Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research
Abstract
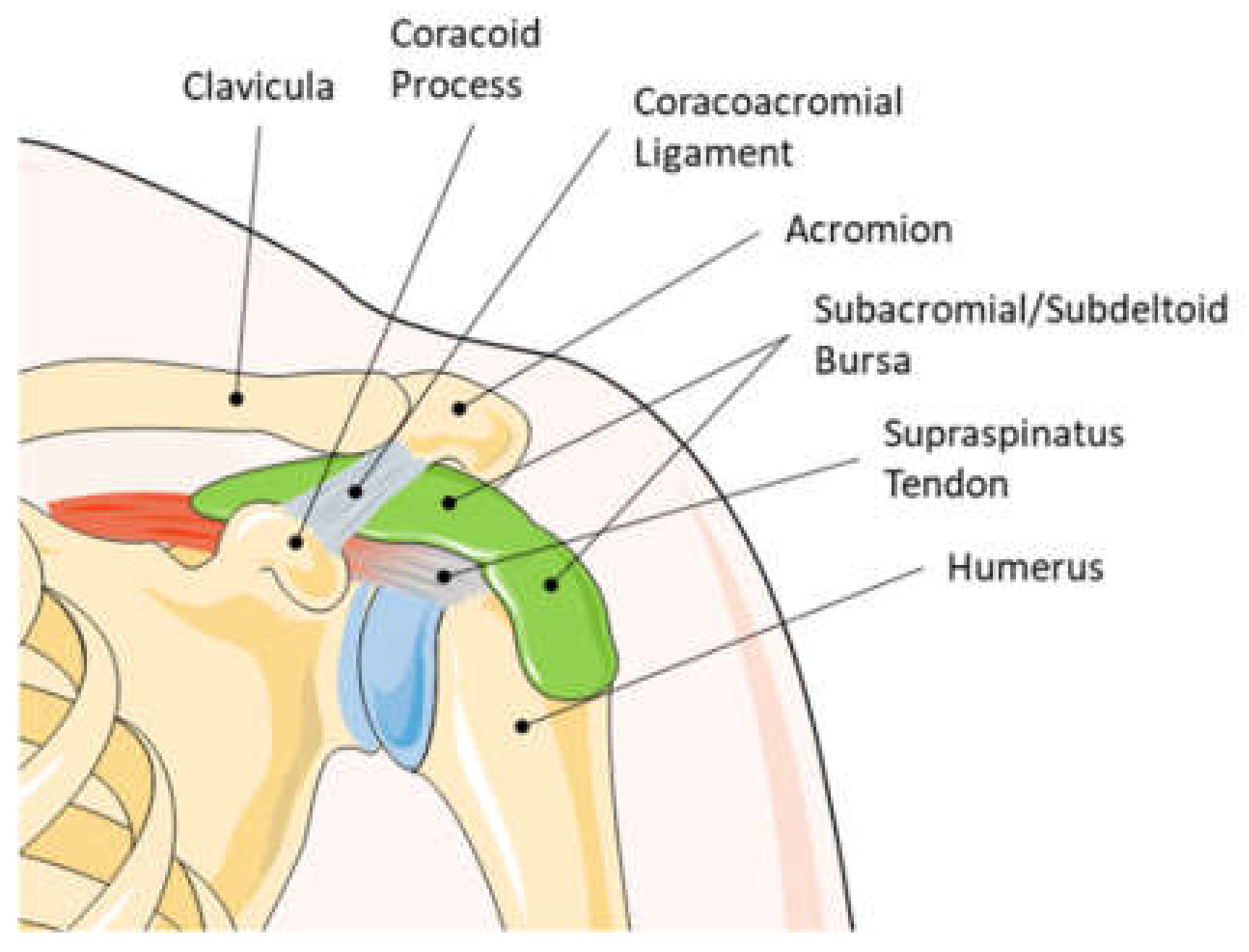
1. Introduction
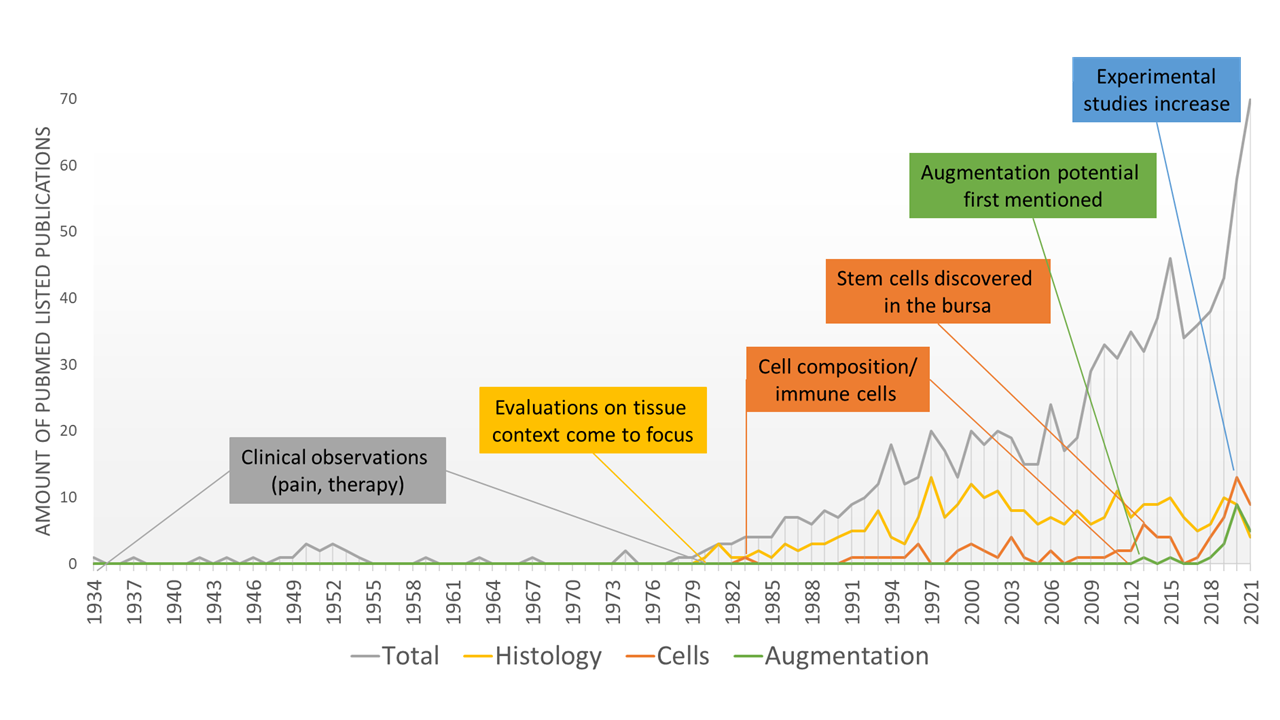
2. History of Attention towards the Subacromial Bursa
3. Cells Come into Focus
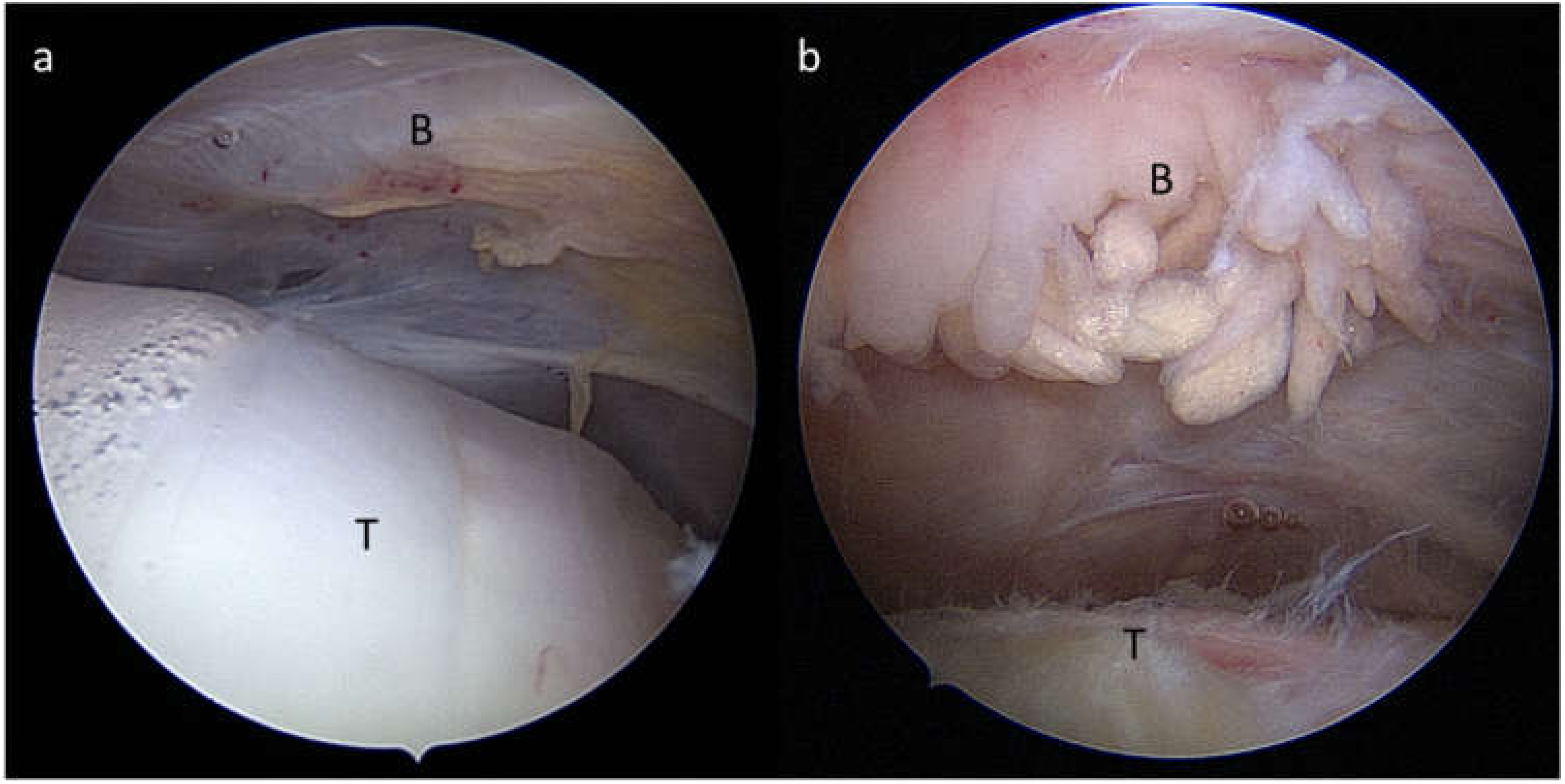
4. Bursitis and Pain: A Clear Correlation
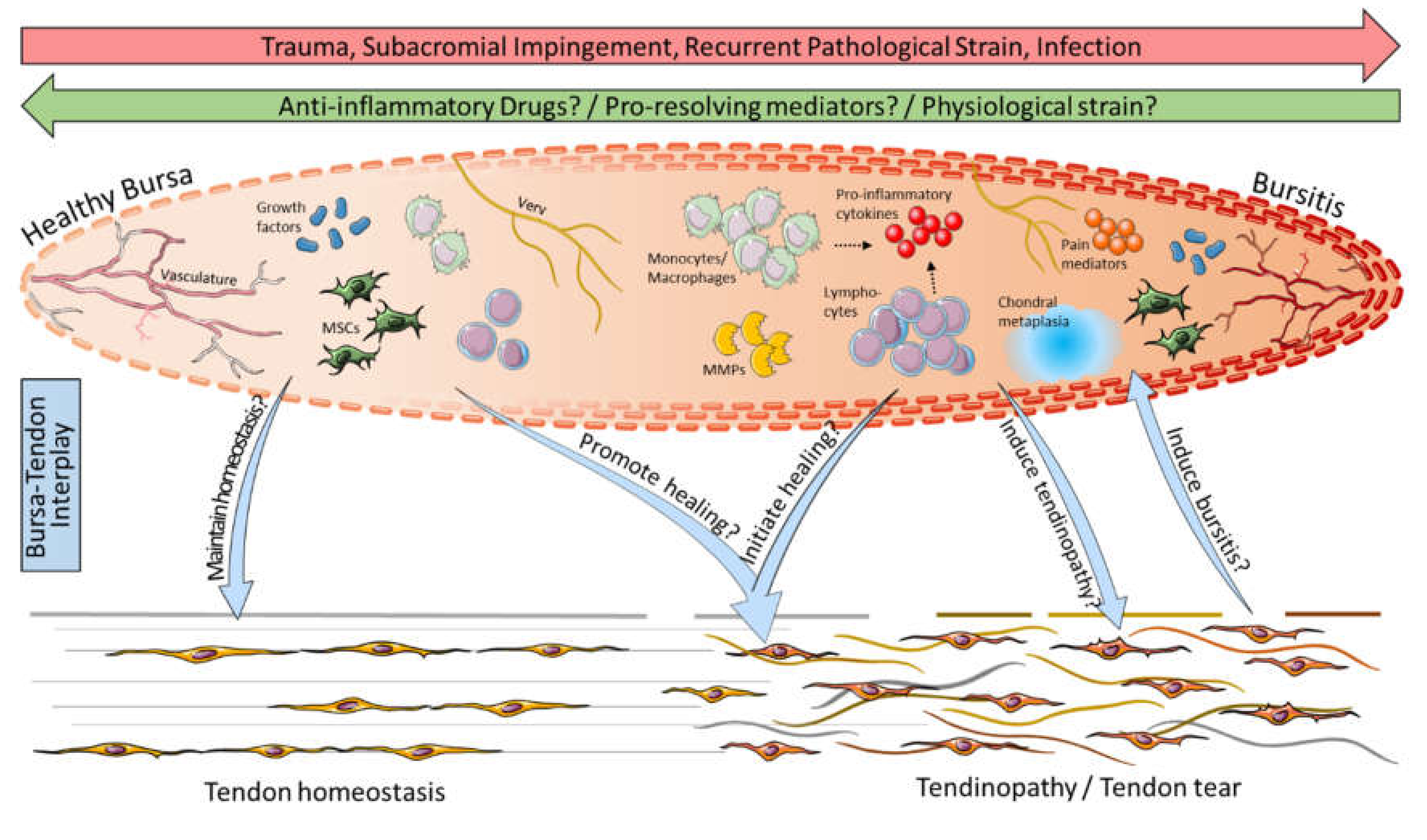
5. Bursa as a Friend or Foe for Rotator Cuff Repair
6. Animal Models for Understanding the Role of the Bursa
7. Possible Therapeutic Targets
8. Clinical Strategies to Use the Augmentation Potential of the Subacromial Bursa
9. Future Perspectives
10. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uhthoff, H.K.; Sarkar, K. Surgical repair of rotator cuff ruptures. The importance of the subacromial bursa. J. Bone Jt. Surg. Br. 1991, 73, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Urwin, M.; Symmons, D.; Allison, T.; Brammah, T.; Busby, H.; Roxby, M.; Simmons, A.; Williams, G. Estimating the burden of musculoskeletal disorders in the community: The comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann. Rheum. Dis. 1998, 57, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Skazalski, C.; Bahr, R.; Whiteley, R. Shoulder complaints more likely in volleyball players with a thickened bursa or supraspinatus tendon neovessels. Scand. J. Med. Sci. Sports 2021, 31, 480–488. [Google Scholar] [CrossRef]
- Luime, J.J.; Koes, B.W.; Hendriksen, I.J.; Burdorf, A.; Verhagen, A.P.; Miedema, H.S.; Verhaar, J.A. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand. J. Rheumatol. 2004, 33, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wiesel, B.B.; Keeling, L.E. Shoulder Injury Related to Vaccine Administration. J. Am. Acad. Orthop. Surg. 2021, 29, 732–739. [Google Scholar] [CrossRef]
- Honarmand, A.R.; Mackey, J.; Hayeri, R. Shoulder injury related to vaccine administration (SIRVA) following mRNA COVID-19 vaccination: Report of 2 cases of subacromial-subdeltoid bursitis. Radiol. Case Rep. 2021, 16, 3631–3634. [Google Scholar] [CrossRef]
- Pearson, M.; Bent, S. Post-vaccination Subacromial Bursitis. J. Gen. Intern. Med. 2021, 37, 467. [Google Scholar] [CrossRef] [PubMed]
- Cantarelli Rodrigues, T.; Hidalgo, P.F.; Skaf, A.Y.; Serfaty, A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: A case of shoulder injury related to vaccine administration (SIRVA). Skeletal. Radiol. 2021, 50, 2293–2297. [Google Scholar] [CrossRef]
- Boonsri, P.; Chuaychoosakoon, C. Combined subacromial-subdeltoid bursitis and supraspinatus tear following a COVID-19 vaccination: A case report. Ann. Med. Surg. 2021, 69, 102819. [Google Scholar] [CrossRef]
- Faruqi, T.; Rizvi, T.J. Subacromial Bursitis. In StatPearls; NCBI: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ishii, H.; Brunet, J.A.; Welsh, R.P.; Uhthoff, H.K. “Bursal reactions” in rotator cuff tearing, the impingement syndrome, and calcifying tendinitis. J. Shoulder Elbow Surg. 1997, 6, 131–136. [Google Scholar] [CrossRef]
