Singular Adult Neural Stem Cells Do Not Exist
Abstract
1. Introduction
2. Adut Neurogenic Niches
3. Neurogenic Rates in Different Niches
4. What Is a Stem Cell?
5. Stem Cell Traits in Progenitor Cells
6. A Limited Warranty of Stemness
7. Stemness as a Phenotype
8. Heterogeneity of Cell Potency
9. Stem Cell Heterogeneity from Single Cell Analyses
10. Stem Cell Identity from Single Cell Analyses
11. Regional Heterogeneity of Stem Cells
12. Heterogeneity of Fate Choice in Stem Cell Progenies
13. Temporal Heterogeneity of Stem Cells
14. Technical Influences on Stem Cell Heterogeneity
15. Regionality of Stem Cell Heterogeneity
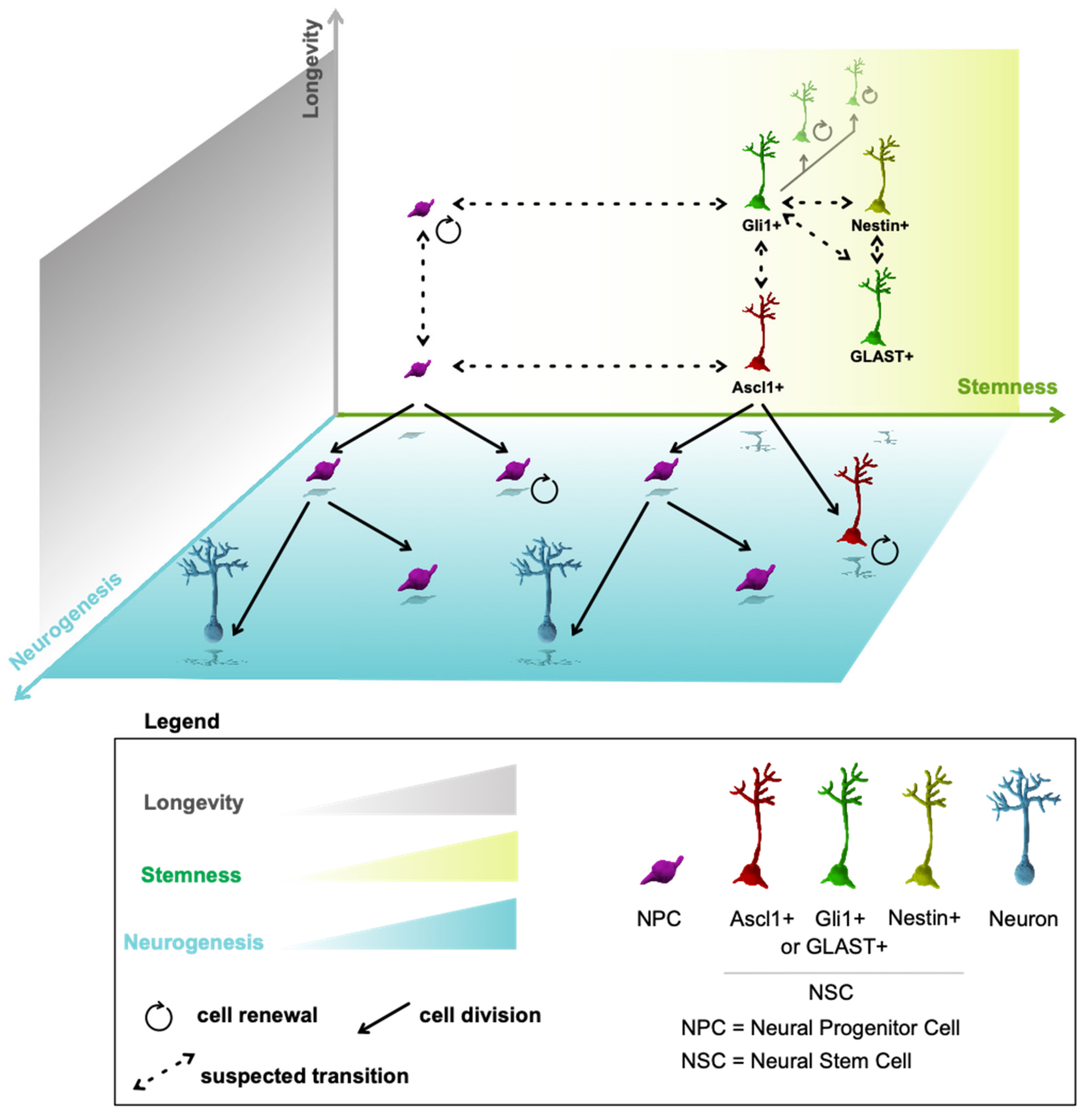
16. Multidimensional Model of Neurogenesis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gage, F.H. Adult neurogenesis in mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Yoo, S.; Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 2018, 170, 53–66. [Google Scholar] [CrossRef]
- Xu, Y.; Tamamaki, N.; Noda, T.; Kimura, K.; Itokazu, Y.; Matsumoto, N.; Dezawa, M.; Ide, C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005, 192, 251–264. [Google Scholar] [CrossRef]
- Batailler, M.; Droguerre, M.; Baroncini, M.; Fontaine, C.; Prevot, V.; Migaud, M. DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: A comparative study between mouse, sheep, and human tissues. J. Comp. Neurol. 2014, 522, 1966–1985. [Google Scholar] [CrossRef]
- Koopman, A.C.M.; Taziaux, M.; Bakker, J. Age-related changes in the morphology of tanycytes in the human female infundibular nucleus/median eminence. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef]
- Sidibe, A.; Mullier, A.; Chen, P.; Baroncini, M.; Boutin, J.A.; Delagrange, P.; Prevot, V.; Jockers, R. Expression of the orphan GPR50 protein in rodent and human dorsomedial hypothalamus, tanycytes and median eminence. J. Pineal Res. 2010, 48, 263–269. [Google Scholar] [CrossRef]
- Baroncini, M.; Allet, C.; Leroy, D.; Beauvillain, J.C.; Francke, J.P.; Prevot, V. Morphological evidence for direct interaction between gonadotrophin-releasing hormone neurones and astroglial cells in the human hypothalamus. J. Neuroendocrinol. 2007, 19, 691–702. [Google Scholar] [CrossRef]
- Pellegrino, G.; Trubert, C.; Terrien, J.; Pifferi, F.; Leroy, D.; Loyens, A.; Migaud, M.; Baroncini, M.; Maurage, C.A.; Fontaine, C.; et al. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). J. Comp. Neurol. 2018, 526, 1419–1443. [Google Scholar] [CrossRef]
- Chaker, Z.; George, C.; Petrovska, M.; Caron, J.B.; Lacube, P.; Caille, I.; Holzenberger, M. Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Neurobiol. Aging 2016, 41, 64–72. [Google Scholar] [CrossRef]
- Haan, N.; Goodman, T.; Najdi-Samiei, A.; Stratford, C.M.; Rice, R.; El Agha, E.; Bellusci, S.; Hajihosseini, M.K. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 2013, 33, 6170–6180. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.C.; Stewart, I.; McNay, D.E.; Taylor, V.; Giachino, C.; Goetz, M.; Ninkovic, J.; Briancon, N.; Maratos-Flier, E.; Flier, J.S.; et al. alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 2013, 4, 2049. [Google Scholar] [CrossRef] [PubMed]
- Saaltink, D.J.; Havik, B.; Verissimo, C.S.; Lucassen, P.J.; Vreugdenhil, E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: Implications for neurogenesis. J. Comp. Neurol. 2012, 520, 2805–2823. [Google Scholar] [CrossRef]
- Saaltink, D.J.; van Zwet, E.W.; Vreugdenhil, E. Doublecortin-Like is Implicated in Adult Hippocampal Neurogenesis and in Motivational Aspects to Escape from an Aversive Environment in Male Mice. eNeuro 2020, 7, ENEURO.0324-19.2020. [Google Scholar] [CrossRef]
- Brus, M.; Keller, M.; Levy, F. Temporal features of adult neurogenesis: Differences and similarities across mammalian species. Front. Neurosci. 2013, 7, 135. [Google Scholar] [CrossRef]
- Petrik, D.; Encinas, J.M. Perspective: Of Mice and Men—How Widespread Is Adult Neurogenesis? Front. Neurosci. 2019, 13, 923. [Google Scholar] [CrossRef]
- Kornack, D.R.; Rakic, P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. USA 1999, 96, 5768–5773. [Google Scholar] [CrossRef]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Bostrom, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef]
