Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements
Abstract
1. Introduction
2. Targeting Numerous Signaling Pathways of Ovarian Cancer
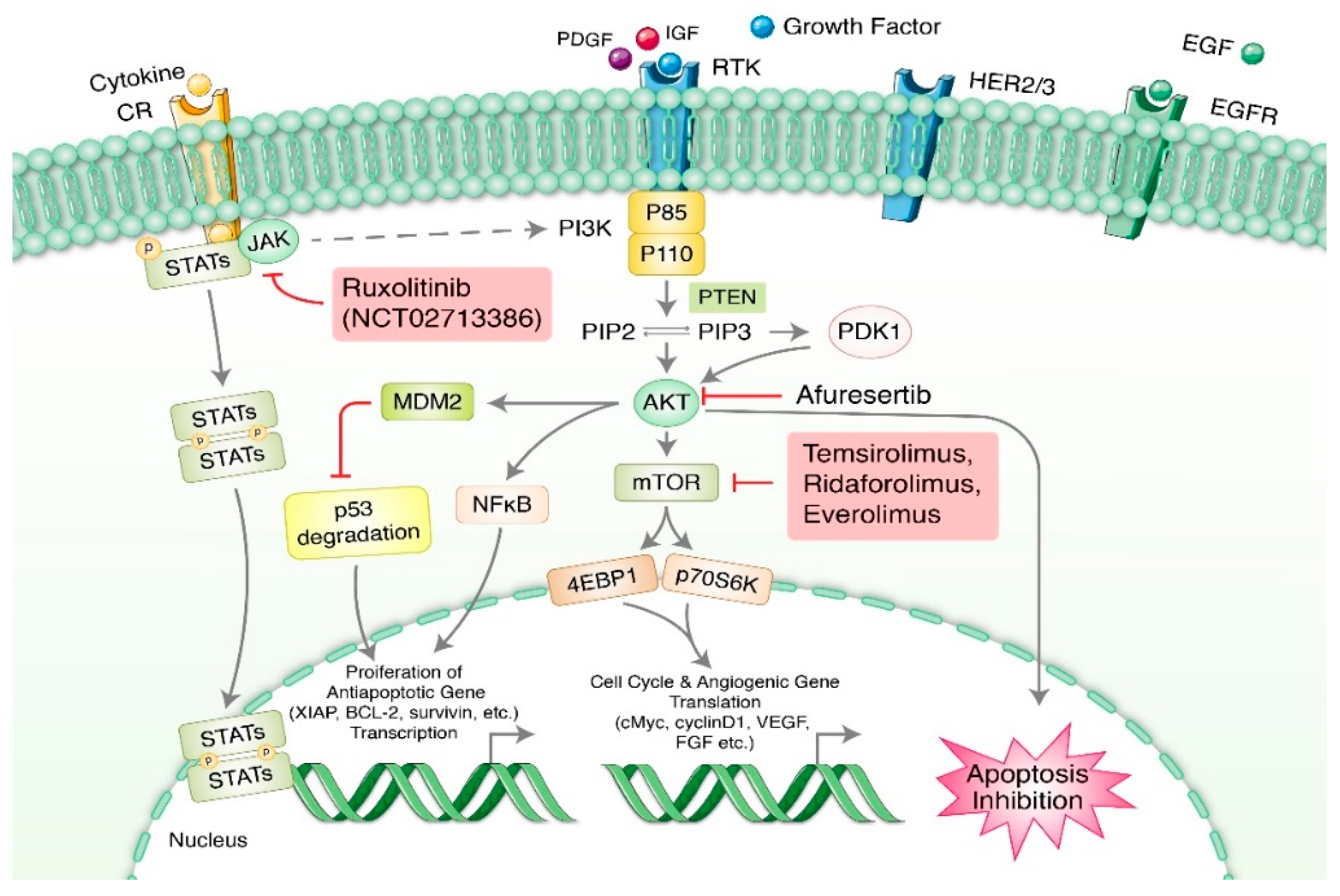
2.1. PI3K/AKT/mTOR Pathway
2.2. JAK/STAT Signaling Pathway
2.3. Wnt/β-Catenin Pathway
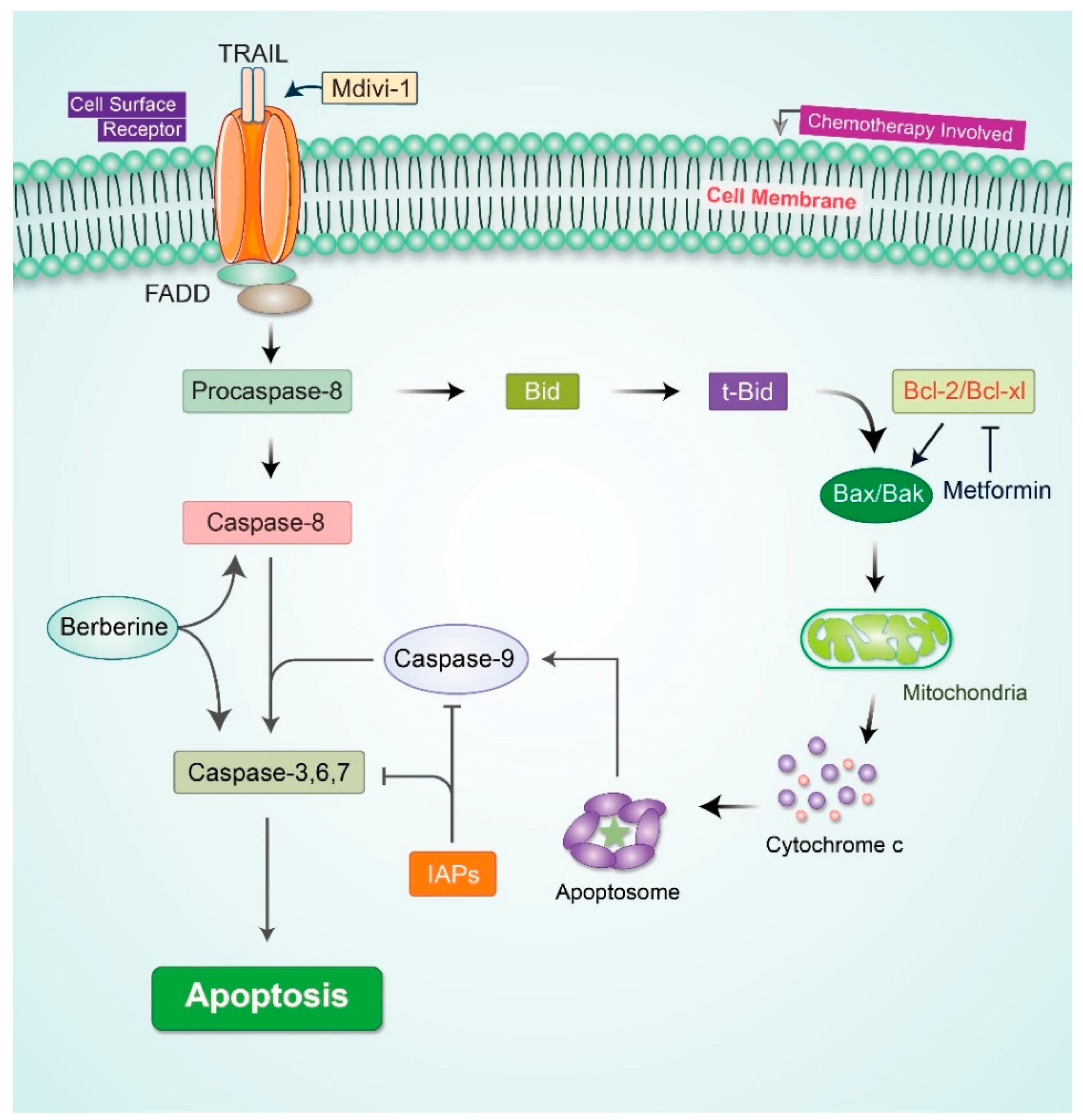
2.4. Apoptotic Signaling Pathway’
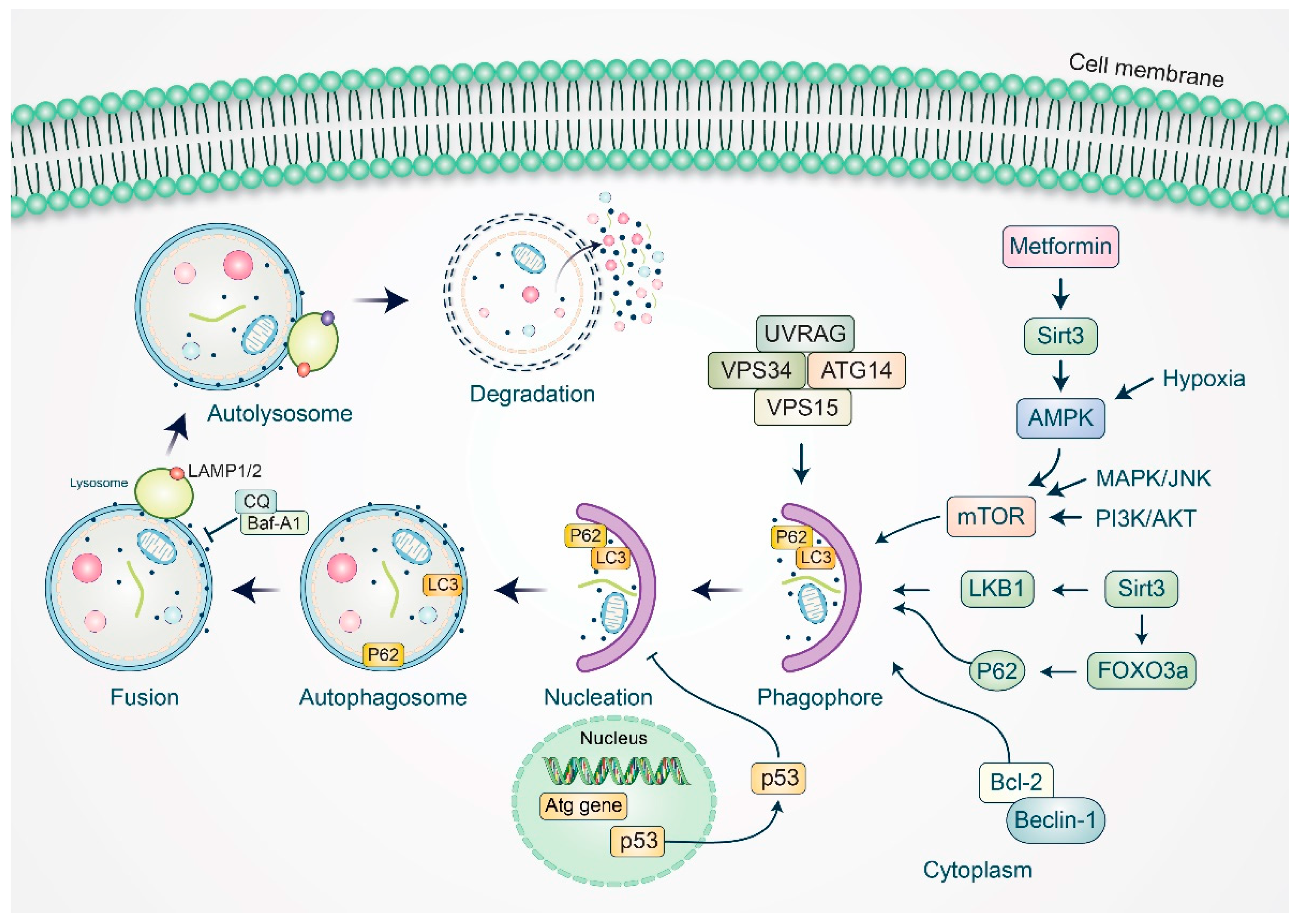
3. Autophagy Modulation in Ovarian Cancer Management
4. Novel Treatment Strategies for Epithelial Ovarian Cancer (EOC)
4.1. Therapeutic Approaches and Targets in Ovarian Cancer
4.2. Angiogenesis and VEGF Signaling Pathway
4.3. ErbB Family Kinases
4.4. Ansamycins and HSP90 Degradation
4.5. 26S Proteosome Inhibition with PS341 (Bortezomib)
4.6. Tubulin-Targeting Molecules
4.7. Ovarian Cancer-Specific Targets: MUC16/CA125
5. Drug-Delivery System for Ovarian Cancer Treatment
5.1. Single-Agent Delivery Systems
5.2. Co-Delivery Nanoparticles
6. Limitations and Chemoresistance of Ovarian Cancer Therapy
7. Technological Advances in Identifying Novel Biomarkers of Ovarian Cancer
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, N.; Kadife, E.; Raza, A.; Short, M.; Jubinsky, P.T.; Kannourakis, G. Ovarian Cancer, Cancer Stem Cells and Current Treatment Strategies: A Potential Role of Magmas in the Current Treatment Methods. Cells 2020, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Q.; Elnitski, L. A Systems Biology Comparison of Ovarian Cancers Implicates Putative Somatic Driver Mutations through Protein-Protein Interaction Models. PLoS ONE 2016, 11, e0163353. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.S.; Maxwell, G.L.; Darcy, K.M.; Hamilton, C.A.; McGuire, W.P. Current Approaches and Challenges in Managing and Monitoring Treatment Response in Ovarian Cancer. J. Cancer 2014, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.V.D.; Mertens, V.; Massuger, L.F.; Brock, R. siRNA in ovarian cancer—Delivery strategies and targets for therapy. J. Control. Release 2018, 283, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.; Colombo, P.-E.; Leaha, C.M.; Rouanet, P.; Carrère, S.; Quenet, F.; Gutowski, M.; Mourregot, A.; D’Hondt, V.; Coupier, I.; et al. Conditional Probability of Survival and Prognostic Factors in Long-Term Survivors of High-Grade Serous Ovarian Cancer. Cancers 2020, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Pisano, C.; Facchini, G.; Bruni, G.S.; Magazzino, F.P.; Losito, S.; Pignata, S. First-line treatment of advanced ovarian cancer: Current research and perspectives. Expert Rev. Anticancer Ther. 2010, 10, 47–60. [Google Scholar] [CrossRef]
- Feliu, J.; Heredia-Soto, V.; Gironés, R.; Jiménez-Munarriz, B.; Saldaña, J.; Guillén-Ponce, C.; Molina-Garrido, M.J. Management of the toxicity of chemotherapy and targeted therapies in elderly cancer patients. Clin. Transl. Oncol. 2020, 22, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Arcaroli, J.J.; Bagby, S.M.; Yahn, R.; Huber, K.M.; Serkova, N.J.; Nguyen, A.; Kim, J.; Thorburn, A.; Vogel, J.; et al. Cabozantinib Exhibits Potent Antitumor Activity in Colorectal Cancer Patient-Derived Tumor Xenograft Models via Autophagy and Signaling Mechanisms. Mol. Cancer Ther. 2018, 17, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Mendelsohn, J.; Fan, Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a hu-man-mouse chimeric anti-EGF receptor mAb C225. Oncogene 1999, 18, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Peracchio, C.; Alabiso, O.; Valente, G.; Isidoro, C. Involvement of autophagy in ovarian cancer: A working hypothesis. J. Ovarian Res. 2012, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted therapies in gynecological cancers: A comprehensive review of clinical evidence. Signal. Transduct. Target. Ther. 2020, 5, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Boku, N.; Onozawa, Y.; Takahashi, K.; Kawaguchi, O.; Ohtsu, A. Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies. Investig. New Drugs 2020, 38, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Thouvenin, L.; Charrier, M.; Clement, S.; Christinat, Y.; Tille, J.-C.; Frigeri, M.; Homicsko, K.; Michielin, O.; Bodmer, A.; Chappuis, P.O.; et al. Ovarian cancer with high-level focal ERBB2 amplification responds to trastuzumab and pertuzumab. Gynecol. Oncol. Rep. 2021, 37, 100787. [Google Scholar] [CrossRef] [PubMed]
- Swiatly, A.; Horala, A.; Matysiak, J.; Hajduk, J.; Nowak-Markwitz, E.; Kokot, Z.J. Understanding Ovarian Cancer: iTRAQ-Based Proteomics for Biomarker Discovery. Int. J. Mol. Sci. 2018, 19, 2240. [Google Scholar] [CrossRef]
- Kim, H.; Xu, H.; George, E.; Hallberg, D.; Kumar, S.; Jagannathan, V.; Medvedev, S.; Kinose, Y.; Devins, K.; Verma, P.; et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Matondo, A.; Jo, Y.H.; Shahid, M.; Choi, T.G.; Nguyen, M.N.; Nguyen, N.N.Y.; Akter, S.; Kang, I.; Ha, J.; Maeng, C.H.; et al. The Prognostic 97 Chemoresponse Gene Signature in Ovarian Cancer. Sci. Rep. 2017, 7, 9689. [Google Scholar] [CrossRef]
