Constitutive Heterochromatin in Eukaryotic Genomes: A Mine of Transposable Elements
Abstract
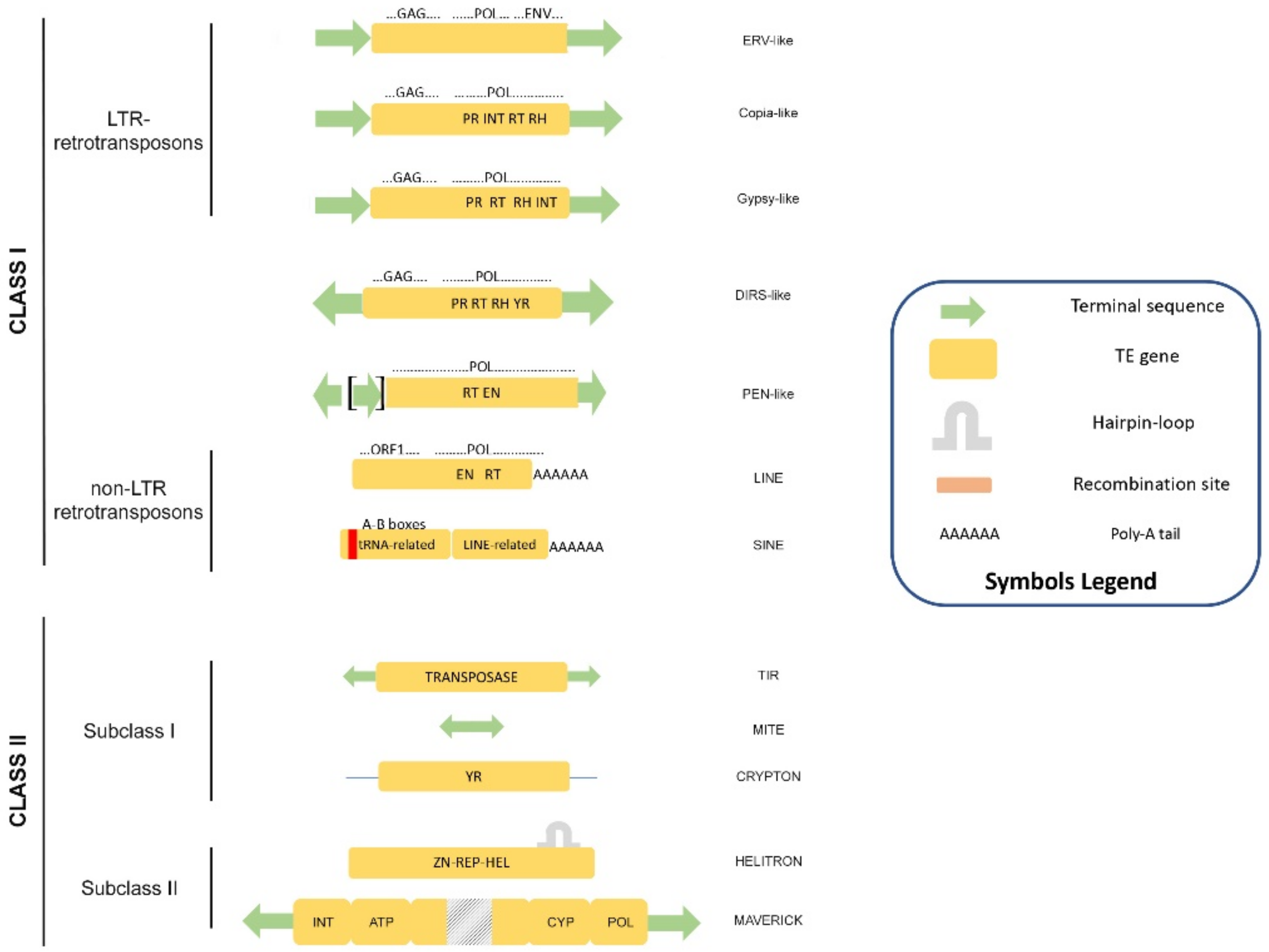
1. Eukaryotic Transposable Elements: A Great Resource of Genome Evolution
2. Accumulation of TEs in Constitutive Heterochromatin of Distantly Related Eukaryotic Genomes
| Accumulation of TEs in the Constitutive Hetrochromatin | ||||
| Organism | TE Type | TE Family | Heterochromatin Link | References |
| Drosophila melanogaster | LINE | I-element | Preferential target | [26,33] |
| Caenorhabditis elegans | TIR (DNA) | Tc1 | Preferential target | [34] |
| Zea mais | TIR (DNA) | Mutator-MuDR | Heterochromatin assembly | [35] |
| Arabidopsis thaliana | LTR | Athila | Preferential target | [36] |
| Saccharomyces cerevisiae | LTR | Ty5 | Telomeric insertion | [37] |
| Dictyostelium discoideum | LTR | Skipper-1 | Centromere insertion | [38] |
| Zea mais | LTR | CR | Centromere insertion | [39] |
| Triticum spp. | LTR | CRW, Quinta | Centromere insertion | [40] |
| Tetrahymena thermophila | TIR (DNA) | PB | Preferential target | [41] |
| Drosophila melanogaster | TIR | 1360 | Preferential target | [42] |
| Mouse ESC | TIR | SB | Preferential target | [43] |
| Tetraodon nigroviridis | TIR | Tol2, Buffy1 | Preferential target | [44] |
| Tetraodon nigroviridis | Non-LTR | Rex3, Babar | Preferential target | [44] |
| Drosophila melanogaster | LTR LINE TIR | Copia Gypsy, Mdg-1 Blood, Doc, F, G Bari1, hobo | Heterochromatic clusters Heterochromatic clusters Heterochromatic clusters | [26] |
| Role of TEs in the Centromere Function | ||||
| Organism | TE Type | TE Family | Centromeric Function | References |
| Drosophila melanogaster | retroelements | Several families | CENP-A recruitment | [45] |
| Dictyostelium dyscoideum | retroelements | DIRS | H3K9me3 and CENH3 histone marks | [38,46] |
| Homo sapiens | retroelements | Several families | Centromeric DNA organization | [47] |
| basidiomycete Cryptococcus genus | LTR | Tcn1–Tcn6 | Centromeric DNA organization | [48] |
| Birth of Satellite DNA | ||||
| Organism | TE Type | TE Family | Satellite | References |
| Aegilops speltoides | LTR | Ty3/gypsy-like | 301 bp repeat | [49] |
| Arabidopsis thaliana | CACTA | Atenspm | Centromeric repeats | [50] |
| Arabidopsis lyrata | LTR | ATCOPIA93 | Centromeric repeats | [51] |
| Drosophila virilis | Not classified | pDv | pvB370 BamHI satellite | [52] |
| Cetaceans | Non-LTR | L1 | Common satellite | [53] |
| Oryza sativa | LTR, DNA | Several families | Diversification of Cen8 | [54] |
| Drosophila melanogaster | TIR | Bari1 | Bari1 satellite | [55,56,57] |
| Exaptation of TEs in Constitutive Heterochromatin | ||||
| Organism | TE Type | TE Family | Evolved Function | References |
| Drosophila melanogaster | Non-LTR | Het-A, TART, TAHRE | Telomere elongation | [58,59,60,61] |
| Rotifers | retroelements | Athena | Telomeric repeats | [62] |
| Many living organisms | TIR | pogo | CENP-B | [63] |
| Ciliates | TIR | PiggyBac | PiggyMac, Pgm-like proteins—macronucleus assembly | [64,65,66] |
| Arabidopsis thaliana | LTR, DNA | Gypsy-like, MULE | Evolved MAIL1 and MAIN genes involved in heterochromatin assembly | [67] |
Dynamics of TE Acculumation in Constitutive Heterochromatin
3. Positive Interactions between Transposable Elements and Constitutive Heterochromatin in Different Host Genomes
Transposable Elements and Transcription of Heterochromatic Genes
4. TE Exaptation in a Heterochromatin-Related Context
4.1. Transposable Elements and Telomeres
4.2. Transposable Elements and Centromeres
4.3. Additional Examples of TE Exaptation in Constitutive Heterochromatin
5. Potential Implications of TE Heterochromatic Copies in Diseases and Aging
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frost, L.S.; Leplae, R.; Summers, A.O.; Toussaint, A. Mobile genetic elements: The agents of open source evolution. Nat. Rev. Microbiol. 2005, 3, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, W.F.; Sapienza, C. Selfish genes, the phenotype paradigm and genome evolution. Nature 1980, 284, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.N.; Feschotte, C. A Field Guide to Eukaryotic Transposable Elements. Annu. Rev. Genet. 2020, 54, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Specchia, V.; Bozzetti, M.P. The Role of HSP90 in Preserving the Integrity of Genomes against Transposons Is Evolutionarily Conserved. Cells 2021, 10, 1096. [Google Scholar] [CrossRef]