- Ninkovic, J.; Mori, T.; Gotz, M. Distinct modes of neuron addition in adult mouse neurogenesis. J. Neurosci. 2007, 27, 10906–10911. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.F.; Malva, J.O.; Crespo-Lopez, M.E. Adult Hippocampal Neurogenesis in Different Taxonomic Groups: Possible Functional Similarities and Striking Controversies. Cells 2019, 8, 125. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Imayoshi, I.; Sakamoto, M.; Ohtsuka, T.; Kageyama, R. Continuous neurogenesis in the adult brain. Dev. Growth Differ. 2009, 51, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Migaud, M.; Batailler, M.; Segura, S.; Duittoz, A.; Franceschini, I.; Pillon, D. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones. Eur. J. Neurosci. 2010, 32, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- McNay, D.E.; Briancon, N.; Kokoeva, M.V.; Maratos-Flier, E.; Flier, J.S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Investig. 2012, 122, 142–152. [Google Scholar] [CrossRef]
- Cameron, H.A.; McKay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Jabes, A.; Lavenex, P.B.; Amaral, D.G.; Lavenex, P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur. J. Neurosci. 2010, 31, 273–285. [Google Scholar] [CrossRef]
- Till, J.E.; Mc, C.E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef]
- Becker, A.J.; Mc, C.E.; Till, J.E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963, 197, 452–454. [Google Scholar] [CrossRef]
- Pilz, G.A.; Bottes, S.; Betizeau, M.; Jorg, D.J.; Carta, S.; Simons, B.D.; Helmchen, F.; Jessberger, S. Live imaging of neurogenesis in the adult mouse hippocampus. Science 2018, 359, 658–662. [Google Scholar] [CrossRef]
- Bottes, S.; Jaeger, B.N.; Pilz, G.A.; Jorg, D.J.; Cole, J.D.; Kruse, M.; Harris, L.; Korobeynyk, V.I.; Mallona, I.; Helmchen, F.; et al. Long-term self-renewing stem cells in the adult mouse hippocampus identified by intravital imaging. Nat. Neurosci. 2021, 24, 225–233. [Google Scholar] [CrossRef]
- Ibrayeva, A.; Bay, M.; Pu, E.; Jorg, D.J.; Peng, L.; Jun, H.; Zhang, N.; Aaron, D.; Lin, C.; Resler, G.; et al. Early stem cell aging in the mature brain. Cell Stem Cell 2021, 28, 955–966.e957. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 1990, 301, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Krish, V.S.; Kirshenbaum, G.S.; Atsak, P.; Lass, T.J.; Lieberman, S.R.; Leonardo, E.D.; Dranovsky, A. Ablation of proliferating neural stem cells during early life is sufficient to reduce adult hippocampal neurogenesis. Hippocampus 2018, 28, 586–601. [Google Scholar] [CrossRef]
- Berg, D.A.; Su, Y.; Jimenez-Cyrus, D.; Patel, A.; Huang, N.; Morizet, D.; Lee, S.; Shah, R.; Ringeling, F.R.; Jain, R.; et al. A Common Embryonic Origin of Stem Cells Drives Developmental and Adult Neurogenesis. Cell 2019, 177, 654–668.e615. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, P.; Hartfuss, E.; Gotz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 2000, 127, 5253–5263. [Google Scholar] [CrossRef]
- Merkle, F.T.; Tramontin, A.D.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 2004, 101, 17528–17532. [Google Scholar] [CrossRef]
- Fuentealba, L.C.; Rompani, S.B.; Parraguez, J.I.; Obernier, K.; Romero, R.; Cepko, C.L.; Alvarez-Buylla, A. Embryonic Origin of Postnatal Neural Stem Cells. Cell 2015, 161, 1644–1655. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Kusne, Y.; Duran-Moreno, M.; Cabrales, E.; Gil-Perotin, S.; Ortiz, C.; Chen, B.; Garcia-Verdugo, J.M.; Sanai, N.; Alvarez-Buylla, A. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat. Commun. 2017, 8, 13759. [Google Scholar] [CrossRef]
- Placzek, M.; Briscoe, J. The floor plate: Multiple cells, multiple signals. Nat. Rev. Neurosci. 2005, 6, 230–240. [Google Scholar] [CrossRef]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef]
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.L.; Song, H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011, 145, 1142–1155. [Google Scholar] [CrossRef]
- Kempermann, G. The pessimist’s and optimist’s views of adult neurogenesis. Cell 2011, 145, 1009–1011. [Google Scholar] [CrossRef][Green Version]
- DeCarolis, N.A.; Mechanic, M.; Petrik, D.; Carlton, A.; Ables, J.L.; Malhotra, S.; Bachoo, R.; Gotz, M.; Lagace, D.C.; Eisch, A.J. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus 2013, 23, 708–719. [Google Scholar] [CrossRef]
