Angiogenesis Driven by the CEBPD–hsa-miR-429–VEGFA Signaling Axis Promotes Urothelial Carcinoma Progression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Exogenous CEBPD Overexpression in BFTC909 and TCCSUP
2.3. Transfection of hsa-miR-429 Mimic and Inhibitor
2.4. Total RNA Isolation and Evaluation by Real-Time Quantitative RT-PCR
2.5. Immunoblotting Assay
2.6. Luciferase Reporter Assays
2.7. HUVECs Tube Formation Assay
2.8. Human Tumor Tissue Specimens
2.9. Immunohistochemistry (IHC)
2.10. In Situ Hybridization Detection of miRNA Using LNA™ Oligonucleotides
2.11. Statistics
3. Results
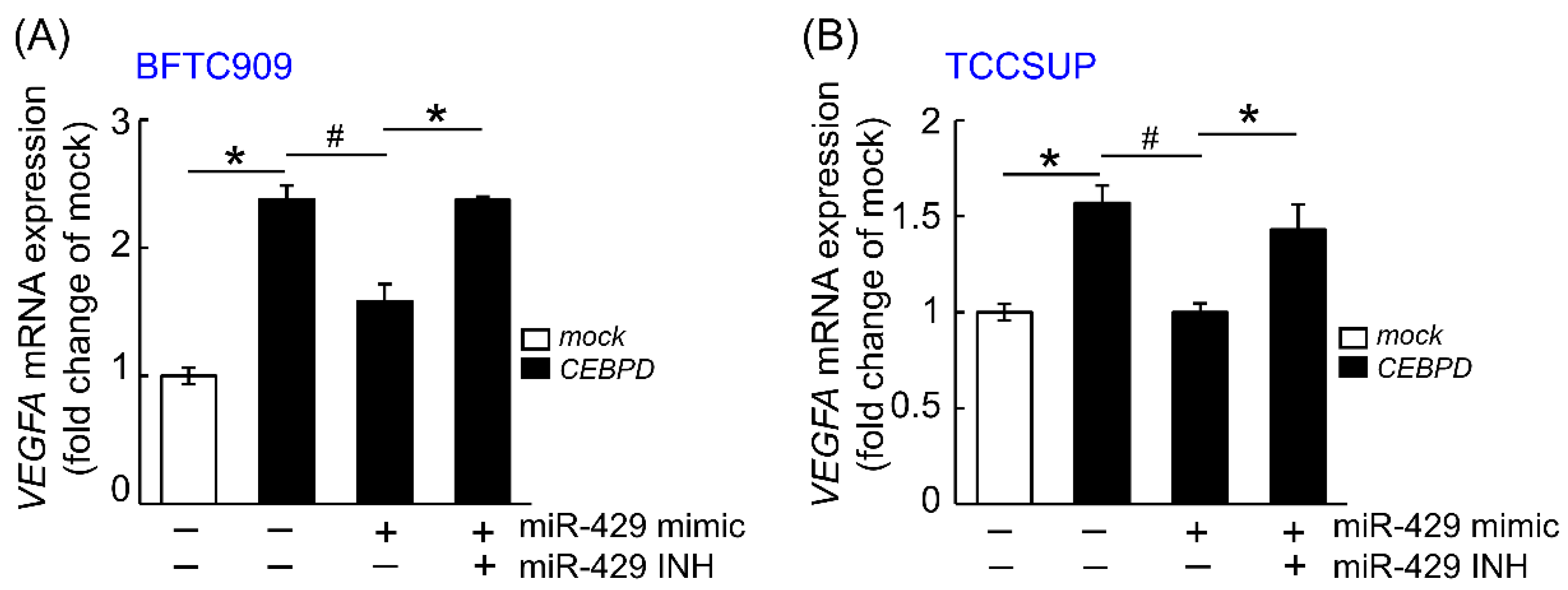
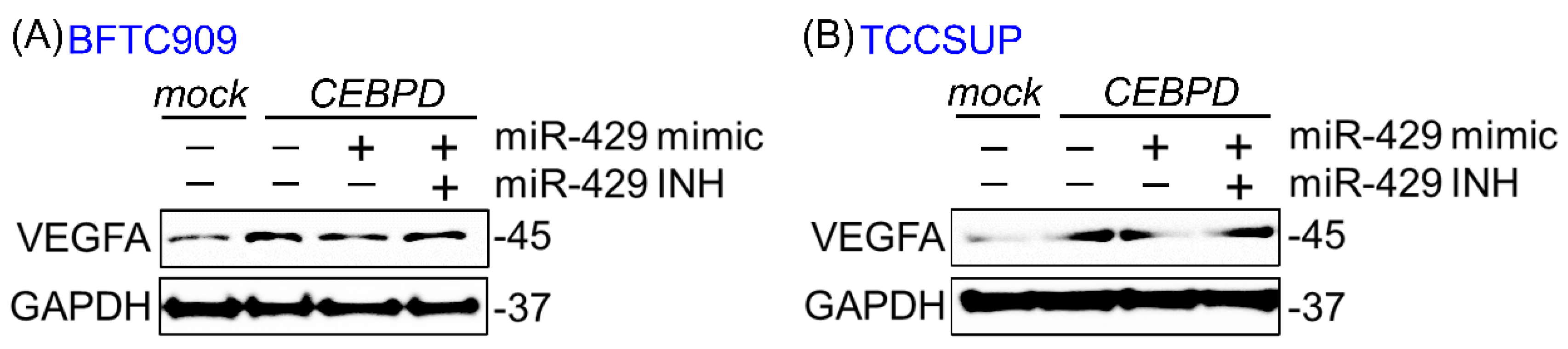
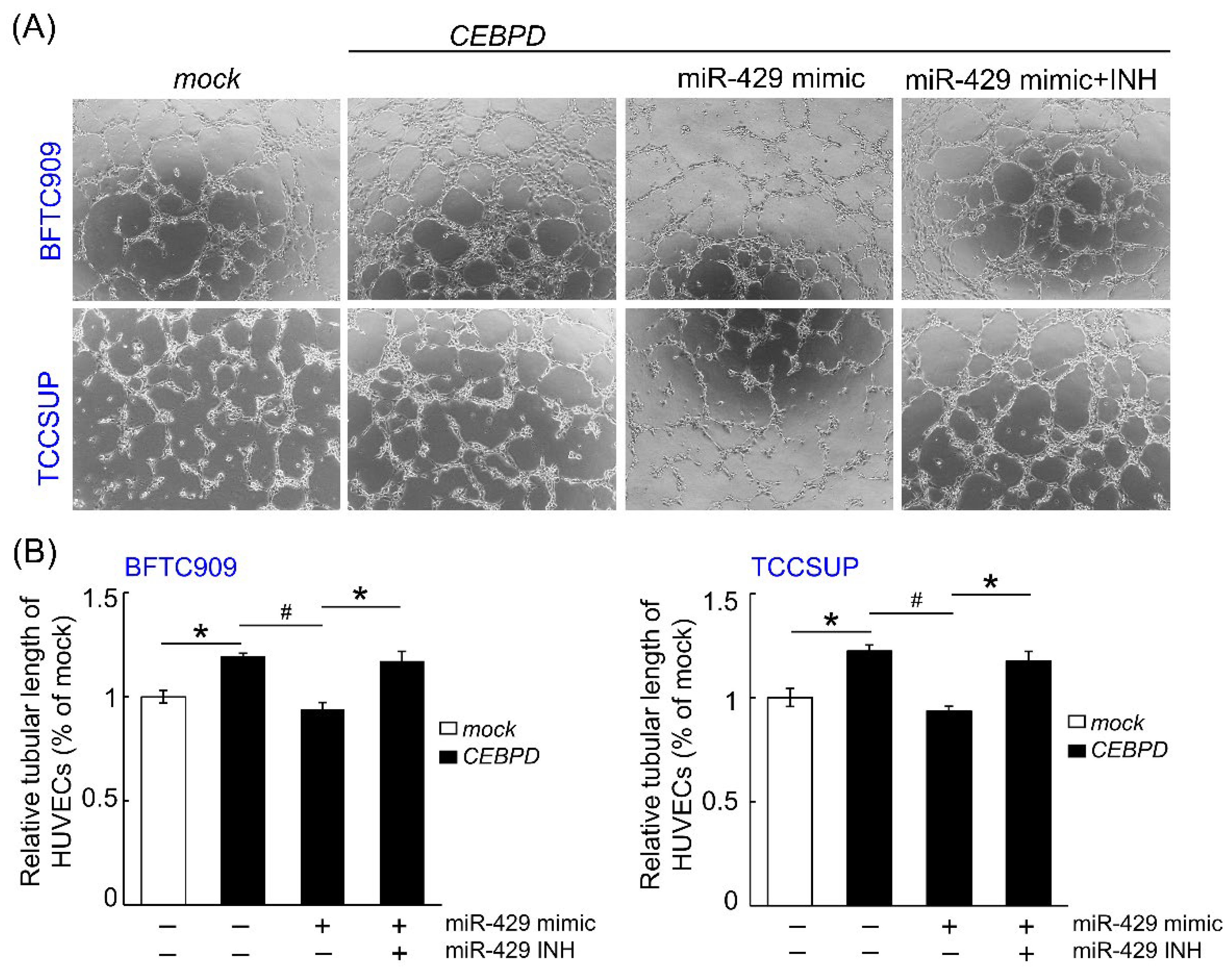
3.1. hsa-miR-429 Inhibits the Expression of VEGFA and Angiogenesis in BFTC909 and TCCSUP by Directly Targeting VEGFA mRNA
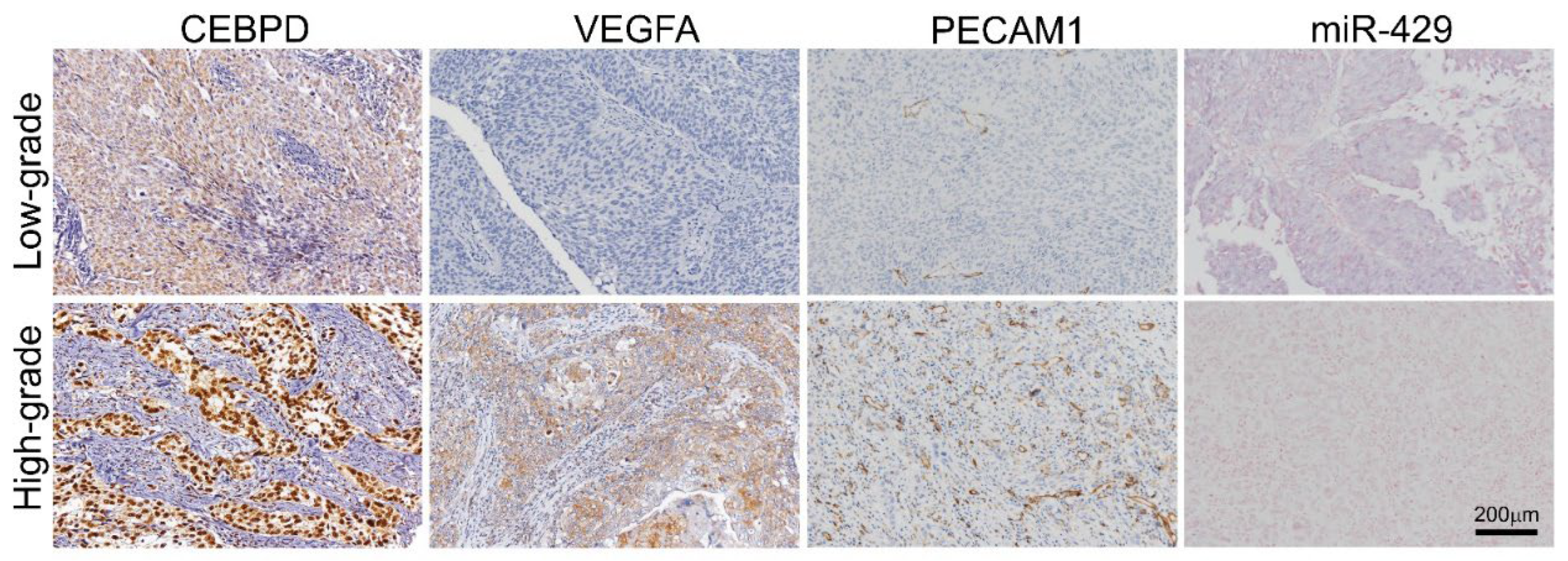
3.2. UBUC and UTUC Patients with High Expression of VEGFA and High Microvascular Density Are Usually Accompanied by Higher CEBPD Expression, Lower hsa-miR-429 and Inferior Survival Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, P.E.; Agarwal, N.; Biagioli, M.C.; Eisenberger, M.A.; Greenberg, R.E.; Herr, H.W.; Inman, B.A.; Kuban, D.A.; Kuzel, T.M.; Lele, S.M.; et al. Bladder cancer. J. Natl. Compr. Canc. Netw. 2013, 11, 446–475. [Google Scholar] [CrossRef] [Green Version]
- Taiwan Cancer Registry; Bureau of Health Promotion, Dept. of Health, Taiwan. The Incidence of Renal Pelvic and Ureteral Tumor in Taiwan. Available online: https://cris.bhp.doh.gov.tw/pagepub/Home.aspx?itemNo=cr.q.10 (accessed on 11 December 2021).
- Chen, C.-H.; Dickman, K.; Moriya, M.; Zavadil, J.; Sidorenko, V.; Edwards, K.; Gnatenko, D.; Wu, L.; Turesky, R.; Wu, X.-R.; et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc. Natl. Acad. Sci. USA 2012, 109, 8241–8246. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Compérat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Rouprêt, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef]
- Cancer Research UK. Bladder Cancer > Types, Stages and Grades. Available online: https://www.cancerresearchuk.org/about-cancer/bladder-cancer/types-stages-grades (accessed on 11 December 2021).
- Seront, E.; Machiels, J.P. Molecular biology and targeted therapies for urothelial carcinoma. Cancer Treat Rev. 2015, 41, 341–353. [Google Scholar] [CrossRef]
- Yassaie, O.; Chehroudi, C.; Black, P.C. Novel and emerging approaches in the management of non-muscle invasive urothelial carcinoma. Adv. Med. Oncol. 2021, 13, 17588359211039052. [Google Scholar] [CrossRef]