- Almojil, D.; Bourgeois, Y.; Falis, M.; Hariyani, I.; Wilcox, J.; Boissinot, S. The Structural, Functional and Evolutionary Impact of Transposable Elements in Eukaryotes. Genes 2021, 12, 918. [Google Scholar] [CrossRef]
- Sundaram, V.; Wysocka, J. Transposable elements as a potent source of diverse cis-regulatory sequences in mammalian genomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190347. [Google Scholar] [CrossRef]
- Moschetti, R.; Palazzo, A.; Lorusso, P.; Viggiano, L.; Marsano, R.M. “What You Need, Baby, I Got It”: Transposable Elements as Suppliers of Cis-Operating Sequences in Drosophila. Biology 2020, 9, 25. [Google Scholar] [CrossRef]
- Ivics, Z.; Izsvák, Z. The expanding universe of transposon technologies for gene and cell engineering. Mob. DNA 2010, 1, 25. [Google Scholar] [CrossRef]
- Sandoval-Villegas, N.; Nurieva, W.; Amberger, M.; Ivics, Z. Contemporary Transposon Tools: A Review and Guide through Mechanisms and Applications of Sleeping Beauty, piggyBac and Tol2 for Genome Engineering. Int. J. Mol. Sci. 2021, 22, 5084. [Google Scholar] [CrossRef]
- Ivics, Z.; Izsvak, Z. Transposons for gene therapy! Curr. Gene Ther. 2006, 6, 593–607. [Google Scholar] [CrossRef]
- Tipanee, J.; VandenDriessche, T.; Chuah, M.K. Transposons: Moving Forward from Preclinical Studies to Clinical Trials. Hum. Gene Ther. 2017, 28, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, A.; Marsano, R.M. Transposable elements: A jump toward the future of expression vectors. Crit. Rev. Biotechnol. 2021, 41, 792–808. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, V.V.; Jurka, J. Rolling-circle transposons in eukaryotes. Proc. Natl. Acad. Sci. USA 2001, 98, 8714–8719. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, V.V.; Jurka, J. Self-synthesizing DNA transposons in eukaryotes. Proc. Natl. Acad. Sci. USA 2006, 103, 4540–4545. [Google Scholar] [CrossRef]
- Wicker, T.; Sabot, F.; Hua-Van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O.; et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Kaminker, J.S.; Bergman, C.M.; Kronmiller, B.; Carlson, J.; Svirskas, R.; Patel, S.; Frise, E.; Wheeler, D.A.; Lewis, S.E.; Rubin, G.M.; et al. The transposable elements of the Drosophila melanogaster euchromatin: A genomics perspective. Genome Biol. 2002, 3, RESEARCH0084. [Google Scholar] [CrossRef]
- Sanmiguel, P.; Bennetzen, J.L. Evidence that a Recent Increase in Maize Genome Size was Caused by the Massive Amplification of Intergene Retrotransposons. Ann. Bot. 1998, 82, 37–44. [Google Scholar] [CrossRef]
- Vicient, C.M.; Suoniemi, A.; Anamthawat-Jonsson, K.; Tanskanen, J.; Beharav, A.; Nevo, E.; Schulman, A.H. Retrotransposon BARE-1 and Its Role in Genome Evolution in the Genus Hordeum. Plant Cell 1999, 11, 1769–1784. [Google Scholar] [CrossRef]
- Meyers, B.C.; Tingey, S.V.; Morgante, M. Abundance, distribution, and transcriptional activity of repetitive elements in the maize genome. Genome Res. 2001, 11, 1660–1676. [Google Scholar] [CrossRef]
- Gilbert, D.M. Replication timing and transcriptional control: Beyond cause and effect. Curr. Opin. Cell Biol. 2002, 14, 377–383. [Google Scholar] [CrossRef]
- Pardue Mary, L.; Gall Joseph, G. Chromosomal Localization of Mouse Satellite DNA. Science 1970, 168, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.J.; Elgin, S.C.R. Epigenetic Codes for Heterochromatin Formation and Silencing: Rounding up the Usual Suspects. Cell 2002, 108, 489–500. [Google Scholar] [CrossRef]
- Charlesworth, B.; Jarne, P.; Assimacopoulos, S. The distribution of transposable elements within and between chromosomes in a population of Drosophila melanogaster. III. Element abundances in heterochromatin. Genet. Res. 2009, 64, 183–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Junakovic, N.; Terrinoni, A.; Di Franco, C.; Vieira, C.; Loevenbruck, C. Accumulation of transposable elements in the heterochromatin and on the Y chromosome of Drosophila simulans and Drosophila melanogaster. J. Mol. Evol. 1998, 46, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Pimpinelli, S.; Berloco, M.; Fanti, L.; Dimitri, P.; Bonaccorsi, S.; Marchetti, E.; Caizzi, R.; Caggese, C.; Gatti, M. Transposable elements are stable structural components of Drosophila melanogaster heterochromatin. Proc. Natl. Acad. Sci. USA 1995, 92, 3804–3808. [Google Scholar] [CrossRef] [PubMed]
- Bennetzen, J.L. The many hues of plant heterochromatin. Genome Biol. 2000, 1, reviews107.101. [Google Scholar] [CrossRef][Green Version]
- Zamudio, N.; Bourc’his, D. Transposable elements in the mammalian germline: A comfortable niche or a deadly trap? Heredity 2010, 105, 92–104. [Google Scholar] [CrossRef]