- Kim, S.I.; Jung, M.; Dan, K.; Lee, S.; Lee, C.; Kim, H.S.; Chung, H.H.; Kim, J.-W.; Park, N.H.; Song, Y.-S.; et al. Proteomic Discovery of Biomarkers to Predict Prognosis of High-Grade Serous Ovarian Carcinoma. Cancers 2020, 12, 790. [Google Scholar] [CrossRef]
- Terraneo, N.; Jacob, F.; Dubrovska, A.; Grünberg, J. Novel Therapeutic Strategies for Ovarian Cancer Stem Cells. Front. Oncol. 2020, 10, 319. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Author Correction: Radiotherapy tox-icity. Nat. Rev. Dis. Primers 2019, 5, 15. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal. Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Xia, Q.; Xu, M.; Zhang, P.; Liu, L.; Meng, X.; Dong, L. Therapeutic Potential of Autophagy in Glioblastoma Treatment With Phosphoinositide 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin Signaling Pathway Inhibitors. Front. Oncol. 2020, 10, 572904. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.B.; Besner, G.E. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 2007, 25, 253–263. [Google Scholar] [CrossRef]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Smith, D.L.; Ram, P.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Bogomolniy, F.; Yee, C.J.; Lash, A.; Barakat, R.R.; Borgen, P.I.; Boyd, J. Frequent Mutation of the PIK3CA Gene in Ovarian and Breast Cancers. Clin. Cancer Res. 2005, 11, 2875–2878. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xu, L.; Tang, H.; Yang, Q.; Yi, X.; Fang, Y.; Zhu, Y.; Wang, Z. The Role of the PTEN/PI3K/Akt Pathway on Prognosis in Epithelial Ovarian Cancer: A Meta-Analysis. Oncol. 2014, 19, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Patch, A.-M.; Christie, E.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole–genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Martorana, F.; Motta, G.; Pavone, G.; Motta, L.; Stella, S.; Vitale, S.R.; Manzella, L.; Vigneri, P. AKT Inhibitors: New Weapons in the Fight Against Breast Cancer? Front. Pharmacol. 2021, 12, 662232. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Deng, J.; Bai, X.; Feng, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expres-sion. BMC Cancer 2019, 19, 618. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.Q.; Sullivan, S.A.; Chan, L.L.; Yin, Y.; Sun, W.; Fang, Z.; Dugar, S.; Zhou, C.; Bae-Jump, V. SPR965, a Dual PI3K/mTOR Inhibitor, as a Targeted Therapy in Ovarian Cancer. Front. Oncol. 2020, 10, 624498. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Liang, W.; Wu, J.; Kowolik, C.M.; Buettner, R.; Scuto, A.; Hsieh, M.Y.; Hong, H.; Brown, C.E.; Forman, S.J.; et al. Targeting JAK1/STAT3 signaling suppresses tumor progression and metastasis in a peritoneal model of human ovarian cancer. Mol. Cancer Ther. 2014, 13, 3037–3048. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.; Danson, S. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef]
- Recio, C.; Guerra, B.; Guerra-Rodríguez, M.; Aranda-Tavío, H.; Martín-Rodríguez, P.; De Mirecki-Garrido, M.; Brito-Casillas, Y.; García-Castellano, J.M.; Estévez-Braun, A.; Fernández-Pérez, L. Signal transducer and activator of transcription (STAT)-5: An opportunity for drug development in oncohematology. Oncogene 2019, 38, 4657–4668. [Google Scholar] [CrossRef]
- Sabaawy, H.E.; Ryan, B.M.; Khiabanian, H.; Pine, S.R. JAK/STAT of all trades: Linking inflammation with cancer development, tumor progression and therapy resistance. Carcinogenesis 2021, 42, 1411–1419. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal. Transduct. Target. Ther. 2021, 6, 1–33. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 1–19. [Google Scholar] [CrossRef]
- Passamonti, F.; Griesshammer, M.; Palandri, F.; Egyed, M.; Benevolo, G.; Devos, T.; Callum, J.; Vannucchi, A.M.; Sivgin, S.; Bensasson, C.; et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): A randomised, open-label, phase 3b study. Lancet Oncol. 2016, 18, 88–99. [Google Scholar] [CrossRef]
- Gritsina, G.; Xiao, F.; O’Brien, S.W.; Gabbasov, R.; Maglaty, M.A.; Xu, R.-H.; Thapa, R.J.; Zhou, Y.; Nicolas, E.; Litwin, S.; et al. Targeted Blockade of JAK/STAT3 Signaling Inhibits Ovarian Carcinoma Growth. Mol. Cancer Ther. 2015, 14, 1035–1047. [Google Scholar] [CrossRef]
- She, S.; Zhao, Y.; Kang, B.; Chen, C.; Chen, X.; Zhang, X.; Chen, W.; Dan, S.; Wang, H.; Wang, Y.J.; et al. Combined inhibition of JAK1/2 and DNMT1 by newly identified small-molecule compounds synergistically suppresses the survival and proliferation of cervical cancer cells. Cell Death Dis. 2020, 11, 724. [Google Scholar] [CrossRef]