- National Collaborating Centre for Cancer (UK). Bladder Cancer: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- Chan, T.-C.; Chen, Y.-T.; Tan, K.T.; Wu, C.-L.; Wu, W.-J.; Li, W.-M.; Wang, J.-M.; Shiue, Y.-L.; Li, C.-F. Biological significance of MYC and CEBPD coamplification in urothelial carcinoma: Multilayered genomic, transcriptional and posttranscriptional positive feedback loops enhance oncogenic glycolysis. Clin. Transl. Med. 2021, 11, e674. [Google Scholar] [CrossRef]
- Balamurugan, K.; Sterneck, E. The Many Faces of C/EBPδ and their Relevance for Inflammation and Cancer. Int. J. Biol. Sci. 2013, 9, 917–933. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.-Y.; Chang, W.-C.; Wang, J.-M. Biological roles of CCAAT/Enhancer-binding protein delta during inflammation. J. Biomed. Sci. 2015, 22, 6. [Google Scholar] [CrossRef] [Green Version]
- Sheshadri, N.; Sharan, S.; Sterneck, E. CEBPD is an early endoplasmic reticulum stress response gene implicated in breast cancer cell survival. FASEB J. 2017, 31. [Google Scholar] [CrossRef]
- Wang, S.-M.; Lin, W.-C.; Lin, H.-Y.; Chen, Y.-L.; Ko, C.-Y.; Wang, J.-M. CCAAT/Enhancer-binding protein delta mediates glioma stem-like cell enrichment and ATP-binding cassette transporter ABCA1 activation for temozolomide resistance in glioblastoma. Cell Death Discov. 2021, 7, 8. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, C.F.; Chu, Y.Y.; Wang, Y.H.; Hour, T.C.; Yen, C.J.; Chang, W.C.; Wang, J.M. Inhibition of the EGFR/STAT3/CEBPD Axis Reverses Cisplatin Cross-resistance with Paclitaxel in the Urothelial Carcinoma of the Urinary Bladder. Clin. Cancer Res. 2017, 23, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhu, Z.; Lin, Z.; Luo, Y.; Liang, Z.; Zhang, C.; Chen, J.; Peng, P. miR-429 suppresses cell proliferation, migration and invasion in nasopharyngeal carcinoma by downregulation of TLN1. Cancer Cell Int. 2019, 19, 115. [Google Scholar] [CrossRef]
- Chan, T.C.; Wu, W.J.; Li, W.M.; Shiao, M.S.; Shiue, Y.L.; Li, C.F. SLC14A1 prevents oncometabolite accumulation and recruits HDAC1 to transrepress oncometabolite genes in urothelial carcinoma. Theranostics 2020, 10, 11775–11793. [Google Scholar] [CrossRef]
- Li, C.F.; Tsai, H.H.; Ko, C.Y.; Pan, Y.C.; Yen, C.J.; Lai, H.Y.; Yuh, C.H.; Wu, W.C.; Wang, J.M. HMDB and 5-AzadC Combination Reverses Tumor Suppressor CCAAT/Enhancer-Binding Protein Delta to Strengthen the Death of Liver Cancer Cells. Mol. Cancer 2015, 14, 2623–2633. [Google Scholar] [CrossRef] [Green Version]