- Seaberg, R.M.; van der Kooy, D. Stem and progenitor cells: The premature desertion of rigorous definitions. Trends Neurosci. 2003, 26, 125–131. [Google Scholar] [CrossRef]
- Slack, J.M.W. What is a stem cell? Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e323. [Google Scholar] [CrossRef]
- Knobloch, M.; Braun, S.M.; Zurkirchen, L.; von Schoultz, C.; Zamboni, N.; Arauzo-Bravo, M.J.; Kovacs, W.J.; Karalay, O.; Suter, U.; Machado, R.A.; et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 2013, 493, 226–230. [Google Scholar] [CrossRef]
- Borrell, V. Recent advances in understanding neocortical development. F1000Res 2019, 8, 1791. [Google Scholar] [CrossRef]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef]
- Siminovitch, L.; McCulloch, E.A.; Till, J.E. The Distribution of Colony-Forming Cells among Spleen Colonies. J. Cell Comp. Physiol. 1963, 62, 327–336. [Google Scholar] [CrossRef]
- Wilson, A.; Laurenti, E.; Oser, G.; van der Wath, R.C.; Blanco-Bose, W.; Jaworski, M.; Offner, S.; Dunant, C.F.; Eshkind, L.; Bockamp, E.; et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 2008, 135, 1118–1129. [Google Scholar] [CrossRef]
- Reeve, R.L.; Yammine, S.Z.; Morshead, C.M.; van der Kooy, D. Quiescent Oct4(+) Neural Stem Cells (NSCs) Repopulate Ablated Glial Fibrillary Acidic Protein(+) NSCs in the Adult Mouse Brain. Stem Cells 2017, 35, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008, 31, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gotz, M.; Sirko, S.; Beckers, J.; Irmler, M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia 2015, 63, 1452–1468. [Google Scholar] [CrossRef] [PubMed]
- Trinchero, M.F.; Giacomini, D.; Schinder, A.F. Dynamic interplay between GABAergic networks and developing neurons in the adult hippocampus. Curr. Opin. Neurobiol. 2021, 69, 124–130. [Google Scholar] [CrossRef]
- Gupta, B.; Errington, A.C.; Jimenez-Pascual, A.; Eftychidis, V.; Brabletz, S.; Stemmler, M.P.; Brabletz, T.; Petrik, D.; Siebzehnrubl, F.A. The transcription factor ZEB1 regulates stem cell self-renewal and cell fate in the adult hippocampus. Cell Rep. 2021, 36, 109588. [Google Scholar] [CrossRef]
- Merkle, F.T.; Fuentealba, L.C.; Sanders, T.A.; Magno, L.; Kessaris, N.; Alvarez-Buylla, A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 2014, 17, 207–214. [Google Scholar] [CrossRef]
- Ninkovic, J.; Steiner-Mezzadri, A.; Jawerka, M.; Akinci, U.; Masserdotti, G.; Petricca, S.; Fischer, J.; von Holst, A.; Beckers, J.; Lie, C.D.; et al. The BAF complex interacts with Pax6 in adult neural progenitors to establish a neurogenic cross-regulatory transcriptional network. Cell Stem Cell 2013, 13, 403–418. [Google Scholar] [CrossRef]
- Delgado, A.C.; Maldonado-Soto, A.R.; Silva-Vargas, V.; Mizrak, D.; von Kanel, T.; Tan, K.R.; Paul, A.; Madar, A.; Cuervo, H.; Kitajewski, J.; et al. Release of stem cells from quiescence reveals gliogenic domains in the adult mouse brain. Science 2021, 372, 1205–1209. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, J.; Lyu, P.; Hoang, T.V.; Ma, A.; Trinh, V.; Dai, W.; Jiang, L.; Leavey, P.; Duncan, L.; et al. Control of neurogenic competence in mammalian hypothalamic tanycytes. Sci. Adv. 2021, 7, eabg3777. [Google Scholar] [CrossRef]
- Mizrak, D.; Levitin, H.M.; Delgado, A.C.; Crotet, V.; Yuan, J.; Chaker, Z.; Silva-Vargas, V.; Sims, P.A.; Doetsch, F. Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages. Cell Rep. 2019, 26, 394–406.e395. [Google Scholar] [CrossRef]
- Cebrian-Silla, A.; Nascimento, M.A.; Redmond, S.A.; Mansky, B.; Wu, D.; Obernier, K.; Romero Rodriguez, R.; Gonzalez-Granero, S.; Garcia-Verdugo, J.M.; Lim, D.A.; et al. Single-cell analysis of the ventricular-subventricular zone reveals signatures of dorsal and ventral adult neurogenesis. Elife 2021, 10, e67436. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Berg, D.A.; Zhu, Y.; Shin, J.Y.; Song, J.; Bonaguidi, M.A.; Enikolopov, G.; Nauen, D.W.; Christian, K.M.; Ming, G.L.; et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell 2015, 17, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Artegiani, B.; Lyubimova, A.; Muraro, M.; van Es, J.H.; van Oudenaarden, A.; Clevers, H. A Single-Cell RNA Sequencing Study Reveals Cellular and Molecular Dynamics of the Hippocampal Neurogenic Niche. Cell Rep. 2017, 21, 3271–3284. [Google Scholar] [CrossRef] [PubMed]
