Glucocorticoids Are Not Associated with Bone Mineral Density in Patients with Polymyalgia Rheumatica, Giant Cell Arteritis and Other Vasculitides—Cross-Sectional Baseline Analysis of the Prospective Rh-GIOP Cohort
Abstract
:1. Introduction
2. Methods
- (1)
- Excluding patients with PMR;
- (2)
- Lumbar spine Ts as dependent variable;
- (3)
- Right femoral neck Ts as dependent variable;
- (4)
- Left femoral neck Ts as dependent variable.
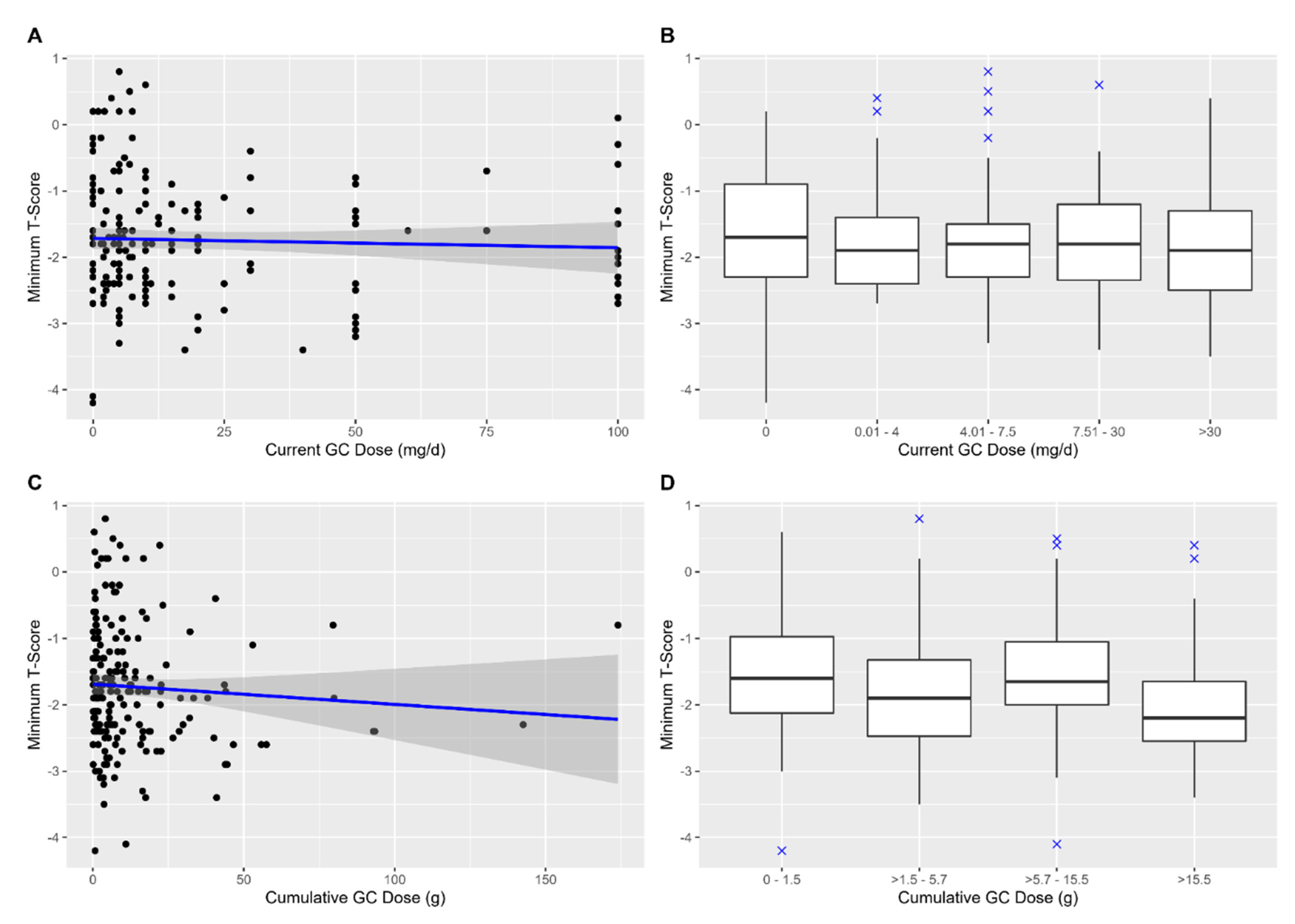
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buttgereit, F. Views on glucocorticoid therapy in rheumatology: The age of convergence. Nat. Rev. Rheumatol. 2020, 16, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Paskins, Z.; Whittle, R.; Sultan, A.A.; Muller, S.; Blagojevic-Bucknall, M.; Helliwell, T.; Hider, S.; Roddy, E.; Mallen, C. Risk of fracture among patients with polymyalgia rheumatica and giant cell arteritis: A population-based study. BMC Med. 2018, 16, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briot, K.; Cortet, B.; Roux, C.; Fardet, L.; Abitbol, V.; Bacchetta, J.; Buchon, D.; Debiais, F.; Guggenbuhl, P.; Laroche, M.; et al. 2014 update of recommendations on the prevention and treatment of glucocorticoid-induced osteoporosis. Jt. Bone Spine 2014, 81, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; De Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Yates, M.; Watts, R.A.; Bajema, I.M.; Cid, M.C.; Crestani, B.; Hauser, T.; Hellmich, B.; Holle, J.U.; Laudien, M.; Little, M.; et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann. Rheum. Dis. 2016, 75, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- Dejaco, C.; Singh, Y.P.; Perel, P.; Hutchings, A.; Camellino, D.; Mackie, S.; Abril, A.; Bachta, A.; Balint, P.; Barraclough, K.; et al. 2015 Recommendations for the management of polymyalgia rheumatica: A European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann. Rheum. Dis. 2015, 74, 1799–1807. [Google Scholar] [CrossRef] [Green Version]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- e.V. DdDWOG. Leitlinie: Prophylaxe, Diagnostik und Therapie der Osteoporose. 2017. Available online: https://dv-osteologie.org/uploads/Leitlinie%202017/Finale%20Version%20Leitlinie%20Osteoporose%202017_end.pdf (accessed on 29 December 2021).
- World Health Organization. Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group; WHO: Geneva, Switzerland, 1994. [Google Scholar]
- Lydersen, S. Statistical review: Frequently given comments. Ann. Rheum. Dis. 2015, 74, 323–325. [Google Scholar] [CrossRef] [Green Version]
- Rosner, B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics 1983, 25, 165–172. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Rabenberg, M.; Mensink, G. Vitamin-D-Status in Deutschland; Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung: Berlin, Germany, 2016. [Google Scholar]