- Sanford, D.C.; DeWille, J.W. C/EBPdelta is a downstream mediator of IL-6 induced growth inhibition of prostate cancer cells. Prostate 2005, 63, 143–154. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, X.; Li, Y.; Guo, M.; Zhao, W.; Qiu, J.; Zheng, Y.; Meng, M.; Ping, X.; Chen, X.; et al. C/EBPδ-Slug-Lox1 axis promotes metastasis of lung adenocarcinoma via oxLDL uptake. Oncogene 2020, 39, 833–848. [Google Scholar] [CrossRef]
- Balamurugan, K.; Mendoza-Villanueva, D.; Sharan, S.; Summers, G.H.; Dobrolecki, L.E.; Lewis, M.T.; Sterneck, E. C/EBPδ links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene 2019, 38, 3765–3780. [Google Scholar] [CrossRef]
- Hartl, L.; Duitman, J.; Aberson, H.L.; Chen, K.; Dijk, F.; Roelofs, J.; Dings, M.P.G.; Hooijer, G.K.J.; Hernanda, P.Y.; Pan, Q.; et al. CCAAT/Enhancer-Binding Protein Delta (C/EBPδ): A Previously Unrecognized Tumor Suppressor that Limits the Oncogenic Potential of Pancreatic Ductal Adenocarcinoma Cells. Cancers 2020, 12, 2546. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wu, W.J.; Wang, W.J.; Huang, H.Y.; Li, W.M.; Yeh, B.W.; Wu, T.F.; Shiue, Y.L.; Sheu, J.J.; Wang, J.M.; et al. CEBPD amplification and overexpression in urothelial carcinoma: A driver of tumor metastasis indicating adverse prognosis. Oncotarget 2015, 6, 31069–31084. [Google Scholar] [CrossRef] [Green Version]
- Chuang, C.H.; Wang, W.J.; Li, C.F.; Ko, C.Y.; Chou, Y.H.; Chuu, C.P.; Cheng, T.L.; Wang, J.M. The combination of the prodrugs perforin-CEBPD and perforin-granzyme B efficiently enhances the activation of caspase signaling and kills prostate cancer. Cell Death Dis. 2014, 5, e1220. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-H.; Huang, H.-S.; Wu, P.-T.; Jou, I.M.; Pan, M.-H.; Chang, W.-C.; Wang, D.D.H.; Wang, J.-M. Role of macrophage CCAAT/enhancer binding protein delta in the pathogenesis of rheumatoid arthritis in collagen-induced arthritic mice. PLoS ONE 2012, 7, e45378. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015, 15, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.L.; Ho, J.Y.; Hung, S.H.; Yu, D.S. miR-429 expression in bladder cancer and its correlation with tumor behavior and clinical outcome. Kaohsiung J. Med. Sci. 2018, 34, 335–340. [Google Scholar] [CrossRef]
- Wu, C.-L.; Ho, J.-Y.; Chou, S.-C.; Yu, D.-S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget 2016, 7, 26593–26603. [Google Scholar] [CrossRef]
