Adult Neurogenesis under Control of the Circadian System
Abstract
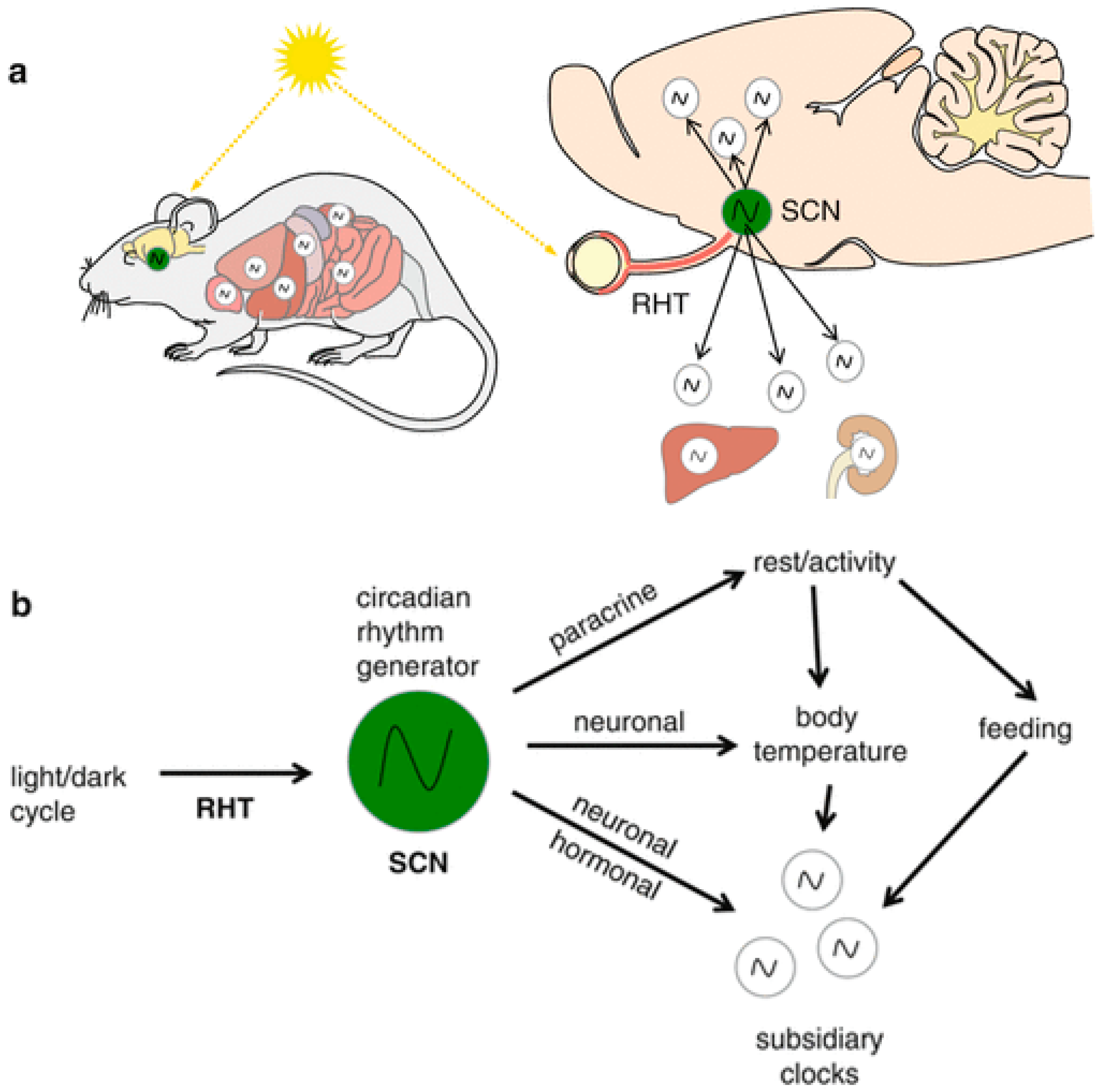
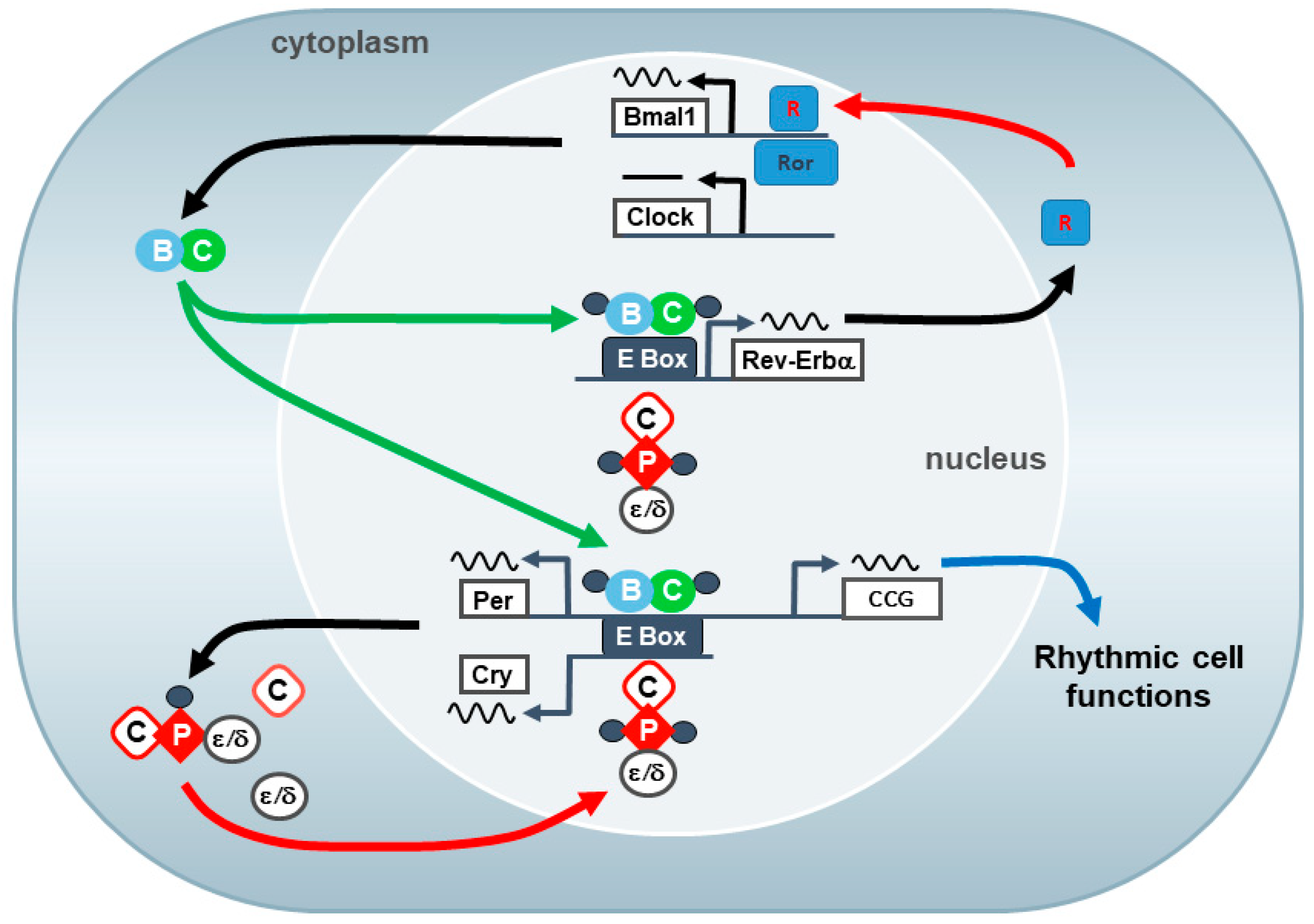
1. The Mammalian Circadian System
2. Adult Neurogenesis
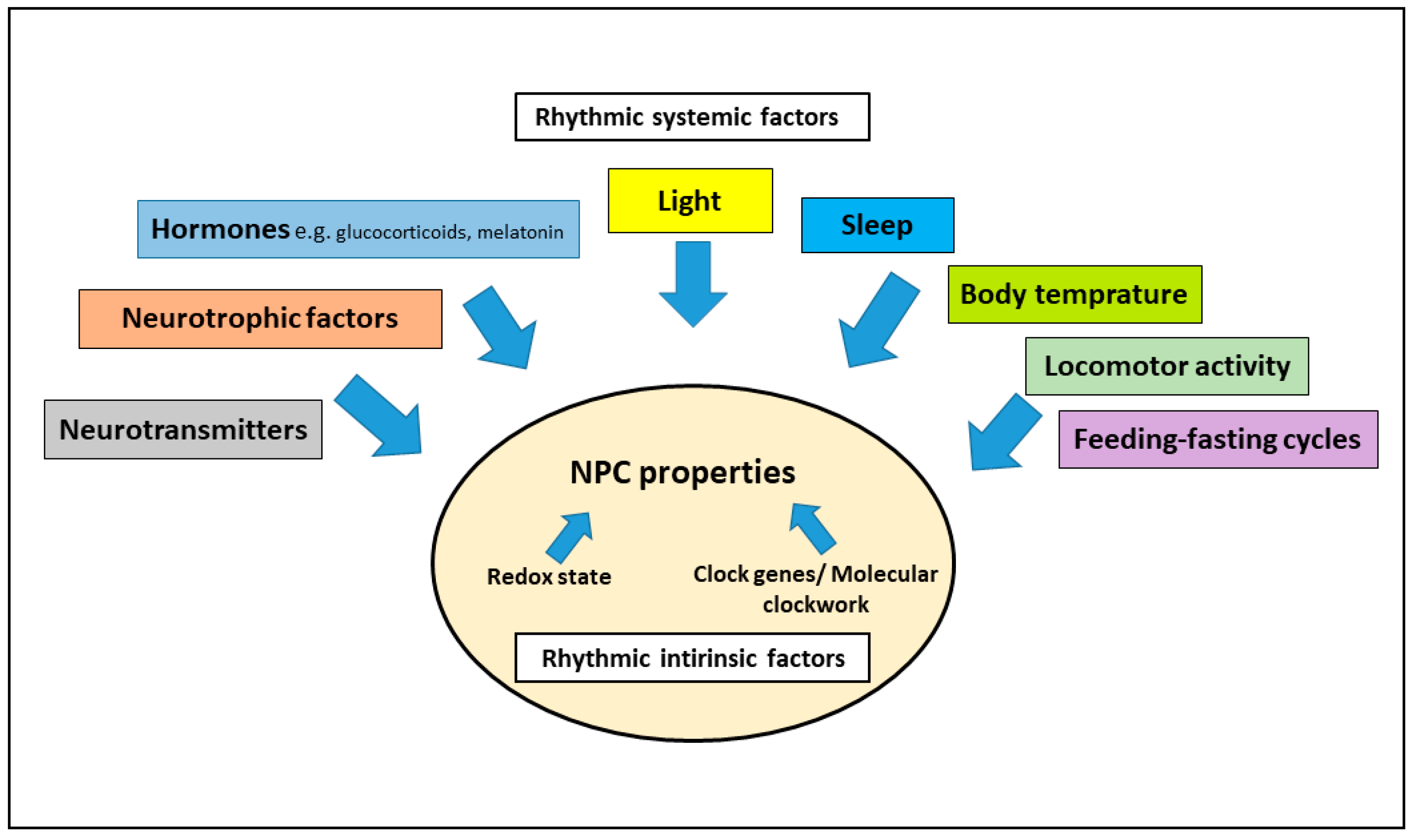
3. Interaction of the Circadian System and Adult Neurogenesis
3.1. Light and Chronodisruption
3.2. Hormones
3.2.1. Glucocorticoids
3.2.2. Melatonin
3.3. Neurotrophic Factors
3.4. Neurotransmitters
3.5. Behavior and Physiology
3.5.1. Sleep
3.5.2. Feeding–Fasting Cycles
3.5.3. Locomotor Activity
3.5.4. Body Temperature
3.6. Redox State
3.7. Clock Genes/Molecular Clockwork
3.7.1. Proliferation and Apoptosis
3.7.2. Differentiation and Migration
3.7.3. Neurogenesis-Related Brain Function
3.7.4. Hypothalamic Neurogenic Niche
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Silver, R.; Kriegsfeld, L.J. Circadian rhythms have broad implications for understanding brain and behavior. Eur. J. Neurosci. 2014, 39, 1866–1880. [Google Scholar] [CrossRef] [PubMed]
- Korf, H.-W.; von Gall, C. Circadian Physiology. In Neuroscience in the 21st Century: From Basic to Clinical; Pfaff, D.W., Ed.; Springer: New York, NY, USA, 2013; pp. 1813–1845. [Google Scholar]
- Kelleher, F.C.; Rao, A.; Maguire, A. Circadian molecular clocks and cancer. Cancer Lett. 2014, 342, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Korf, H.-W.; von Gall, C. Circadian Physiology. In Neuroscience in the 21st Century, 3rd ed.; Pfaff, D.W., Volkow, N.D., Eds.; Springer: New York, NY, USA, 2022; in press. [Google Scholar]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef]
- Evans, J.; Silver, R. The Suprachiasmatic Nucleus and the Circadian Timekeeping System of the Body. In Neuroscience in the 21st Century; Pfaff, D.W., Volkow, N.D., Eds.; Springer: New York, NY, USA, 2016; pp. 1–49. [Google Scholar]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Michel, S.; Colwell, C.S. Cellular communication and coupling within the suprachiasmatic nucleus. Chronobiol. Int. 2001, 18, 579–600. [Google Scholar] [CrossRef]
- Ali, A.A.H.; Stahr, A.; Ingenwerth, M.; Theis, M.; Steinhäuser, C.; von Gall, C. Connexin30 and Connexin43 show a time-of-day dependent expression in the mouse suprachiasmatic nucleus and modulate rhythmic locomotor activity in the context of chronodisruption. Cell Commun. Signal 2019, 17, 61. [Google Scholar] [CrossRef]
- Vosko, A.M.; Schroeder, A.; Loh, D.H.; Colwell, C.S. Vasoactive intestinal peptide and the mammalian circadian system. Gen. Comp. Endocrinol. 2007, 152, 165–175. [Google Scholar] [CrossRef]
- Moriya, T.; Horikawa, K.; Akiyama, M.; Shibata, S. Correlative association between N-methyl-D-aspartate receptor-mediated expression of period genes in the suprachiasmatic nucleus and phase shifts in behavior with photic entrainment of clock in hamsters. Mol. Pharmacol. 2000, 58, 1554–1562. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Fogerson, P.M.; Lazzerini Ospri, L.; Thomsen, M.B.; Layne, R.M.; Severin, D.; Zhan, J.; Singer, J.H.; Kirkwood, A.; Zhao, H.; et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell 2018, 175, 71–84.e18. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Gerfen, C.R.; Young, W.S., 3rd. Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol. 2013, 521, 1844–1866. [Google Scholar] [CrossRef] [PubMed]
- Fisk, A.S.; Tam, S.K.E.; Brown, L.A.; Vyazovskiy, V.V.; Bannerman, D.M.; Peirson, S.N. Light and Cognition: Roles for Circadian Rhythms, Sleep, and Arousal. Front. Neurol. 2018, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.-L.; Guo, H.; Song, N.-N.; Jia, Z.-P.; Hu, X.-T.; Huang, J.-F.; Ding, Y.-Q.; Richter-Levin, G.; Zhou, Q.-X.; Xu, L. Light exposure before learning improves memory consolidation at night. Sci. Rep. 2015, 5, 15578. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, M.; Lin, R.; Wang, Y.; Zhuo, Z.; Cheng, N.; Wang, M.; Tang, Y.; Wang, L.; Hou, S.-T. Rhythmic light flicker rescues hippocampal low gamma and protects ischemic neurons by enhancing presynaptic plasticity. Nat. Commun. 2020, 11, 3012. [Google Scholar] [CrossRef]
- Stehle, J.H.; von Gall, C.; Korf, H.W. Melatonin: A clock-output, a clock-input. J. Neuroendocrinol. 2003, 15, 383–389. [Google Scholar] [CrossRef]
- Son, G.H.; Chung, S.; Kim, K. The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Front. Neuroendocrinol. 2011, 32, 451–465. [Google Scholar] [CrossRef]
- Evans, J.A.; Davidson, A.J. Chapter Ten—Health Consequences of Circadian Disruption in Humans and Animal Models. In Progress in Molecular Biology and Translational Science; Gillette, M.U., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 283–323. [Google Scholar]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold. Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Leal-Galicia, P.; Saldívar-González, A.; Morimoto, S.; Arias, C. Exposure to environmental enrichment elicits differential hippocampal cell proliferation: Role of individual responsiveness to anxiety. Dev. Neurobiol. 2007, 67, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.M. Social regulation of adult neurogenesis: A comparative approach. Front. Neuroendocrinol. 2016, 41, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Kuhn, H.G.; Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997, 386, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.L.; Ma, X.T.; Wang, J.J.; Liu, H.; Chen, Y.F.; Yang, Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res 2017, 317, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Apple, D.M.; Solano-Fonseca, R.; Kokovay, E. Neurogenesis in the aging brain. Biochem. Pharmacol. 2017, 141, 77–85. [Google Scholar] [CrossRef]
