Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
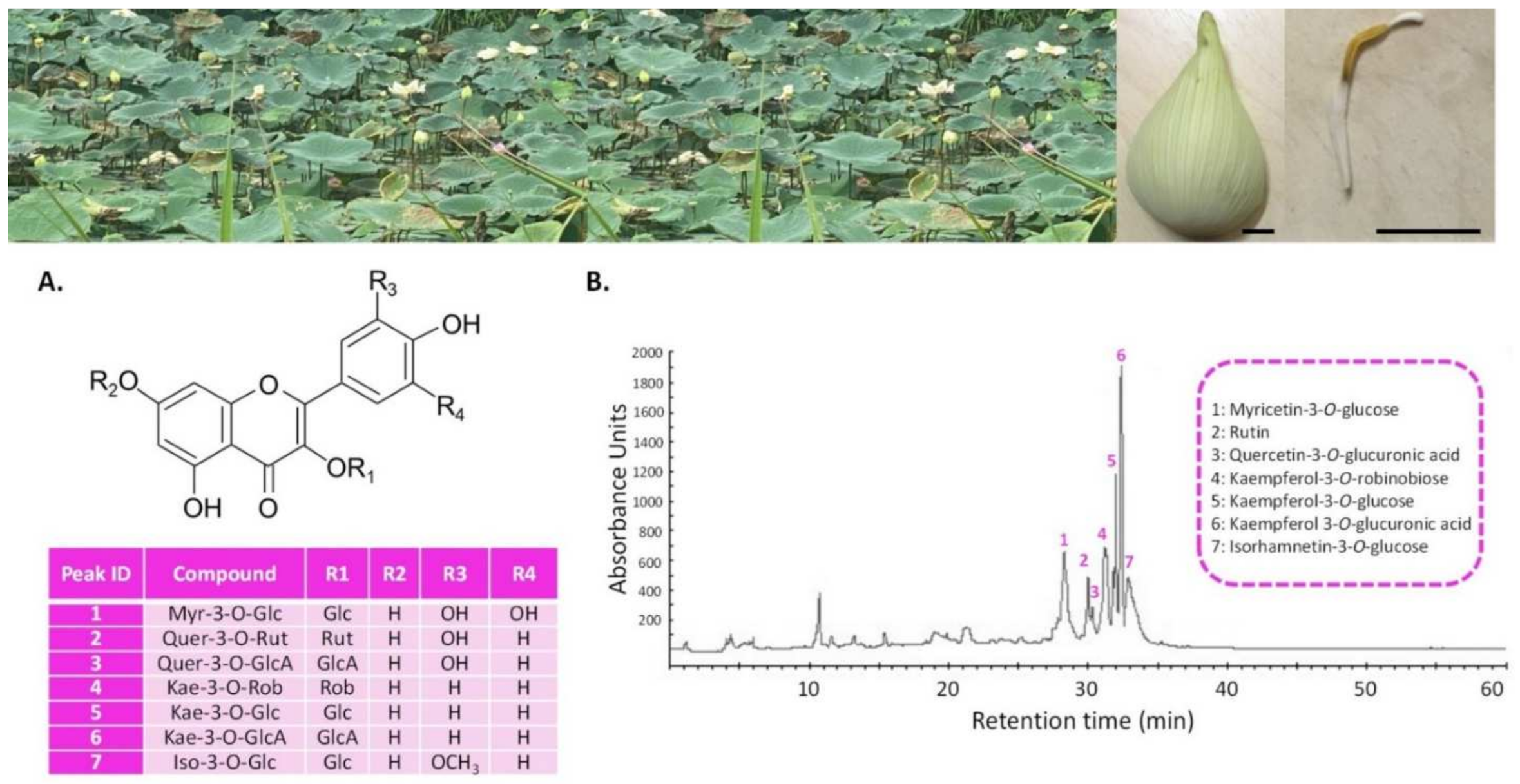
2.2. Plant Materials
2.3. Extraction
2.4. HPLC Analysis
2.5. In Vitro Cell Free Antioxidant Assays
2.6. Yeast Culture Conditions
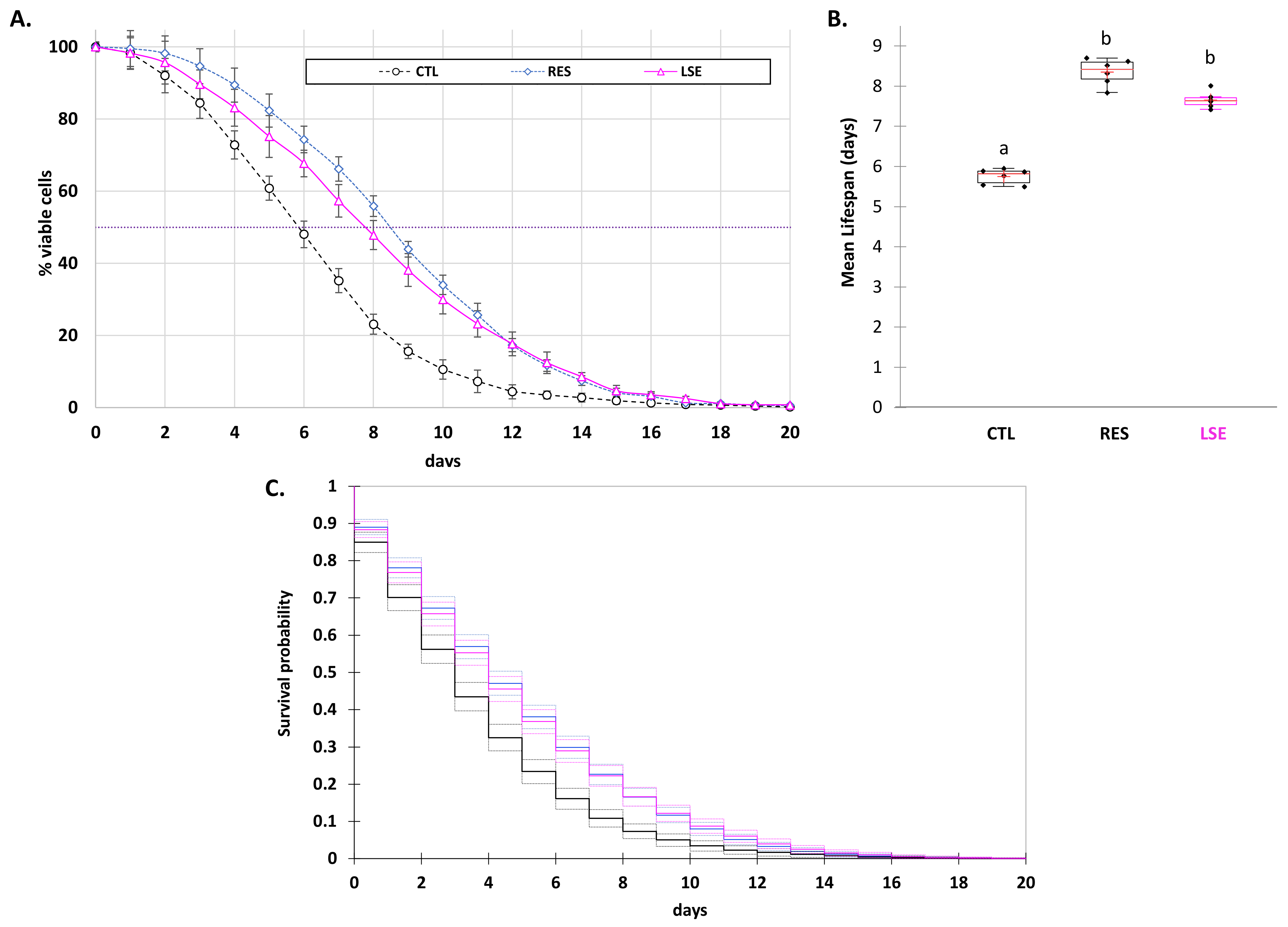
2.7. Chronological Aging Evaluation
2.8. Reactive Oxygen and Nitrogen Species Measurement
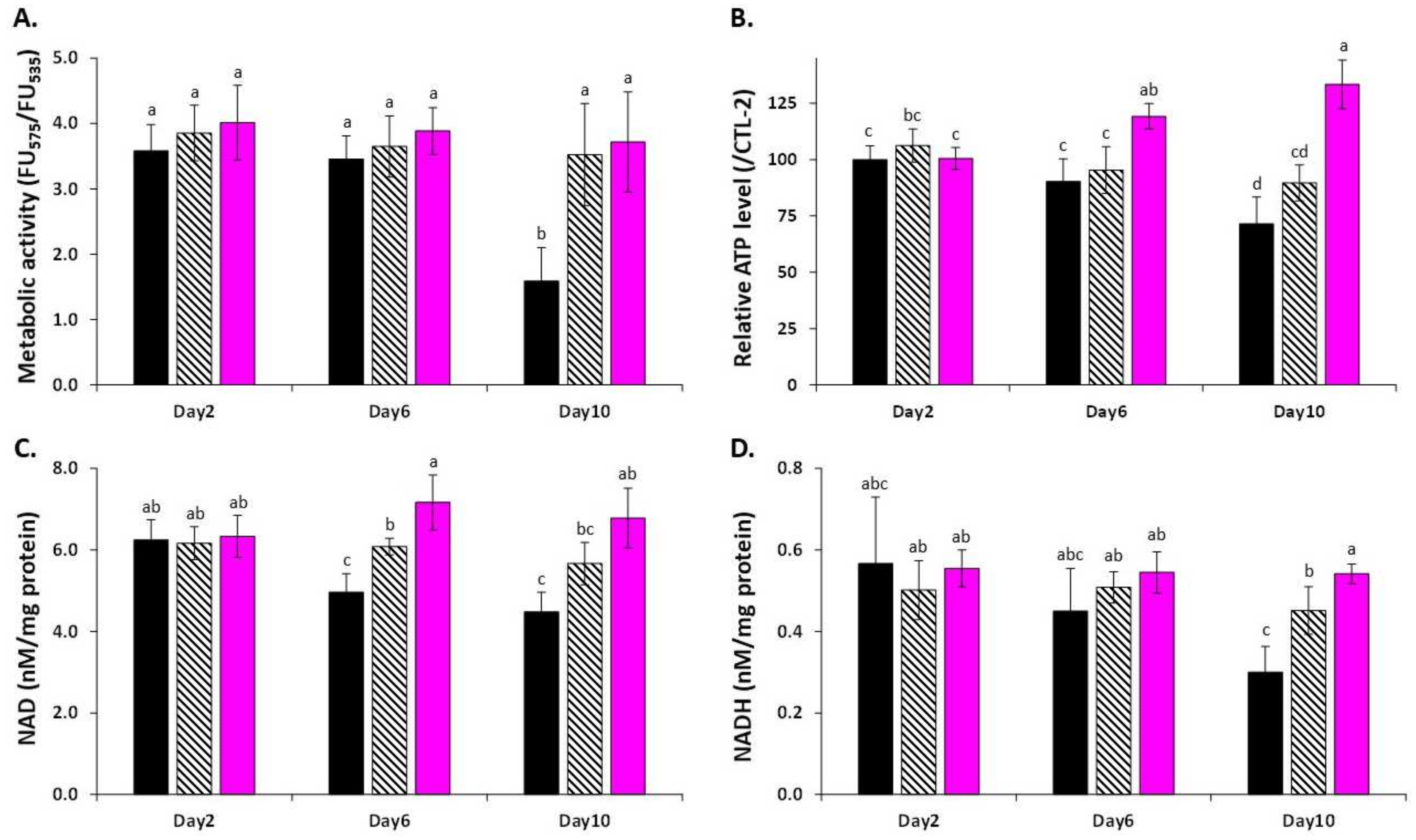
2.9. Estimation of NAD, NADH, and ATP Contents
2.10. Mitochondrial Membrane Potential and Metabolic Activity Evaluation
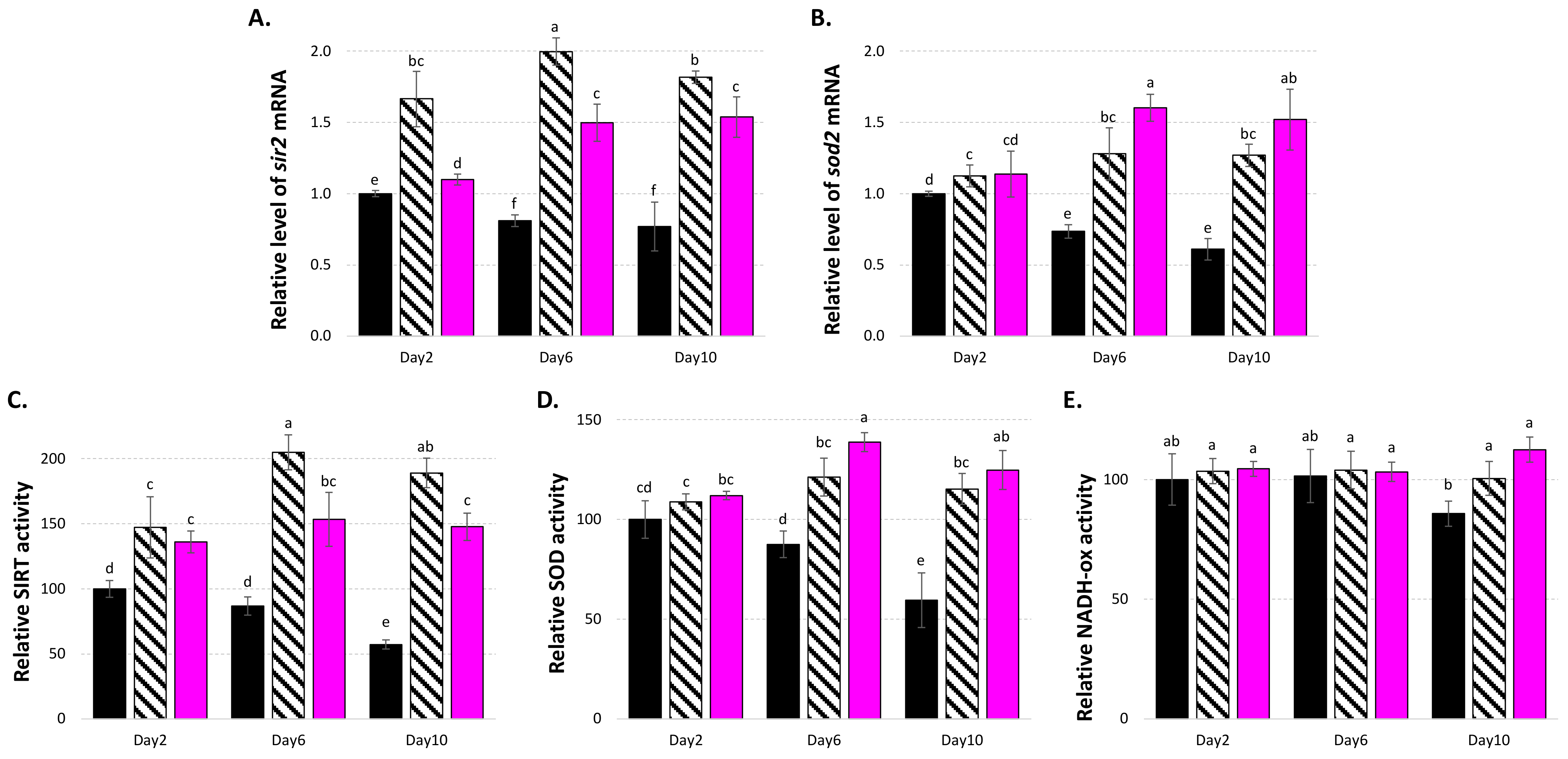
2.11. Gene Expression by RT-qPCR Analysis
- sod1, forward: 5′-CACCATTTTCGTCCGTCTTT-3′, and reverse: 5′-TGGTTGTGTCTCTGCTGGTC-3′;
- sod2, forward: 5′-CTCCGGTCAAATCAACGAAT-3′, and reverse: 5′-CCTTGGCCAGAAGATCTGAG-3′;
- sir2, forward: 5′-CGTTCCCCAAGTCCTGATTA-3′, and reverse: 5′-CCACATTTTTGGGCTACCAT-3′;
- ndi-1, forward: 5′-GGTGGTGGGCCTACTGGTGT-3′, and reverse: 5′-TTCAAAACGATGGGCAGAGC-3′;
- tub1, forward: 5′-CCAAGGGCTATTTACGTGGA-3′, and reverse: 5′-GGTGTAATGGCCTCTTGCAT-3′.
2.12. Enzymatic Activities Determinations
2.13. Membrane Lipid Peroxidation Evaluation
2.14. Protein Carbonylation Level Estimation
2.15. 8-Oxo-Guanine Level Estimation
2.16. Statistical Analysis
3. Results and Discussion
3.1. LSE (Sacred Lotus Stamen Extract) Is Rich in Antioxidant Flavonoids
3.2. LSE Delays Yeast Chronological Aging
3.3. LSE Protects Cells from Oxidative Stress Damage by Maintaining Mitochondrial Functions
3.4. LSE Acts on Central Metabolism, Gene Expressions, and Enzyme Regulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chayamarit, K.; Balslav, H.; Esser, H.J. Flora of Thailand; Chayamarit, K., Balslav, H., Eds.; The Forest Herbarium, Royal Forest Department: Bangkok, Thailand, 2020; Volume 14, ISBN 9786163165923. [Google Scholar]