- Abubaker, K.; Luwor, R.B.; Escalona, R.; McNally, O.; Quinn, M.A.; Thompson, E.W.; Findlay, J.K.; Ahmed, N. Targeted Disrup-tion of the JAK2/STAT3 Pathway in Combination with Systemic Administration of Paclitaxel Inhibits the Priming of Ovarian Cancer Stem Cells Leading to a Reduced Tumor Burden. Front. Oncol. 2014, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, M.; Sacchetti, A.; Cansoy, M.; Joosten, R.; Teeuwssen, M.; Heijmans-Antonissen, C.; Ewing-Graham, P.C.; Burger, C.W.; Blok, L.J.; Fodde, R. IL6/JAK1/STAT3 Signaling Blockade in Endometrial Cancer Affects the ALDHhi/CD126+ Stem-like Component and Reduces Tumor Burden. Cancer Res. 2015, 75, 3608–3622. [Google Scholar] [CrossRef]
- Nakayamada, S.; Kubo, S.; Iwata, S.; Tanaka, Y. Recent Progress in JAK Inhibitors for the Treatment of Rheumatoid Arthritis. BioDrugs 2016, 30, 407–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond beta-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef]
- Nguyen, V.H.L.; Hough, R.; Bernaudo, S.; Peng, C. Wnt/beta-catenin signalling in ovarian cancer: Insights into its hyperacti-vation and function in tumorigenesis. J. Ovarian Res. 2019, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-W.; Yang, S.-T.; Chien, M.-H.; Hua, K.-T.; Wu, C.-J.; Hsiao, S.M.; Lin, H.; Hsiao, M.; Su, J.-L.; Wei, L.-H. The STAT3-miRNA-92-Wnt Signaling Pathway Regulates Spheroid Formation and Malignant Progression in Ovarian Cancer. Cancer Res. 2017, 77, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, G.F.; Angelico, G.; Santoro, A. Aberrant non-canonical WNT pathway as key-driver of high-grade serous ovarian cancer development. Virchows Arch. 2020, 477, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Doo, D.W.; Meza-Perez, S.; Londono, A.I.; Goldsberry, W.N.; Katre, A.A.; Boone, J.D.; Moore, D.J.; Hudson, C.T.; Betella, I.; McCaw, T.R.; et al. Inhibition of the Wnt/beta-catenin pathway enhances antitumor immunity in ovarian cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920913798. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Obeng, E. Apoptosis (programmed cell death) and its signals—A review. Braz. J. Biol. 2021, 81, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Lowe, S.W.; Cepero, E.; Evan, G. Intrinsic tumour suppression. Nature 2004, 432, 307–315. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Finger, L.R.; Yunis, J.; Nowell, P.C.; Croce, C.M. Cloning of the Chromosome Breakpoint of Neoplastic B Cells with the t(14;18) Chromosome Translocation. Science 1984, 226, 1097–1099. [Google Scholar] [CrossRef]
- Hengartner, M.O. Apoptosis: Corralling the corpses. Cell 2001, 104, 325–328. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Al-Alem, L.F.; Baker, A.T.; Pandya, U.M.; Eisenhauer, E.L.; Rueda, B.R. Understanding and Targeting Apoptotic Pathways in Ovarian Cancer. Cancers 2019, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.T.; Bartholomeusz, C.; Herrmann, J.L.; Estrov, Z.; Shao, R.; Andreeff, M.; Price, J.; Paul, R.W.; Anklesaria, P.; Yu, D.; et al. E1A-mediated paclitaxel sensitization in HER-2/neu-overexpressing ovarian cancer SKOV3.ip1 through apoptosis in-volving the caspase-3 pathway. Clin. Cancer Res. 2000, 6, 250–259. [Google Scholar]
- Brautigam, K.; Bauerschlag, D.O.; Weigel, M.T.; Biernath-Wupping, J.; Bauknecht, T.; Arnold, N.; Maass, N.; Meinhold-Heerlein, I. Combination of enzastaurin and pemetrexed inhibits cell growth and induces apoptosis of chemoresistant ovarian cancer cells regulating extracellular signal-regulated kinase 1/2 phosphorylation. Transl. Oncol. 2009, 2, 164–173. [Google Scholar] [CrossRef]
- Yasmeen, A.; Beauchamp, M.-C.; Piura, E.; Segal, E.; Pollak, M.; Gotlieb, W.H. Induction of apoptosis by metformin in epithelial ovarian cancer: Involvement of the Bcl-2 family proteins. Gynecol. Oncol. 2011, 121, 492–498. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef]
- Wang, J.; Hansen, K.; Edwards, R.; Van Houten, B.; Qian, W. Mitochondrial division inhibitor 1 (mdivi-1) enhances death re-ceptor-mediated apoptosis in human ovarian cancer cells. Biochem Biophys Res. Commun 2015, 456, 7–12. [Google Scholar] [CrossRef]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.S.; Rahman, M.H.; Rasheduzzaman, M.; Mamun-Or-Rashid, A.N.M.; Uddin, M.J.; Rahman, M.R.; Hwang, H.; Pang, M.G.; Rhim, H. Modulatory Effects of Autophagy on APP Processing as a Potential Treatment Target for Alzheimer’s Disease. Biomedicines 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rhim, H. Therapeutic implication of autophagy in neurodegenerative diseases. Bmb. Rep. 2017, 50, 345–354. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Hossain, M.S.; Biswas, P.; Islam, R.; Uddin, M.J.; Rahman, M.H.; Rhim, H. Molecular Insights into the Multifunctional Role of Natural Compounds: Autophagy Modulation and Cancer Prevention. Biomedicines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Saha, S.K.; Rahman, M.S.; Uddin, M.J.; Uddin, M.S.; Pang, M.G.; Rhim, H.; Cho, S.G. Molecular Insights Into Therapeutic Potential of Autophagy Modulation by Natural Products for Cancer Stem Cells. Front. Cell. Dev. Biol. 2020, 8, 283. [Google Scholar] [CrossRef]