- Hochgerner, H.; Zeisel, A.; Lonnerberg, P.; Linnarsson, S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 2018, 21, 290–299. [Google Scholar] [CrossRef]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Dulken, B.W.; Leeman, D.S.; Boutet, S.C.; Hebestreit, K.; Brunet, A. Single-Cell Transcriptomic Analysis Defines Heterogeneity and Transcriptional Dynamics in the Adult Neural Stem Cell Lineage. Cell Rep. 2017, 18, 777–790. [Google Scholar] [CrossRef]
- Xie, X.P.; Laks, D.R.; Sun, D.; Poran, A.; Laughney, A.M.; Wang, Z.; Sam, J.; Belenguer, G.; Farinas, I.; Elemento, O.; et al. High-resolution mouse subventricular zone stem-cell niche transcriptome reveals features of lineage, anatomy, and aging. Proc. Natl. Acad. Sci. USA 2020, 117, 31448–31458. [Google Scholar] [CrossRef]
- Mizrak, D.; Bayin, N.S.; Yuan, J.; Liu, Z.; Suciu, R.M.; Niphakis, M.J.; Ngo, N.; Lum, K.M.; Cravatt, B.F.; Joyner, A.L.; et al. Single-Cell Profiling and SCOPE-Seq Reveal Lineage Dynamics of Adult Ventricular-Subventricular Zone Neurogenesis and NOTUM as a Key Regulator. Cell Rep. 2020, 31, 107805. [Google Scholar] [CrossRef]
- Yuzwa, S.A.; Borrett, M.J.; Innes, B.T.; Voronova, A.; Ketela, T.; Kaplan, D.R.; Bader, G.D.; Miller, F.D. Developmental Emergence of Adult Neural Stem Cells as Revealed by Single-Cell Transcriptional Profiling. Cell Rep. 2017, 21, 3970–3986. [Google Scholar] [CrossRef]
- Seri, B.; Garcia-Verdugo, J.M.; McEwen, B.S.; Alvarez-Buylla, A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001, 21, 7153–7160. [Google Scholar] [CrossRef]
- Egeland, M.; Guinaudie, C.; Du Preez, A.; Musaelyan, K.; Zunszain, P.A.; Fernandes, C.; Pariante, C.M.; Thuret, S. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl. Psychiatry 2017, 7, e1101. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Mizumatsu, S.; Fike, J.R.; Palmer, T.D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002, 8, 955–962. [Google Scholar] [CrossRef]
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Chen, X.; Tolkovsky, A.M.; Herbert, J. Cell origin and culture history determine successful integration of neural precursor transplants into the dentate gyrus of the adult rat. PLoS ONE 2011, 6, e17072. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Theise, N.D.; Collector, M.I.; Henegariu, O.; Hwang, S.; Gardner, R.; Neutzel, S.; Sharkis, S.J. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001, 105, 369–377. [Google Scholar] [CrossRef]
- Buenrostro, J.D.; Corces, M.R.; Lareau, C.A.; Wu, B.; Schep, A.N.; Aryee, M.J.; Majeti, R.; Chang, H.Y.; Greenleaf, W.J. Integrated Single-Cell Analysis Maps the Continuous Regulatory Landscape of Human Hematopoietic Differentiation. Cell 2018, 173, 1535–1548.e1516. [Google Scholar] [CrossRef]
- Calzolari, F.; Michel, J.; Baumgart, E.V.; Theis, F.; Gotz, M.; Ninkovic, J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 2015, 18, 490–492. [Google Scholar] [CrossRef]
- Bast, L.; Calzolari, F.; Strasser, M.K.; Hasenauer, J.; Theis, F.J.; Ninkovic, J.; Marr, C. Increasing Neural Stem Cell Division Asymmetry and Quiescence Are Predicted to Contribute to the Age-Related Decline in Neurogenesis. Cell Rep. 2018, 25, 3231–3240.e3238. [Google Scholar] [CrossRef]
- Basak, O.; Krieger, T.G.; Muraro, M.J.; Wiebrands, K.; Stange, D.E.; Frias-Aldeguer, J.; Rivron, N.C.; van de Wetering, M.; van Es, J.H.; van Oudenaarden, A.; et al. Troy+ brain stem cells cycle through quiescence and regulate their number by sensing niche occupancy. Proc. Natl. Acad. Sci. USA 2018, 115, E610–E619. [Google Scholar] [CrossRef]
- Wang, D.Y.; Luo, A.F.; Bai, Q.R.; Gong, X.L.; Zheng, Y.; Shen, Q.; Hu, X.L.; Wang, X.M. VCAM1 Labels a Subpopulation of Neural Stem Cells in the Adult Hippocampus and Contributes to Spatial Memory. Stem Cell Rep. 2020, 14, 1093–1106. [Google Scholar] [CrossRef]