- Chillemi, C.; Petrozza, V.; Franceschini, V.; Garro, L.; Pacchiarotti, A.; Porta, N.; Cirenza, M.; Salate Santone, F.; Castagna, A. The role of tendon and subacromial bursa in rotator cuff tear pain: A clinical and histopathological study. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3779–3786. [Google Scholar] [CrossRef] [PubMed]
- Poldoja, E.; Rahu, M.; Kask, K.; Weyers, I.; Kolts, I. Blood supply of the subacromial bursa and rotator cuff tendons on the bursal side. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Fujita, K.; Sakai, Y.; Mizuno, K. Immunolocalization of cytokines and growth factors in subacromial bursa of rotator cuff tear patients. Kobe J. Med. Sci. 2001, 47, 25–34. [Google Scholar] [PubMed]
- Steinert, A.F.; Gohlke, F. Editorial Commentary: Subacromial Bursa-Friend or Foe Within the Shoulder? An Old Debate with New Insights. Arthroscopy 2019, 35, 2989–2991. [Google Scholar] [CrossRef]
- Lanham, N.S.; Swindell, H.W.; Levine, W.N. The Subacromial Bursa: Current Concepts Review. JBJS Rev. 2021, 9, e21. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, D.; Johnson, J.D.; Kia, C.; McCarthy, M.B.R.; Macken, C.; Bellas, N.; Baldino, J.B.; Cote, M.P.; Mazzocca, A.D. Examining the Potency of Subacromial Bursal Cells as a Potential Augmentation for Rotator Cuff Healing: An In Vitro Study. Arthroscopy 2019, 35, 2978–2988. [Google Scholar] [CrossRef]
- Song, N.; Armstrong, A.D.; Li, F.; Ouyang, H.; Niyibizi, C. Multipotent mesenchymal stem cells from human subacromial bursa: Potential for cell based tendon tissue engineering. Tissue Eng. Part A 2014, 20, 239–249. [Google Scholar] [CrossRef]
- Codman, E.A. The Shoulder: Rupture of the Supraspinatus Tendon and Other Lesions in or about the Subacromial Bursa; Thomas Todd Company, Printers: Boston, MA, USA, 1934. [Google Scholar]
- Caldwell, G.A.; Unkauf, B.M. Results of treatment of subacromial bursitis in three hundred forty cases. Ann. Surg. 1950, 132, 432–442. [Google Scholar] [CrossRef]
- Guido, F.R. Acute Calcified Subacromial or Subdeltoid Bursitis. Cal. West. Med. 1944, 60, 69–72. [Google Scholar]
- Olin, H.A. Subacromial bursitis: Clinical and roentgen observations. Radiology 1946, 47, 593–596. [Google Scholar] [CrossRef]
- Ferguson, L.K. Painful Shoulder: Arising from Lesions of the Subacromial Bursa and Supraspinatus Tendon. Ann. Surg. 1937, 105, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.M.; Singer, G.; Kaech, A.; Ziegler, U.; Eid, K. Characterization of Deposits in Calcific Tendinitis of the Shoulder: Deposits Are Composed of Large Aggregates of Highly Crystalline, Rod-Like Crystals. Orthop. J. Sports Med. 2021, 9, 23259671211044715. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.; Delprat, G.D. Acute Subdeltoid or Subacromial Bursitis-A Suggestion. Cal. West. Med. 1934, 41, 273. [Google Scholar] [PubMed]
- Chapman, J.F. Subacromial Bursitis and Supraspinatus Tendinitis: Its Roentgen Treatment. Cal. West. Med. 1942, 56, 248–251. [Google Scholar]
- Mc, C.A.; Norton, G.I.; Bouchard, J. Subacromial bursitis; a classification and an evaluation of the results of roentgen therapy. Can. Med. Assoc. J. 1949, 61, 39–44. [Google Scholar]
- Aldes, J.H.; Jadeson, W.J.; Grabinski, S. A new approach to the treatment of subdeltoid bursitis. Am. J. Phys. Med. 1954, 33, 79–88. [Google Scholar] [PubMed]
