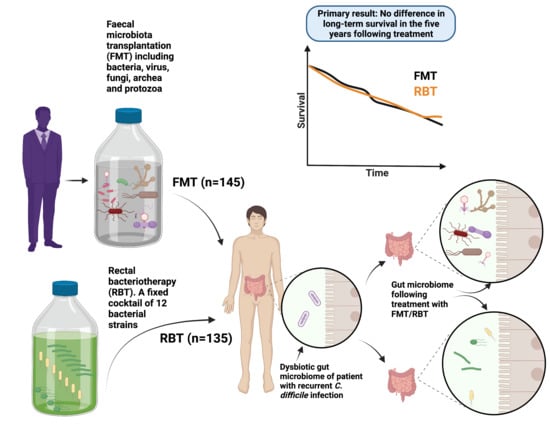
Long-Term Safety Following Faecal Microbiota Transplantation as a Treatment for Recurrent Clostridioides difficile Infection Compared with Patients Treated with a Fixed Bacterial Mixture: Results from a Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
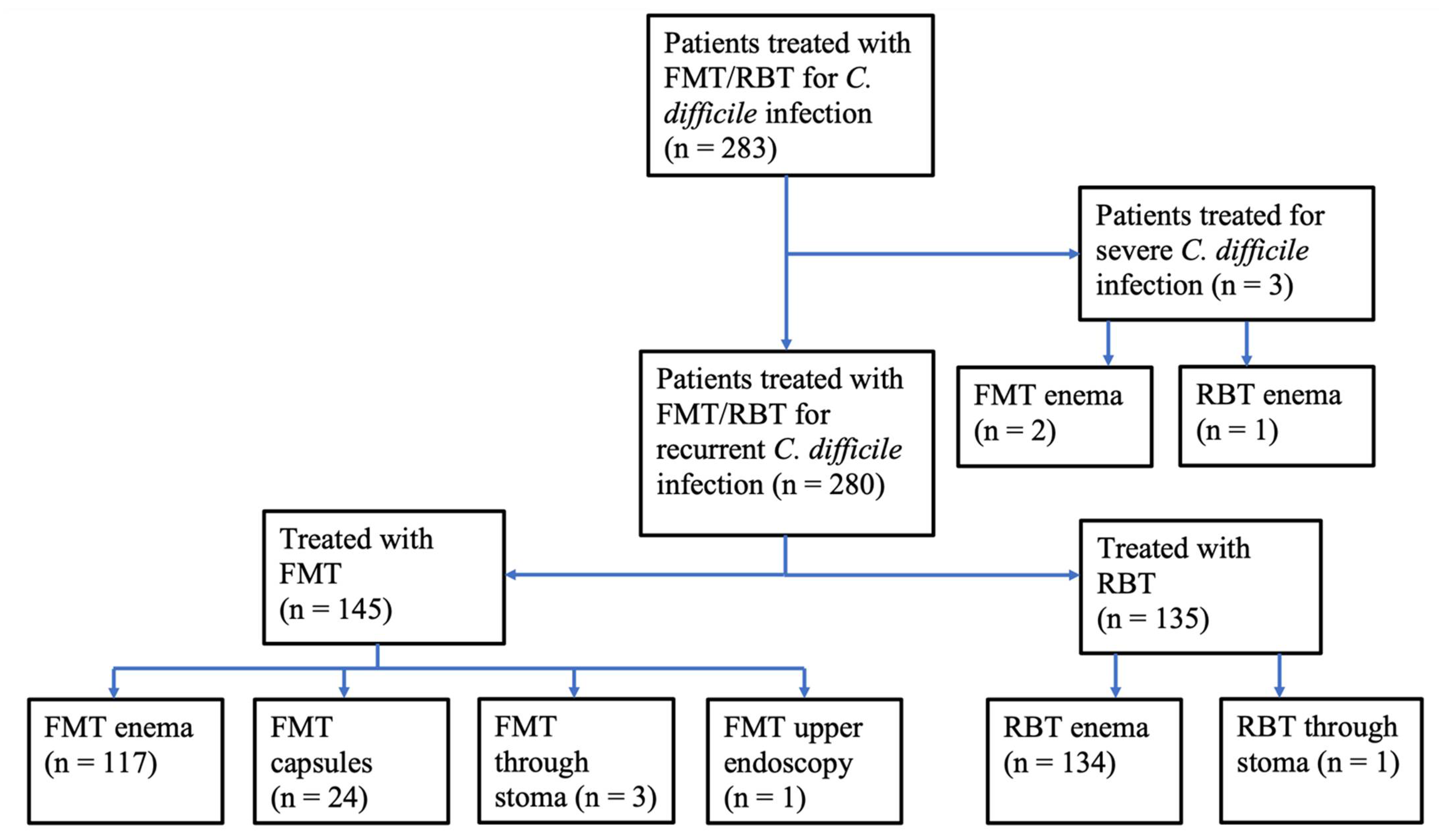
2.1. Treated Patients
2.2. Treatments
2.2.1. Rectal Bacteriotherapy
2.2.2. Faecal Microbiota Transplantation
2.3. Recorded Data
2.3.1. Survival
2.3.2. Onset of Disease
2.3.3. Disappearance of Disease/Cessation of Treatment
2.3.4. Cancer
2.3.5. Diabetes Mellitus, Hypertension and Inflammatory Bowel Disease
2.3.6. Multidrug-Resistant Organisms
2.3.7. Day of First Hospital Admission Following Treatment
2.4. Statistical Analyses
3. Results
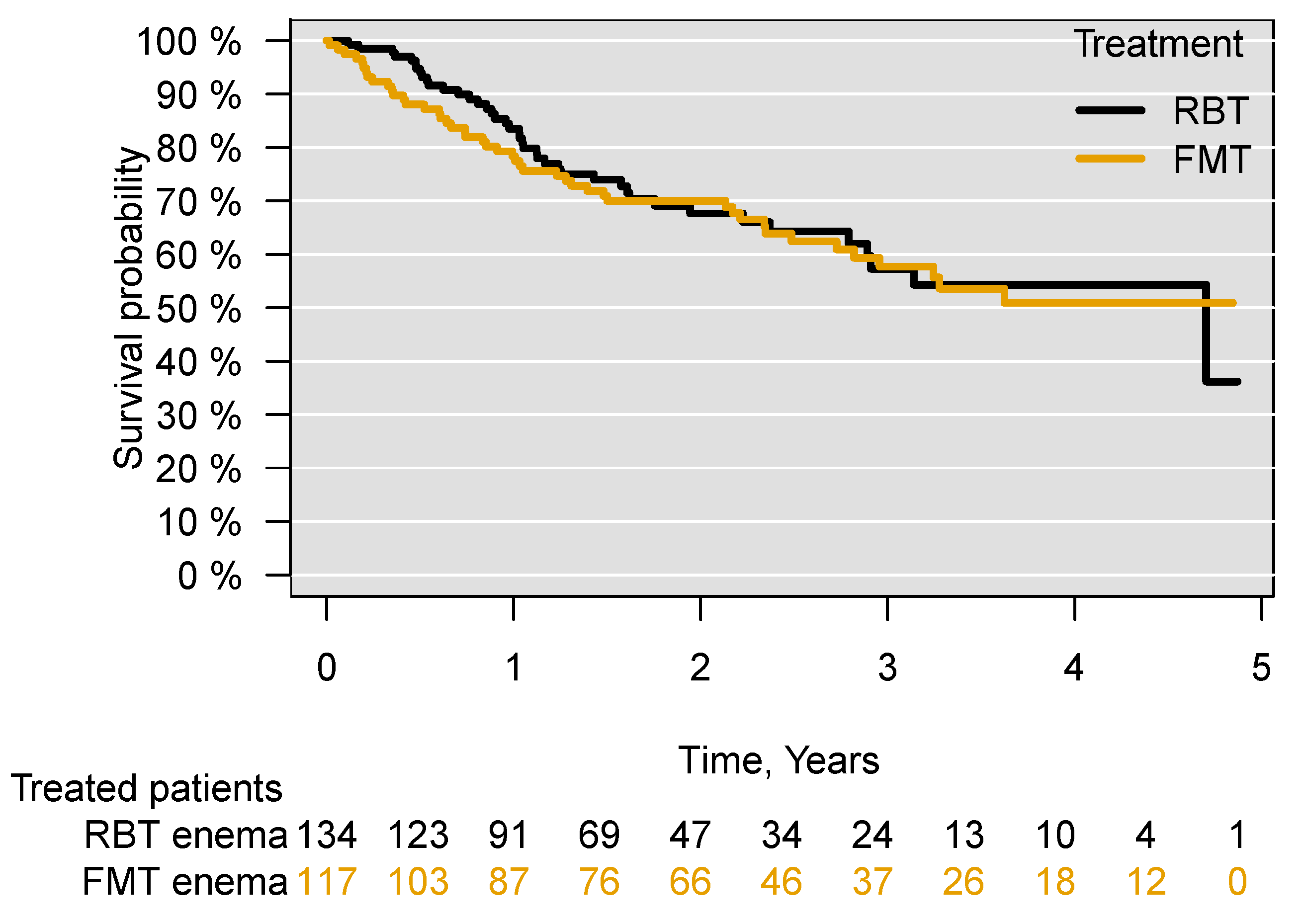
3.1. Survival
3.2. Onset of Disease and Disappearance of Disease/cessation of Treatment
3.2.1. Cancer
3.2.2. Diabetes Mellitus
3.2.3. Hypertension
3.2.4. Inflammatory Bowel Disease
3.2.5. Multidrug-Resistant Organisms
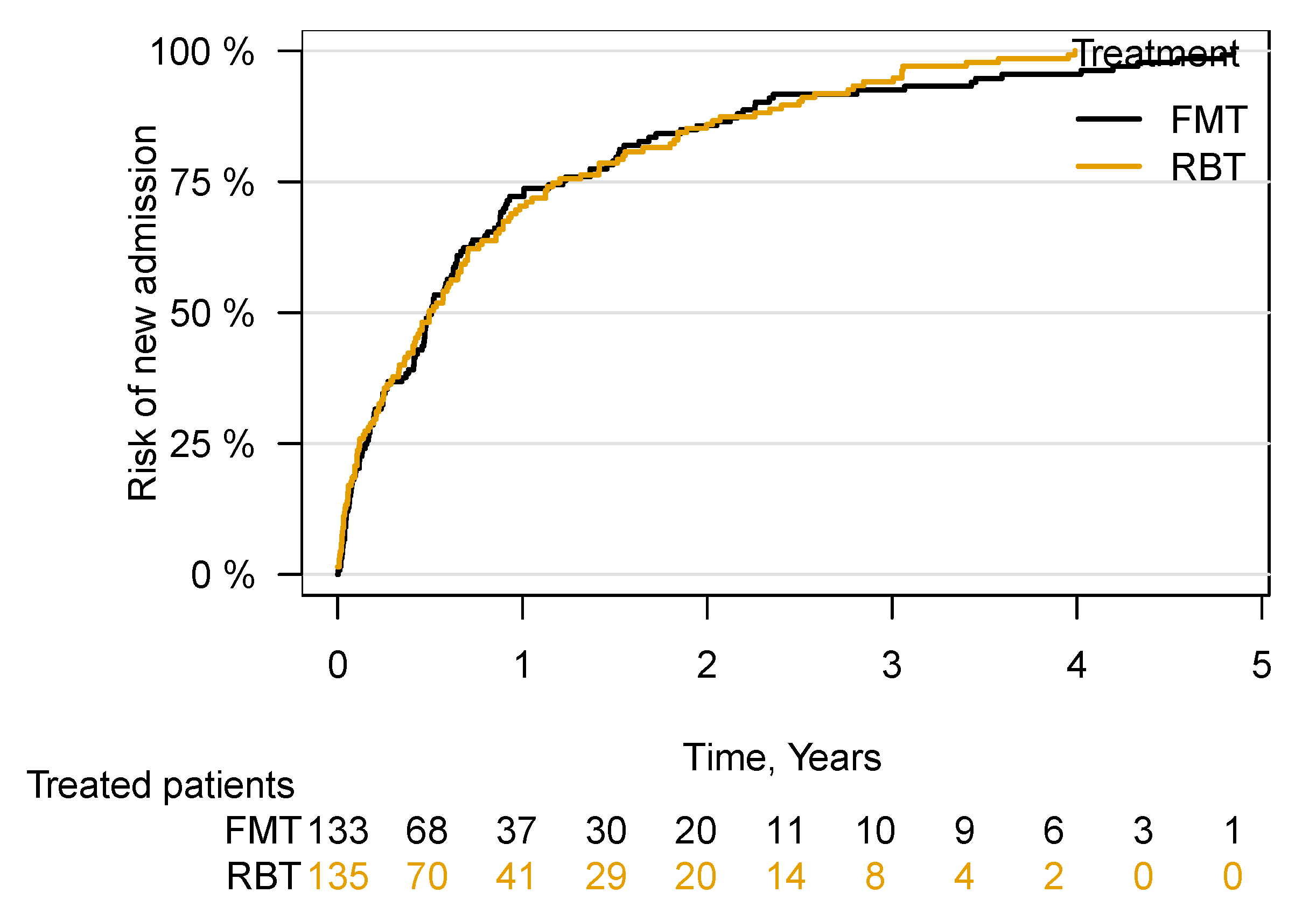
3.3. Hospital Admission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Graphics
References
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; Laplante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef] [PubMed]
- Baunwall, S.M.D.; Dahlerup, J.F.; Engberg, J.H.; Erikstrup, C.; Helms, M.; Juel, M.A.; Kjeldsen, J.; Nielsen, H.L.; Nilsson, A.C.; Rode, A.A.; et al. Danish national guideline for the treatment of Clostridioides difficile infection and use of faecal microbiota transplantation (FMT). Scand. J. Gastroenterol. 2021, 56, 1056–1077. [Google Scholar] [CrossRef] [PubMed]
- Van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27, S1–S21. [Google Scholar] [CrossRef] [PubMed]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef]
- Proenca, I.M.; Allegretti, J.R.; Bernardo, W.M.; De Moura, D.T.H.; Ponte Neto, A.M.; Matsubayashi, C.O.; Flor, M.M.; Kotinda, A.P.S.T.; De Moura, E.G.H. Fecal microbiota transplantation improves metabolic syndrome parameters: Systematic review with meta-analysis based on randomized clinical trials. Nutr. Res. 2020, 83, 1–14. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut Microbiota in Ulcerative Colitis: Insights on Pathogenesis and Treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Louca, P.; Nogal, A.; Wells, P.M.; Asnicar, F.; Wolf, J.; Steves, C.J.; Spector, T.D.; Segata, N.; Berry, S.E.; Valdes, A.M.; et al. Gut microbiome diversity and composition is associated with hypertension in women. J. Hypertens. 2021, 39, 1810–1816. [Google Scholar] [CrossRef]