- Miner, R.W. The New York Academy of Sciences. Section of Biology. Science 1907, 26, 870. [Google Scholar] [CrossRef]
- Randolph, L.A. Types of supernumerary chromosomes in maize. Anat. Rec. 1928, 41, 102. [Google Scholar]
- Beukeboom, L.W. Bewildering Bs: An impression of the 1st B-Chromosome Conference. Heredity 1994, 73, 328–336. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Martins, C. The Modern View of B Chromosomes Under the Impact of High Scale Omics Analyses. Cells 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, P.; Arcà, B.; Berghella, L.; Mei, E. High genetic instability of heterochromatin after transposition of the LINE-like I factor in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1997, 94, 8052–8057. [Google Scholar] [CrossRef] [PubMed]
- Sijen, T.; Plasterk, R.H.A. Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature 2003, 426, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, R.K.; Freeling, M.; Lisch, D. Heritable transposon silencing initiated by a naturally occurring transposon inverted duplication. Nat. Genet. 2005, 37, 641–644. [Google Scholar] [CrossRef]
- Pereira, V. Insertion bias and purifying selection of retrotransposons in the Arabidopsis thaliana genome. Genome Biol. 2004, 5, R79. [Google Scholar] [CrossRef]
- Zou, S.; Ke, N.; Kim, J.M.; Voytas, D.F. The Saccharomyces retrotransposon Ty5 integrates preferentially into regions of silent chromatin at the telomeres and mating loci. Genes Dev. 1996, 10, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Dubin, M.; Fuchs, J.; Gräf, R.; Schubert, I.; Nellen, W. Dynamics of a novel centromeric histone variant CenH3 reveals the evolutionary ancestral timing of centromere biogenesis. Nucleic Acids Res. 2010, 38, 7526–7537. [Google Scholar] [CrossRef][Green Version]
- Wolfgruber, T.K.; Sharma, A.; Schneider, K.L.; Albert, P.S.; Koo, D.-H.; Shi, J.; Gao, Z.; Han, F.; Lee, H.; Xu, R.; et al. Maize Centromere Structure and Evolution: Sequence Analysis of Centromeres 2 and 5 Reveals Dynamic Loci Shaped Primarily by Retrotransposons. PLoS Genet. 2009, 5, e1000743. [Google Scholar] [CrossRef]
- Li, B.; Choulet, F.; Heng, Y.; Hao, W.; Paux, E.; Liu, Z.; Yue, W.; Jin, W.; Feuillet, C.; Zhang, X. Wheat centromeric retrotransposons: The new ones take a major role in centromeric structure. Plant J. 2013, 73, 952–965. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Vogt, A.; Mochizuki, K.; Yao, M.-C. A Domesticated piggyBac Transposase Plays Key Roles in Heterochromatin Dynamics and DNA Cleavage during Programmed DNA Deletion in Tetrahymena thermophila. Mol. Biol. Cell 2010, 21, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Huisinga, K.L.; Riddle, N.C.; Leung, W.; Shimonovich, S.; McDaniel, S.; Figueroa-Clarevega, A.; Elgin, S.C.R. Targeting of P-Element Reporters to Heterochromatic Domains by Transposable Element 1360 in Drosophila melanogaster. Genetics 2016, 202, 565–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikeda, R.; Kokubu, C.; Yusa, K.; Keng, V.W.; Horie, K.; Takeda, J. Sleeping beauty transposase has an affinity for heterochromatin conformation. Mol. Cell. Biol. 2007, 27, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Bouneau, L.; Coutanceau, J.P.; Weissenbach, J.; Volff, J.N.; Ozouf-Costaz, C. Global heterochromatic colocalization of transposable elements with minisatellites in the compact genome of the pufferfish Tetraodon nigroviridis. Gene 2004, 336, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Chavan, A.; Palladino, J.; Wei, X.; Martins, N.M.C.; Santinello, B.; Chen, C.-C.; Erceg, J.; Beliveau, B.J.; Wu, C.-T.; et al. Islands of retroelements are major components of Drosophila centromeres. PLoS Biol. 2019, 17, e3000241. [Google Scholar] [CrossRef]
- Glöckner, G.; Heidel, A.J. Centromere sequence and dynamics in Dictyostelium discoideum. Nucleic Acids Res. 2009, 37, 1809–1816. [Google Scholar] [CrossRef][Green Version]
- Logsdon, G.A.; Vollger, M.R.; Hsieh, P.; Mao, Y.; Liskovykh, M.A.; Koren, S.; Nurk, S.; Mercuri, L.; Dishuck, P.C.; Rhie, A.; et al. The structure, function and evolution of a complete human chromosome 8. Nature 2021, 593, 101–107. [Google Scholar] [CrossRef]
- Yadav, V.; Sun, S.; Billmyre, R.B.; Thimmappa, B.C.; Shea, T.; Lintner, R.; Bakkeren, G.; Cuomo, C.A.; Heitman, J.; Sanyal, K. RNAi is a critical determinant of centromere evolution in closely related fungi. Proc. Natl. Acad. Sci. USA 2018, 115, 3108. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Murata, M. A centromeric tandem repeat family originating from a part of Ty3/gypsy-retroelement in wheat and its relatives. Genetics 2003, 164, 665–672. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Jurka, J. Molecular paleontology of transposable elements in the Drosophila melanogaster genome. Proc. Natl. Acad. Sci. USA 2003, 100, 6569–6574. [Google Scholar] [CrossRef]