- Fuchs, J.; Scheidt-Nave, C.; Kuhnert, R. 12-Monats-Prävalenz von Osteoporose in Deutschland; Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung: Berlin, Germany, 2017. [Google Scholar]
- Hadji, P.; Klein, S.; Gothe, H.; Häussler, B.; Kless, T.; Schmidt, T.; Steinle, T.; Verheyen, F.; Linder, R. Epidemiologie der Osteoporose—Bone Evaluation Study. Dtsch Arztebl Int. 2013, 110, 52–57. [Google Scholar] [PubMed] [Green Version]
- Lems, W.F.; Baak, M.M.; van Tuyl, L.H.; Lodder, M.C.; Dijkmans, B.A.C.; Boers, M. One-year effects of glucocorticoids on bone density: A meta-analysis in cohorts on high and low-dose therapy. RMD Open 2016, 2, e000313. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Wang, J.; Ju, B.; Wang, Y.; Luo, J.; Tian, J.; He, L. Assessment of Low Bone Mineral Density in Untreated Patients with Takayasu’s Arteritis. Biomed Res. Int. 2021, 2021, 6489631. [Google Scholar] [CrossRef] [PubMed]
- Petri, H.; Nevitt, A.; Sarsour, K.; Napalkov, P.; Collinson, N. Incidence of giant cell arteritis and characteristics of patients: Data-driven analysis of comorbidities. Arthritis Care Res. 2015, 67, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.F.; Sinha, R.; Bukhari, M. AB0575 Steroid Use Is Protective of Osteoporosis in Polymyalgia Rheumatica Patients: A Case-Control Study. Ann. Rheum. Dis 2016, 75 (Suppl. 2), 1101. [Google Scholar] [CrossRef]
- Dolan, A.L.; Moniz, C.; Li, F.; Mackintosh, C.; Todd, P.; Dasgupta, B.; Corrigall, V.; Panayi, G.S. Effects of inflammation and treatment on bone turnover and bone mass in polymyalgia rheumatica. Arthritis Rheum. 1997, 40, 2022–2029. [Google Scholar] [CrossRef]
- Haugeberg, G. GMHDBMJTG. No permanent reduction in bone mineral density during treatment of polymyalgia rheumatica and temporal arteritis using low dose corticosteroids: A cross sectional study. Scand. J. Rheumatol. 2000, 29, 163–169. [Google Scholar] [CrossRef]
- Boomsma, M.M.; Stegeman, C.A.; Kramer, A.B.; Karsijns, M.; Piers, D.A.; Tervaert, J.W.C. Prevalence of reduced bone mineral density in patients with anti-neutrophil cytoplasmic antibody associated vasculitis and the role of immunosuppressive therapy: A cross-sectional study. Osteoporos Int. 2002, 13, 74–82. [Google Scholar] [CrossRef]
- Schett, G.; Kiechl, S.; Weger, S.; Pederiva, A.; Mayr, A.; Petrangeli, M.; Oberhollenzer, F.; Lorenzini, R.; Redlich, K.; Axmann, R.; et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch. Intern. Med. 2006, 166, 2495–2501. [Google Scholar] [CrossRef] [Green Version]
- Miyano, S.; Michihata, N.; Sada, K.-E.; Uda, K.; Matsui, H.; Fushimi, K.; Nangaku, M.; Yasunaga, H. Comparison of fracture risk between proton pump inhibitors and histamine-2 receptor antagonists in ANCA-associated vasculitis patients: A nested case–control study. Rheumatology 2020, 60, 1717–1723. [Google Scholar] [CrossRef]
- Weinstein, R.S. Glucocorticoid-Induced Bone Disease. N. Engl. J. Med. 2011, 365, 62–70. [Google Scholar] [CrossRef] [PubMed]
| Model No. | Dependent Variable | Main Independent Variable |