- Lee, K.A.; Luong, M.K.; Shaw, H.; Nathan, P.; Bataille, V.; Spector, T.D. The gut microbiome: What the oncologist ought to know. Br. J. Cancer 2021, 125, 1197–1209. [Google Scholar] [CrossRef]
- Cold, F.; Browne, P.D.; Gunther, S.; Halkjaer, S.I.; Petersen, A.M.; Al-Gibouri, Z.; Hansen, L.H.; Christensen, A.H. Multidonor FMT capsules improve symptoms and decrease fecal calprotectin in ulcerative colitis patients while treated—An open-label pilot study. Scand. J. Gastroenterol. 2019, 54, 289–296. [Google Scholar] [CrossRef]
- Saha, S.; Tariq, R.; Tosh, P.K.; Pardi, D.S.; Khanna, S. Faecal microbiota transplantation for eradicating carriage of multidrug-resistant organisms: A systematic review. Clin. Microbiol. Infect. 2019, 25, 958–963. [Google Scholar] [CrossRef]
- Green, J.E.; Davis, J.A.; Berk, M.; Hair, C.; Loughman, A.; Castle, D.; Athan, E.; Nierenberg, A.A.; Cryan, J.F.; Jacka, F.; et al. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: A systematic review and meta-analysis. Gut Microbes 2020, 12, 1854640. [Google Scholar] [CrossRef] [PubMed]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal microbiota transplantation therapy in Crohn’s disease: Systematic review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef] [PubMed]
- Ghani, R.; Mullish, B.H.; Mcdonald, J.a.K.; Ghazy, A.; Williams, H.R.T.; Brannigan, E.T.; Mookerjee, S.; Satta, G.; Gilchrist, M.; Duncan, N.; et al. Disease Prevention Not Decolonization: A Model for Fecal Microbiota Transplantation in Patients Colonized With Multidrug-resistant Organisms. Clin. Infect. Dis. 2021, 72, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Ahmad, T.; Colville, A.; Sheridan, R. Fatal Aspiration Pneumonia as a Complication of Fecal Microbiota Transplant. Clin. Infect. Dis. 2015, 61, 136–137. [Google Scholar] [CrossRef] [Green Version]
- Merrick, B.; Allen, L.; Masirah M Zain, N.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, risk and safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2021, 53, 33–42. [Google Scholar] [CrossRef]
- Defilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Zellmer, C.; Sater, M.R.A.; Huntley, M.H.; Osman, M.; Olesen, S.W.; Ramakrishna, B. Shiga Toxin-Producing Escherichia coli Transmission via Fecal Microbiota Transplant. Clin. Infect. Dis. 2021, 72, e876–e880. [Google Scholar] [CrossRef]