- Lagace, D.C.; Whitman, M.C.; Noonan, M.A.; Ables, J.L.; DeCarolis, N.A.; Arguello, A.A.; Donovan, M.H.; Fischer, S.J.; Farnbauch, L.A.; Beech, R.D.; et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 2007, 27, 12623–12629. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Rigo, P.; Stiehl, T.; Gaber, Z.B.; Austin, S.H.L.; Masdeu, M.D.M.; Edwards, A.; Urban, N.; Marciniak-Czochra, A.; Guillemot, F. Coordinated changes in cellular behavior ensure the lifelong maintenance of the hippocampal stem cell population. Cell Stem Cell 2021, 28, 863–876.e866. [Google Scholar] [CrossRef]
- Kim, E.J.; Ables, J.L.; Dickel, L.K.; Eisch, A.J.; Johnson, J.E. Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS ONE 2011, 6, e18472. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Leung, C.T.; Reed, R.R.; Johnson, J.E. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J. Neurosci. 2007, 27, 12764–12774. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hebert, J.M. A Sox2 BAC transgenic approach for targeting adult neural stem cells. PLoS ONE 2012, 7, e49038. [Google Scholar] [CrossRef]
- Venere, M.; Han, Y.G.; Bell, R.; Song, J.S.; Alvarez-Buylla, A.; Blelloch, R. Sox1 marks an activated neural stem/progenitor cell in the hippocampus. Development 2012, 139, 3938–3949. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Takeda, N.; Jain, R.; Manderfield, L.J.; Liu, F.; Li, L.; Anderson, S.A.; Epstein, J.A. Hopx distinguishes hippocampal from lateral ventricle neural stem cells. Stem Cell Res. 2015, 15, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Lugert, S.; Vogt, M.; Tchorz, J.S.; Muller, M.; Giachino, C.; Taylor, V. Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat. Commun. 2012, 3, 670. [Google Scholar] [CrossRef]
- Giachino, C.; Basak, O.; Lugert, S.; Knuckles, P.; Obernier, K.; Fiorelli, R.; Frank, S.; Raineteau, O.; Alvarez-Buylla, A.; Taylor, V. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells 2014, 32, 70–84. [Google Scholar] [CrossRef]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef]
- Sirko, S.; Behrendt, G.; Johansson, P.A.; Tripathi, P.; Costa, M.; Bek, S.; Heinrich, C.; Tiedt, S.; Colak, D.; Dichgans, M.; et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected]. Cell Stem Cell 2013, 12, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Faiz, M.; Sachewsky, N.; Gascon, S.; Bang, K.W.; Morshead, C.M.; Nagy, A. Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke. Cell Stem Cell 2015, 17, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; He, Y.; Park, J.S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015, 21, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014, 344, 630–634. [Google Scholar] [CrossRef] [PubMed]
- White, C.W., 3rd; Fan, X.; Maynard, J.C.; Wheatley, E.G.; Bieri, G.; Couthouis, J.; Burlingame, A.L.; Villeda, S.A. Age-related loss of neural stem cell O-GlcNAc promotes a glial fate switch through STAT3 activation. Proc. Natl. Acad. Sci. USA 2020, 117, 22214–22224. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Tanaka, K.; Buffo, A.; Wurst, W.; Kuhn, R.; Gotz, M. Inducible gene deletion in astroglia and radial glia--a valuable tool for functional and lineage analysis. Glia 2006, 54, 21–34. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Cai, D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 2012, 14, 999–1012. [Google Scholar] [CrossRef]
- Pak, T.; Yoo, S.; Miranda-Angulo, A.L.; Wang, H.; Blackshaw, S. Rax-CreERT2 knock-in mice: A tool for selective and conditional gene deletion in progenitor cells and radial glia of the retina and hypothalamus. PLoS ONE 2014, 9, e90381. [Google Scholar] [CrossRef] [PubMed]
- Denoth-Lippuner, A.; Jaeger, B.N.; Liang, T.; Royall, L.N.; Chie, S.E.; Buthey, K.; Machado, D.; Korobeynyk, V.I.; Kruse, M.; Munz, C.M.; et al. Visualization of individual cell division history in complex tissues using iCOUNT. Cell Stem Cell 2021, 28, 2020–2034.e12. [Google Scholar] [CrossRef]
- Stapornwongkul, K.S.; Vincent, J.P. Generation of extracellular morphogen gradients: The case for diffusion. Nat. Rev. Genet. 2021, 22, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R.; Zhang, C.L.; Lie, D.C. Transcription-Factor-Dependent Control of Adult Hippocampal Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a018879. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J. The Jackson Lab: B6;129S4-Eomestm1.1(cre/ERT2)Zjh/J. JAX Depository. 2021. Available online: https://www.jax.org/strain/036301 (accessed on 30 November 2021).