- Newman, M.K.; Kill, M.; Frampton, G. Effects of ultrasound alone and combined with hydrocortisone injections by needle or hypo-spray. Am. J. Phys. Med. 1958, 37, 206–209. [Google Scholar]
- Steinberg, C.L.; Roodenburg, A.I. Corticotropin (ACTH) in the treatment of acute subacromial bursitis. J. Am. Med. Assoc. 1952, 149, 1458–1460. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R. Use of X-rays to treat shoulder tendonitis/bursitis: A historical assessment. Arch. Toxicol. 2014, 88, 1503–1517. [Google Scholar] [CrossRef]
- Young, H.H.; Ward, L.E.; Henderson, E.D. The use of hydrocortisone acetate (compound F acetate) in the treatment of some common orthopaedic conditions. J. Bone Jt. Surg. Am. 1954, 36, 602–609. [Google Scholar] [CrossRef]
- Neer, C.S., 2nd. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: A preliminary report. J. Bone Jt. Surg. Am. 1972, 54, 41–50. [Google Scholar] [CrossRef]
- Ellman, H. Arthroscopic subacromial decompression: Analysis of one- to three-year results. Arthroscopy 1987, 3, 173–181. [Google Scholar] [CrossRef]
- Rossi, L.A.; Piuzzi, N.; Giunta, D.; Tanoira, I.; Brandariz, R.; Pasqualini, I.; Ranalletta, M. Subacromial Platelet-Rich Plasma Injections Decrease Pain and Improve Functional Outcomes in Patients with Refractory Rotator Cuff Tendinopathy. Arthroscopy 2021, 37, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Thepsoparn, M.; Thanphraisan, P.; Tanpowpong, T.; Itthipanichpong, T. Comparison of a Platelet-Rich Plasma Injection and a Conventional Steroid Injection for Pain Relief and Functional Improvement of Partial Supraspinatus Tears. Orthop. J. Sports Med. 2021, 9, 23259671211024937. [Google Scholar] [CrossRef]
- Schwitzguebel, A.J.; Kolo, F.C.; Tirefort, J.; Kourhani, A.; Nowak, A.; Gremeaux, V.; Saffarini, M.; Ladermann, A. Efficacy of Platelet-Rich Plasma for the Treatment of Interstitial Supraspinatus Tears: A Double-Blinded, Randomized Controlled Trial. Am. J. Sports Med. 2019, 47, 1885–1892. [Google Scholar] [CrossRef]
- Hurley, E.T.; Hannon, C.P.; Pauzenberger, L.; Fat, D.L.; Moran, C.J.; Mullett, H. Nonoperative Treatment of Rotator Cuff Disease with Platelet-Rich Plasma: A Systematic Review of Randomized Controlled Trials. Arthroscopy 2019, 35, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr. Polym. 2022, 276, 118699. [Google Scholar] [CrossRef]
- Hsieh, L.F.; Lin, Y.J.; Hsu, W.C.; Kuo, Y.C.; Liu, Y.C.; Chiang, Y.P.; Wang, C.P. Comparison of the corticosteroid injection and hyaluronate in the treatment of chronic subacromial bursitis: A randomized controlled trial. Clin. Rehabil. 2021, 35, 1305–1316. [Google Scholar] [CrossRef]
- Penning, L.I.; de Bie, R.A.; Walenkamp, G.H. Subacromial triamcinolone acetonide, hyaluronic acid and saline injections for shoulder pain an RCT investigating the effectiveness in the first days. BMC Musculoskelet. Disord. 2014, 15, 352. [Google Scholar] [CrossRef]
- Henrotin, Y.; Dierckxsens, Y.; Delisse, G.; Seidel, L.; Albert, A. Curcuminoids and Boswellia serrata extracts combination decreases tendinopathy symptoms: Findings from an open-label post-observational study. Curr. Med. Res. Opin. 2021, 37, 423–430. [Google Scholar] [CrossRef]