- Qazi, T.; Amaratunga, T.; Barnes, E.L.; Fischer, M.; Kassam, Z.; Allegretti, J.R. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: Systematic review and meta-analysis. Gut Microbes 2017, 8, 574–588. [Google Scholar] [CrossRef] [Green Version]
- Youngster, I.; Mahabamunuge, J.; Systrom, H.K.; Sauk, J.; Khalili, H.; Levin, J.; Kaplan, J.L.; Hohmann, E.L. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016, 14, 134. [Google Scholar] [CrossRef] [Green Version]
- Costello, S.P.; Soo, W.; Bryant, R.V.; Jairath, V.; Hart, A.L.; Andrews, J.M. Systematic review with meta-analysis: Faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 46, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bahl, M.I.; Roager, H.M.; Fonvig, C.E.; Hellgren, L.I.; Frandsen, H.L.; Pedersen, O.; Holm, J.C.; Hansen, T.; Licht, T.R. Environmental spread of microbes impacts the development of metabolic phenotypes in mice transplanted with microbial communities from humans. ISME J. 2017, 11, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mara, K.; Pardi, D.S.; Khanna, S. Long-term Safety of Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection. Gastroenterology 2021, 160, 1961–1969.e1963. [Google Scholar] [CrossRef] [PubMed]
- Perler, B.K.; Chen, B.; Phelps, E.; Allegretti, J.R.; Fischer, M.; Ganapini, V.; Krajiceck, E.; Kumar, V.; Marcus, J.; Nativ, L.; et al. Long-Term Efficacy and Safety of Fecal Microbiota Transplantation for Treatment of Recurrent Clostridioides difficile Infection. J. Clin. Gastroenterol. 2020, 54, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; Van Nood, E.; Goorhuis, A.; Terveer, E.M.; Van Prehn, J.; Verspaget, H.W.; Van Beurden, Y.H.; Dijkgraaf, M.G.W.; Keller, J.J. Ten-Year Follow-Up of Patients Treated with Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection from a Randomized Controlled Trial and Review of the Literature. Microorganisms 2021, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Rode, A.A.; Chehri, M.; Krogsgaard, L.R.; Heno, K.K.; Svendsen, A.T.; Ribberholt, I.; Helms, M.; Engberg, J.; Schønning, K.; Tvede, M.; et al. Randomised clinical trial: A 12-strain bacterial mixture versus faecal microbiota transplantation versus vancomycin for recurrent Clostridioides difficile infections. Aliment. Pharmacol. Ther. 2021, 53, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Dahl Jorgensen, S.M.; Jorgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332.e1323. [Google Scholar] [CrossRef] [Green Version]