- Tsukahara, S.; Kawabe, A.; Kobayashi, A.; Ito, T.; Aizu, T.; Shin-i, T.; Toyoda, A.; Fujiyama, A.; Tarutani, Y.; Kakutani, T. Centromere-targeted de novo integrations of an LTR retrotransposon of Arabidopsis lyrata. Genes Dev. 2012, 26, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, E.; Launonen, V.; Müller, E.; Bachmann, L. The pvB370 BamHI satellite DNA family of the Drosophila virilis group and its evolutionary relation to mobile dispersed genetic pDv elements. J. Mol. Evol. 1995, 41, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, V.V.; Holmquist, G.P.; Jurka, J. L1 repeat is a basic unit of heterochromatin satellites in cetaceans. Mol. Biol. Evol. 1998, 15, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Jiang, N.; Wing, R.A.; Jiang, J.; Jackson, S.A. Transposons play an important role in the evolution and diversification of centromeres among closely related species. Front. Plant Sci. 2015, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, A.; Lovero, D.; D’Addabbo, P.; Caizzi, R.; Marsano, R.M. Identification of Bari Transposons in 23 Sequenced Drosophila Genomes Reveals Novel Structural Variants, MITEs and Horizontal Transfer. PLoS ONE 2016, 11, e0156014. [Google Scholar] [CrossRef] [PubMed]
- Marsano, R.M.; Milano, R.; Minervini, C.; Moschetti, R.; Caggese, C.; Barsanti, P.; Caizzi, R. Organization and possible origin of the Bari-1 cluster in the heterochromatic h39 region of Drosophila melanogaster. Genetica 2003, 117, 281–289. [Google Scholar] [CrossRef]
- McGurk, M.P.; Barbash, D.A. Double insertion of transposable elements provides a substrate for the evolution of satellite DNA. Genome Res. 2018, 28, 714–725. [Google Scholar] [CrossRef]
- Casacuberta, E. Drosophila: Retrotransposons Making up Telomeres. Viruses 2017, 9, 192. [Google Scholar] [CrossRef]
- Pardue, M.L.; DeBaryshe, P.G. Drosophila telomeres: A variation on the telomerase theme. Fly 2008, 2, 101–110. [Google Scholar] [CrossRef]
- Pardue, M.L.; Rashkova, S.; Casacuberta, E.; DeBaryshe, P.G.; George, J.A.; Traverse, K.L. Two retrotransposons maintain telomeres in Drosophila. Chromosome Res. 2005, 13, 443–453. [Google Scholar] [CrossRef]
- Villasante, A.; de Pablos, B.; Mendez-Lago, M.; Abad, J.P. Telomere maintenance in Drosophila: Rapid transposon evolution at chromosome ends. Cell Cycle 2008, 7, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, E.A.; Arkhipova, I.R. Telomere-associated endonuclease-deficient Penelope-like retroelements in diverse eukaryotes. Proc. Natl. Acad. Sci. USA 2007, 104, 9352–9357. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Nakano, M.; Ohzeki, J.-I. The role of CENP-B and α-satellite DNA: De novo assembly and epigenetic maintenance of human centromeres. Chromosome Res. 2004, 12, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Baudry, C.; Malinsky, S.; Restituito, M.; Kapusta, A.; Rosa, S.; Meyer, E.; Bétermier, M. PiggyMac, a domesticated piggyBac transposase involved in programmed genome rearrangements in the ciliate Paramecium tetraurelia. Genes Dev. 2009, 23, 2478–2483. [Google Scholar] [CrossRef] [PubMed]
- Dubois, E.; Mathy, N.; Régnier, V.; Bischerour, J.; Baudry, C.; Trouslard, R.; Bétermier, M. Multimerization properties of PiggyMac, a domesticated piggyBac transposase involved in programmed genome rearrangements. Nucleic Acids Res. 2017, 45, 3204–3216. [Google Scholar] [CrossRef] [PubMed]
- Bischerour, J.; Bhullar, S.; Denby Wilkes, C.; Régnier, V.; Mathy, N.; Dubois, E.; Singh, A.; Swart, E.; Arnaiz, O.; Sperling, L.; et al. Six domesticated PiggyBac transposases together carry out programmed DNA elimination in Paramecium. eLife 2018, 7, e37927. [Google Scholar] [CrossRef]
- Ikeda, Y.; Pélissier, T.; Bourguet, P.; Becker, C.; Pouch-Pélissier, M.-N.; Pogorelcnik, R.; Weingartner, M.; Weigel, D.; Deragon, J.-M.; Mathieu, O. Arabidopsis proteins with a transposon-related domain act in gene silencing. Nat. Commun. 2017, 8, 15122. [Google Scholar] [CrossRef]
- Bartolomé, C.; Maside, X.; Charlesworth, B. On the Abundance and Distribution of Transposable Elements in the Genome of Drosophila melanogaster. Mol. Biol. Evol. 2002, 19, 926–937. [Google Scholar] [CrossRef]