- Dezhi, F.; Wiersema, J.H. Nelumbo nucifera. In Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; p. 1. [Google Scholar]
- Tungmunnithum, D.; Pinthong, D.; Hano, C. Flavonoids from Nelumbo nucifera Gaertn., a Medicinal Plant: Uses in Traditional Medicine, Phytochemistry and Pharmacological Activities. Medicines 2018, 5, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungmunnithum, D.; Renouard, S.; Drouet, S.; Blondeau, J.-P.; Hano, C. A Critical Cross-Species Comparison of Pollen from Nelumbo nucifera Gaertn. vs. Nymphaea lotus L. for Authentication of Thai Medicinal Herbal Tea. Plants 2020, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Shen-Miller, J.; Mudgett, M.B.; Schopf, J.W.; Clarke, S.; Berger, R. Exceptional Seed Longevity and Robust Growth: Ancient Sacred Lotus from China. Am. J. Bot. 1995, 82, 1367–1380. [Google Scholar] [CrossRef]
- Sikarwar, R.L.S. Angiosperm diversity assessment of Chitrakootthe legendary place of Vindhyan range. India J. Econ. Taxon. Bot. 2014, 38, 563–619. [Google Scholar]
- Lin, H.Y.; Kuo, Y.H.; Lin, Y.L.; Chiang, W. Antioxidative effect and active components from leaves of lotus (Nelumbo nucifera). J. Agric. Food Chem. 2009, 57, 6623–6629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shukla, S.; Kim, J.A.; Kim, M. Anti-angiogenic effect of nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potential. PLoS ONE 2015, 10, e0118552. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Wu, W.; Jiao, L.L.; Yang, P.F.; Guo, M.Q. Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Kim, J.E.; Chung, H.Y.; Choi, J.S. Antioxidant principles of Nelumbo nucifera stamens. Arch Pharm Res 2003, 26, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.A. Ethno-medicinal uses and pharmacological activities of lotus (Nelumbo nucifera). J. Med. Plants Stud. 2014, 2, 42–46. [Google Scholar]