- Du Preez, A.; Onorato, D.; Eiben, I.; Musaelyan, K.; Egeland, M.; Zunszain, P.A.; Fernandes, C.; Thuret, S.; Pariante, C.M. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behav. Immun 2021, 91, 24–47. [Google Scholar] [CrossRef]
- Ali, A.A.; Schwarz-Herzke, B.; Stahr, A.; Prozorovski, T.; Aktas, O.; von Gall, C. Premature aging of the hippocampal neurogenic niche in adult Bmal1-deficient mice. Aging 2015, 7, 435–449. [Google Scholar] [CrossRef]
- Ali, A.A.H.; Schwarz-Herzke, B.; Mir, S.; Sahlender, B.; Victor, M.; Görg, B.; Schmuck, M.; Dach, K.; Fritsche, E.; Kremer, A.; et al. Deficiency of the clock gene Bmal1 affects neural progenitor cell migration. Brain Struct. Funct. 2019, 224, 373–386. [Google Scholar] [CrossRef]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef]
- Platel, J.C.; Stamboulian, S.; Nguyen, I.; Bordey, A. Neurotransmitter signaling in postnatal neurogenesis: The first leg. Brain Res. Rev. 2010, 63, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Gilhooley, M.J.; Pinnock, S.B.; Herbert, J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: Implications for neurogenesis. Neurosci. Lett. 2011, 489, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Matanzo, A.; Llorens-Martín, M.; Hernández, F.; Avila, J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: Therapeutic approaches. Mediat. Inflamm. 2013, 2013, 260925. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kondratov, R.V.; Jamasbi, R.J.; Geusz, M.E. Circadian Clock Genes Are Essential for Normal Adult Neurogenesis, Differentiation, and Fate Determination. PLoS ONE 2015, 10, e0139655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Leiter, O.; Seidemann, S.; Overall, R.W.; Ramasz, B.; Rund, N.; Schallenberg, S.; Grinenko, T.; Wielockx, B.; Kempermann, G.; Walker, T.L. Exercise-Induced Activated Platelets Increase Adult Hippocampal Precursor Proliferation and Promote Neuronal Differentiation. Stem Cell Rep. 2019, 12, 667–679. [Google Scholar] [CrossRef]
- Rojczyk-Gołębiewska, E.; Pałasz, A.; Wiaderkiewicz, R. Hypothalamic Subependymal Niche: A Novel Site of the Adult Neurogenesis. Cell. Mol. Ecular Neurobiol. 2014, 34, 631–642. [Google Scholar] [CrossRef]
- Leal-Galicia, P.; Chávez-Hernández, M.E.; Mata, F.; Mata-Luévanos, J.; Rodríguez-Serrano, L.M.; Tapia-de-Jesús, A.; Buenrostro-Jáuregui, M.H. Adult Neurogenesis: A Story Ranging from Controversial New Neurogenic Areas and Human Adult Neurogenesis to Molecular Regulation. Int. J. Mol. Sci. 2021, 22, 11489. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Jessberger, S.; Clark, R.E.; Broadbent, N.J.; Clemenson, G.D.; Consiglio, A., Jr.; Lie, D.C.; Squire, L.R.; Gage, F.H. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009, 16, 147–154. [Google Scholar] [CrossRef]
- Terranova, J.I.; Ogawa, S.K.; Kitamura, T. Adult hippocampal neurogenesis for systems consolidation of memory. Behav. Brain Res. 2019, 372, 112035. [Google Scholar] [CrossRef] [PubMed]
- Nakashiba, T.; Cushman Jesse, D.; Pelkey Kenneth, A.; Renaudineau, S.; Buhl Derek, L.; McHugh Thomas, J.; Barrera Vanessa, R.; Chittajallu, R.; Iwamoto Keisuke, S.; McBain Chris, J.; et al. Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion. Cell 2012, 149, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Glover, L.R. Adult neurogenesis: Beyond learning and memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshihara, S.; Tsuboi, A. The Functional Role of Olfactory Bulb Granule Cell Subtypes Derived From Embryonic and Postnatal Neurogenesis. Front. Mol. Neurosci. 2018, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef]
- Li Puma, D.D.; Piacentini, R.; Grassi, C. Does Impairment of Adult Neurogenesis Contribute to Pathophysiology of Alzheimer’s Disease? A Still Open Question. Front. Mol. Neurosci. 2020, 13, 578211. [Google Scholar] [CrossRef]
- Dallmann, R.; DeBruyne, J.P.; Weaver, D.R. Photic resetting and entrainment in CLOCK-deficient mice. J. Biol. Rhythm. 2011, 26, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Husse, J.; Eichele, G.; Oster, H. Synchronization of the mammalian circadian timing system: Light can control peripheral clocks independently of the SCN clock. BioEssays 2015, 37, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Mrosovsky, N.; Lucas, R.J.; Foster, R.G. Persistence of masking responses to light in mice lacking rods and cones. J. Biol. Rhythm. 2001, 16, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Namgyal, D.; Chandan, K.; Ali, S.; Ahmad, A.; Hashim, M.J.; Sarwat, M. Aberrant Lighting Causes Anxiety-like Behavior in Mice but Curcumin Ameliorates the Symptoms. Animals 2021, 11, 2590. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.; Anderson David, J. A Cre-Dependent, Anterograde Transsynaptic Viral Tracer for Mapping Output Pathways of Genetically Marked Neurons. Neuron 2011, 72, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.E.; Robison, A.J.; Núñez, A.A.; Yan, L. Light modulates hippocampal function and spatial learning in a diurnal rodent species: A study using male nile grass rat (Arvicanthis niloticus). Hippocampus 2018, 28, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [CrossRef]