- Loiacono, C.; Palermi, S.; Massa, B.; Belviso, I.; Romano, V.; Gregorio, A.D.; Sirico, F.; Sacco, A.M. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina 2019, 55, 447. [Google Scholar] [CrossRef] [PubMed]
- Fusini, F.; Bisicchia, S.; Bottegoni, C.; Gigante, A.; Zanchini, F.; Busilacchi, A. Nutraceutical supplement in the management of tendinopathies: A systematic review. Muscles Ligaments Tendons J. 2016, 6, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Oberg, B.; Adolfsson, L.; Foldevi, M. A combination of systematic review and clinicians’ beliefs in interventions for subacromial pain. Br. J. Gen. Pract. 2002, 52, 145–152. [Google Scholar]
- Farfaras, S.; Sernert, N.; Rostgard Christensen, L.; Hallstrom, E.K.; Kartus, J.T. Subacromial Decompression Yields a Better Clinical Outcome Than Therapy Alone: A Prospective Randomized Study of Patients with a Minimum 10-Year Follow-up. Am. J. Sports Med. 2018, 46, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Butt, U.; Whiteman, A.; Wilson, J.; Paul, E.; Roy, B. Does arthroscopic subacromial decompression improve quality of life. Ann. R Coll. Surg. Engl. 2015, 97, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Beard, D.J.; Rees, J.L.; Cook, J.A.; Rombach, I.; Cooper, C.; Merritt, N.; Shirkey, B.A.; Donovan, J.L.; Gwilym, S.; Savulescu, J.; et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): A multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 2017, 391, 10118. [Google Scholar] [CrossRef]
- Ketola, S.; Lehtinen, J.T.; Arnala, I. Arthroscopic decompression not recommended in the treatment of rotator cuff tendinopathy: A final review of a randomised controlled trial at a minimum follow-up of ten years. Bone Jt. J. 2017, 99-B, 799–805. [Google Scholar] [CrossRef]
- Lahdeoja, T.; Karjalainen, T.; Jokihaara, J.; Salamh, P.; Kavaja, L.; Agarwal, A.; Winters, M.; Buchbinder, R.; Guyatt, G.; Vandvik, P.O.; et al. Subacromial decompression surgery for adults with shoulder pain: A systematic review with meta-analysis. Br. J. Sports Med. 2020, 54, 665–673. [Google Scholar] [CrossRef]
- Sarkar, K.; Uhthoff, H.K. Ultrastructure of the subacromial bursa in painful shoulder syndromes. Virchows Arch. A Pathol. Anat. Histopathol. 1983, 400, 107–117. [Google Scholar] [CrossRef]
- Santavirta, S.; Konttinen, Y.T.; Antti-Poika, I.; Nordstrom, D. Inflammation of the subacromial bursa in chronic shoulder pain. Arch. Orthop. Trauma Surg. 1992, 111, 336–340. [Google Scholar] [CrossRef]
- Minkwitz, S.; Thiele, K.; Schmock, A.; Bormann, N.; Nguyen, T.H.; Moroder, P.; Scheibel, M.; Wildemann, B.; Plachel, F.; Klatte-Schulz, F. Histological and molecular features of the subacromial bursa of rotator cuff tears compared to non-tendon defects: A pilot study. BMC Musculoskelet. Disord. 2021, 22, 877. [Google Scholar] [CrossRef]