|---|---|---|
| 1 | Minimum T-score | Current GC dose, continuous |
| 2 | Current GC dose, categorical | |
| 3 | Cumulative GC dose, continuous | |
| 4 | Cumulative GC dose, categorical |
| Potential Confounders Included in Multivariable Regression Models |
| Age |
| Sex |
| Type of vasculitis |
| Smoking status (current, former, no smoking) |
| Body mass index |
| History of osteoporotic fractures (yes/no) |
| Family history of osteoporotic fractures (yes/no) |
| Alcohol consumption (none, irregular/infrequent, occasional, frequent) |
| Menopause (yes/no) |
| History of vertebral fractures (yes/no) |
| Health Assessment Questionnaire (HAQ) |
| Alkaline phosphatase |
| Gamma-glutamyltransferase |
| Proton pump inhibitor use (yes/no) |
| C-reactive protein |
| Disease duration |
| Bisphosphonate use (yes/no) |
| Denosumab use (yes/no) |
| Vitamin D deficiency (no deficiency/subclinical/clinically relevant) |
| Tocilizumab intake (yes/no) |
| Cumulative duration of GC use 1 |
| Interaction Terms Included in Multivariable Regression Models |
| CRP: Tocilizumab intake |
| CRP: GC 1 |
| GC: Type of vasculitis 1 |
| GC: Disease duration 1 |
| GC: Cumulative duration of GC use 1 |
| GC: HAQ 1 |
| Menopause: Sex |
| Overall | n = 198 |
|---|---|
| Age, years n (%) | 67.69 (11.4) |
| Sex (male) n (%) | 66 (33.3) |
| Type of vasculitis n (%) | |
| Polymyalgia rheumatica | 71 (35.9) |
| Giant cell arteritis | 51 (25.8) |
| c-ANCA-associated vasculitis * | 36 (18.2) |
| p-ANCA-associated vasculitis ** | 21 (10.6) |
| Takayasu’s arteritis | 8 (4.9) |
| Undifferentiated vasculitis | 6 (3.0) |
| Polyarteritis nodosa | 4 (2.0) |
| Cogan’s syndrome | 1 (0.5) |
| Disease duration, years mean (SD) | 5.31 (6.33) |
| Glucocorticoid intake | |
| Current intake n (%) | 173 (87.4) |
| Current dose, mg/d mean (SD) | 30.79 (67.5) |
| 0 | 25 (12.6) |
| 0.01–4 | 27 (13.6) |
| 4.01–7.5 | 49 (24.7) |
| 7.51–30 | 53 (26.8) |
| >30 | 44 (22.2) |
| Cumulative dose, g mean (SD) | 13.21 (22.15) |
| Cumulative duration of use, years mean (SD) | 4.80 (6.61) |
| Disease-modifying anti-rheumatic drugs n (%) | 118 (59.6) |
| Conventional synthetic | 87 (43.9) |
| Biological | 31 (15.7) |
| Minimum T-score mean (SD) | −1.74 (0.9) |
| Osteoporosis by DXA | 39 (19.7) |
| Family history of osteoporosis n (%) | 27 (19.1) |
| Family history of osteoporotic fracture | 18 (12.8) |
| Prior osteoporotic fracture n (%) | 65 (32.8) |
| Prior vertebral fracture n (%) | 19 (9.6) |
| Anti-osteoporotic therapy n (%) | |
| Bisphosphonates | 29 (14.6) |
| Denosumab | 6 (3) |
| 25-OH vitamin D3 deficiency n (%) | |
| Subclinical (25–50 nmol/L) | 9 (4.5) |
| Clinically relevant (<25 nmol/L) | 5 (2.5) |
| Vitamin D supplementation n (%) | 176 (88.9) |
| Calcium supplementation n (%) | 8 (4.0) |
| C-reactive protein, mg/L mean (SD) | 13.15 (26.05) |
| Body mass index mean (SD) | 26.44 (4.44) |
| Smoking status n (%) | |
| Never | 99 (50.8) |