- Fujioka, A.; Fujioka, T.; Tsuruta, R.; Izumi, T.; Kasaoka, S.; Maekawa, T. Effects of a constant light environment on hippocampal neurogenesis and memory in mice. Neurosci. Lett. 2011, 488, 41–44. [Google Scholar] [CrossRef]
- Walker, W.H.; 2nd Borniger, J.C.; Gaudier-Diaz, M.M.; Hecmarie Meléndez-Fernández, O.; Pascoe, J.L.; Courtney DeVries, A.; Nelson, R.J. Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol. Psychiatry 2020, 25, 1080–1093. [Google Scholar] [CrossRef]
- Horsey, E.A.; Maletta, T.; Turner, H.; Cole, C.; Lehmann, H.; Fournier, N.M. Chronic Jet Lag Simulation Decreases Hippocampal Neurogenesis and Enhances Depressive Behaviors and Cognitive Deficits in Adult Male Rats. Front. Behav. Neurosci. 2020, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Kott, J.; Leach, G.; Yan, L. Direction-dependent effects of chronic “jet-lag” on hippocampal neurogenesis. Neurosci. Lett. 2012, 515, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Sellix, M.T.; Daniel, J.; Yamazaki, S.; Menaker, M.; Block, G.D. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006, 16, R914–R916. [Google Scholar] [CrossRef] [PubMed]
- Yan, L. Expression of clock genes in the suprachiasmatic nucleus: Effect of environmental lighting conditions. Rev. Endocr. Metab. Disord. 2009, 10, 301–310. [Google Scholar] [CrossRef]
- Gibson, E.M.; Wang, C.; Tjho, S.; Khattar, N.; Kriegsfeld, L.J. Experimental ‘jet lag’ inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS ONE 2010, 5, e15267. [Google Scholar] [CrossRef]
- Iggena, D.; Winter, Y.; Steiner, B. Melatonin restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice. J. Pineal. Res. 2017, 62, 440. [Google Scholar] [CrossRef]
- Kino, T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: Implications to mood disorders. Front. Physiol. 2015, 6, 230. [Google Scholar] [CrossRef]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar]
- Todd, W.D.; Venner, A.; Anaclet, C.; Broadhurst, R.Y.; De Luca, R.; Bandaru, S.S.; Issokson, L.; Hablitz, L.M.; Cravetchi, O.; Arrigoni, E.; et al. Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat. Commun. 2020, 11, 4410. [Google Scholar] [CrossRef]
- Buijs, R.M.; la Fleur, S.E.; Wortel, J.; Van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009, 200, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Nader, N.; Chrousos, G.P.; Kino, T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. FASEB J. 2009, 23, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Agasse, F.; Mendez-David, I.; Christaller, W.; Carpentier, R.; Braz, B.Y.; David, D.J.; Saudou, F.; Humbert, S. Chronic Corticosterone Elevation Suppresses Adult Hippocampal Neurogenesis by Hyperphosphorylating Huntingtin. Cell 2020, 32, 107865. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Patchev, A.V.; Wu, Y.; Lu, J.; Holsboer, F.; Zhang, J.Z.; Sousa, N.; Almeida, O.F. Depletion of the neural precursor cell pool by glucocorticoids. Ann. Neurol. 2010, 67, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.; Bielefeld, P.; Garcia-Corzo, L.; Passchier, E.M.J.; Gradari, S.; Jungenitz, T.; Pons-Espinal, M.; Gebara, E.; Martín-Suárez, S.; Lucassen, P.J.; et al. Circadian glucocorticoid oscillations preserve a population of adult hippocampal neural stem cells in the aging brain. Mol. Psychiatry 2020, 25, 1382–1405. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Kumagai, H.; Skach, A.; Sato, M.; Yanagisawa, M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell 2013, 155, 1323–1336. [Google Scholar] [CrossRef]
- Nagamine, M.; Noguchi, H.; Takahashi, N.; Kim, Y.; Matsuoka, Y. Effect of cortisol diurnal rhythm on emotional memory in healthy young adults. Sci. Rep. 2017, 7, 10158. [Google Scholar] [CrossRef]
- Stawski, R.S.; Almeida, D.M.; Lachman, M.E.; Tun, P.A.; Rosnick, C.B.; Seeman, T. Associations Between Cognitive Function and Naturally Occurring Daily Cortisol During Middle Adulthood: Timing Is Everything. J. Gerontol. Ser. B 2011, 66B, i71–i81. [Google Scholar] [CrossRef]
- Fiocco, A.J.; Wan, N.; Weekes, N.; Pim, H.; Lupien, S.J. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and/or depressive symptoms: Relation to cognitive functioning. Stress 2006, 9, 143–152. [Google Scholar] [CrossRef]
- Gilpin, H.; Whitcomb, D.; Cho, K. Atypical evening cortisol profile induces visual recognition memory deficit in healthy human subjects. Mol. Brain 2008, 1, 4. [Google Scholar] [CrossRef]
- Korf, H.W.; von Gall, C. Mice, melatonin and the circadian system. Mol. Cell Endocrinol. 2006, 252, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Borjigin, J.; Zhang, L.S.; Calinescu, A.A. Circadian regulation of Pineal. gland rhythmicity. Mol. Cell Endocrinol. 2012, 349, 13–19. [Google Scholar] [CrossRef] [PubMed]
- von Gall, C.; Lewy, A.; Schomerus, C.; Vivien-Roels, B.; Pevet, P.; Korf, H.W.; Stehle, J.H. (Transcription factor dynamics and neuroendocrine signalling in the mouse Pineal. gland: A comparative analysis of melatonin-deficient C57BL mice and melatonin-proficient C3H mice. Eur. J. Neurosci. 2000, 12, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8 (Suppl. 3), 34–42. [Google Scholar] [CrossRef]