- Lhee, S.H.; Jo, Y.H.; Kim, B.Y.; Nam, B.M.; Nemeno, J.G.; Lee, S.; Yang, W.; Lee, J.I. Novel supplier of mesenchymal stem cell: Subacromial bursa. Transplant. Proc. 2013, 45, 3118–3121. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Uchida, S.; Sekiya, I.; Sakai, A.; Moridera, K.; Nakamura, T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am. J. Sports Med. 2013, 41, 657–668. [Google Scholar] [CrossRef]
- Steinert, A.F.; Kunz, M.; Prager, P.; Gobel, S.; Klein-Hitpass, L.; Ebert, R.; Noth, U.; Jakob, F.; Gohlke, F. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res. Ther. 2015, 6, 114. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Bormann, N.; Voss, I.; Melzer, J.; Schmock, A.; Bucher, C.H.; Thiele, K.; Moroder, P.; Haffner-Luntzer, M.; Ignatius, A.; et al. Bursa-Derived Cells Show a Distinct Mechano-Response to Physiological and Pathological Loading in vitro. Front. Cell Dev. Biol. 2021, 9, 657166. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Duruksu, G.; Erman, G.; Subasi, C.; Aksoy, A.; Unal, Z.S.; Karaoz, E. Neurogenic differentiation capacity of subacromial bursal tissue-derived stem cells. J. Orthop. Res. 2014, 32, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, D.; Muench, L.N.; Baldino, J.B.; Kia, C.; Johnson, J.; Otto, A.; Pauzenberger, L.; Dyrna, F.; McCarthy, M.B.R.; Mazzocca, A.D. Comparison of Preparation Techniques for Isolating Subacromial Bursa-Derived Cells as a Potential Augment for Rotator Cuff Repair. Arthroscopy 2020, 36, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Landry, A.; Levy, B.J.; McCarthy, M.B.; Muench, L.N.; Uyeki, C.; Berthold, D.P.; Cote, M.P.; Mazzocca, A.D. Analysis of Time to Form Colony Units for Connective Tissue Progenitor Cells (Stem Cells) Harvested From Concentrated Bone Marrow Aspirate and Subacromial Bursa Tissue in Patients Undergoing Rotator Cuff Repair. Arthrosc. Sports Med. Rehabil. 2020, 2, e629–e636. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Pauly, S.; Scheibel, M.; Greiner, S.; Gerhardt, C.; Hartwig, J.; Schmidmaier, G.; Wildemann, B. Characteristics and stimulation potential with BMP-2 and BMP-7 of tenocyte-like cells isolated from the rotator cuff of female donors. PLoS ONE 2013, 8, e67209. [Google Scholar] [CrossRef]
- Klatte-Schulz, F.; Pauly, S.; Scheibel, M.; Greiner, S.; Gerhardt, C.; Schmidmaier, G.; Wildemann, B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur. Cell Mater. 2012, 24, 74–89. [Google Scholar] [CrossRef]
- Morikawa, D.; Hawthorne, B.C.; McCarthy, M.B.R.; Bellas, N.; Johnson, J.D.; Trudeau, M.T.; Murphy, K.V.; Mancini, M.R.; LeVasseur, M.R.; Cote, M.P.; et al. Analysis of Patient Factors Affecting In Vitro Characteristics of Subacromial Bursal Connective Tissue Progenitor Cells during Rotator Cuff Repair. J. Clin. Med. 2021, 10, 4006. [Google Scholar] [CrossRef] [PubMed]
- Aszmann, O.C.; Dellon, A.L.; Birely, B.T.; McFarland, E.G. Innervation of the human shoulder joint and its implications for surgery. Clin. Orthop. Relat. Res. 1996, 330, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Soifer, T.B.; Levy, H.J.; Soifer, F.M.; Kleinbart, F.; Vigorita, V.; Bryk, E. Neurohistology of the subacromial space. Arthroscopy 1996, 12, 182–186. [Google Scholar] [CrossRef]