| Former smoker | 78 (40.0) |
| Current smoker | 18 (9.2) |
| Alcohol consumption n (%) | |
| None | 83 (43.0) |
| Irregular/infrequent | 81 (42.0) |
| Occasional | 24 (12.4) |
| Frequent | 5 (2.6) |
| Slope β | 97.5% CI | Adjusted R² | p | |
|---|---|---|---|---|
| Current GC dose (continuous) | −0.01 | −0.02 to 0.01 | 22.70% | 0.49 |
| Current GC dose (categorical) | 20.00% | |||
| 0 mg/d (reference) | - | - | - | |
| >0–4 mg/d | −0.03 | −1.11 to 1.04 | 0.96 * | |
| >4–7.5 mg/d | 0.32 | −0.69 to 1.32 | 0.53 * | |
| >7.5–30 mg/d | −0.14 | −1.09 to 0.81 | 0.77 * | |
| >30 mg/d | 0.21 | −1.28 to 1.69 | 0.78 * | |
| Cumulative GC dose (continuous) | 0.01 | −0.04 to 0.07 | 23.10% | 0.59 |
| Cumulative GC dose (categorical) | 26.00% | |||
| >1.5–5.7 g | −0.30 | −1.05 to 0.44 | 0.42 * | |
| >5.7–15.5 g | 0.38 | −0.43 to 1.20 | 0.35 * | |
| >15.5 g | −0.00 | −1.29 to 1.29 | 1.00 * |
| Polymyalgia Rheumatica Excluded | Lumbar Spine Ts as Dependent Variable | Right Femoral Neck Ts as Dependent Variable | Left Femoral Neck TS as Dependent Variable | |
|---|---|---|---|---|
| p | ||||
| Current GC dose (continuous) | 0.90 | 0.54 | 0.92 | 0.41 |
| Current GC dose (categorical) | ≥0.47 | ≥0.47 | ≥0.44 | ≥0.06 |
| Cumulative GC dose (continuous) | 0.31 | 0.71 | 0.36 | 0.30 |
| Cumulative GC dose (categorical) | ≥0.12 | ≥0.33 | ≥0.11 | ≥0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmowski, A.; Wiebe, E.; Muche, B.; Hermann, S.; Dejaco, C.; Matteson, E.L.; Buttgereit, F. Glucocorticoids Are Not Associated with Bone Mineral Density in Patients with Polymyalgia Rheumatica, Giant Cell Arteritis and Other Vasculitides—Cross-Sectional Baseline Analysis of the Prospective Rh-GIOP Cohort. Cells 2022, 11, 536. https://doi.org/10.3390/cells11030536
Palmowski A, Wiebe E, Muche B, Hermann S, Dejaco C, Matteson EL, Buttgereit F. Glucocorticoids Are Not Associated with Bone Mineral Density in Patients with Polymyalgia Rheumatica, Giant Cell Arteritis and Other Vasculitides—Cross-Sectional Baseline Analysis of the Prospective Rh-GIOP Cohort. Cells. 2022; 11(3):536. https://doi.org/10.3390/cells11030536
Chicago/Turabian StylePalmowski, Andriko, Edgar Wiebe, Burkhard Muche, Sandra Hermann, Christian Dejaco, Eric L. Matteson, and Frank Buttgereit. 2022. "Glucocorticoids Are Not Associated with Bone Mineral Density in Patients with Polymyalgia Rheumatica, Giant Cell Arteritis and Other Vasculitides—Cross-Sectional Baseline Analysis of the Prospective Rh-GIOP Cohort" Cells 11, no. 3: 536. https://doi.org/10.3390/cells11030536
APA StylePalmowski, A., Wiebe, E., Muche, B., Hermann, S., Dejaco, C., Matteson, E. L., & Buttgereit, F. (2022). Glucocorticoids Are Not Associated with Bone Mineral Density in Patients with Polymyalgia Rheumatica, Giant Cell Arteritis and Other Vasculitides—Cross-Sectional Baseline Analysis of the Prospective Rh-GIOP Cohort. Cells, 11(3), 536. https://doi.org/10.3390/cells11030536