- Tvede, M.; Tinggaard, M.; Helms, M. Rectal bacteriotherapy for recurrent Clostridium difficile-associated diarrhoea: Results from a case series of 55 patients in Denmark 2000–2012. Clin. Microbiol. Infect. 2015, 21, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Emanuelsson, F.; Claesson, B.E.; Ljungström, L.; Tvede, M.; Ung, K.A. Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: A retrospective evaluation of 31 patients. Scand. J. Infect. Dis. 2014, 46, 89–97. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilic-Stojanovic, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Tvede, M.; Rask-Madsen, J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1989, 1, 1156–1160. [Google Scholar] [CrossRef]
- Rode, A.A.; Bytzer, P.; Pedersen, O.B.; Engberg, J. Establishing a donor stool bank for faecal microbiota transplantation: Methods and feasibility. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.M.D.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation: Establishment of a clinical application framework. Eur. J. Gastroenterol. Hepatol. 2017, 29, e36–e45. [Google Scholar] [CrossRef] [PubMed]
- Chehri, M.; Christensen, A.H.; Halkjaer, S.I.; Gunther, S.; Petersen, A.M.; Helms, M. Case series of successful treatment with fecal microbiota transplant (FMT) oral capsules mixed from multiple donors even in patients previously treated with FMT enemas for recurrent Clostridium difficile infection. Medicine 2018, 97, e11706. [Google Scholar] [CrossRef]
- Jorgensen, S.M.D.; Rubak, T.M.M.; Damsgaard, E.M.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation as a home therapy to frail older people. Age Ageing 2020, 49, 1093–1096. [Google Scholar] [CrossRef]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A. Oral Fecal Microbiota Transplant Capsules Are Safe and Effective for Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2021, 55, 300–308. [Google Scholar] [CrossRef]
- Mendelsohn, R.B.; Kaltsas, A.; King, S.; Hwang, C.; Kassam, Z.; Abend, A.M.; Kramer, E.; Kamboj, M. Fecal Microbiota Transplantation Is Safe for Clostridiodies difficile Infection in Patients with Solid Tumors Undergoing Chemotherapy. Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef]
- Navalkele, B.D.; Polistico, J.; Sandhu, A.; Awali, R.; Krishna, A.; Chandramohan, S.; Tillotson, G.; Chopra, T. Clinical outcomes after faecal microbiota transplant by retention enema in both immunocompetent and immunocompromised patients with recurrent Clostridioides difficile infections at an academic medical centre. J. Hosp. Infect. 2020, 106, 643–648. [Google Scholar] [CrossRef]