- Vangsness, C.T., Jr.; Ennis, M.; Taylor, J.G.; Atkinson, R. Neural anatomy of the glenohumeral ligaments, labrum, and subacromial bursa. Arthroscopy 1995, 11, 180–184. [Google Scholar] [CrossRef]
- Kennedy, M.S.; Nicholson, H.D.; Woodley, S.J. The morphology of the subacromial and related shoulder bursae. An anatomical and histological study. J. Anat. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Hamada, K.; Yamakawa, H.; Inoue, A.; Fukuda, H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J. Orthop. Res. 1998, 16, 618–621. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Hamada, K.; Gotoh, M.; Tokunaga, T.; Oshika, Y.; Tomisawa, M.; Lee, Y.H.; Handa, A.; Kijima, H.; Yamazaki, H.; et al. Vascular endothelial growth factor (VEGF) expression in the subacromial bursa is increased in patients with impingement syndrome. J. Orthop. Res. 2001, 19, 448–455. [Google Scholar] [CrossRef]
- Gotoh, M.; Hamada, K.; Yamakawa, H.; Yanagisawa, K.; Nakamura, M.; Yamazaki, H.; Inoue, A.; Fukuda, H. Interleukin-1-induced glenohumeral synovitis and shoulder pain in rotator cuff diseases. J. Orthop. Res. 2002, 20, 1365–1371. [Google Scholar] [CrossRef]
- Neuwirth, J.; Fuhrmann, R.A.; Veit, A.; Aurich, M.; Stonans, I.; Trommer, T.; Hortschansky, P.; Chubinskaya, S.; Mollenhauer, J.A. Expression of bioactive bone morphogenetic proteins in the subacromial bursa of patients with chronic degeneration of the rotator cuff. Arthritis Res. Ther. 2006, 8, R92. [Google Scholar] [CrossRef]
- Blaine, T.A.; Kim, Y.S.; Voloshin, I.; Chen, D.; Murakami, K.; Chang, S.S.; Winchester, R.; Lee, F.Y.; O′Keefe, R.J.; Bigliani, L.U. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J. Shoulder Elbow Surg. 2005, 14 (Suppl. S1), 84S–89S. [Google Scholar] [CrossRef]
- Blaine, T.A.; Cote, M.A.; Proto, A.; Mulcahey, M.; Lee, F.Y.; Bigliani, L.U. Interleukin-1beta stimulates stromal-derived factor-1alpha expression in human subacromial bursa. J. Orthop. Res. 2011, 29, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Bigliani, L.U.; Fujisawa, M.; Murakami, K.; Chang, S.S.; Lee, H.J.; Lee, F.Y.; Blaine, T.A. Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J. Orthop. Res. 2006, 24, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, I.; Gelinas, J.; Maloney, M.D.; O′Keefe, R.J.; Bigliani, L.U.; Blaine, T.A. Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthroscopy 2005, 21, 1076.e1–1076.e9. [Google Scholar] [CrossRef] [PubMed]
- Lho, Y.M.; Ha, E.; Cho, C.H.; Song, K.S.; Min, B.W.; Bae, K.C.; Lee, K.J.; Hwang, I.; Park, H.B. Inflammatory cytokines are overexpressed in the subacromial bursa of frozen shoulder. J. Shoulder Elbow Surg. 2013, 22, 666–672. [Google Scholar] [CrossRef]
- Ko, J.Y.; Wang, F.S.; Huang, H.Y.; Wang, C.J.; Tseng, S.L.; Hsu, C. Increased IL-1beta expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J. Orthop. Res. 2008, 26, 1090–1097. [Google Scholar] [CrossRef]
- Feng, H.; He, Z.; Twomey, K.; Ilaltdinov, A.W.; Leong, D.; Wang, Y.; Li, J.; Gonzalez, D.; Sun, H.B. Epigallocatechin-3-gallate suppresses pain-related and proinflammatory mediators in the subacromial bursa in rotator cuff tendinopathy. Discov. Med. 2019, 27, 63–77. [Google Scholar]