- Charlesworth, B.; Sniegowski, P.; Stephan, W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 1994, 371, 215–220. [Google Scholar] [CrossRef]
- Dimitri, P. Constitutive heterochromatin and transposable elements in Drosophila melanogaster. Genetica 1997, 100, 85–93. [Google Scholar] [CrossRef]
- Dimitri, P.; Junakovic, N. Revising the selfish DNA hypothesis: New evidence on accumulation of transposable elements in heterochromatin. Trends Genet. 1999, 15, 123–124. [Google Scholar] [CrossRef]
- Lansman, R.A.; Shade, R.O.; Grigliatti, T.A.; Brock, H.W. Evolution of P transposable elements: Sequences of Drosophila nebulosa P elements. Proc. Natl. Acad. Sci. USA 1987, 84, 6491. [Google Scholar] [CrossRef] [PubMed]
- Maside, X.; BartolomÉ, C.; Assimacopoulos, S.; Charlesworth, B. Rates of movement and distribution of transposable elements in Drosophila melanogaster: In situ hybridization vs Southern blotting data. Genet. Res. 2001, 78, 121–136. [Google Scholar] [CrossRef][Green Version]
- Rizzon, C.; Marais, G.; Gouy, M.; Biémont, C. Recombination rate and the distribution of transposable elements in the Drosophila melanogaster genome. Genome Res. 2002, 12, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.A.; Huang, S.M.; Langley, C.H.; Judd, B.H. Chromosome rearrangement by ectopic recombination in Drosophila melanogaster: Genome structure and evolution. Genetics 1991, 129, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Weil, C.F.; Wessler, S.R. Molecular evidence that chromosome breakage by Ds elements is caused by aberrant transposition. Plant Cell 1993, 5, 515–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wicker, T.; Yu, Y.; Haberer, G.; Mayer, K.F.X.; Marri, P.R.; Rounsley, S.; Chen, M.; Zuccolo, A.; Panaud, O.; Wing, R.A.; et al. DNA transposon activity is associated with increased mutation rates in genes of rice and other grasses. Nat. Commun. 2016, 7, 12790. [Google Scholar] [CrossRef]
- Montgomery, E.; Charlesworth, B.; Langley, C.H. A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster. Genet. Res. 2008, 89, 435–445. [Google Scholar] [CrossRef]
- Charlesworth, B.; Langley, C.H. The Population Genetics of Drosophila Transposable Elements. Annu. Rev. Genet. 1989, 23, 251–287. [Google Scholar] [CrossRef]
- Jensen-Seaman, M.I.; Furey, T.S.; Payseur, B.A.; Lu, Y.; Roskin, K.M.; Chen, C.-F.; Thomas, M.A.; Haussler, D.; Jacob, H.J. Comparative recombination rates in the rat, mouse, and human genomes. Genome Res. 2004, 14, 528–538. [Google Scholar] [CrossRef]
- Daron, J.; Glover, N.; Pingault, L.; Theil, S.; Jamilloux, V.; Paux, E.; Barbe, V.; Mangenot, S.; Alberti, A.; Wincker, P.; et al. Organization and evolution of transposable elements along the bread wheat chromosome 3B. Genome Biol. 2014, 15, 546. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, R.A.; Carlson, J.W.; Kennedy, C.; Acevedo, D.; Evans-Holm, M.; Frise, E.; Wan, K.H.; Park, S.; Mendez-Lago, M.; Rossi, F.; et al. Sequence finishing and mapping of Drosophila melanogaster heterochromatin. Science 2007, 316, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Marsano, R.M.; Giordano, E.; Messina, G.; Dimitri, P. A New Portrait of Constitutive Heterochromatin: Lessons from Drosophila melanogaster. Trends Genet. 2019, 35, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Crozatier, M.; Vaury, C.; Busseau, I.; Pelisson, A.; Bucheton, A. Structure and genomic organization of I elements involved in I-R hybrid dysgenesis in Drosophila melanogaster. Nucleic Acids Res. 1988, 16, 9199–9213. [Google Scholar] [CrossRef]
- Bucheton, A. I transposable elements and I-R hybrid dysgenesis in Drosophila. Trends Genet. 1990, 6, 16–21. [Google Scholar] [CrossRef]
- Orsi, G.A.; Joyce, E.F.; Couble, P.; McKim, K.S.; Loppin, B. Drosophila I-R hybrid dysgenesis is associated with catastrophic meiosis and abnormal zygote formation. J. Cell Sci. 2010, 123, 3515–3524. [Google Scholar] [CrossRef]
- Grewal, S.I.S.; Jia, S. Heterochromatin revisited. Nat. Rev. Genet. 2007, 8, 35–46. [Google Scholar] [CrossRef]
- Caizzi, R.; Moschetti, R.; Piacentini, L.; Fanti, L.; Marsano, R.M.; Dimitri, P. Comparative Genomic Analyses Provide New Insights into the Evolutionary Dynamics of Heterochromatin in Drosophila. PLoS Genet. 2016, 12, e1006212. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Wang, H. The Contributions of Transposable Elements to the Structure, Function, and Evolution of Plant Genomes. Annu. Rev. Plant Biol. 2014, 65, 505–530. [Google Scholar] [CrossRef]