- Bahl, M.I.; Jørgensen, S.M.D.; Skriver, A.H.; Larsen, N.A.; Wang, M.; Licht, T.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for eradication of co-infection with Clostridioides difficile and extensively drug-resistant KPC-producing Klebsiella pneumoniae. Scand. J. Gastroenterol. 2020, 55, 626–630. [Google Scholar] [CrossRef] [Green Version]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.R.; Kim, A.M.; Laine, L.; Wu, G.D. The AGA’s Fecal Microbiota Transplantation National Registry: An Important Step Toward Understanding Risks and Benefits of Microbiota Therapeutics. Gastroenterology 2017, 152, 681–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, C.R.; Laine, L.A.; Wu, G.D. Monitoring Fecal Microbiota Transplantation Practice in a Rapidly Evolving Health and Regulatory Environment. Gastroenterology 2020, 159, 2004–2006. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.D.; Cold, F.; Petersen, A.M.; Halkjær, S.I.; Christensen, A.H.; Günther, S.; Hestbjerg Hansen, L. Engraftment of strictly anaerobic oxygen-sensitive bacteria in irritable bowel syndrome patients following fecal microbiota transplantation does not improve symptoms. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; Mcdonald, J.a.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Li, P.; Zhang, T.; Cui, B.; Ji, G.; Lu, X.; Zhang, F. Long-Term Safety and Efficacy of Fecal Microbiota Transplant in Active Ulcerative Colitis. Drug Saf. 2019, 42, 869–880. [Google Scholar] [CrossRef]
- Aggarwala, V.; Mogno, I.; Li, Z.; Yang, C.; Britton, G.J.; Chen-Liaw, A.; Mitcham, J.; Bongers, G.; Gevers, D.; Clemente, J.C.; et al. Precise quantification of bacterial strains after fecal microbiota transplantation delineates long-term engraftment and explains outcomes. Nat. Microbiol. 2021, 6, 1309–1318. [Google Scholar] [CrossRef]
- Segal, J.P.; Mullish, B.H.; Quraishi, M.N.; Iqbal, T.; Marchesi, J.R.; Sokol, H. Mechanisms underpinning the efficacy of faecal microbiota transplantation in treating gastrointestinal disease. Therap. Adv. Gastroenterol. 2020, 13, 1756284820946904. [Google Scholar] [CrossRef]
- Khoruts, A.; Staley, C.; Sadowsky, M.J. Faecal microbiota transplantation for Clostridioides difficile: Mechanisms and pharmacology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 67–80. [Google Scholar] [CrossRef]