- Pu, Z.; Wu, L.; Guo, Y.; Li, G.; Xiang, M.; Liu, L.; Zhan, H.; Zhou, X.; Tan, H. LncRNA MEG3 contributes to adenosine-induced cytotoxicity in hepatoma HepG2 cells by downregulated ILF3 and autophagy inhibition via regulation PI3K-AKT-mTOR and beclin-1 signaling pathway. J. Cell Biochem. 2019, 120, 18172–18185. [Google Scholar] [CrossRef]
- Cai, M.; Hu, Z.; Liu, J.; Gao, J.; Liu, C.; Liu, D.; Tan, M.; Zhang, D.; Lin, B. Beclin 1 Expression in Ovarian Tissues and Its Effects on Ovarian Cancer Prognosis. Int. J. Mol. Sci. 2014, 15, 5292–5303. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, E.; Maiuri, M.C.; Galluzzi, L.; Vitale, I.; Djavaheri-Mergny, M.; D’Amelio, M.; Criollo, A.; Morselli, E.; Zhu, C.; Harper, F.; et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 2008, 10, 676–687. [Google Scholar] [CrossRef]
- He, C.; Levine, B. The Beclin 1 interactome. Curr. Opin. Cell Biol. 2010, 22, 140–149. [Google Scholar] [CrossRef]
- Liang, M.; Zhao, J. Protein expressions of AIB1, p53 and Bcl-2 in epithelial ovarian cancer and their correlations with the clinical pathological features and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5134–5139. [Google Scholar]
- Lu, Z.; Luo, R.Z.; Lu, Y.; Zhang, X.; Yu, Q.; Khare, S.; Kondo, S.; Kondo, Y.; Yu, Y.; Mills, G.B.; et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Investig. 2008, 118, 3917–3929. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Zhang, Y.; Wang, W.Y.; Song, E.X.; Fan, Y.J.; Li, J.; Wei, B. Autophagy as an emerging therapy target for ovarian car-cinoma. Oncotarget 2016, 7, 83476–83487. [Google Scholar] [CrossRef]
- Abdallah, M.; El-Readi, M.; Althubiti, M.; Almaimani, R.; Ismail, A.; Idris, S.; Refaat, B.; Almalki, W.; Babakr, A.; Mukhtar, M.; et al. Tamoxifen and the PI3K Inhibitor: LY294002 Synergistically Induce Apoptosis and Cell Cycle Arrest in Breast Cancer MCF-7 Cells. Molecules 2020, 25, 3355. [Google Scholar] [CrossRef]
- Shariati, M.; Meric-Bernstam, F. Targeting AKT for cancer therapy. Expert Opin. Investig. Drugs 2019, 28, 977–988. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, W.N.; Xue, Y.N.; Zhang, L.C.; Zhang, J.J.; Lu, S.Y.; Yan, X.Y.; Yu, H.M.; Su, J.; Sun, L.K. SIRT3 aggravates metfor-min-induced energy stress and apoptosis in ovarian cancer cells. Exp. Cell Res. 2018, 367, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.-Y.; Kang, J.-S.; Xue, Y.-N.; Yang, X.-C.; Su, J.; Wu, Y.; Yan, X.-Y.; Xu, Y.; Liu, Y.-H.; Yu, C.-Y.; et al. SIRT3 participates in glucose metabolism interruption and apoptosis induced by BH3 mimetic S1 in ovarian cancer cells. Int. J. Oncol. 2016, 49, 773–784. [Google Scholar] [CrossRef]
- Banerjee, S.; Kaye, S.B. New Strategies in the Treatment of Ovarian Cancer: Current Clinical Perspectives and Future Potential. Clin. Cancer Res. 2013, 19, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Bonello, M.; Sims, A.H.; Langdon, S.P. Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer. Cancer Biol. Med. 2018, 15, 375–388. [Google Scholar] [PubMed]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Diaz-Flores, L.; Gutierrez, R.; Garcia-Suarez, M.P.; Saez, F.J.; Gutierrez, E.; Valladares, F.; Carrasco, J.L.; Diaz-Flores, L., Jr.; Madrid, J.F. Morphofunctional basis of the different types of angiogenesis and formation of postnatal angiogenesis-related secondary structures. Histol. Histopathol 2017, 32, 1239–1279. [Google Scholar]
- Siveen, K.S.; Prabhu, K.; Krishnankutty, R.; Kuttikrishnan, S.; Tsakou, M.; Alali, F.; Dermime, S.; Mohammad, R.M.; Uddin, S. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumour Vascularization: Potential and Challenges. Curr. Vasc. Pharmacol. 2017, 15, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Tvorogov, D.; Anisimov, A.; Zheng, W.; Leppänen, V.-M.; Tammela, T.; Laurinavicius, S.; Holnthoner, W.; Heloterä, H.; Holopainen, T.; Jeltsch, M.; et al. Effective Suppression of Vascular Network Formation by Combination of Antibodies Blocking VEGFR Ligand Binding and Receptor Dimerization. Cancer Cell 2010, 18, 630–640. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.-P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Gourley, C.; McNeish, I.; Ledermann, J.; Gore, M.; Jayson, G.; Perren, T.; Rustin, G.; Kaye, S. Targeted anti-vascular therapies for ovarian cancer: Current evidence. Br. J. Cancer 2013, 108, 250–258. [Google Scholar] [CrossRef]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab Combined With Chemotherapy for Platinum-Resistant Recurrent Ovarian Cancer: The AURELIA Open-Label Randomized Phase III Trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Chemotherapy With or Without Bevacizumab in Patients With Platinum-Sensitive Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. J. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Lockhart, A.C.; Rothenberg, M.L.; Dupont, J.; Cooper, W.; Chevalier, P.; Sternas, L.; Buzenet, G.; Koehler, E.; Sosman, J.A.; Schwartz, L.H.; et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J. Clin. Oncol. 