- Kazazian, H.H., Jr. Genetics. L1 retrotransposons shape the mammalian genome. Science 2000, 289, 1152–1153. [Google Scholar] [CrossRef]
- Graham, T.; Boissinot, S. The genomic distribution of L1 elements: The role of insertion bias and natural selection. J. Biomed. Biotechnol. 2006, 2006, 75327. [Google Scholar] [CrossRef] [PubMed]
- Vergara, Z.; Sequeira-Mendes, J.; Morata, J.; Peiró, R.; Hénaff, E.; Costas, C.; Casacuberta, J.M.; Gutierrez, C. Retrotransposons are specified as DNA replication origins in the gene-poor regions of Arabidopsis heterochromatin. Nucleic Acids Res. 2017, 45, 8358–8368. [Google Scholar] [CrossRef] [PubMed]
- Gorinsek, B.; Gubensek, F.; Kordis, D. Evolutionary genomics of chromoviruses in eukaryotes. Mol. Biol. Evol. 2004, 21, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Brehm, A.; Tufteland, K.R.; Aasland, R.; Becker, P.B. The many colours of chromodomains. BioEssays 2004, 26, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hou, Y.; Ebina, H.; Levin, H.L.; Voytas, D.F. Chromodomains direct integration of retrotransposons to heterochromatin. Genome Res. 2008, 18, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Voytas, D.F. A single amino acid change in the yeast retrotransposon Ty5 abolishes targeting to silent chromatin. Mol. Cell 1998, 1, 1051–1055. [Google Scholar] [CrossRef]
- Xie, W.; Gai, X.; Zhu, Y.; Zappulla, D.C.; Sternglanz, R.; Voytas, D.F. Targeting of the yeast Ty5 retrotransposon to silent chromatin is mediated by interactions between integrase and Sir4p. Mol. Cell. Biol. 2001, 21, 6606–6614. [Google Scholar] [CrossRef] [PubMed]
- Cary, L.C.; Goebel, M.; Corsaro, B.G.; Wang, H.-G.; Rosen, E.; Fraser, M.J. Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 1989, 172, 156–169. [Google Scholar] [CrossRef]
- Vogt, A.; Mochizuki, K. A Domesticated PiggyBac Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in Tetrahymena. PLoS Genet. 2013, 9, e1004032. [Google Scholar] [CrossRef]
- Ivics, Z.; Hackett, P.B.; Plasterk, R.H.; Izsvak, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997, 91, 501–510. [Google Scholar] [CrossRef]
- Dasilva, C.; Hadji, H.; Ozouf-Costaz, C.; Nicaud, S.; Jaillon, O.; Weissenbach, J.; Roest Crollius, H. Remarkable compartmentalization of transposable elements and pseudogenes in the heterochromatin of the Tetraodon nigroviridis genome. Proc. Natl. Acad. Sci. USA 2002, 99, 13636–13641. [Google Scholar] [CrossRef] [PubMed]
- Creasey, K.M.; Martienssen, R.A. Germline reprogramming of heterochromatin in plants. Cold Spring Harb. Symp. Quant. Biol. 2010, 75, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Mishra, R.K. Heterochromatic hues of transcription—The diverse roles of noncoding transcripts from constitutive heterochromatin. FEBS J. 2019, 286, 4626–4641. [Google Scholar] [CrossRef] [PubMed]
- Allshire, R.C.; Madhani, H.D. Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol. 2018, 19, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Bingham, B.; Wakimoto, B.T. The organization and expression of the light gene, a heterochromatic gene of Drosophila melanogaster. Genetics 1990, 125, 129–140. [Google Scholar] [CrossRef]
- Dimitri, P.; Corradini, N.; Rossi, F.; Vernì, F.; Cenci, G.; Belloni, G.; Zhimulev, I.F.; Koryakov, D.E. Vital Genes in the Heterochromatin of Chromosomes 2 and 3 of Drosophila Melanogaster. Genetica 2003, 117, 209–215. [Google Scholar] [CrossRef]
- Carvalho, A.B.; Vibranovski, M.D.; Carlson, J.W.; Celniker, S.E.; Hoskins, R.A.; Rubin, G.M.; Sutton, G.G.; Adams, M.D.; Myers, E.W.; Clark, A.G. Y Chromosome and Other Heterochromatic Sequences of the Drosophila Melanogaster Genome: How Far can we go? Genetica 2003, 117, 227–237. [Google Scholar] [CrossRef]
- Dimitri, P.; Junakovic, N.; Arcà, B. Colonization of Heterochromatic Genes by Transposable Elements in Drosophila. Mol. Biol. Evol. 2003, 20, 503–512. [Google Scholar] [CrossRef]
- Gatti, M.; Pimpinelli, S. Cytological and genetic analysis of the Y chromosome of Drosophila melanogaster. Chromosoma 1983, 88, 349–373. [Google Scholar] [CrossRef]
- Hoskins, R.A.; Carlson, J.W.; Wan, K.H.; Park, S.; Mendez, I.; Galle, S.E.; Booth, B.W.; Pfeiffer, B.D.; George, R.A.; Svirskas, R.; et al. The Release 6 reference sequence of the Drosophila melanogaster genome. Genome Res. 2015, 25, 445–458. [Google Scholar] [CrossRef]