- Staley, C.; Kaiser, T.; Vaughn, B.P.; Graiziger, C.T.; Hamilton, M.J.; Rehman, T.U.; Song, K.; Khoruts, A.; Sadowsky, M.J. Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation. Microbiome 2018, 6, 166. [Google Scholar] [CrossRef] [Green Version]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018, 67, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Mullish, B.H.; Allegretti, J.R. The contribution of bile acid metabolism to the pathogenesis of Clostridioides difficile infection. Therap. Adv. Gastroenterol. 2021, 14, 17562848211017725. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Flemer, B.; Joyce, S.A.; Zulquernain, A.; Sheehan, D.; Shanahan, F.; O’toole, P.W. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018, 18, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Bloemendaal, M.; Szopinska-Tokov, J.; Belzer, C.; Boverhoff, D.; Papalini, S.; Michels, F.; Van Hemert, S.; Arias Vasquez, A.; Aarts, E. Probiotics-induced changes in gut microbial composition and its effects on cognitive performance after stress: Exploratory analyses. Transl. Psychiatry 2021, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Lee, S.K.; Cheon, J.H.; Yong, D.E.; Koh, H.; Kang, Y.K.; Jeong, W.Y.; Lee, W.J.; Sohn, Y.; Cho, Y.; et al. Fecal Microbiota Transplantation for multidrug-resistant organism: Efficacy and Response prediction. J. Infect. 2020, 81, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Huttner, B.D.; De Lastours, V.; Wassenberg, M.; Maharshak, N.; Mauris, A.; Galperine, T.; Zanichelli, V.; Kapel, N.; Bellanger, A.; Olearo, F.; et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: A randomized clinical trial. Clin. Microbiol. Infect. 2019, 25, 830–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerum, A.M.; Justesen, U.S.; Pinholt, M.; Roer, L.; Kaya, H.; Worning, P.; Nygaard, S.; Kemp, M.; Clausen, M.E.; Nielsen, K.L.; et al. Surveillance of vancomycin-resistant enterococci reveals shift in dominating clones and national spread of a vancomycin-variable vanA Enterococcus faecium ST1421-CT1134 clone, Denmark, 2015 to March 2019. Eurosurveillance 2019, 24, 1900503. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.; Hicks, L.; Ali, I.; Salzman, E.; Wang, J.; Snitkin, E.; Gibson, K.; Cassone, M.; Mody, L.; Foxman, B. Epidemiology of Vancomycin-Resistant Enterococcus faecium and Enterococcus faecalis Colonization in Nursing Facilities. Open Forum Infect. Dis. 2020, 7, ofz553. [Google Scholar] [CrossRef]
- Kjær Hansen, S.; Andersen, L.; Detlefsen, M.; Holm, A.; Roer, L.; Antoniadis, P.; Skov, M.N.; Hammerum, A.M.; Kemp, M. Using core genome multilocus sequence typing (cgMLST) for vancomycin-resistant Enterococcus faecium isolates to guide infection control interventions and end an outbreak. J. Glob. Antimicrob. Resist. 2021, 24, 418–423. [Google Scholar] [CrossRef]