- von Gall, C.; Stehle, J.H.; Weaver, D.R. Mammalian melatonin receptors: Molecular biology and signal transduction. Cell Tissue Res. 2002, 309, 151–162. [Google Scholar] [CrossRef]
- Leung, J.W.; Cheung, K.K.; Ngai, S.P.; Tsang, H.W.; Lau, B.W. Protective Effects of Melatonin on Neurogenesis Impairment in Neurological Disorders and Its Relevant Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5654. [Google Scholar] [CrossRef]
- Fredrich, M.; Hampel, M.; Seidel, K.; Christ, E.; Korf, H.W. Impact of melatonin receptor-signaling on Zeitgeber time-dependent changes in cell proliferation and apoptosis in the adult murine hippocampus. Hippocampus 2017, 27, 495–506. [Google Scholar] [CrossRef]
- Rennie, K.; De Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the Pineal.ectomized rat. J. Pineal. Res. 2009, 47, 313–317. [Google Scholar] [CrossRef]
- Motta-Teixeira, L.C.; Machado-Nils, A.V.; Battagello, D.S.; Diniz, G.B.; Andrade-Silva, J.; Silva, S., Jr.; Matos, R.A.; do Amaral, F.G.; Xavier, G.F.; Bittencourt, J.C.; et al. The absence of maternal Pineal. melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm. Behav. 2018, 105, 146–156. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin Modulates Cell Survival of New Neurons in the Hippocampus of Adult Mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef]
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal. Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J. Pineal. Res. 2013, 54, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.; Vega-Rivera, N.M.; Benítez-King, G.; Castro-García, M.; Ortíz-López, L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012, 530, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Figueiro-Silva, J.; Antequera, D.; Pascual, C.; de la Fuente Revenga, M.; Volt, H.; Acuña-Castroviejo, D.; Rodríguez-Franco, M.I.; Carro, E. The Melatonin Analog IQM316 May Induce Adult Hippocampal Neurogenesis and Preserve Recognition Memories in Mice. Cell Transplant. 2018, 27, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin Ameliorates Valproic Acid-Induced Neurogenesis Impairment: The Role of Oxidative Stress in Adult Rats. Oxid. Med. Cell. Longev. 2021, 2021, 9997582. [Google Scholar] [CrossRef] [PubMed]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef]
- Lee, E.; Son, H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009, 42, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Liang, F.Q.; Walline, R.; Earnest, D.J. Circadian rhythm of brain-derived neurotrophic factor in the rat suprachiasmatic nucleus. Neurosci Lett. 1998, 242, 89–92. [Google Scholar] [CrossRef]
- Pollock, G.S.; Vernon, E.; Forbes, M.E.; Yan, Q.; Ma, Y.T.; Hsieh, T.; Robichon, R.; Frost, D.O.; Johnson, J.E. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J. Neurosci. 2001, 21, 3923–3931. [Google Scholar] [CrossRef]
- Cain, S.W.; Chang, A.-M.; Vlasac, I.; Tare, A.; Anderson, C.; Czeisler, C.A.; Saxena, R. Circadian Rhythms in Plasma Brain-derived Neurotrophic Factor Differ in Men and Women. J. Biol. Rhythm. 2017, 32, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Bhang, S.; Ahn, J.H. Diurnal variation and gender differences of plasma brain-derived neurotrophic factor in healthy human subjects. Psychiatry Res. 2011, 186, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Begliuomini, S.; Lenzi, E.; Ninni, F.; Casarosa, E.; Merlini, S.; Pluchino, N.; Valentino, V.; Luisi, S.; Luisi, M.; Genazzani, A.R. Plasma brain-derived neurotrophic factor daily variations in men: Correlation with cortisol circadian rhythm. J. Endocrinol. 2008, 197, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Katoh-Semba, R.; Tsuzuki, M.; Miyazaki, N.; Matsuda, M.; Nakagawa, C.; Ichisaka, S.; Sudo, K.; Kitajima, S.; Hamatake, M.; Hata, Y.; et al. A phase advance of the light-dark cycle stimulates production of, BDNF.; but not of other neurotrophins, in the adult rat cerebral cortex: Association with the activation of CREB. J. Neurochem. 2008, 106, 2131–2142. [Google Scholar] [CrossRef]
- von Gall, C.; Duffield, G.E.; Hastings, M.H.; Kopp, M.D.; Dehghani, F.; Korf, H.W.; Stehle, J.H. CREB in the mouse SCN: A molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J. Neurosci. 1998, 18, 10389–10397. [Google Scholar] [CrossRef]
- Gau, D.; Lemberger, T.; von Gall, C.; Kretz, O.; Le Minh, N.; Gass, P.; Schmid, W.; Schibler, U.; Korf, H.W.; Schutz, G. Phosphorylation of CREB Ser142 regulates light-induced phase shifts of the circadian clock. Neuron 2002, 34, 245–253. [Google Scholar] [CrossRef]
- Mendoza-Viveros, L.; Chiang, C.-K.; Ong, J.; Hegazi, S.; Cheng, A.; Bouchard Cannon, P.; Fana, M.; Lowden, C.; Zhang, P.; Bothorel, B.; et al. miR-132/212 Modulates Seasonal Adaptation and Dendritic Morphology of the Central Circadian Clock. Cell Rep. 2017, 19, 505–520. [Google Scholar] [CrossRef]
- Westwood, A.J.; Beiser, A.; Decarli, C.; Harris, T.B.; Chen, T.C.; He, X.M.; Roubenoff, R.; Pikula, A.; Au, R.; Braverman, L.E.; et al. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology 2014, 82, 1613–1619. [Google Scholar] [CrossRef]
- Roman, O.; Seres, J.; Herichova, I.; Zeman, M.; Jurcovicova, J. Daily profiles of plasma prolactin (PRL), growth hormone (GH), insulin-like growth factor-1 (IGF-1), luteinizing hormone (LH), testosterone, and melatonin, and of pituitary PRL mRNA and GH mRNA in male Long Evans rats in acute phase of adjuvant arthritis. Chronobiol. Int. 2003, 20, 823–836. [Google Scholar] [CrossRef]