- Guo, C.; Zhao, D.; Zhang, Q.; Liu, S.; Sun, M.-Z. miR-429 suppresses tumor migration and invasion by targeting CRKL in hepatocellular carcinoma via inhibiting Raf/MEK/ERK pathway and epithelial-mesenchymal transition. Sci. Rep. 2018, 8, 2375. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.-C.; Huang, Y.; Mu, Z.-Y.; Lv, H.; Xie, Q.-P.; Wang, K.; Hu, B. Efficacy and Safety of Chemotherapy Regimens in Advanced or Metastatic Bladder and Urothelial Carcinomas: An Updated Network Meta-Analysis. Front. Pharmacol. 2020, 10. [Google Scholar] [CrossRef]
- Chen, B.; Jin, H.; Wu, K. Potential role of vascular targeted therapy to combat against tumor. Expert Opin. Drug Deliv. 2009, 6, 719–726. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2012, 153, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Int. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, A.; Gruber, D.; Pisarsky, L.; Heck, C.; Kunita, A.; Yilmaz, M.; Meyer-Schaller, N.; Cornille, K.; Hopfer, U.; Bentires-alj, M.; et al. VEGF-Mediated Angiogenesis Links EMT-Induced Cancer Stemness to Tumor Initiation. Cancer Res. 2014, 74, 1566–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.J.; Qi, W.X.; He, A.N.; Sun, Y.J.; Shen, Z.; Yao, Y. Prognostic value of tissue vascular endothelial growth factor expression in bladder cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 645–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaravinos, A.; Volanis, D.; Lambrou, G.I.; Delakas, D.; Spandidos, D.A. Role of the angiogenic components, VEGFA, FGF2, OPN and RHOC, in urothelial cell carcinoma of the urinary bladder. Oncol. Rep. 2012, 28, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.; Srinivas, S. Incorporating VEGF-targeted therapy in advanced urothelial cancer. Ther. Adv. Med. Oncol. 2017, 9, 33–45. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Tebbutt, N.C.; Kabbinavar, F.; Giantonio, B.J.; Guan, Z.-Z.; Mitchell, L.; Waterkamp, D.; Tabernero, J. Efficacy and safety of bevacizumab in metastatic colorectal cancer: Pooled analysis from seven randomized controlled trials. Oncologist 2013, 18, 1004–1012. [Google Scholar] [CrossRef] [Green Version]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- Petrylak, D.P.; de Wit, R.; Chi, K.N.; Drakaki, A.; Sternberg, C.N.; Nishiyama, H.; Castellano, D.; Hussain, S.A.; Fléchon, A.; Bamias, A.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): Overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2020, 21, 105–120. [Google Scholar] [CrossRef]