2010, 28, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Muthuswamy, S.K.; Gilman, M.; Brugge, J.S. Controlled dimerization of ErbB receptors provides evidence for differential sig-naling by homo- and heterodimers. Mol. Cell Biol. 1999, 19, 6845–6857. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Darcy, K.M.; Clarke-Pearson, D.; Boothby, R.A.; Horowitz, I.R. Evaluation of monoclonal humanized an-ti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with over-expression of HER2: A phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Mamot, C.; Ritschard, R.; Wicki, A.; Stehle, G.; Dieterle, T.; Bubendorf, L.; Hilker, C.; Deuster, S.; Herrmann, R.; Rochlitz, C. Tol-erability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tu-mours: A phase 1 dose-escalation study. Lancet. Oncol. 2012, 13, 1234–1241. [Google Scholar] [CrossRef]
- Cai, W.-Q.; Zeng, L.-S.; Wang, L.-F.; Wang, Y.-Y.; Cheng, J.-T.; Zhang, Y.; Han, Z.-W.; Zhou, Y.; Huang, S.-L.; Wang, X.-W.; et al. The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells. Front. Oncol. 2020, 10, 1249. [Google Scholar] [CrossRef]
- Santos, E.D.S.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; Neto, J.D.B.V.; Júnior, I.J.D.S.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int. J. Pharm. 2020, 592, 120082. [Google Scholar] [CrossRef]
- Vergote, I.B.; Jimeno, A.; Joly, F.; Katsaros, D.; Coens, C.; Despierre, E.; Marth, C.; Hall, M.; Steer, C.B.; Colombo, N.; et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: A European Organisation for Research and Treatment of Can-cer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J. Clin. Oncol 2014, 32, 320–326. [Google Scholar]
- Mak, V.C.; Li, X.; Rao, L.; Zhou, Y.; Tsao, S.W.; Cheung, L.W. p85beta alters response to EGFR inhibitor in ovarian cancer through p38 MAPK-mediated regulation of DNA repair. Neoplasia 2021, 23, 718–730. [Google Scholar] [CrossRef]
- Stebbins, E.; Russo, A.A.; Schneider, C.; Rosen, N.; Hartl, F.; Pavletich, N.P. Crystal Structure of an Hsp90–Geldanamycin Complex: Targeting of a Protein Chaperone by an Antitumor Agent. Cell 1997, 89, 239–250. [Google Scholar] [CrossRef]
- Garcia-Morales, P.; Carrasco-Garcia, E.; Ruiz-Rico, P.; Martinez-Mira, R.; Menendez-Gutierrez, M.P.; Ferragut, J.A.; Saceda, M.; Martinez-Lacaci, I. Inhibition of Hsp90 function by ansamycins causes downregulation of cdc2 and cdc25c and G(2)/M arrest in glioblastoma cell lines. Oncogene 2007, 26, 7185–7193. [Google Scholar] [CrossRef]
- Schulte, T.W.; Neckers, L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998, 42, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Munster, P.N.; Marchion, D.C.; Basso, A.D.; Rosen, N. Degradation of HER2 by ansamycins induces growth arrest and apop-tosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res. 2002, 62, 3132–3137. [Google Scholar] [PubMed]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt Forms an Intracellular Complex with Heat Shock Protein 90 (Hsp90) and Cdc37 and Is Destabilized by Inhibitors of Hsp90 Function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Battelli, C.; Watson, J.; Liu, J.; Curtis, J.; Morse, A.N.; Matulonis, U.A.; Chowdhury, D.; Konstantinopoulos, P.A. Sub-lethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget 2014, 5, 2678–2687. [Google Scholar] [CrossRef]
- Palombella, V.J.; Rando, O.J.; Goldberg, A.L.; Maniatis, T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 1994, 78, 773–785. [Google Scholar] [CrossRef]
- Langer, C.J.; O’Byrne, K.J.; Socinski, M.A.; Mikhailov, S.M.; Lesniewski-Kmak, K.; Smakal, M.; Ciuleanu, T.E.; Orlov, S.V.; Dediu, M.; Heigener, D.; et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 623–630. [Google Scholar] [CrossRef]
- Vermunt, M.A.; Bergman, A.M.; van der Putten, E.; Beijnen, J.H. The intravenous to oral switch of taxanes: Strategies and current clinical developments. Futur. Oncol. 2021, 17, 1379–1399. [Google Scholar] [CrossRef]
- Scholler, N.; Urban, N. CA125 in ovarian cancer. Biomark. Med. 2007, 1, 513–523. [Google Scholar] [CrossRef]
- Funston, G.; Hamilton, W.; Abel, G.; Crosbie, E.J.; Rous, B.; Walter, F.M. The diagnostic performance of CA125 for the detection of ovarian and non-ovarian cancer in primary care: A population-based cohort study. PLoS Med. 2020, 17, e1003295. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.W.; Dnistrian, A.; Lloyd, K.O. Ovarian cancer antigen CA125 is encoded by theMUC16 mucin gene. Int. J. Cancer 2002, 98, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Frietze, K.M.; Roden, R.B.; Lee, J.-H.; Shi, Y.; Peabody, D.S.; Chackerian, B. Identification of Anti-CA125 Antibody Responses in Ovarian Cancer Patients by a Novel Deep Sequence–Coupled Biopanning Platform. Cancer Immunol. Res. 2015, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.; Köhler, S.; Reinartz, S.; Giffels, P.; Huober, J.; Renke, K.; Schlebusch, H.; Biersack, H.J.; Möbus, V.; Kreienberg, R.; et al. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA125: Immune responses and survival in palliative treatment. See The biology behind: K. A. Foon and M. Bhattacharya-Chatterjee, Are solid tumor anti-idiotype vaccines ready for prime time? Clin. Cancer Res. 2001, 7, 1112–1115. [Google Scholar]