- Gebara, E.; Bonaguidi, M.A.; Beckervordersandforth, R.; Sultan, S.; Udry, F.; Gijs, P.J.; Lie, D.C.; Ming, G.L.; Song, H.; Toni, N. Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells 2016, 34, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Villalba, A.; Gotz, M.; Borrell, V. The regulation of cortical neurogenesis. Curr. Top. Dev. Biol. 2021, 142, 1–66. [Google Scholar] [CrossRef]
| Reference | Region | Isolation and Sequencing | aNSC Hetero-Geneity | Notes |
|---|---|---|---|---|
| [63] | DG | Microdissection; negative selection (GluR1-, Cd24-); SORT-seq | quiescent v activated | Populations of quiescent and activated NSCs could be defined, but no other heterogeneity |
| [30] | DG | Intravital imaging, Microdissection, Gli1/Ascl1-CreERT2; TdTomato, Smart-seq2 | quiescent v activated | Transcriptional differences partly overlapping amongst two subpopulations of NSCs (quiescent/activated) with a differential self-renewal capacity. |
| [62] | DG | Microdissection, Nestin-CFPnuc, SMART-seq | quiescent v activated | Identified two super-groups with six subgroups of NSC immediate progeny, corresponding to quiescent/activated states. |
| [64] | DG from embryonic and adult between E16.5 to P132 | Microdissection, positive selection (hGFAP-GFP+), Fluidigm C1, 10X Chromium V1/2, Illumina HiSeq2000/2500/4000 | developmental; lineage; young v aged | Single cell profiling of cell types in DG across prenatal, juvenile and adult. Neuronal intermediate progenitors (nIPCs), neuroblasts and immature granule cells did not form separate clusters in the transition from perinatal to adult, but radial glia molecularly switch at P5 |
| [65] | DG | Whole hippocampus dissection, positive selection (ACSA-2+), modified SMART-Seq2, Illumina NextSeq 500 | regional | Astrocyte clustering into 5 subgroups reveals intra- and inter-regional heterogeneity. Two distinct clusters are defined, one cluster spatially mapped to most GLAST+ cells in SGZ and an intermediate GLAST+ progenitor population mapped in subpial, stratum lacunosum moleculare, and DG |
| [66] | SVZ | Microdissection, positive and negative selection (Gfap-Gfp+, Prom1+, Egfr+/−, Cd31−, Cd24-, Cd45−); Fluidigm C1 Single-Cell Auto Prep chip and SMARTer-seq | quiescent v activated | Three NSC subpopulations across a spectrum of activation/differentiation states. Identified rare intermediate states with unique molecular fingerprints. |
| [67] | SVZ | Microdissection, Nestin-CreERT2 Histone H2B-Gfp, Diphtheria toxin receptor, positive and negative selection Glast+, Cd133+, Cd45− Cd95 | quiescent v activated; young v aged | Analysis of NSCs from infancy to old age to identifies transition from quiescence to proliferation and uncovers NSC heterogeneity. |
| [61] | SVZ | Microdissection; hGFAP-GFP; 10X Chromium V3 | regional | Two populations of NSPCs in dorsal vs. ventral V-SVZ are transcriptionally distinguishable. |
| [60] | SVZ Lateral v Septal walls | Microdissection, hGFAP::CreERT2; R26RCAG-tdTomato, Microwell and DROP-seq | regional; male v female | Regional and sex differences between lateral and septal wall NSCs. Distinct spatiotemporal TF expression profiles of dormancy and lineage progression across neurogenesis and oligodendrogenesis. |
| [68] | SVZ OB | Microdissection GFAP-CreERT2 Nestin-FlpER, Microwell/DROP-seq and SCOPE-seq | lineage | Heterogeneous qNSCs with distinct OB interneuron and astrocyte lineages. Identified novel V-SVZ proliferation marker in a transitory intermediate NSC population. |
| [69] | Embryonic cortex from 4 developmental timepoints between E11.5 to E17.5 | Microdissection, CD1 mice, DROP-seq, and FISH and immunostaining Of adult V-SVZ | developmental time | Identification of embryonic cortical radial precursors with distinct transcriptional identity which is maintained through their transition to quiescence. A distinct E17.5 radial precursor population transcriptionally similar to adult V-SVZ qNSCs. |
| Driver | Reference | Mouse Line | Region | Quiescence | Active | Exhaustive (Short Term Maintenance) | Maintenance (Long Term Maintenance) | Self-Renewal | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Nestin | [41] | Nestin-CreERT2: Z/EG | SGZ | ↑↓ | ↓↑ | ↓ Assumed–activated RGLs maintained at 12 months | ↑ (12 mo) * | ↑ | Reporter-positive radial glia-like cells displayed both self-renewal properties and multipotent differentiation at 2 mpi. Radial glial like cells can alternate between an active and quiescent state. * Maintenance of some activated radial glia-like cells up to 1 year. |
| Nestin-CreERT2: MADM | ↑↓ | ↓↑ | ↓ Assumed—activated RGLs maintained at 12 months | ↑ (12 mo) | ↑ | Frequencies of all types of clones (quiescent, symmetrically self-renewed, asymmetrically self-renewed, and differentiated) were comparable between the Z/EG and MADM reporters. However, the MADM reporter allowed for a more rigorous clonal analysis of quiescent radial glia-like cells. | |||
| [31] | Nestin-CreERT2: Confetti | SGZ | ↑ (4 mpi in 6 mo old mice) “by calculating the time to cell-cycle entry and re-entry according to power-law decay fitting of clonal tracings” | ↑ (Slow) | ↓ | ↑ (4 mpi in 12 mo old mice) | ↑ | Nestin-NSCs are longer lived and slowly generate new neurons, astrocytes and NSCs. Nestin-NSCs prolong their quiescence with each division and switch to symmetric cell fate choice after NSC homeostasis has been lost in mice around 4–6 mo of age. | |
| Ascl1-CreERT2 | n.d. | ↑ (Fast) | ↑ | ↓ (6 mo) | ↓ * | Ascl1-NSCs demonstrated short term stem cell maintenance for approximately 1 week followed by rapid initial depletion that slowed with time. * No significant expansion (symmetric self-renewal) over time was observed in the Ascl1- NSC population. | |||