- Li, M.M.; Huang, X.Y.; Liang, Q.K.; Li, Y.X.; Yan, Y.; Tian, Z.F.; Qiu, X.Q.; Zhang, X.W.; Song, Q.; Li, H. Effects and mechanisms of lotus leaf water extract on lipid metabolism of adult experimental obesity rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2017, 8, 476–480. [Google Scholar] [CrossRef]
- Fukui, H.; Mizuguchi, H.; Kitamura, Y.; Takeda, N. Patho-Pharmacological Research of Anti-allergic Natural Products Targeting Antihistamine-Sensitive and -Insensitive Allergic Mechanisms. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Qiu, M.; Xiao, F.; Wang, T.; Piao, S.; Zhao, W.; Shao, S.; Yan, M.; Zhao, D. Protective effect of Hedansanqi Tiaozhi Tang against non-alcoholic fatty liver disease in vitro and in vivo through activating Nrf2/HO-1 antioxidant signaling pathway. Phytomedicine 2020, 67, 153140. [Google Scholar] [CrossRef]
- Zeng, M.; Li, L.; Wu, Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019(COVID-19): Meta-analysis of randomized controlled trials. PLoS ONE 2020, 11, e0238828. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Anh, P.T.; Yang, S.H. Polysaccharide Derived from Nelumbo nucifera Lotus Plumule Shows Potential Prebiotic Activity and Ameliorates Insulin Resistance in HepG2 Cells. Polymers 2021, 13, 1780. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, K.; Chen, Y.; Ouyang, Y.; Feng, Y.; Li, S.; Zhang, L.; Feng, N. Effect of lotus seedpod oligomeric procyanidins on AGEs formation in simulated gastrointestinal tract and cytotoxicity in Caco-2 cells. Food Funct. 2021, 12, 3527–3538. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, K.; Yang, Z.; Guo, X.; Wei, S. Identification of Antioxidant and Anti- α -amylase Components in Lotus (Nelumbo nucifera, Gaertn.) Seed Epicarp. Appl. Biochem. Biotechnol. 2019, 187, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, T.; Zhang, C.; Guo, M. Flavonoids of Lotus (Nelumbo nucifera) Seed Embryos and Their Antioxidant Potential. J. Food Sci. 2017, 82, 1834–1841. [Google Scholar] [CrossRef]