- Ebert, A.; Lein, S.; Schotta, G.; Reuter, G. Histone modification and the control of heterochromatic gene silencing in Drosophila. Chromosome Res. 2006, 14, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.; Pasternak, M.; El Marjou, F.; Le Baccon, P.; Probst, A.V.; Almouzni, G. Heterochromatin Reorganization during Early Mouse Development Requires a Single-Stranded Noncoding Transcript. Cell Rep. 2013, 4, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Brosius, J.; Gould, S.J. On “genomenclature”: A comprehensive (and respectful) taxonomy for pseudogenes and other “junk DNA”. Proc. Natl. Acad. Sci. USA 1992, 89, 10706–10710. [Google Scholar] [CrossRef] [PubMed]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory activities of transposable elements: From conflicts to benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. A loopy view of telomere evolution. Front. Genet. 2015, 6, 321. [Google Scholar] [CrossRef]
- Zou, S.; Kim, J.M.; Voytas, D.F. The Saccharomyces retrotransposon Ty5 influences the organization of chromosome ends. Nucleic Acids Res. 1996, 24, 4825–4831. [Google Scholar] [CrossRef]
- Gentles, A.J.; Wakefield, M.J.; Kohany, O.; Gu, W.; Batzer, M.A.; Pollock, D.D.; Jurka, J. Evolutionary dynamics of transposable elements in the short-tailed opossum Monodelphis domestica. Genome Res. 2007, 17, 992–1004. [Google Scholar] [CrossRef]
- Arkhipova, I.R.; Morrison, H.G. Three retrotransposon families in the genome of Giardia lamblia: Two telomeric, one dead. Proc. Natl. Acad. Sci. USA 2001, 98, 14497–14502. [Google Scholar] [CrossRef]
- De Felice, B.; Wilson, R.R.; Argenziano, C.; Kafantaris, I.; Conicella, C. A transcriptionally active copia-like retroelement in Citrus limon. Cell. Mol. Biol. Lett. 2009, 14, 289–304. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. What makes a centromere? Exp. Cell Res. 2020, 389, 111895. [Google Scholar] [CrossRef]
- Casola, C.; Hucks, D.; Feschotte, C. Convergent domestication of pogo-like transposases into centromere-binding proteins in fission yeast and mammals. Mol. Biol. Evol. 2008, 25, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Irelan, J.T.; Gutkin, G.I.; Clarke, L. Functional redundancies, distinct localizations and interactions among three fission yeast homologs of centromere protein-B. Genetics 2001, 157, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Fachinetti, D.; Han, J.S.; McMahon, M.A.; Ly, P.; Abdullah, A.; Wong, A.J.; Cleveland, D.W. DNA Sequence-Specific Binding of CENP-B Enhances the Fidelity of Human Centromere Function. Dev. Cell 2015, 33, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Fachinetti, D.; Folco, H.D.; Nechemia-Arbely, Y.; Valente, L.P.; Nguyen, K.; Wong, A.J.; Zhu, Q.; Holland, A.J.; Desai, A.; Jansen, L.E.T.; et al. A two-step mechanism for epigenetic specification of centromere identity and function. Nat. Cell Biol. 2013, 15, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Tudor, M.; Lobocka, M.; Goodell, M.; Pettitt, J.; O’Hare, K. The pogo transposable element family of Drosophila melanogaster. Mol. Gen. Genet. MGG 1992, 232, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.F.; Riggs, A.D. Tiggers and DNA transposon fossils in the human genome. Proc. Natl. Acad. Sci. USA 1996, 93, 1443–1448. [Google Scholar] [CrossRef]
- Mateo, L.; González, J. Pogo-like transposases have been repeatedly domesticated into CENP-B-related proteins. Genome Biol. Evol. 2014, 6, 2008–2016. [Google Scholar] [CrossRef]
- Sun, X.; Wahlstrom, J.; Karpen, G. Molecular structure of a functional Drosophila centromere. Cell 1997, 91, 1007–1019. [Google Scholar] [CrossRef]
- Blower, M.D.; Sullivan, B.A.; Karpen, G.H. Conserved organization of centromeric chromatin in flies and humans. Dev. Cell 2002, 2, 319–330. [Google Scholar] [CrossRef]
- Garavís, M.; Méndez-Lago, M.; Gabelica, V.; Whitehead, S.L.; González, C.; Villasante, A. The structure of an endogenous Drosophila centromere reveals the prevalence of tandemly repeated sequences able to form i-motifs. Sci. Rep. 2015, 5, 13307. [Google Scholar] [CrossRef]
- Niu, Y.; Teng, X.; Shi, Y.; Li, Y.; Tang, Y.; Zhang, P.; Luo, H.; Kang, Q.; Xu, T.; He, S. Genome-wide analysis of mobile element insertions in human genomes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Nurk, S.; Walenz, B.P.; Rhie, A.; Vollger, M.R.; Logsdon, G.A.; Grothe, R.; Miga, K.H.; Eichler, E.E.; Phillippy, A.M.; Koren, S. HiCanu: Accurate assembly of segmental duplications, satellites, and allelic variants from high-fidelity long reads. Genome Res. 2020, 30, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Concepcion, G.T.; Feng, X.; Zhang, H.; Li, H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat. Methods 2021, 18, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, G.A.; Vollger, M.R.; Eichler, E.E. Long-read human genome sequencing and its applications. Nat. Rev. Genet. 2020, 21, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dong, F.; Langdon, T.; Ouyang, S.; Buell, C.R.; Gu, M.; Blattner, F.R.; Jiang, J. Functional rice centromeres are marked by a satellite repeat and a centromere-specific retrotransposon. Plant Cell 2002, 14, 1691–1704. [Google Scholar] [CrossRef]