- Robertson, C.M.; Chen, C.T.; Shindle, M.K.; Cordasco, F.A.; Rodeo, S.A.; Warren, R.F. Failed healing of rotator cuff repair correlates with altered collagenase and gelatinase in supraspinatus and subscapularis tendons. Am. J. Sports Med. 2012, 40, 1993–2001. [Google Scholar] [CrossRef]
- Benson, R.T.; McDonnell, S.M.; Rees, J.L.; Athanasou, N.A.; Carr, A.J. The morphological and immunocytochemical features of impingement syndrome and partial-thickness rotator-cuff tear in relation to outcome after subacromial decompression. J. Bone Jt. Surg. Br. 2009, 91, 119–123. [Google Scholar] [CrossRef][Green Version]
- Rahme, H.; Nordgren, H.; Hamberg, H.; Westerberg, C.E. The subacromial bursa and the impingement syndrome. A clinical and histological study of 30 cases. Acta Orthop. Scand. 1993, 64, 485–488. [Google Scholar] [CrossRef]
- Chillemi, C.; Petrozza, V.; Garro, L.; Sardella, B.; Diotallevi, R.; Ferrara, A.; Gigante, A.; Di Cristofano, C.; Castagna, A.; Della Rocca, C. Rotator cuff re-tear or non-healing: Histopathological aspects and predictive factors. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1588–1596. [Google Scholar] [CrossRef]
- Marshall, B.P.; Song, L.; Kovacevic, D.; Thomopoulos, S. The Role of the Subacromial Bursa in Rotator Cuff Disease in a Rat Model. In Proceedings of the ORS 2021 Annual Meeting, Online, 12 February 2021. [Google Scholar]
- Kriscenski, D.E.; Lebaschi, A.; Tamburini, L.M.; McCarthy, M.B.R.; Cote, M.P.; Kumbar, S.G.; Mazzocca, A.D. Characterization of murine subacromial bursal-derived cells. Connect. Tissue Res. 2021, 1–11, in press. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Alaee, F.; Dyrna, F.; Kronenberg, M.S.; Maye, P.; Kalajzic, I.; Rowe, D.W.; Mazzocca, A.D.; Dyment, N.A. Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect. Tissue Res. 2016, 57, 507–515. [Google Scholar] [CrossRef]
- Uhthoff, H.K.; Sano, H.; Trudel, G.; Ishii, H. Early reactions after reimplantation of the tendon of supraspinatus into bone. A study in rabbits. J. Bone Jt. Surg. Br. 2000, 82, 1072–1076. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hamada, K.; Gotoh, M.; Handa, A.; Yamakawa, H.; Fukuda, H. Healing of full-thickness tears of avian supracoracoid tendons: In situ hybridization of alpha1(I) and alpha1(III) procollagen mRNA. J. Orthop. Res. 2001, 19, 862–868. [Google Scholar] [CrossRef]
- Ko, J.Y.; Lian, W.S.; Tsai, T.C.; Chen, Y.S.; Hsieh, C.K.; Kuo, C.W.; Wang, F.S. MicroRNA-29a Mitigates Subacromial Bursa Fibrosis in Rotator Cuff Lesion with Shoulder Stiffness. Int. J. Mol. Sci. 2019, 20, 5742. [Google Scholar] [CrossRef]
- Dyrna, F.; Zakko, P.; McCarthy, M.B.; Rowe, D.W.; Mazzocca, A.; Dyment, N.A. Human subacromial bursa cells display superior engraftment vs. bone marrow stromal cells in murine tendon repair. In Proceedings of the ORS 2017 Annual Meeting, San Diego, CA, USA, 19 March 2017. [Google Scholar]