- Tan, S.-J.; Lee, C.-K.; Gan, C.-Y.; Olalere, O.A. Statistical Optimization of Flavonoid and Antioxidant Recovery from Macerated Chinese and Malaysian Lotus Root (Nelumbo nucifera) Using Response Surface Methodology. Molecules 2021, 26, 2014. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. Adaptive homeostasis and the free radical theory of ageing. Free Radic. Biol. Med. 2018, 124, 420–430. [Google Scholar] [CrossRef]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radical chemistry. J Gerontol. 1956, 11, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Hofer, S.; Pendl, T.; Kainz, K.; Madeo, F.; Carmona-Gutierrez, D. Yeast as a tool to identify anti-aging compounds. FEMS Yeast Res. 2018, 18, foy020. [Google Scholar] [CrossRef]
- He, C.; Zhou, C.; Kennedy, B.K. The yeast replicative aging model. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2690–2696. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Abid, M.; Elamrani, A.; Drouet, S.; Addi, M.; Hano, C. Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast. Life 2020, 10, 80. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Sun, K.; Lu, J.; Weng, Y.; Taoka, A.; Sakagami, Y.; Qi, J. Anti-aging effects of phloridzin, an apple polyphenol, on yeast via the SOD and Sir2 genes. Biosci. Biotechnol. Biochem. 2011, 75, 854–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Xiang, L.; Ishihara, S.; Matsuura, A.; Sakagami, Y.; Qi, J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Biosci. Biotechnol. Biochem. 