organoids Maintain the Expression of Neural Stem Cell-Associated Glycoepitopes and Extracellular Matrix. Cells. 2022; 11(5):760. https://doi.org/10.3390/cells11050760
Chicago/Turabian StyleRoll, Lars, Katrin Lessmann, Oliver Brüstle, and Andreas Faissner. 2022. "Cerebral Organoids Maintain the Expression of Neural Stem Cell-Associated Glycoepitopes and Extracellular Matrix" Cells 11, no. 5: 760. https://doi.org/10.3390/cells11050760
APA StyleRoll, L., Lessmann, K., Brüstle, O., & Faissner, A. (2022). Cerebral Organoids Maintain the Expression of Neural Stem Cell-Associated Glycoepitopes and Extracellular Matrix. Cells, 11(5), 760. https://doi.org/10.3390/cells11050760