- Nagura, N.; Uchida, K.; Kenmoku, T.; Inoue, G.; Nakawaki, M.; Miyagi, M.; Takaso, M. IL-1beta mediates NGF and COX-2 expression through transforming growth factor-activating kinase 1 in subacromial bursa cells derived from rotator cuff tear patients. J. Orthop. Sci. 2019, 24, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Wei, X.; Xu, J.; Chen, Q.; Song, S.; Lu, Z.; Wang, Z. Targeted knockout of TNF-alpha by injection of lentivirus-mediated siRNA into the subacromial bursa for the treatment of subacromial bursitis in rats. Mol. Med. Rep. 2015, 12, 4389–4395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muench, L.N.; Tamburini, L.; Kriscenski, D.; Landry, A.; Berthold, D.P.; Kia, C.; Cote, M.P.; McCarthy, M.B.; Mazzocca, A.D. The Effect of Insulin and Insulin-like Growth Factor 1 (IGF-1) on Cellular Proliferation and Migration of Human Subacromial Bursa Tissue. Arthrosc. Sports Med. Rehabil. 2021, 3, e781–e789. [Google Scholar] [CrossRef]
- Freislederer, F.; Dittrich, M.; Scheibel, M. Biological Augmentation with Subacromial Bursa in Arthroscopic Rotator Cuff Repair. Arthrosc. Tech. 2019, 8, e741–e747. [Google Scholar] [CrossRef]
- Bhatia, D.N. Arthroscopic Bursa-Augmented Rotator Cuff Repair: A Vasculature-preserving Technique for Subacromial Bursal Harvest and Tendon Augmentation. Arthrosc. Tech. 2021, 10, e1203–e1209. [Google Scholar] [CrossRef]
- Pancholi, N.; Gregory, J.M. Biologic Augmentation of Arthroscopic Rotator Cuff Repair Using Minced Autologous Subacromial Bursa. Arthrosc. Tech. 2020, 9, e1519–e1524. [Google Scholar] [CrossRef]
- Dei Giudici, L.; Castricini, R. Local Autologous Stem Cells Application in Rotator Cuff Repairs: "LASCA" Technique. Arthrosc. Tech. 2020, 9, e1571–e1575. [Google Scholar] [CrossRef] [PubMed]
- Muench, L.N.; Kia, C.; Berthold, D.P.; Uyeki, C.; Otto, A.; Cote, M.P.; McCarthy, M.B.; Beitzel, K.; Arciero, R.A.; Mazzocca, A.D. Preliminary Clinical Outcomes Following Biologic Augmentation of Arthroscopic Rotator Cuff Repair Using Subacromial Bursa, Concentrated Bone Marrow Aspirate, and Platelet-Rich Plasma. Arthrosc. Sports Med. Rehabil. 2020, 2, e803–e813. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.G.; Dudhia, J.; Smith, R.K. Resolving an inflammatory concept: The importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 2014, 158, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.; Smith, R.D.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klatte-Schulz, F.; Thiele, K.; Scheibel, M.; Duda, G.N.; Wildemann, B. Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research. Cells 2022, 11, 663. https://doi.org/10.3390/cells11040663
Klatte-Schulz F, Thiele K, Scheibel M, Duda GN, Wildemann B. Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research. Cells. 2022; 11(4):663. https://doi.org/10.3390/cells11040663
Chicago/Turabian StyleKlatte-Schulz, Franka, Kathi Thiele, Markus Scheibel, Georg N. Duda, and Britt Wildemann. 2022. "Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research" Cells 11, no. 4: 663. https://doi.org/10.3390/cells11040663
APA StyleKlatte-Schulz, F., Thiele, K., Scheibel, M., Duda, G. N., & Wildemann, B. (2022). Subacromial Bursa: A Neglected Tissue Is Gaining More and More Attention in Clinical and Experimental Research. Cells, 11(4), 663. https://doi.org/10.3390/cells11040663