- Hahn, N.M.; Stadler, W.M.; Zon, R.T.; Waterhouse, D.; Picus, J.; Nattam, S.; Johnson, C.S.; Perkins, S.M.; Waddell, M.J.; Sweeney, C.J. Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J. Clin. Oncol. 2011, 29, 1525–1530. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Ballman, K.A.; Halabi, S.; Atherton, P.J.; Mortazavi, A.; Sweeney, C.; Stadler, W.M.; Teply, B.A.; Picus, J.; Tagawa, S.T.; et al. Randomized Phase III Trial of Gemcitabine and Cisplatin With Bevacizumab or Placebo in Patients With Advanced Urothelial Carcinoma: Results of CALGB 90601 (Alliance). J. Clin. Oncol. 2021, 39, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Pisarsky, L.; Bill, R.; Fagiani, E.; Dimeloe, S.; Goosen, W.R.; Hagmann, J.; Hess, C.; Christofori, G. Targeting Metabolic Symbiosis to Overcome Resistance to Anti-angiogenic Therapy. Cell Rep. 2016, 15, 1161–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]

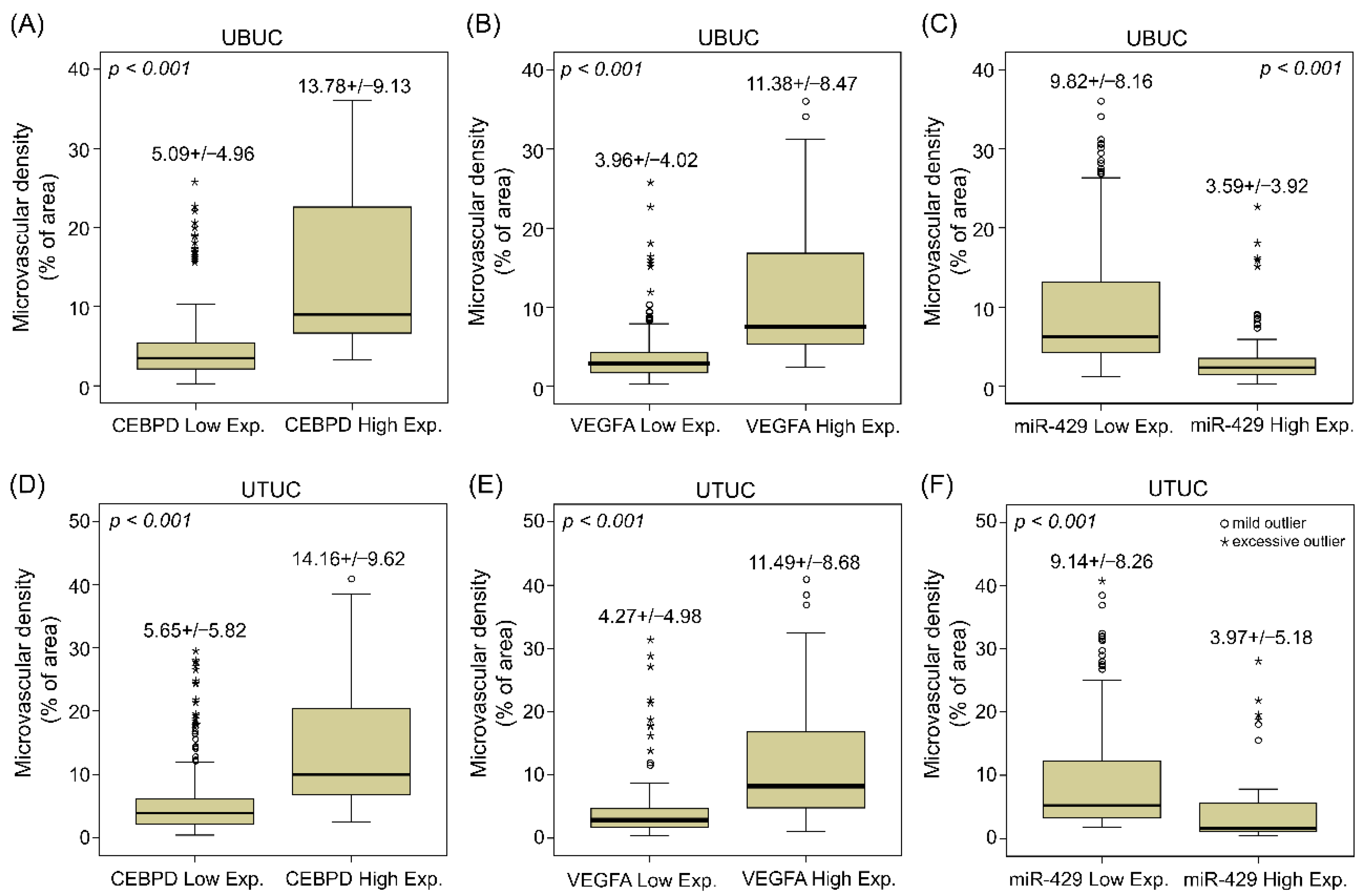
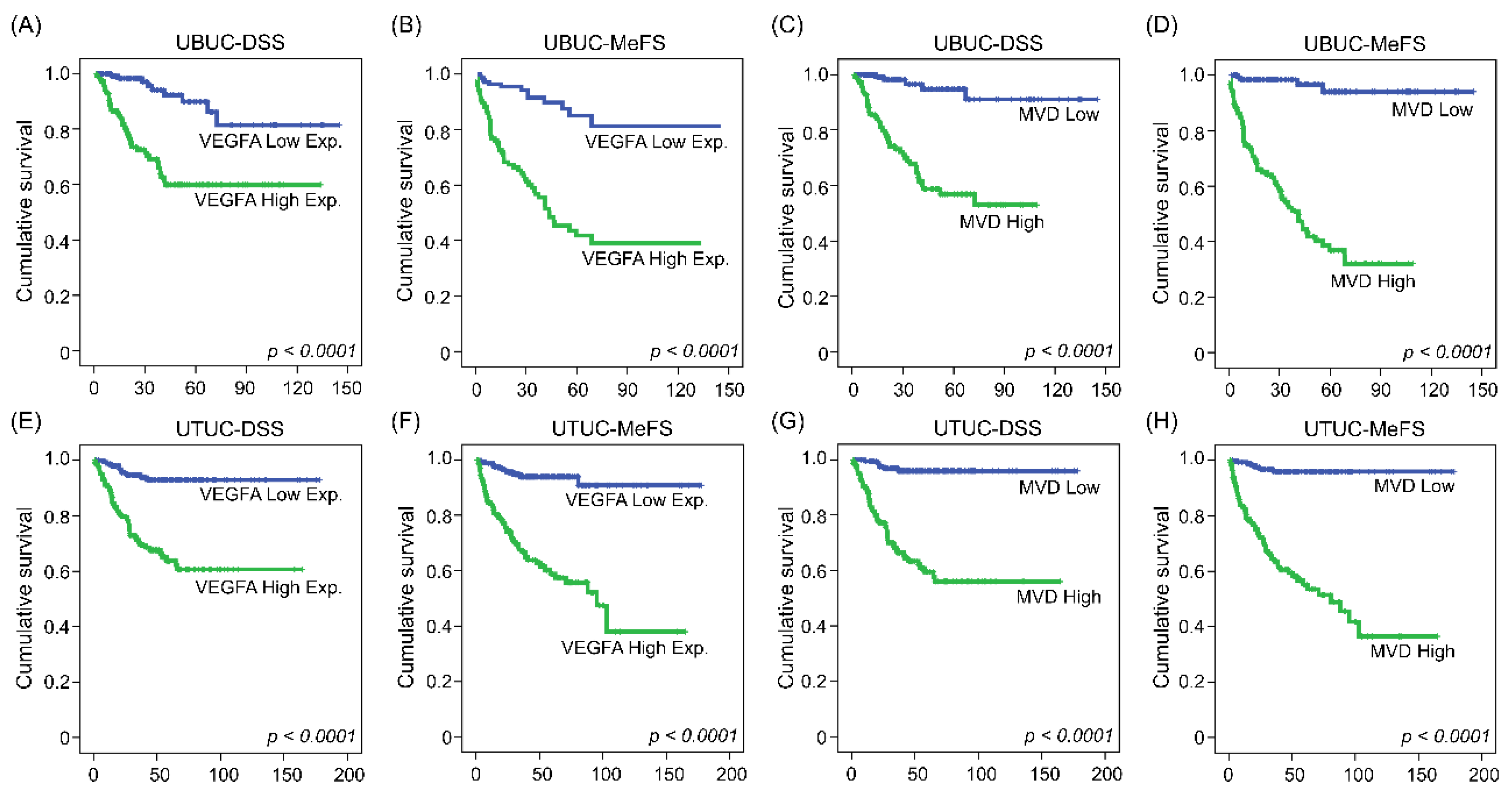
| Parameter | Category | Case No. | VEGFA Expression | Microvascular Density | ||||
|---|---|---|---|---|---|---|---|---|
| Low | High | p Value | Low | High | p Value | |||
| Sex & | Male | 216 | 111 | 105 | 0.376 | 108 | 108 | 0.923 |
| Female | 79 | 36 | 43 | 39 | 40 | |||
| Age (years) & | <65 | 121 | 60 | 61 | 0.944 | 61 | 60 | 0.867 |
| ≥65 | 174 | 87 | 87 | 86 | 88 | |||
| Primary tumor (T) & | Ta | 84 | 70 | 14 | <0.001 * | 73 | 11 | <0.001 * |
| T1 | 88 | 51 | 37 | 51 | 37 | |||
| T2–T4 | 123 | 26 | 97 | 23 | 100 | |||
| Nodal metastasis & | Negative (N0) | 266 | 143 | 123 | <0.001 * | 147 | 119 | <0.001 * |
| Positive (N1–N2) | 29 | 4 | 25 | 0 | 29 | |||
| Histological grade & | Low grade | 56 | 46 | 10 | <0.001 * | 43 | 13 | <0.001 * |
| High grade | 239 | 101 | 138 | 104 | 135 | |||
| Vascular invasion & | Absent | 246 | 140 | 106 | <0.001 * | 140 | 106 | <0.001 * |
| Present | 49 | 7 | 42 | 7 | 42 | |||
| Perineural invasion & | Absent | 275 | 145 | 130 | <0.001 * | 145 | 130 | <0.001 * |
| Present | 20 | 2 | 18 | 2 | 18 | |||
| Mitotic rate (per 10 high power fields) # | 295 | 11.3 ± 14.12 | 17.5 ± 13.30 | <0.001 * | 13.2 ± 15.64 | 15.6 ± 12.18 | 0.138 | |
| CEBPD expression & | Low expression | 207 | 140 | 67 | <0.001 * | 144 | 63 | <0.001 * |
| High expression | 88 | 7 | 81 | - | 3 | 85 | ||
| miR-429 expression & | High expression | 194 | 64 | 130 | <0.001 * | 66 | 128 | <0.001 * |
| Low expression | 101 | 83 | 18 | 81 | 20 | |||
| VEGFA expression & | Low expression | 147 | - | - | - | 120 | 27 | <0.001 * |
| High expression | 148 | - | - | - | 27 | 121 | ||
| Parameter | Category | Case No. | VEGFA Expression | Microvascular Density | ||||
|---|---|---|---|---|---|---|---|---|