2012, 76, 640–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Cao, S.; Pei, L.; Matsuura, A.; Xiang, L.; Qi, J.A. A steroidal saponin from Ophiopogon japonicus extends the lifespan of yeast via the pathway involved in SOD and UTH1. Int. J. Mol. Sci. 2013, 14, 4461–4475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierman, M.B.; Smith, J.S. Yeast sirtuins and the regulation of aging. FEMS Yeast Res. 2014, 14, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merksamer, P.I.; Liu, Y.; He, W.; Hirschey, M.D.; Chen, D.; Verdin, E. The sirtuins, oxidative stress and aging: An emerging link. Aging 2013, 5, 144. [Google Scholar] [CrossRef] [Green Version]
- Temviriyanukul, P.; Sritalahareuthai, V.; Promyos, N.; Thangsiri, S.; Pruesapan, K.; Srinuanchai, W.; Nuchuchua, O.; Siriwan, D.; On-nom, N.; Suttisansanee, U. The Effect of Sacred Lotus (Nelumbo nucifera) and Its Mixtures on Phenolic Profiles, Antioxidant Activities, and Inhibitions of the Key Enzymes Relevant to Alzheimer’s Disease. Molecules 2020, 25, 3713. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Thiers, B.; Thiers, B.H.; Cokic, B.B.B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff; New York Botanical Garden’s Virtual Herbarium: New York, NY, USA, 2009. [Google Scholar]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; World Health Organization: Geneva, Switzerland, 1998; ISBN 9241545100. [Google Scholar]
- Drouet, S.; Abbasi, B.H.; Falguières, A.; Ahmad, W.S.; Ferroud, C.; Doussot, J.; Vanier, J.R.; Lainé, E.; Hano, C. Single Laboratory Validation of a Quantitative Core Shell-Based LC Separation for the Evaluation of Silymarin Variability and Associated Antioxidant Activity of Pakistani Ecotypes of Milk Thistle (Silybum marianum L.). Molecules 2018, 23, 904. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wei, M.; Mirisola, M.G.; Longo, V.D. Assessing chronological aging in Saccharomyces cerevisiae. In Cell Senescence; (Methods in Molecular Biology (Methods and Protocols)); Galluzzi, L., Vitale, I., Kepp, O., Kroemer, G., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 463–472. [Google Scholar]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; Lebas de Lacour, J.