- Wu, J.; Fujisawa, M.; Tian, Z.; Yamagata, H.; Kamiya, K.; Shibata, M.; Hosokawa, S.; Ito, Y.; Hamada, M.; Katagiri, S.; et al. Comparative analysis of complete orthologous centromeres from two subspecies of rice reveals rapid variation of centromere organization and structure. Plant J. 2009, 60, 805–819. [Google Scholar] [CrossRef]
- Tower, J.; Karpen, G.H.; Craig, N.; Spradling, A.C. Preferential transposition of Drosophila P elements to nearby chromosomal sites. Genetics 1993, 133, 347–359. [Google Scholar] [CrossRef]
- Miller, W.J.; Hagemann, S.; Reiter, E.; Pinsker, W. P-element homologous sequences are tandemly repeated in the genome of Drosophila guanche. Proc. Natl. Acad. Sci. USA 1992, 89, 4018–4022. [Google Scholar] [CrossRef]
- Caizzi, R.; Caggese, C.; Pimpinelli, S. Bari-1, a new transposon-like family in Drosophila melanogaster with a unique heterochromatic organization. Genetics 1993, 133, 335–345. [Google Scholar] [CrossRef]
- Ahmed, M.; Liang, P. Transposable elements are a significant contributor to tandem repeats in the human genome. Comp. Funct. Genom. 2012, 2012, 947089. [Google Scholar] [CrossRef]
- Meštrović, N.; Mravinac, B.; Pavlek, M.; Vojvoda-Zeljko, T.; Šatović, E.; Plohl, M. Structural and functional liaisons between transposable elements and satellite DNAs. Chromosome Res. 2015, 23, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.H.C.; Chan, C.; Bachtrog, D. Establishment of H3K9me3-dependent heterochromatin during embryogenesis in Drosophila miranda. eLife 2021, 10, e55612. [Google Scholar] [CrossRef] [PubMed]
- Fabry, M.H.; Falconio, F.A.; Joud, F.; Lythgoe, E.K.; Czech, B.; Hannon, G.J. Maternally inherited piRNAs direct transient heterochromatin formation at active transposons during early Drosophila embryogenesis. eLife 2021, 10, e68573. [Google Scholar] [CrossRef] [PubMed]
- Wasserzug-Pash, P.; Rothman, R.; Reich, E.; Schonberger, O.; Weiss, Y.; Srebnik, N.; Cohen-Hadad, Y.; Weintraub, A.; Ben-Ami, I.; Holzer, H.; et al. Loss of heterochromatin and retrotransposon silencing constitute an early phase in oocyte aging. bioRxiv 2020. [Google Scholar] [CrossRef]
- Solovei, I.; Wang, A.S.; Thanisch, K.; Schmidt, C.S.; Krebs, S.; Zwerger, M.; Cohen, T.V.; Devys, D.; Foisner, R.; Peichl, L.; et al. LBR and Lamin A/C Sequentially Tether Peripheral Heterochromatin and Inversely Regulate Differentiation. Cell 2013, 152, 584–598. [Google Scholar] [CrossRef]
- Prudencio, M.; Gonzales, P.K.; Cook, C.N.; Gendron, T.F.; Daughrity, L.M.; Song, Y.; Ebbert, M.T.W.; van Blitterswijk, M.; Zhang, Y.-J.; Jansen-West, K.; et al. Repetitive element transcripts are elevated in the brain of C9orf72 ALS/FTLD patients. Hum. Mol. Genet. 2017, 26, 3421–3431. [Google Scholar] [CrossRef]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 2014, 17, 357–366. [Google Scholar] [CrossRef]
- Sun, W.; Samimi, H.; Gamez, M.; Zare, H.; Frost, B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat. Neurosci. 2018, 21, 1038–1048. [Google Scholar] [CrossRef]
- Gerlitz, G. The Emerging Roles of Heterochromatin in Cell Migration. Front. Cell Dev. Biol. 2020, 8, 394. [Google Scholar] [CrossRef]
- Andrenacci, D.; Cavaliere, V.; Lattanzi, G. The role of transposable elements activity in aging and their possible involvement in laminopathic diseases. Ageing Res. Rev. 2020, 57, 100995. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsano, R.M.; Dimitri, P. Constitutive Heterochromatin in Eukaryotic Genomes: A Mine of Transposable Elements. Cells 2022, 11, 761. https://doi.org/10.3390/cells11050761
Marsano RM, Dimitri P. Constitutive Heterochromatin in Eukaryotic Genomes: A Mine of Transposable Elements. Cells. 2022; 11(5):761. https://doi.org/10.3390/cells11050761
Chicago/Turabian StyleMarsano, René Massimiliano, and Patrizio Dimitri. 2022. "Constitutive Heterochromatin in Eukaryotic Genomes: A Mine of Transposable Elements" Cells 11, no. 5: 761. https://doi.org/10.3390/cells11050761
APA StyleMarsano, R. M., & Dimitri, P. (2022). Constitutive Heterochromatin in Eukaryotic Genomes: A Mine of Transposable Elements. Cells, 11(5), 761. https://doi.org/10.3390/cells11050761