- Palaia, I.; Tomao, F.; Sassu, C.M.; Musacchio, L.; Panici, P.B. Immunotherapy For Ovarian Cancer: Recent Advances And Combination Therapeutic Approaches. OncoTargets Ther. 2020, 13, 6109–6129. [Google Scholar] [CrossRef] [PubMed]
- Raave, R.; de Vries, R.B.; Massuger, L.F.; van Kuppevelt, T.H.; Daamen, W.F. Drug delivery systems for ovarian cancer treat-ment: A systematic review and meta-analysis of animal studies. PeerJ. 2015, 3, e1489. [Google Scholar] [CrossRef]
- Zeng, Q.; Saha, S.; Lee, L.A.; Barnhill, H.; Oxsher, J.; Dreher, T.; Wang, Q. Chemoselective modification of turnip yellow mosaic virus by Cu(I) catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction and its application in cell binding. Bioconjug. Chem. 2011, 22, 58–66. [Google Scholar] [CrossRef]
- Ye, H.; Karim, A.A.; Loh, X.J. Current treatment options and drug delivery systems as potential therapeutic agents for ovarian cancer: A review. Mater. Sci. Eng. C 2014, 45, 609–619. [Google Scholar] [CrossRef]
- Manchester, M.; Singh, P. Virus-based nanoparticles (VNPs): Platform technologies for diagnostic imaging. Adv. Drug Deliv. Rev. 2006, 58, 1505–1522. [Google Scholar] [CrossRef]
- Levit, S.; Tang, C. Polymeric Nanoparticle Delivery of Combination Therapy with Synergistic Effects in Ovarian Cancer. Nanomaterials 2021, 11, 1048. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, S.; Sharma, V.; Singh, C. Poly (Lactic-co-Glycolic Acid) & Tocopheryl Polyethylene Glycol Succinate Nanoparticles for the Treatment of Different Brain Cancers. Anti-Cancer Agents Med. Chem. 2021, 21, 1977–1986. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rother, M.; Nussbaumer, M.G.; Renggli, K.; Bruns, N. Protein cages and synthetic polymers: A fruitful symbiosis for drug delivery applications, bionanotechnology and materials science. Chem. Soc. Rev. 2016, 45, 6213–6249. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef]
- Paraskar, A.S.; Soni, S.; Chin, K.T.; Chaudhuri, P.; Muto, K.W.; Berkowitz, J.; Handlogten, M.W.; Alves, N.J.; Bilgicer, B.; Dinulescu, D.M.; et al. Harnessing structure-activity relationship to engineer a cisplatin nanoparticle for enhanced antitumor efficacy. Proc. Natl. Acad. Sci. USA 2010, 107, 12435–12440. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cho, T.J.; Tsai, D.H.; Liu, J.; Pettibone, J.M.; You, R.; Hackley, V.A.; Zachariah, M.R. Surface Modification of Cispla-tin-Complexed Gold Nanoparticles and Its Influence on Colloidal Stability, Drug Loading, and Drug Release. Langmuir 2018, 34, 154–163. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Yuan, D.-M.; Lv, Y.-L.; Yao, Y.-W.; Miao, X.-H.; Wang, Q.; Xiao, X.-W.; Yin, J.; Shi, Y.; Shi, M.-Q.; Zhang, X.-W.; et al. Efficacy and safety of Abraxane in treatment of progressive and recurrent non-small cell lung cancer patients: A retrospective clinical study. Thorac. Cancer 2012, 3, 341–347. [Google Scholar] [CrossRef]
- Kundranda, M.N.; Niu, J. Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. Dev. Ther. 2015, 9, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhao, G.; Yu, L.; Gough, D.; Howell, S.B. Preclinical efficacy studies of a novel nanoparticle-based formulation of paclitaxel that out-performs Abraxane. Cancer Chemother. Pharmacol. 2009, 65, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wen, H.; Wen, Q.; Chen, X.; Wang, Y.; Xuan, W.; Liang, J.; Wan, S. Cucumber mosaic virus as drug delivery vehicle for doxorubicin. Biomaterials 2013, 34, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Khan, R.U.; Asif, H.; Alamgeer; Khalid, S.H.; Asghar, S.; Saleem, M.; Shah, K.U.; Shah, S.U.; Rizvi, S.A.A.; et al. Co-delivery strategies to overcome multidrug resistance in ovarian cancer. Int. J. Pharm. 2017, 533, 111–124. [Google Scholar] [CrossRef]
- Devalapally, H.; Duan, Z.; Seiden, M.V.; Amiji, M.M. Paclitaxel and ceramide co-administration in biodegradable polymeric nanoparticulate delivery system to overcome drug resistance in ovarian cancer. Int. J. Cancer 2007, 121, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Md, S.; Kesharwani, P. RGD engineered dendrimer nanotherapeutic as an emerging targeted approach in cancer therapy. J. Control. Release 2021, 340, 221–242. [Google Scholar] [CrossRef]
- Shah, V.; Taratula, O.; Garbuzenko, O.B.; Taratula, O.R.; Rodriguez-Rodriguez, L.; Minko, T. Targeted nanomedicine for sup-pression of CD44 and simultaneous cell death induction in ovarian cancer: An optimal delivery of siRNA and anticancer drug. Clin. Cancer Res. 2013, 19, 6193–6204. [Google Scholar] [CrossRef]