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential Production of Phenylpropanoid Metabolites in Callus Cultures of Ocimum basilicum L. With Distinct in Vitro Antioxidant Activities and in Vivo Protective Effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Manchester, J.K.; Gordon, J.I. Enhanced gluconeogenesis and increased energy storage as hallmarks of aging in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 36000–36007. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.M.; Lee, H.L.; Kwon, Y.Y.; Kang, M.S.; Lee, S.K.; Lee, C.K. Enhancement of mitochondrial function correlates with the extension of lifespan by caloric restriction and caloric restriction mimetics in yeast. Biochem. Biophys. Res. Commun. 2013, 441, 236–242. [Google Scholar] [CrossRef]
- Hano, C.; Addi, M.; Fliniaux, O.; Bensaddek, L.; Duverger, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Molecular characterization of cell death induced by a compatible interaction between Fusarium oxysporum f. sp. linii and flax (Linum usitatissimum) cells. Plant Physiol. Biochem. 2008, 46, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Petit, P.X.; O’Connor, J.E.; Grunwald, D.; Brown, S.C. Analysis of the membrane potential of rat-and mouse-liver mitochondria by flow cytometry and possible applications. Eur. J. Biochem. 1990, 194, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Czachor, J.; Miłek, M.; Galiniak, S.; Stępień, K.; Dżugan, M.; Mołoń, M. Coffee Extends Yeast Chronological Lifespan through Antioxidant Properties. Int. J. Mol. Sci. 2020, 21, 9510. [Google Scholar] [CrossRef]