- Kimura, S.; Toyoura, M.; Toyota, Y.; Takaoka, Y. Serum concentrations of insulin-like growth factor-1 as a biomarker of improved circadian rhythm sleep-wake disorder in school-aged children. J. Clin. Sleep Med. 2020, 16, 2073–2078. [Google Scholar] [CrossRef]
- Banks, R.E.; Forbes, M.A.; Kinsey, S.E.; Stanley, A.; Ingham, E.; Walters, C.; Selby, P.J. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: Significance for VEGF measurements and cancer biology. Br. J. Cancer 1998, 77, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, B.D.; Harik, S.I.; Hudetz, A.G. VEGF mRNA expressed in microvessels of neonatal and adult rat cerebral cortex. Mol. Brain Res. 2002, 101, 103–108. [Google Scholar] [CrossRef]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the nervous system. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.D.; Cao, Y. Clock controls angiogenesis. Cell Cycle 2013, 12, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Engelmann, K.; Appelt, D.; Sandner, D.; Weigmann, I.; Ganz, X.; Pistrosch, F.; Köhler, C.; Gasparic, A.; Birkenfeld, A.L. Intra-individual variability and circadian rhythm of vascular endothelial growth factors in subjects with normal glucose tolerance and type 2 diabetes. PLoS ONE 2017, 12, e0184234. [Google Scholar] [CrossRef]
- Berg, D.A.; Belnoue, L.; Song, H.; Simon, A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar] [CrossRef]
- Kikuchi, T.; Tan, H.; Mihara, T.; Uchimoto, K.; Mitsushima, D.; Takase, K.; Morita, S.; Goto, T.; Andoh, T.; Kamiya, Y. Effects of volatile anesthetics on the circadian rhythms of rat hippocampal acetylcholine release and locomotor activity. Neuroscience 2013, 237, 151–160. [Google Scholar] [CrossRef]
- Kafka, M.S.; Benedito, M.A.; Roth, R.H.; Steele, L.K.; Wolfe, W.W.; Catravas, G.N. Circadian rhythms in catecholamine metabolites and cyclic nucleotide production. Chronobiol. Int. 1986, 3, 101–115. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Golombek, D.A. The Rhythmic GABAergic System. Neurochem. Res. 1998, 23, 607–614. [Google Scholar] [CrossRef]
- Holmes, M.C.; French, K.L.; Seckl, J.R. Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Brain Res. Mol. Brain Res. 1995, 28, 186–192. [Google Scholar] [CrossRef]
- Parekh, P.K.; Ozburn, A.R.; McClung, C.A. Circadian clock genes: Effects on dopamine, reward and addiction. Alcohol 2015, 49, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Fukushima, H.; Hosoda, H.; Serita, T.; Ishikawa, R.; Rokukawa, T.; Kawahara-Miki, R.; Zhang, Y.; Ohta, M.; Okada, S.; et al. Hippocampal clock regulates memory retrieval via Dopamine and PKA-induced GluA1 phosphorylation. Nat. Commun. 2019, 10, 5766. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Desplats, P.; Hagl, C.; Klucken, J.; Aigner, R.; Ploetz, S.; Laemke, J.; Karl, A.; Aigner, L.; Masliah, E.; et al. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp. Neurol. 2009, 219, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.; O’Brien, W.T.; Manlove, J.; Krizman, E.N.; Fang, B.; Gerhart-Hines, Z.; Robinson, M.B.; Klein, P.S.; Lazar, M.A. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor Rev-erbα. Mol. Endocrinol. 2014, 28, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.E.; Kwolek, E.M.; Bischof, J.M.; van Eede, M.; Henkelman, R.M.; Wevrick, R. Regionally reduced brain volume, altered serotonin neurochemistry, and abnormal behavior in mice null for the circadian rhythm output gene Magel2. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009, 150, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Kostin, A.; Alam, M.A.; McGinty, D.; Alam, M.N. Adult hypothalamic neurogenesis and sleep-wake dysfunction in aging. Sleep 2021, 44, zsaa173. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.; Helgadóttir, H.; Mölle, M.; Born, J. Boosting slow oscillations during sleep potentiates memory. Nature 2006, 444, 610–613. [Google Scholar] [CrossRef]
- Yang, G.; Lai, C.S.; Cichon, J.; Ma, L.; Li, W.; Gan, W.B. Sleep promotes branch-specific formation of dendritic spines after learning. Science 2014, 344, 1173–1178. [Google Scholar] [CrossRef]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Alhola, P.; Polo-Kantola, P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar]
- Akers, K.G.; Chérasse, Y.; Fujita, Y.; Srinivasan, S.; Sakurai, T.; Sakaguchi, M. Concise Review: Regulatory Influence of Sleep and Epigenetics on Adult Hippocampal Neurogenesis and Cognitive and Emotional Function. Stem Cells 2018, 36, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.D.; Pollock, M.S.; Lieblich, S.E.; Epp, J.R.; Galea, L.A.; Mistlberger, R.E. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1693–R1703. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Koyanagi, I.; Carrier-Ruiz, A.; Vergara, P.; Srinivasan, S.; Sugaya, Y.; Kasuya, M.; Yu, T.S.; Vogt, K.E.; Muratani, M.; et al. Sparse Activity of Hippocampal Adult-Born Neurons during REM Sleep Is Necessary for Memory Consolidation. Neuron 2020, 107, 552–565.e510. [Google Scholar] [CrossRef] [PubMed]
- Carrier-Ruiz, A.; Sugaya, Y.; Kumar, D.; Vergara, P.; Koyanagi, I.; Srinivasan, S.; Naoi, T.; Kano, M.; Sakaguchi, M. Calcium imaging of adult-born neurons in freely moving mice. STAR Protoc. 2021, 2, 100238. [Google Scholar] [CrossRef] [PubMed]