| GLAST | [77] | GLAST- CreERT2: Confetti | SVZ | ↓ | ↑ | ↑ | ↓ (4–6 mo) | n.d. Suggested limited self-renewal | The NSC population underwent multiple rounds of division in a short time span, generating progeny before becoming exhausted. While other previously quiescent NSCs becomes activated to counteract the decline in adult neurogenesis. |
| [78] | GLAST-CreERT2: Confetti | SVZ | n.d. Not determined in this mouse model | ↑ | ↑ | ↓ (56 d) | n.d. Not determined in this mouse model | By 21 dpi, most clones consisted of progenitor cells or progenitor cells and neurons. By 56 dpi, the proportion of clones comprised by only neurons had increased. These clones were rarely found in the same hemisphere as a radial astrocyte, indicating NSC exhaustion to be the major terminating mechanism of OB neurogenesis. | |
| [55] | GLAST-CreERT2 | SGZ | ↔ * | ↓ ** | ↑ | ↓ (4 w) | ↓ | * Depletion of Zeb1 does not directly alter the quiescent population. ** Active clones containing radial glia-like cell and non-radial glia-like cell progenies were significantly reduced, while depleted clones containing only differentiated progeny were significantly increased. | |
| Troy | [79] | TroyGFPiresCreER | SVZ | ↔ * | ↑↓ Active NSCs can return to quiescence after one or more rounds of cell division | ↓ | ↔ (32 w) | ↑ ** | From 14 dpi, and in subsequent timed points, both the density of NSC retaining clones and their stem cell content remained stable. * Most clones consisted of a single qNSC through all time points. ** At early time points, clones consisted of multiple Troy+ cells. Suggesting symmetric division upon activation. |
| Ki67iresCreER | SVZ | ↔ | ↑↓ * | ↓ | ↑ (1 y) | ↑ | * Majority of active NSCs exit the cell cycle quickly, however some expand before returning to quiescence (qNSCs). These qNSCs may remain long-term to later contribute to ongoing adult neurogenesis. | ||
| VCAM1 | [80] | VP lentivirus injection in Ai14 mice | SGZ | ↔ | ↑ (Slow) | ↓ | ↔ (28 d) * | ↓ | * Reporter-positive cells exhibited slow proliferation with some VCAM1-expressing NSCs remaining quiescent. |
| Hopx | [34] | Hopx-CreERT2 | SGZ | ↑ | ↑ * | ↓ * | ↑ (12 mo) | ↑ | Reporter-positive radial glia-like cells were quiescent neural progenitors with some capacity to self-renew. Notably, these qNSCs retain the capacity to re-enter the cell cycle up to a year post induction. * At 4 mpi, there was a large shift toward clones consisting of only mature neurons, indicating that some radial glia-like cells were depleted. |
| Driver | Reference | Mouse Line | Region | Quiescence | Active | Exhaustive (Short Term Maintenance) | Maintenance (Long Term Maintenance) | Self-Renewal | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Nestin | [81] | Nestin-CreERT2 | SGZ | n.d. | ↑ | ↓ * | ↔ (100 d) | n.d. | Stem-like recombined cells with radial glia morphology was present in the SGZ up to 100 dpi * 50% of YFP+ cells expressed NeuN by 65 d and plateaued over subsequent time points. |
| [40] | Nestin-CreER | SGZ | ↔ | ↑ | ↓ * | ↔ (45 d) | n.d. | Production of mature astrocytes detected after 20 d. * The fraction of labelled quiescent NPs, new astrocytes, and newly generated neurons remained constant over all time points (45 d). | |
| GLAST | [19] | GLAST-CreERT2 | SGZ | n.d. | ↑ (4 mo) | ↓ * | ↔ (9 mo) * | n.d. | Reporter-positive mature neurons reached a plateau after 4 mo in the DG (also observed in the GCL of the OB). * Reporter-positive slow-dividing stem cells remained stable over months. |
| SVZ | n.d. | ↑ | n.d. | n.d. | n.d. | The proportion of neurons in the GL of the OB increases linearly due to the net addition of inhibitory interneurons. | |||
| [82] | GLAST- CreERT2 | SGZ | n.d. Quiescence established not in this mouse line | ↑ 1 to 3 self—renewing div. | ↓ * Assumed non exhaustive as 28% of NSCs self-renew | ↑ (30 d) ** | ↑ | * 28% of stem cells underwent 3 or more self-renewing divisions before losing their stem cell identity in adults compared to 12% in juvenile mice. ** Increased self-renewal in adult mice is a mechanism contributing to preserving the NSC pool. | |
| Ki67-CreERT2 | ↑ (5 d) | n.d. | n.d. | n.d. | n.d. | In 1-month old mice NSCs remain proliferating (Ki67+). In contrast, in 6-month-old animals a significant proportion of NSCs returns to quiescence (Ki67-). | |||
| [43] | GLAST-CreERT2 | SGZ | n.d. | ↑ | ↑ | ↓ (180 d) | n.d. | At 180 d post induction (dpi), many reporter-positive cells matured into neurons with a corresponding decrease in proportion of radial glia-like cells. | |
| Nestin-CreERT2 | n.d. | ↑ | ↓ | ↔ (180 d) | n.d. | There was an initial surge of reporter-positive cells through 30 dpi, which was followed by a plateau at later time points. Most reporter-positive cells were early progenitors at 12–60 dpi. At 180 dpi, cells were almost exclusively neurons or radial glia-like cells. | |||
| [55] | GLAST- CreERT2 | SGZ | ↓ | ↑ | ↑ | ↓ (12 w) | ↓ | Steady decline of activated radial glia-like cells lead to the continuous recruitment of quiescent radial glia-like cells. In turn, resulting in exhaustion of the cell pool. | |
| NG2 | [12] | NG2-CreER | HVZ | n.d. | ↑ | ↓ | ↔ (60 d) | ↑ * | The absolute number of reporter positive NG2 glia remained constant up to 60 dpi. However, by day 60 the proportion of oligodendrocytes increased while the NG2 positive glia decreased. * The absolute number of NG2 glia remained constant between 7 d-60 dpi, indicating that the rate of cell death or differentiation was roughly the same, as they were generated by self-renewing divisions. |