- Vemuri, G.N.; Eiteman, M.A.; McEwen, J.E.; Olsson, L.; Nielsen, J. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 104, 2402–2407. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, P.; Longo, V.D. The chronological life span of Saccharomyces cerevisiae. Aging Cell 2003, 2, 73–81. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus flavonoids and phenolic acids: Health promotion and safe consumption dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.; Xiang, L.; Matsuura, A.; Zhang, Y.; Huang, Q.; Qi, J. Ganodermasides A and B, two novel antiaging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg. Med. Chem. 2010, 18, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, Y.; Cao, X.; Xiang, L.; Qi, J. Sterols from Mytilidae show anti-aging and neuroprotective effects via anti-oxidative activity. Int. J. Mol. Sci. 2014, 15, 21660–21673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Amer. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.; Ren, Z.; Morciano, G.; Perrone, M.; Wieckowski, M.R. Mitochondria and reactive oxygen species in aging and age-related diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- De Alves, G.A.D.; de Souza, R.O.; Rogez, H.L.G.; Masaki, H.; Fonseca, M.J.V. Cecropia obtusa extract and chlorogenic acid exhibit anti aging effect in human fibroblasts and keratinocytes cells exposed to UV radiation. PLoS ONE 2019, 14, e0216501. [Google Scholar] [CrossRef]
- Belenky, P.; Racette, F.G.; Bogan, K.L.; McClure, J.M.; Smith, J.S.; Brenner, C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 2007, 129, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Ondracek, C.R.; Frappier, V.; Ringel, A.E.; Wolberger, C.; Guarente, L. Mutations that allow SIR2 orthologs to function in a NAD(+)-depleted environment. Cell Rep. 2017, 18, 2310–2319. [Google Scholar] [CrossRef] [Green Version]