- Sippel, D.; Schwabedal, J.; Snyder, J.C.; Oyanedel, C.N.; Bernas, S.N.; Garthe, A.; Tröndle, A.; Storch, A.; Kempermann, G.; Brandt, M.D. Disruption of NREM sleep and sleep-related spatial memory consolidation in mice lacking adult hippocampal neurogenesis. Sci. Rep. 2020, 10, 16467. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- López-Armas, G.; Flores-Soto, M.E.; Chaparro-Huerta, V.; Jave-Suarez, L.F.; Soto-Rodríguez, S.; Rusanova, I.; Acuña-Castroviejo, D.; González-Perez, O.; González-Castañeda, R.E. Prophylactic Role of Oral Melatonin Administration on Neurogenesis in Adult Balb/C Mice during REM Sleep Deprivation. Oxid. Med. Cell. Longev. 2016, 2016, 2136902. [Google Scholar] [CrossRef]
- Kostin, A.; Alam, M.A.; McGinty, D.; Szymusiak, R.; Alam, M.N. Chronic Suppression of Hypothalamic Cell Proliferation and Neurogenesis Induces Aging-Like Changes in Sleep-Wake Organization in Young Mice. Neuroscience 2019, 404, 541–556. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi In, Y.; Wei, M.; Cheng Chia, W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap Li, P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Kaptan, Z.; Akgün-Dar, K.; Kapucu, A.; Dedeakayoğulları, H.; Batu, Ş.; Üzüm, G. Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res. 2015, 1618, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Association between Time Restricted Feeding and Cognitive Status in Older Italian Adults. Nutrients 2021, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Ralph, M.R.; Foster, R.G.; Davis, F.C.; Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990, 247, 975–978. [Google Scholar] [CrossRef]
- Cormier, H.C.; Della-Maggiore, V.; Karatsoreos, I.N.; Koletar, M.M.; Ralph, M.R. Suprachiasmatic vasopressin and the circadian regulation of voluntary locomotor behavior. Eur. Neurosci. 2015, 41, 79–88. [Google Scholar] [CrossRef]
- Gremmelspacher, T.; Gerlach, J.; Hubbe, A.; Haas, C.A.; Häussler, U. Neurogenic Processes Are Induced by Very Short Periods of Voluntary Wheel-Running in Male Mice. Front. Neurosci. 2017, 11, 385. [Google Scholar] [CrossRef]
- Bolijn, S.; Lucassen, P.J. How the Body Talks to the Brain; Peripheral Mediators of Physical Activity-Induced Proliferation in the Adult Hippocampus. Brain Plast. 2015, 1, 5–27. [Google Scholar] [CrossRef]
- Nakagawa, T.; Koan, I.; Chen, C.; Matsubara, T.; Hagiwara, K.; Lei, H.; Hirotsu, M.; Yamagata, H.; Nakagawa, S. Regular Moderate- to Vigorous-Intensity Physical Activity Rather Than Walking Is Associated with Enhanced Cognitive Functions and Mental Health in Young Adults. Int. Environ. Res. Public Health 2020, 17, 614. [Google Scholar] [CrossRef]
- Patten, A.R.; Yau, S.Y.; Fontaine, C.J.; Meconi, A.; Wortman, R.C.; Christie, B.R. The Benefits of Exercise on Structural and Functional Plasticity in the Rodent Hippocampus of Different Disease Models. Brain Plast. 2015, 1, 97–127. [Google Scholar] [CrossRef]
- Holmes, M.M.; Galea, L.A.; Mistlberger, R.E.; Kempermann, G. Adult hippocampal neurogenesis and voluntary running activity: Circadian and dose-dependent effects. J. Neurosci. Res. 2004, 76, 216–222. [Google Scholar] [CrossRef]
- Refinetti, R. Circadian rhythmicity of body temperature and metabolism. Temperature 2020, 7, 321–362. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R. Relationship between the daily rhythms of locomotor activity and body temperature in eight mammalian species. Am. J. Physiol. 1999, 277, R1493–R1500. [Google Scholar] [CrossRef] [PubMed]
- Ingenwerth, M.; Noichl, E.; Stahr, A.; Korf, H.W.; Reinke, H.; von Gall, C. Heat Shock Factor 1 Deficiency Affects Systemic Body Temperature Regulation. Neuroendocrinology 2016, 103, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Bregy, A.; Nixon, R.; Lotocki, G.; Alonso, O.F.; Atkins, C.M.; Tsoulfas, P.; Bramlett, H.M.; Dietrich, W.D. Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp. Neurol. 2012, 233, 821–828. [Google Scholar] [CrossRef]
- Kanagawa, T.; Fukuda, H.; Tsubouchi, H.; Komoto, Y.; Hayashi, S.; Fukui, O.; Shimoya, K.; Murata, Y. A decrease of cell proliferation by hypothermia in the hippocampus of the neonatal rat. Brain Res. 2006, 1111, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Ichikawa, J.; Matsuki, N.; Ikegaya, Y.; Koyama, R. Experimental febrile seizures induce age-dependent structural plasticity and improve memory in mice. Neuroscience 2016, 318, 34–44. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Guo, N.-W.; Huang, C.-C.; Wang, S.-T.; Tsai, J.-J. Neurocognitive Attention and Behavior Outcome of School-Age Children with a History of Febrile Convulsions: A Population Study. Epilepsia 2000, 41, 412–420. [Google Scholar] [CrossRef]
- Umschweif, G.; Shabashov, D.; Alexandrovich, A.G.; Trembovler, V.; Horowitz, M.; Shohami, E. Neuroprotection after Traumatic Brain Injury in Heat-Acclimated Mice Involves Induced Neurogenesis and Activation of Angiotensin Receptor Type 2 Signaling. J. Cereb. Blood Flow Metab. 2014, 34, 1381–1390. [Google Scholar] [CrossRef]
- Koyama, Y.; Mukuda, T.; Hamasaki, S.; Nakane, H.; Kaidoh, T. Short-term Heat Exposure Promotes Hippocampal Neurogenesis via Activation of Angiotensin II Type 1 Receptor in Adult Rats. Neuroscience 2018, 385, 121–132. [Google Scholar] [CrossRef]