- Bholakant, R.; Qian, H.; Zhang, J.; Huang, X.; Huang, D.; Feijen, J.; Zhong, Y.; Chen, W. Recent Advances of Polycationic siRNA Vectors for Cancer Therapy. Biomacromolecules 2020, 21, 2966–2982. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer popu-lation screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strat-egies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Luvero, D.; Milani, A.; Ledermann, J.A. Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 229–239. [Google Scholar] [CrossRef]
- Sazonova, E.V.; Kopeina, G.S.; Imyanitov, E.N.; Zhivotovsky, B. Platinum drugs and taxanes: Can we overcome resistance? Cell Death Discov. 2021, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, Y.K. Cancer Stem Cells as a Potential Target to Overcome Multidrug Resistance. Front. Oncol. 2020, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, C.; Huang, Y.; He, M.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zeng, W.; Wan, C.; Duan, S.; Jiang, S. microRNA-137 promotes apoptosis in ovarian cancer cells via the regu-lation of XIAP. Br. J. Cancer 2017, 116, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Mu, T.; Jiang, J.; Sun, Q.; Hou, X.; Sun, Y.; Zhong, L.; Wang, C.; Sun, C. Identification of Potential Biomarkers and Metabolic Profiling of Serum in Ovarian Cancer Patients Using UPLC/Q-TOF MS. Cell. Physiol. Biochem. 2018, 51, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Choi, T.G.; Nguyen, M.N.; Matondo, A.; Kim, J.-H.; Jo, Y.H.; Jo, A.; Shahid, M.; Jun, D.Y.; Yoo, J.Y.; et al. Prognostic value of a 92-probe signature in breast cancer. Oncotarget 2015, 6, 15662–15680. [Google Scholar] [CrossRef][Green Version]
- Soto, J.A.; Rodriguez-Antolin, C.; Vera, O.; Pernia, O.; Esteban-Rodriguez, I.; Dolores Diestro, M.; Benitez, J.; Sanchez-Cabo, F.; Alvarez, R.; De Castro, J.; et al. Transcriptional epigenetic regulation of Fkbp1/Pax9 genes is associated with impaired sensitivity to platinum treatment in ovarian cancer. Clin. Epigenetics 2021, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Häfner, N.; Steinbach, D.; Jansen, L.; Diebolder, H.; Dürst, M.; Runnebaum, I.B. RUNX3 and CAMK2N1 hypermethylation as prognostic marker for epithelial ovarian cancer. Int. J. Cancer 2016, 138, 217–228. [Google Scholar] [CrossRef]
- Tassi, R.A.; Todeschini, P.; Siegel, E.R.; Calza, S.; Cappella, P.; Ardighieri, L.; Cadei, M.; Bugatti, M.; Romani, C.; Bandiera, E.; et al. FOXM1 expression is significantly as-sociated with chemotherapy resistance and adverse prognosis in non-serous epithelial ovarian cancer patients. J. Exp. Clin. Cancer Res. 2017, 36, 63. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martin, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Ad-vanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Oza, A.M.; Matulonis, U.A.; Malander, S.; Hudgens, S.; Sehouli, J.; Del Campo, J.M.; Berton-Rigaud, D.; Banerjee, S.; Scambia, G.; Berek, J.S. er al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (EN-GOT-OV16/NOVA): Results from a double-blind, phase 3, randomised controlled trial. Lancet. Oncol. 2018, 19, 1117–1125. [Google Scholar] [CrossRef]
- Longo, D.L. Personalized Medicine for Primary Treatment of Serous Ovarian Cancer. New Engl. J. Med. 2019, 381, 2471–2474. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Shay, J.; Zeng, T.-C.; Chou, J.-L.; Huang, T.H.-M.; Lai, H.-C.; Chan, M. Epigenetic silencing of ARNTL, a circadian gene and potential tumor suppressor in ovarian cancer. Int. J. Oncol. 2014, 45, 2101–2107. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Liu, X.; Ma, Z.; Lv, L. PER1 Is a Prognostic Biomarker and Correlated With Immune Infiltrates in Ovarian Cancer. Front. Genet. 2021, 12, 984. [Google Scholar] [CrossRef]
- Heinze, K.; Rengsberger, M.; Gajda, M.; Jansen, L.; Osmers, L.; Oliveira-Ferrer, L.; Schmalfeldt, B.; Durst, M.; Hafner, N.; Runnebaum, I.B. CAMK2N1/RUNX3 methylation is an independent prognostic biomarker for progression-free and overall survival of platinum-sensitive epithelial ovarian cancer patients. Clin. Epigenetics 2021, 13, 15. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Chang, T.-H.; Huang, Y.-F.; Huang, H.-D.; Chou, C.-Y. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene 2013, 33, 3432–3440. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, J.; Quan, C.; Wen, H.; Feng, Z.; Hu, Q.; Zhu, J.; Huang, Y.; Wu, X. circCELSR1 (hsa_circ_0063809) Contributes to Paclitaxel Resistance of Ovarian Cancer Cells by Regulating FOXR2 Expression via miR-1252. Mol. Ther. Nucleic Acids 2020, 19, 718–730. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Oh, Y.; Kim, J.; Lee, S.; Chae, H. Increased Expression of Retinol-Binding Protein 4 in Ovarian Endometrioma and Its Possible Role in the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2021, 22, 5827. [Google Scholar] [CrossRef] [PubMed]