| Fgf10 | [11] | Fgf10—CreERT2 | HVZ | n.d. | n.d. | ↑ Number of Xgal+ tanycyes drops in adult | ↓ (83 d) Number of Xgal+ tanycytes drops in adult | n.d. | The total number of reporter-positive cells found in adult mice showed a small but nonsignificant drop at 39–83 d compared to 24–27 d. |
| Ascl1 | [29] | Ascl1-tdTomato | SGZ | ↓ | ↑ | ↑ | ↓ (2 mo) | ↓ “self-renewal capacity of Ascl1-targeted R cells is temporally limited” | By implanting a cortical window that allowed for 2-photon imaging, it was shown that, once activated, Ascl1-targeted radial glia-like progenitor cells generateA a burst of neurogenic activity to then commit to differentiation and loss. These cells did not re-enter long term quiescence. |
| [83] | Ascl1-CreERT2 | SGZ | ↔ * No change in Sox2+ cells | ↓ By 180 d | ↓ Number of Sox2+ cells (180 vs. 30 d) is maintained and NeuN+ cells increase | ↔ (180 d) * | ↑ | At 180 dpi, 65% of reporter-positive cells were NeuN positive granule neurons. However, 25% of reporter-positive cells also expressed markers of progenitor cells. *No obvious loss of Sox2+ cells indicate labeling of quiescent Type-1 cells. | |
| SVZ | n.d. | ↑ | ↓ Sox2+ cells remained in the SVZ at 180 d | ↑ (180 d) * | ↑ | At 30 dpi, many reporter-positive cells in the OB co-expressed NeuN, demonstrating that labelled cells are migrating and maturing. * Reporter-positive cells still remained in the SVZ and expressed Sox2, DCX or Ki67 up to 180 d after induction. | |||
| [84] | Ascl1-CreERTM | SGZ | n.d. | ↑ | ↑ | ↓ (180 d) | ↓ | 30 d after induction, 86% of reporter-positive cells were mature neurons. This increased to 98% after 6 mo. Ascl1+ cells were mostly identified as Type 2a progenitor cells, but also a subset of stem cells with limited self-renewal potential. | |
| Sox | [85] | Sox2-CreER | SVZ, SGZ | n.d. | ↑ | ↓ | ↑ (4 mo) | ↑ | Reporter-positive cells with morphological characteristics of radial glia stem cells remained abundant in both brain regions up to 4 mo after induction. |
| [86] | Sox1-tTA; LC-1; R26eYFP | SGZ | ↓↑ * | ↑↓ * Some NSCs diff., some remain NSCs | ↓ Some Sox1+ remain NSCs | ↑ (18 w) | n.d. | * A continuous, long term (3 mo) production of progenitors and NBs from Sox1+ cells is consistent with a stem cell population with long term neurogenic potential that alternate between an activated and a quiescent state. However, the decline of Sox1+ radial astrocytes after a 12-week chase period indicates that some cells permanently exit the stem cell pool. | |
| Hopx | [87] | HopxCreER/+ | SGZ | n.d. Not determined in this mouse model | ↑ | ↓ * | ↑ (2 mo) * | n.d. | At 2 mpi, many reporter-positive cells differentiate into granule neurons and the proportion of NSCs declined. * At 2 mpi Sox+ and GFAP+ NSCs derived from Hopx+ NSCs were still identifiable. |
| Hes5 | [88] | Hes5-CreERT2 | SGZ | n.d. | ↑ | ↓ * | ↔ (100 d) * | n.d. | 29% of reporter-positive NSCs remained 100 d post induction, with a corresponding increase in proportion of neuroblasts and postmitotic neurons. * The number of NSCs remained constant over 100 d post induction. |
| [89] | Hes5-CreERT2 | SVZ | n.d. Not determined in this mouse model | ↑ | ↓ * | ↑ (100 d) | n.d. Not determined in this mouse model | Reporter-positive cells in the SVZ continued to generate mitotic progenitors and neuroblasts 100 d after induction. * The neural stem cell population remained in the niche over months and retained long term neurogenic potential. | |
| Troy | [79] | TroyGFPiresCreER | SVZ | n.d. | ↑ | ↓ | ↑ (1 y) | n.d. | Reporter-positive cells remained in the SVZ up to 1 y post labelling while generating new neuroblasts. |
| PDGFRb | [58] | PDGFRb-P2A-CreERT2 | SVZ | n.d. Not determined under physiological conditions | ↑ | ↓ | ↑ (4 mo) | n.d. | Reporter-positive radial cells (GFP+GFAP+), TAPs, and migrating neuroblasts could be found within the SVZ at both 30 and 120 dpi, indicating that reporter-positive stem cells in the SVZ generate progeny up to 4 mpi. |
| VCAM1 | [80] | Ai14 Cre (VP lentivirus injection) | SGZ | ↔ * | ↑ Analysed only at 28 dpi | ↓ Quiescent NSCs remain constant | ↔ (28 d) * Quiescent NSCs remain constant | n.d. | At 28 dpi 31% of reporter-positive cells were co -labelled with S100β+ and 67% were NeuN positive. * The ratio of quiescent NSCs that display a radial and horizontal morphology remained constant from 14 dpi to 28 dpi. |
| Spot14 | [46] | Spot14-CreERT2 | SGZ | n.d. Not determined in this mouse model | ↑ | ↑ | ↓ (3 mo)* | n.d. Not determined in this mouse model | At 3 mpi, 62% of reporter-positive cells were mature neurons compared to 0% at 10 dpi. * The proportion of radial NSPCs declined from 48% to 24% (10 d vs. 3 mo) and non-radial NSPCs declined from 50% to 8%. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrik, D.; Jörgensen, S.; Eftychidis, V.; Siebzehnrubl, F.A. Singular Adult Neural Stem Cells Do Not Exist. Cells 2022, 11, 722. https://doi.org/10.3390/cells11040722
Petrik D, Jörgensen S, Eftychidis V, Siebzehnrubl FA. Singular Adult Neural Stem Cells Do Not Exist. Cells. 2022; 11(4):722. https://doi.org/10.3390/cells11040722
Chicago/Turabian StylePetrik, David, Sara Jörgensen, Vasileios Eftychidis, and Florian A. Siebzehnrubl. 2022. "Singular Adult Neural Stem Cells Do Not Exist" Cells 11, no. 4: 722. https://doi.org/10.3390/cells11040722
APA StylePetrik, D., Jörgensen, S., Eftychidis, V., & Siebzehnrubl, F. A. (2022). Singular Adult Neural Stem Cells Do Not Exist. Cells, 11(4), 722. https://doi.org/10.3390/cells11040722