- Pan, S.C.; Wang, J.T.; Chen, Y.C.; Chang, Y.Y.; Chen, M.L.; Chang, S.C. Incidence of and risk factors for infection or colonization of vancomycin-resistant enterococci in patients in the intensive care unit. PLoS ONE 2012, 7, e47297. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Villodres, Á.; Martín-Gandul, C.; Peñalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics 2021, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Yadav, D.; Khanna, S. The interplay of Clostridioides difficile infection and inflammatory bowel disease. Therap. Adv. Gastroenterol. 2021, 14, 17562848211020285. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]


| FMT | RBT | p-Value | |
|---|---|---|---|
| Number | 145 | 135 | |
| Age, mean (SD) | 66.9 (18.8) | 66.7 (19.7) | 0.89 |
| Sex: | |||
| female | 85 | 81 | 0.91 |
| male | 60 | 54 | |
| Charlson Comorbidity Index, mean (SD) | 4.3 (2.7) | 4.4 (2.9) | 0.69 |
| Recurrences of CDI, mean (SD) | 2.9 (1.5) | 2.7 (1.3) | 0.29 |
| Location of treatment: | |||
| outpatient | 124 | 112 | 0.04 |
| hospital admission | 6 | 22 | <0.01 |
| at home | 4 | 0 | 0.12 |
| not recorded | 11 | 1 | |
| Previous FMT or RBT treatment (all delivered by enemas): | |||
| FMT | 2 | 3 | 0.67 |
| RBT | 2 | 0 | 0.49 |
| both | 2 | 1 | 1 |
| Both FMT and RBT for current episode of CDI | 5 | 21 | <0.01 |
| Cancer: | |||
| all | 18 | 13 | 0.41 |
| colorectal | 1 | 0 | 0.49 |
| Diabetes mellitus: | |||
| DM1 | 0 | 6 | 0.03 |
| DM2 | 16 | 21 | 0.41 |
| Hypertension | 66 | 53 | 0.12 |
| IBD: | |||
| Crohn’s disease | 1 | 5 | 0.21 |
| Ulcerative colitis | 12 | 10 | 0.79 |
| IBD unknown type | 1 | 1 | 1 |
| MDRO: | |||
| all | 7 | 9 | 0.90 |
| VRE | 5 | 8 | 0.66 |
| ESBL-producing K. pneumoniae | 1 | 1 | 1 |
| MDR E. coli | 1 | 0 | 1 |
| FMT (New Diagnosis/Patients at Risk) | RBT (New Diagnosis/Patients at Risk) | p-Value | |
|---|---|---|---|
| Cancer: | |||
| first year | 2/115 | 2/122 | 1 |
| second and third year | 5/80 | 2/82 | 0.27 |
| fourth and fifth year | 1/34 | 0/20 | 1 |
| Multidrug-resistant organisms: | |||
| first month | 3/126 | 1/125 | 0.62 |
| second to sixth month | 4/120 | 1/124 | 0.35 |
| Diabetes mellitus type 2: | |||
| first year | 2/117 | 0/108 | 0.5 |
| second and third year | 1/76 | 1/72 | 1 |
| fourth and fifth year | 1/31 | 0/19 | 1 |
| Hypertension: | |||
| first year | 2/66 | 1/82 | 0.59 |
| second and third year | 1/44 | 1/51 | 1 |
| fourth and fifth year | 0/18 | 0/14 | 1 |