| Low | High | p Value | Low | High | p Value | |||
| Sex & | Male | 158 | 76 | 82 | 0.514 | 76 | 82 | 0.514 |
| Female | 182 | 94 | 88 | 94 | 88 | |||
| Age (years) & | <65 | 138 | 80 | 58 | 0.015 * | 77 | 61 | 0.077 |
| ≥65 | 202 | 90 | 112 | 93 | 109 | |||
| Multifocality & | Single | 278 | 145 | 133 | 0.092 | 141 | 137 | 0.574 |
| Multifocal | 62 | 25 | 37 | 29 | 33 | |||
| Primary tumor (T) & | Ta | 89 | 68 | 21 | <0.001 * | 70 | 19 | <0.001 * |
| T1 | 92 | 61 | 31 | 63 | 29 | |||
| T2–T4 | 159 | 41 | 118 | 37 | 122 | |||
| Nodal metastasis & | Negative (N0) | 312 | 168 | 144 | <0.001 * | 168 | 144 | <0.001 * |
| Positive (N1–N2) | 28 | 2 | 26 | 2 | 26 | |||
| Histological grade & | Low grade | 56 | 42 | 14 | 0.015 * | 40 | 16 | <0.001 * |
| High grade | 284 | 128 | 156 | 130 | 154 | |||
| Vascular invasion & | Absent | 234 | 150 | 84 | <0.001 * | 157 | 77 | 0.001 * |
| Present | 106 | 20 | 86 | 13 | 93 | |||
| Perineural invasion & | Absent | 321 | 169 | 152 | <0.001 * | 169 | 152 | <0.001 * |
| Present | 19 | 1 | 18 | 1 | 18 | |||
| Mitotic rate (per 10 high power fields) # | 340 | 10.8 ± 12.02 | 13.8 ± 12.38 | 0.022 * | 10.5 ± 11.58 | 14.16 ± 12.71 | 0.005 * | |
| CEBPD expression & | Low expression | 251 | 158 | 93 | <0.001 * | 153 | 98 | <0.001 * |
| High expression | 89 | 12 | 77 | 17 | 72 | |||
| miR-429 expression & | High expression | 257 | 109 | 148 | <0.001 * | 113 | 144 | <0.001 * |
| Low expression | 83 | 61 | 22 | - | 57 | 26 | ||
| VEGFA expression & | Low expression | 170 | - | - | - | 129 | 41 | <0.001 * |
| High expression | 170 | - | - | 41 | 129 | |||
| Parameter | Category | Case No. | Disease-Specific Survival | Metastasis-Free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| No. of Events | p Value | RR | 95% CI | p Value | No. of Events | p-Value | RR | 95% CI | p Value | |||
| Sex | Male | 216 | 41 | 0.4906 | - | - | - | 61 | 0.2745 | - | - | - |
| Female | 79 | 11 | - | - | - | 16 | - | - | - | |||
| Age (years) | <65 | 121 | 17 | 0.1315 | - | - | - | 32 | 0.8786 | - | - | - |
| ≥65 | 174 | 35 | - | - | - | 45 | - | - | - | |||
| Primary tumor (T) | Ta | 84 | 1 | <0.0001 * | 1 | - | 0.003 * | 4 | <0.0001 * | 1 | - | 0.491 |
| T1 | 88 | 9 | 3.532 | 0.343–36.331 | 23 | 2.126 | 0.533–8.176 | |||||
| T2–T4 | 123 | 42 | 12.308 | 1.194–126.906 | 50 | 2.344 | 0.576–9.529 | |||||
| Nodal metastasis | Negative (N0) | 266 | 41 | 0.0001 * | 1 | - | 0.923 | 61 | <0.0001 * | 1 | - | 0.123 |
| Positive (N1–N2) | 29 | 11 | 1.036 | 0.507–2.115 | 16 | 1.614 | 0.878–2.966 | |||||