- Fanjul-Moles, M.L.; López-Riquelme, G.O. Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxid. Med. Cell. Longev. 2016, 2016, 7420637. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Zou, Y.; Corniola, R. Oxidative stress and adult neurogenesis--effects of radiation and superoxide dismutase deficiency. Semin. Cell Dev. Biol 2012, 23, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Pérez Estrada, C.; Covacu, R.; Sankavaram, S.R.; Svensson, M.; Brundin, L. Oxidative stress increases neurogenesis and oligodendrogenesis in adult neural progenitor cells. Stem Cells Dev. 2014, 23, 2311–2327. [Google Scholar] [CrossRef] [PubMed]
- Prozorovski, T.; Schulze-Topphoff, U.; Glumm, R.; Baumgart, J.; Schröter, F.; Ninnemann, O.; Siegert, E.; Bendix, I.; Brüstle, O.; Nitsch, R.; et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 2008, 10, 385–394. [Google Scholar] [CrossRef]
- O’Neill, J.S.; van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.Y.; Reddy, A.B.; Millar, A.J. Circadian rhythms persist without transcription in a eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef]
- Wang, T.A.; Yu, Y.V.; Govindaiah, G.; Ye, X.; Artinian, L.; Coleman, T.P.; Sweedler, J.V.; Cox, C.L.; Gillette, M.U. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 2012, 337, 839–842. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef]
- Kondratova, A.A.; Dubrovsky, Y.V.; Antoch, M.P.; Kondratov, R.V. Circadian clock proteins control adaptation to novel environment and memory formation. Aging 2010, 2, 285–297. [Google Scholar] [CrossRef]
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef]
- Farshadi, E.; van der Horst, G.T.J.; Chaves, I. Molecular Links between the Circadian Clock and the Cell Cycle. J. Mol. Ecular. Biol. 2020, 432, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-H.; Leem, S.-H. Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res. 2014, 42, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Cannon, P.; Mendoza-Viveros, L.; Yuen, A.; Kærn, M.; Cheng, H.Y. The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell Rep. 2013, 5, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Hechtman, A.; Swann, J. Fluctuations in cellular proliferation across the light/dark cycle in the subgranular zone of the dentate gyrus in the adult male Syrian hamster. Neurosci. Lett. 2010, 473, 192–195. [Google Scholar] [CrossRef]
- Schnell, A.; Chappuis, S.; Schmutz, I.; Brai, E.; Ripperger, J.A.; Schaad, O.; Welzl, H.; Descombes, P.; Alberi, L.; Albrecht, U. The nuclear receptor REV-ERBα regulates Fabp7 and modulates adult hippocampal neurogenesis. PLoS ONE 2014, 9, e99883. [Google Scholar] [CrossRef]
- Hancock, A.; Priester, C.; Kidder, E.; Keith, J.R. Does 5-bromo-2′-deoxyuridine (BrdU) disrupt cell proliferation and neuronal maturation in the adult rat hippocampus in vivo? Behav. Brain Res. 2009, 199, 218–221. [Google Scholar] [CrossRef]
- Kochman, L.J.; Weber, E.T.; Fornal, C.A.; Jacobs, B.L. Circadian variation in mouse hippocampal cell proliferation. Neurosci. Lett. 2006, 406, 256–259. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Tsunekawa, Y.; Nomura, T.; Suto, F.; Matsumata, M.; Tsuchiya, S.; Osumi, N. Differential proliferation rhythm of neural progenitor and oligodendrocyte precursor cells in the young adult hippocampus. PLoS ONE 2011, 6, e27628. [Google Scholar] [CrossRef]
- Tamai, S.; Sanada, K.; Fukada, Y. Time-of-day-dependent enhancement of adult neurogenesis in the hippocampus. PLoS ONE 2008, 3, e3835. [Google Scholar] [CrossRef]
- Yun, S.W.; Leong, C.; Zhai, D.; Tan, Y.L.; Lim, L.; Bi, X.; Lee, J.J.; Kim, H.J.; Kang, N.Y.; Ng, S.H.; et al. Neural stem cell specific fluorescent chemical probe binding to FABP7. Proc. Natl. Acad. Sci. USA 2012, 109, 10214–10217. [Google Scholar] [CrossRef]
- Borgs, L.; Beukelaers, P.; Vandenbosch, R.; Nguyen, L.; Moonen, G.; Maquet, P.; Albrecht, U.; Belachew, S.; Malgrange, B. Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci. 2009, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Hou, L.; Xia, H.; Li, H.; Fan, H.; Jia, X.; Niu, Z. PER2 inhibits proliferation and stemness of glioma stem cells via the Wnt/β-catenin signaling pathway. Oncol. Rep. 2020, 44, 533–542. [Google Scholar] [CrossRef]
- Cheng, A.H.; Bouchard-Cannon, P.; Hegazi, S.; Lowden, C.; Fung, S.W.; Chiang, C.K.; Ness, R.W.; Cheng, H.M. SOX2-Dependent Transcription in Clock Neurons Promotes the Robustness of the Central Circadian Pacemaker. Cell Rep. 2019, 26, 3191–3202.e8. [Google Scholar] [CrossRef]
- Rakai, B.D.; Chrusch, M.J.; Spanswick, S.C.; Dyck, R.H.; Antle, M.C. Survival of adult generated hippocampal neurons is altered in circadian arrhythmic mice. PLoS ONE 2014, 9, e99527. [Google Scholar]
- Kimiwada, T.; Sakurai, M.; Ohashi, H.; Aoki, S.; Tominaga, T.; Wada, K. Clock genes regulate neurogenic transcription factors, including NeuroD1, and the neuronal differentiation of adult neural stem/progenitor cells. Neurochem. Int. 2009, 54, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Moriggi, E.; Bauer, C.; Dibner, C.; Brown, S.A. The circadian clock starts ticking at a developmentally early stage. J. Biol. Rhythm. 2010, 25, 442–449. [Google Scholar] [CrossRef]
- Wang, R.; Liu, K.; Chen, L.; Aihara, K. Neural fate decisions mediated by trans-activation and cis-inhibition in Notch signaling. Bioinformatics 2011, 27, 3158–3165. [Google Scholar] [CrossRef]