- Farooq, U.; Pan, Y.; Lin, Y.; Wang, Y.; Osada, H.; Xiang, L.; Qi, J. Structure Characterization and Action Mechanism of an Antiaging New Compound from Gastrodia elata Blume. Oxid. Med. Cell. Longev. 2019, 2019, 5459862. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Sun, Y.; Lin, Y.; Pan, Y.; Farooq, U.; Xiang, L.; Qi, J. Antiaging of Cucurbitane Glycosides from Fruits of Momordica charantia L. Oxid. Med. Cell. Longev. 2018, 2018, 1538632. [Google Scholar] [CrossRef] [Green Version]
- Semchyshyn, H.M.; Lozinska, L.M. Fructose protects baker’s yeast against peroxide stress: Potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012, 12, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, P.; Pletcher, S.D.; Minois, N.; Vaupel, J.W.; Longo, V.D. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004, 557, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Tanno, M.; Kuno, A.; Yano, T.; Miura, T.; Hisahara, S.; Ishikawa, S.; Shimamoto, K.; Horio, Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010, 285, 8375–8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daitoku, H.; Hatta, M.; Matsuzaki, H.; Aratani, S.; Ohshima, T.; Miyagishi, M.; Nakajima, T.; Fukamizu, A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10042–10047. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungmunnithum, D.; Drouet, S.; Hano, C. Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism. Cells 2022, 11, 599. https://doi.org/10.3390/cells11040599
Tungmunnithum D, Drouet S, Hano C. Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism. Cells. 2022; 11(4):599. https://doi.org/10.3390/cells11040599
Chicago/Turabian StyleTungmunnithum, Duangjai, Samantha Drouet, and Christophe Hano. 2022. "Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism" Cells 11, no. 4: 599. https://doi.org/10.3390/cells11040599
APA StyleTungmunnithum, D., Drouet, S., & Hano, C. (2022). Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism. Cells, 11(4), 599. https://doi.org/10.3390/cells11040599