| Histological grade | Low grade | 56 | 2 | 0.0016 * | 1 | - | 0.809 | 5 | 0.0007 * | 1 | - | 0.576 |
| High grade | 239 | 50 | 0.814 | 0.153–4.326 | 72 | 1.363 | 0.460–4.039 | |||||
| Vascular invasion | Absent | 246 | 37 | 0.0010 * | 1 | - | 0.108 | 54 | <0.0001 * | 1 | - | 0.726 |
| Present | 49 | 15 | 0.558 | 0.274–1.137 | 23 | 0.898 | 0.492–1.640 | |||||
| Perineural invasion | Absent | 275 | 44 | <0.0001 * | 1 | - | 0.065 | 67 | 0.0003 * | 1 | - | 0.311 |
| Present | 20 | 8 | 2.252 | 0.952–5.327 | 10 | 1.491 | 0.689–3.227 | |||||
| Mitotic rate(per 10 high power fields) | <10 | 139 | 12 | 0.0001 * | 1 | - | 0.026 * | 23 | <0.0002 * | 1 | - | 0.036 * |
| ≥10 | 156 | 40 | 2.173 | 1.097–4.304 | - | 54 | 1.721 | 1.035–2.864 | - | |||
| CEBPD expression | Low | 207 | 22 | <0.0001 * | 1 | - | 0.259 | 32 | <0.0001 * | 1 | - | 0.452 |
| High | 88 | 30 | 1.446 | 0.762–2.742 | 45 | 1.216 | 0.730–2.026 | |||||
| miR-429 expression | Low | 194 | 48 | <0.0001* | 1 | - | 0.273 | 66 | <0.0001 * | 1 | - | 0.987 |
| High | 101 | 4 | 0.542 | 0.182–1.618 | 11 | 1.006 | 0.499–2.026 | |||||
| VEGFA expression & | Low expression | 147 | 9 | <0.0001 * | - | - | 0.980 | 13 | <0.0001 * | - | - | 0.258 |
| High expression | 148 | 43 | 1.012 | 0.414–2.471 | - | 64 | 1.501 | 0.742–3.035 | - | |||
| Microvascular density | Low | 147 | 5 | <0.0001 * | 1 | - | 0.021 * | 4 | <0.0001 * | 1 | - | <0.001 |
| High | 148 | 47 | 3.509 | 1.208–10.196 | 73 | 11.494 | 3.825–34.542 | |||||
| Parameter | Category | Case No. | Disease-Specific Survival | Metastasis-Free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| No. of Events | p Value | RR | 95% CI | p Value | No. of Events | p Value | RR | 95% CI | p Value | |||
| Sex | Male | 158 | 28 | 0.9301 | - | - | - | 32 | 0.7904 | - | - | - |
| Female | 182 | 33 | - | - | - | 38 | - | - | - | |||
| Age (years) | <65 | 138 | 26 | 0.8660 | - | - | - | 30 | 0.8470 | - | - | - |
| ≥65 | 202 | 35 | - | - | - | 40 | - | - | - | |||
| Multifocality | Single | 273 | 43 | 0.0042 * | 1 | - | 0.001 * | 52 | 0.0196 * | 1 | - | 0.002 * |
| Multifocal | 62 | 18 | 2.748 | 1.516–4.984 | 18 | 2.488 | 1.175–3.665 | |||||
| Primary tumor (T) | Ta | 89 | 2 | <0.0001 * | 1 | - | 0.088 | 4 | <0.0001 * | 1 | - | 0.050 * |
| T1 | 92 | 9 | 3.642 | 0.726–18.220 | 15 | 1.924 | 0.580–6.378 | |||||
| T2–T4 | 159 | 50 | 4.162 | 0.877–19.757 | 51 | 3.731 | 1.141–12.197 | |||||
| Nodal metastasis | Negative ((N0) | 312 | 42 | <0.0001 * | 1 | - | <0.001 * | 55 | <0.0001 * | 1 | - | 0.104 |
| Positive (N1–N2) | 28 | 19 | 3.802 | 1.998–7.234 | 15 | 1.711 | 0.895–3.271 | |||||