- Lucas, D.; Battista, M.; Shi, P.A.; Isola, L.; Frenette, P.S. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 2008, 3, 364–366. [Google Scholar] [CrossRef]
- Feng, L.; Hatten, M.E.; Heintz, N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron 1994, 12, 895–908. [Google Scholar] [CrossRef]
- Fontenot, M.R.; Berto, S.; Liu, Y.; Werthmann, G.; Douglas, C.; Usui, N.; Gleason, K.; Tamminga, C.A.; Takahashi, J.S.; Konopka, G. Novel transcriptional networks regulated by CLOCK in human neurons. Genes Dev. 2017, 31, 2121–2135. [Google Scholar] [CrossRef]
- Mendoza, J.; Vanotti, G. Circadian neurogenetics of mood disorders. Cell Tissue Res. 2019, 377, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, O.; Jilg, A.; Jedlicka, P.; Slawska, J.; Thomas, L.; Saade, A.; Schwarzacher, S.W.; Stehle, J.H. PERIOD1 coordinates hippocampal rhythms and memory processing with daytime. Hippocampus 2014, 24, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Sei, H.; Oishi, K.; Sano, A.; Seno, H.; Ohmori, T.; Morita, Y.; Ishida, N. Clock mutant mice with Jcl/ICR background shows an impaired learning ability in water maze, but not in passive avoidance, at the beginning of dark phase. Congenit. Anom. 2006, 46, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, E.A.; Havekes, R.; Barf, R.P.; Hut, R.A.; Nijholt, I.M.; Jacobs, E.H.; Gerkema, M.P. Circadian time-place learning in mice depends on Cry genes. Curr. Biol. 2008, 18, 844–848. [Google Scholar] [CrossRef]
- Granados-Fuentes, D.; Ben-Josef, G.; Perry, G.; Wilson, D.A.; Sullivan-Wilson, A.; Herzog, E.D. Daily rhythms in olfactory discrimination depend on clock genes but not the suprachiasmatic nucleus. J. Biol. Rhythm. 2011, 26, 552–560. [Google Scholar] [CrossRef]
- Zueger, M.; Urani, A.; Chourbaji, S.; Zacher, C.; Lipp, H.P.; Albrecht, U.; Spanagel, R.; Wolfer, D.P.; Gass, P. mPer1 and mPer2 mutant mice show regular spatial and contextual learning in standardized tests for hippocampus-dependent learning. J. Neural. Transm. 2006, 113, 347–356. [Google Scholar] [CrossRef]
- Mulder, C.; Van Der Zee, E.A.; Hut, R.A.; Gerkema, M.P. Time-Place Learning and Memory Persist in Mice Lacking Functional Per1 and Per2 Clock Genes. J. Biol. Rhythm. 2013, 28, 367–379. [Google Scholar] [CrossRef]
- Snider, K.H.; Dziema, H.; Aten, S.; Loeser, J.; Norona, F.E.; Hoyt, K.; Obrietan, K. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav. Brain Res. 2016, 308, 222–235. [Google Scholar] [CrossRef]
- Ali, A.A.H.; Tundo-Lavalle, F.; Hassan, S.A.; Pfeffer, M.; Stahr, A.; von Gall, C. Impact of Targeted Deletion of the Circadian Clock Gene Bmal1 in Excitatory Forebrain Neurons on Adult Neurogenesis and Olfactory Function. Int. J. Mol. Sci. 2020, 21, 1394. [Google Scholar] [CrossRef]
- Maggi, R.; Zasso, J.; Conti, L. Neurodevelopmental origin and adult neurogenesis of the neuroendocrine hypothalamus. Front. Cell. Neurosci. 2015, 8, 440. [Google Scholar] [CrossRef]
- Pierce, A.A.; Xu, A.W. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J. Comp. Neurol. 2007, 505, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Bedont, J.L.; Pak, T.; Wang, H.; Song, J.; Miranda-Angulo, A.; Takiar, V.; Charubhumi, V.; Balordi, F.; Takebayashi, H.; et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 2012, 15, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Saaltink, D.J.; Håvik, B.; Verissimo, C.S.; Lucassen, P.J.; Vreugdenhil, E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: Implications for neurogenesis. J. Comp. Neurol. 2012, 520, 2805–2823. [Google Scholar] [CrossRef]
- Geoghegan, D.; Carter, D.A. A novel site of adult doublecortin expression: Neuropeptide neurons within the suprachiasmatic nucleus circadian clock. BMC Neurosci. 2008, 9, 2. [Google Scholar] [CrossRef]
- Haan, N.; Goodman, T.; Najdi-Samiei, A.; Stratford, C.M.; Rice, R.; El Agha, E.; Bellusci, S.; Hajihosseini, M.K. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 2013, 33, 6170–6180. [Google Scholar] [CrossRef]
- Korf, H.W.; Møller, M. Arcuate nucleus, median eminence, and hypophysial pars tuberalis. Handb. Clin. Neurol. 2021, 180, 227–251. [Google Scholar]
- Robins, S.C.; Stewart, I.; McNay, D.E.; Taylor, V.; Giachino, C.; Goetz, M.; Ninkovic, J.; Briancon, N.; Maratos-Flier, E.; Flier, J.S.; et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 2013, 4, 2049. [Google Scholar] [CrossRef]
- Beligala, D.H.; De, A.; Geusz, M.E. A Meta-Analysis Characterizing Stem-Like Gene Expression in the Suprachiasmatic Nucleus and Its Circadian Clock. Biomed. Res. Int. 2018, 2018, 3610603. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.A.H.; von Gall, C. Adult Neurogenesis under Control of the Circadian System. Cells 2022, 11, 764. https://doi.org/10.3390/cells11050764
Ali AAH, von Gall C. Adult Neurogenesis under Control of the Circadian System. Cells. 2022; 11(5):764. https://doi.org/10.3390/cells11050764
Chicago/Turabian StyleAli, Amira A. H., and Charlotte von Gall. 2022. "Adult Neurogenesis under Control of the Circadian System" Cells 11, no. 5: 764. https://doi.org/10.3390/cells11050764
APA StyleAli, A. A. H., & von Gall, C. (2022). Adult Neurogenesis under Control of the Circadian System. Cells, 11(5), 764. https://doi.org/10.3390/cells11050764