- Avril, S.; Dincer, Y.; Malinowsky, K.; Wolff, C.; Gündisch, S.; Hapfelmeier, A.; Boxberg, M.; Bronger, H.; Becker, K.-F.; Schmalfeldt, B. Increased PDGFR-beta and VEGFR-2 protein levels are associated with resistance to platinum-based chemotherapy and adverse outcome of ovarian cancer patients. Oncotarget 2017, 8, 97851–97861. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sundfeldt, K.; Borrebaeck, C.; Jakobsson, M. Comprehending the Proteomic Landscape of Ovarian Cancer: A Road to the Discovery of Disease Biomarkers. Proteomes 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Natanzon, Y.; Goode, E.L.; Cunningham, J.M. Epigenetics in ovarian cancer. Semin. Cancer Biol. 2017, 51, 160–169. [Google Scholar] [CrossRef]
| Biomarker/ Drug/ Inhibitor | Treatment Strategies/ Components | Therapeutic Response | Features/ Properties/ Nature | Detection Level | Supported Technologies | Refs. |
|---|---|---|---|---|---|---|
| PARP inhibitor | Extended PFS | OC, phase 3 trial | Personalized medicine | HRD-positive tumors | [164,165,166] | |
| PARP inhibitor, bevacizumab | PFS benefit, anti-VEGF |
OC, phase 3 trial | Antiangiogenic | HRD-positive tumors, BRCA mutation | [100] | |
| Combination of PARP and ATR inhibitor | Overcomes PARPi and platinum resistance | OC, PDX models | Stabilize stressed replication fork and apoptosis | DNA, protein | Western blot, IHC, NGS, RPPA | [17] |
| ARNTL | Epi-biomarker by reducing promoter methylation | OC | Circadian and tumor-suppressor gene | DNA | CpG island microarray, COBRA, ChIP-PCR | [167,168] |
| RUNX3/CAMK2N1 | Epigenetic prognostic marker | EOC | Hypermethylation of CpG island reduces PFS | DNA | GWA and targeted NGBS confirming array | [162,169] |
| Fkbp1/Pax9 | Epi-biomarker for platinum-resistant therapeutic target | OC | PAX9 hypermethylation causes a poor prognosis for OS | DNA, RNA | Sanger sequencing, RT-PCR | [161] |
| COL11A1 | Promotes tumor progression through TGF-β1–MMP3 axis and predicts poor prognosis | OC | Disease-progression-associated gene | mRNA | Microarray, RT-PCR, casein zymography, and ChIP assay | [170] |
| circCELSR1 | Increases paclitaxel resistance and poor prognosis | OC | Circular RNA | miRNA | Microarray analysis and RT-qPCR | [171] |
| microRNA-137 | Promotes apoptosis; represses mRNA translation | Improves drug resistance | Regulating RNA | Short non-coding RNA | Dual-luciferase reporter assay | [158] |
| FOXM1 | Prognostic and chemoresistant therapeutic target | Non-serous EOC | Oncogene | mRNA, protein | Microarray, RT-qPCR, and IHC | [163] |
| RBP4 | Diagnostic or prognostic biomarker | Ovarian endometrioma | Adipokine RBP4 involved in the pathogenesis of endometriosis | Protein | Human XL proteome profile assay, IHC, cell viability, and invasiveness assay | [172] |
| AAT, NFKB, PMVK, VAP1, FABP4, and PF4 | Predicts prognosis | HGSOC | Differentially expressed proteins | Protein | Hierarchical clustering, bioinformatics, LC-MS, and IHC | [19] |
| Serotransferrin, amyloid A1, hemopexin, C-reactive protein, albumin | Multimarker test specific for screening and detection of OC | OC | Molecular signaling pathways of OC | Protein | ITRAQ-tagging coupled with mass spectrometry | [16] |
| PDGFR-beta and VEGFR-2 | Predictive biomarker for treatment response | OC | Angiogenesis-related growth factor receptors | mRNA Protein | Quantitative RPPA, bioinformatic analysis | [173] |
| Circulatory protein | Personalized therapy for early diagnosis and prediction of drug resistance | OC | Proteomic landscape | Protein | Proteomic | [174] |
| 2-piperidinone and 1-heptadecanoylglycerophosphoethanolamine | Clinical diagnosis and treatment | OC | Candidate biomarker | Metabolites | UPLC/Q-TOF MS | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, S.; Rahman, M.A.; Hasan, M.N.; Akhter, H.; Noor, P.; Islam, R.; Shin, Y.; Rahman, M.H.; Gazi, M.S.; Huda, M.N.; et al. Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements. Cells 2022, 11, 650. https://doi.org/10.3390/cells11040650
Akter S, Rahman MA, Hasan MN, Akhter H, Noor P, Islam R, Shin Y, Rahman MH, Gazi MS, Huda MN, et al. Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements. Cells. 2022; 11(4):650. https://doi.org/10.3390/cells11040650
Chicago/Turabian StyleAkter, Salima, Md. Ataur Rahman, Mohammad Nazmul Hasan, Hajara Akhter, Priya Noor, Rokibul Islam, Yoonhwa Shin, MD. Hasanur Rahman, Md. Shamim Gazi, Md Nazmul Huda, and et al. 2022. "Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements" Cells 11, no. 4: 650. https://doi.org/10.3390/cells11040650
APA StyleAkter, S., Rahman, M. A., Hasan, M. N., Akhter, H., Noor, P., Islam, R., Shin, Y., Rahman, M. H., Gazi, M. S., Huda, M. N., Nam, N. M., Chung, J., Han, S., Kim, B., Kang, I., Ha, J., Choe, W., Choi, T. G., & Kim, S. S. (2022). Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements. Cells, 11(4), 650. https://doi.org/10.3390/cells11040650