| Histological grade | Low grade | 56 | 4 | 0.0171 * | 1 | - | 0.105 | 3 | 0.0019 * | 1 | - | 0.398 |
| 2.580 | 0.820–8.122 | 1.733 | 0.484–6.206 | |||||||||
| Vascular invasion | Absent | 234 | 24 | <0.0001 * | 1 | - | 0.458 | 26 | <0.0001 * | 1 | - | 0.010 * |
| Present | 106 | 37 | 1.268 | 0.677–2.376 | 44 | 2.346 | 1.231–4.469 | |||||
| Perineural invasion | Absent | 321 | 50 | <0.0001 * | 1 | - | 0.009 * | 61 | <0.0001 * | 1 | - | 0.238 |
| Present | 19 | 11 | 2.799 | 1.296–6.048 | 9 | 1.599 | 0.733–3.487 | |||||
| Mitotic rate(per 10 high power fields) | <10 | 173 | 27 | 0.1268 | - | - | - | 30 | 0.0581 | - | - | - |
| ≥10 | 167 | 34 | - | - | - | 40 | - | - | - | |||
| CEBPD expression | Low | 251 | 26 | <0.0001 * | 1 | - | 0.081 | 24 | <0.0001 * | 1 | - | <0.001 * |
| High | 89 | 35 | 1.740 | 0.934–3.243 | 46 | 3.886 | 2.119–7.125 | |||||
| miR-429 expression | Low | 257 | 55 | 0.0024 * | 1 | - | 0.908 | 64 | <0.0001 * | 1 | - | 0.633 |
| High | 83 | 6 | 1.055 | 0.423–2.633 | 6 | 1.250 | 0.500–3.127 | |||||
| VEGFA expression & | Low expression | 170 | 10 | <0.0001 * | - | - | 0.743 | 10 | <0.0001 * | - | - | 0.660 |
| High expression | 170 | 51 | 0.868 | 0.371–2.029 | 60 | 1.202 | .530–2.724 | |||||
| Microvascular density | Low | 170 | 6 | <0.0001 * | 1 | - | 0.003 * | 6 | <0.0001 * | 1 | 0.001 * | |
| High | 170 | 55 | 4.596 | 1.666–12.679 | 64 | 5.611 | 2.102–14.976 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, T.-C.; Hsing, C.-H.; Shiue, Y.-L.; Huang, S.K.; Hsieh, K.-L.; Kuo, Y.-H.; Li, C.-F. Angiogenesis Driven by the CEBPD–hsa-miR-429–VEGFA Signaling Axis Promotes Urothelial Carcinoma Progression. Cells 2022, 11, 638. https://doi.org/10.3390/cells11040638
Chan T-C, Hsing C-H, Shiue Y-L, Huang SK, Hsieh K-L, Kuo Y-H, Li C-F. Angiogenesis Driven by the CEBPD–hsa-miR-429–VEGFA Signaling Axis Promotes Urothelial Carcinoma Progression. Cells. 2022; 11(4):638. https://doi.org/10.3390/cells11040638
Chicago/Turabian StyleChan, Ti-Chun, Chung-Hsi Hsing, Yow-Ling Shiue, Steven K. Huang, Kun-Lin Hsieh, Yu-Hsuan Kuo, and Chien-Feng Li. 2022. "Angiogenesis Driven by the CEBPD–hsa-miR-429–VEGFA Signaling Axis Promotes Urothelial Carcinoma Progression" Cells 11, no. 4: 638. https://doi.org/10.3390/cells11040638
APA StyleChan, T.-C., Hsing, C.-H., Shiue, Y.-L., Huang, S. K., Hsieh, K.-L., Kuo, Y.-H., & Li, C.-F. (2022). Angiogenesis Driven by the CEBPD–hsa-miR-429–VEGFA Signaling Axis Promotes Urothelial Carcinoma Progression. Cells, 11(4), 638. https://doi.org/10.3390/cells11040638