| IBD: | |||
| first year | 1/113 | 0/119 | 0.49 |
| second and third year | 0/74 | 0/76 | 1 |
| fourth and fifth year | 0/29 | 0/22 | 1 |
| FMT (Disappearance of Disease/Patients with a Diagnosis) | RBT (Disappearance of Disease/Patients with a Diagnosis) | p-Value | |
|---|---|---|---|
| Cancer: | |||
| first year | 0/18 | 0/13 | 1 |
| second and third year | 0/8 | 0/7 | 1 |
| fourth and fifth year | 0/3 | 0/3 | 1 |
| Multidrug-resistant organisms: | |||
| first month | 0/7 | 0/9 | 1 |
| second to sixth month | 2/5 | 0/8 | 0.25 |
| Diabetes mellitus type 2: | |||
| first year | 0/13 | 0/20 | 1 |
| second and third year | 0/10 | 1/11 | 1 |
| fourth and fifth year | 0/6 | 0/3 | 1 |
| Hypertension: | |||
| first year | 2/61 | 4/49 | 0.49 |
| second and third year | 0/37 | 2/32 | 0.21 |
| fourth and fifth year | 0/17 | 0/14 | 1 |
| IBD: | |||
| first year | 0/12 | 0/13 | 1 |
| second and third year | 0/8 | 0/10 | 1 |
| fourth and fifth year | 0/4 | 0/2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cold, F.; Svensson, C.K.; Petersen, A.M.; Hansen, L.H.; Helms, M. Long-Term Safety Following Faecal Microbiota Transplantation as a Treatment for Recurrent Clostridioides difficile Infection Compared with Patients Treated with a Fixed Bacterial Mixture: Results from a Retrospective Cohort Study. Cells 2022, 11, 435. https://doi.org/10.3390/cells11030435
Cold F, Svensson CK, Petersen AM, Hansen LH, Helms M. Long-Term Safety Following Faecal Microbiota Transplantation as a Treatment for Recurrent Clostridioides difficile Infection Compared with Patients Treated with a Fixed Bacterial Mixture: Results from a Retrospective Cohort Study. Cells. 2022; 11(3):435. https://doi.org/10.3390/cells11030435
Chicago/Turabian StyleCold, Frederik, Camilla Kara Svensson, Andreas Munk Petersen, Lars Hestbjerg Hansen, and Morten Helms. 2022. "Long-Term Safety Following Faecal Microbiota Transplantation as a Treatment for Recurrent Clostridioides difficile Infection Compared with Patients Treated with a Fixed Bacterial Mixture: Results from a Retrospective Cohort Study" Cells 11, no. 3: 435. https://doi.org/10.3390/cells11030435
APA StyleCold, F., Svensson, C. K., Petersen, A. M., Hansen, L. H., & Helms, M. (2022). Long-Term Safety Following Faecal Microbiota Transplantation as a Treatment for Recurrent Clostridioides difficile Infection Compared with Patients Treated with a Fixed Bacterial Mixture: Results from a Retrospective Cohort Study. Cells, 11(3), 435. https://doi.org/10.3390/cells11030435