Protein Arginine Methyltransferases in Neuromuscular Function and Diseases
Abstract
1. Introduction
2. Protein Arginine Methyltransferases (PRMTs)
3. PRMTs in Muscle Homeostasis and Remodeling
4. PRMTs in Neuromuscular Function
5. PRMTs in Neuromuscular Diseases (NMDs)
5.1. Regulation of Cytoplasmic Ribonucleoprotein (RNP) Granules
5.2. Transcriptional Regulation
5.3. Regulation of Protein-Protein and Protein-RNA Interactions
6. Therapeutic Strategies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rygiel, K.A.; Picard, M.; Turnbull, D. The ageing neuromuscular system and sarcopenia: A mitochondrial perspective. J. Physiol. 2016, 594, 4499–4512. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.K.; de Souto Barreto, P.; Fougère, B.; Rolland, Y.; Vellas, B.; Cesari, M. Chronic inflammation and sarcopenia: A regenerative cell therapy perspective. Exp. Gerontol. 2018, 103, 115–123. [Google Scholar] [CrossRef]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2014, 14, 58–74. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachex Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef]
- Paik, W.K.; Kim, S. Protein methylase I. Purification and properties of the enzyme. J. Biol. Chem. 1968, 243, 2108–2114. [Google Scholar] [CrossRef]
- Bedford, M.T.; Clarke, S.G. Protein Arginine Methylation in Mammals: Who, What, and Why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef]
- Pal, S.; Sif, S. Interplay between chromatin remodelers and protein arginine methyltransferases. J. Cell. Physiol. 2007, 213, 306–315. [Google Scholar] [CrossRef]
- Yang, Y.; Bedford, M.T. Protein arginine methyltransferases and cancer. Nat. Rev. Cancer 2013, 13, 37–50. [Google Scholar] [CrossRef]
- Feng, Y.; Maity, R.; Whitelegge, J.P.; Hadjikyriacou, A.; Li, Z.; Zurita-Lopez, C.; Al-Hadid, Q.; Clark, A.T.; Bedford, M.T.; Masson, J.Y.; et al. Mammalian protein arginine methyltransferase 7 (PRMT7) specifically targets RXR sites in lysine- and arginine-rich regions. J. Biol. Chem. 2013, 288, 37010–37025. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kao, P.N.; Herschman, H.R. Protein-arginine Methyltransferase I, the Predominant Protein-arginine Methyltransferase in Cells, Interacts with and Is Regulated by Interleukin Enhancer-binding Factor 3. J. Biol. Chem. 2000, 275, 19866–19876. [Google Scholar] [CrossRef]
- Thandapani, P.; O’Connor, T.R.; Bailey, T.L.; Richard, S. Defining the RGG/RG Motif. Mol. Cell 2013, 50, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Blanc, R.S.; Richard, S. Arginine Methylation: The Coming of Age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef]
- Thompson, P.R.; Fast, W. Histone citrullination by protein arginine deiminase: Is arginine methylation a green light or a roadblock? ACS Chem. Biol. 2006, 1, 433–441. [Google Scholar] [CrossRef]
- Chang, B.; Chen, Y.; Zhao, Y.; Bruick, R.K. JMJD6 is a histone arginine demethylase. Science 2007, 318, 444–447. [Google Scholar] [CrossRef]
- Stouth, D.W.; VanLieshout, T.L.; Shen, N.Y.; Ljubicic, V. Regulation of Skeletal Muscle Plasticity by Protein Arginine Methyltransferases and Their Potential Roles in Neuromuscular Disorders. Front. Physiol. 2017, 8, 870. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.R.; Scherer, C.A.; Chen, J.; Roshon, M.J.; Ruley, H.E. Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol. Cell. Biol. 2000, 20, 4859–4869. [Google Scholar] [CrossRef] [PubMed]
- Tee, W.W.; Pardo, M.; Theunissen, T.W.; Yu, L.; Choudhary, J.S.; Hajkova, P.; Surani, M.A. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 2010, 24, 2772–2777. [Google Scholar] [CrossRef]
- Ying, Z.; Mei, M.; Zhang, P.; Liu, C.; He, H.; Gao, F.; Bao, S. Histone Arginine Methylation by PRMT7 Controls Germinal Center Formation via Regulating Bcl6 Transcription. J. Immunol. 2015, 195, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Torres-Padilla, M.-E.; Parfitt, D.-E.; Kouzarides, T.; Zernicka-Goetz, M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 2007, 445, 214–218. [Google Scholar] [CrossRef]
- Swiercz, R.; Cheng, D.; Kim, D.; Bedford, M.T. Ribosomal Protein rpS2 Is Hypomethylated in PRMT3-deficient Mice. J. Biol. Chem. 2007, 282, 16917–16923. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-H.; Fan, X.-J.; Hu, Y.; Tian, X.-X.; Guo, M.; Mao, M.-W.; Fang, Z.-Y.; Wu, P.; Gao, S.-X.; Peng, C.; et al. A systematic survey of PRMT interactomes reveals the key roles of arginine methylation in the global control of RNA splicing and translation. Sci. Bull. 2021, 66, 1342–1357. [Google Scholar] [CrossRef]
- Guccione, E.; Richard, S. The regulation, functions and clinical relevance of arginine methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Schapira, M.; Arrowsmith, C.H.; Barsyte-Lovejoy, D. Protein arginine methylation: From enigmatic functions to therapeutic targeting. Nat. Rev. Drug Discov. 2021, 20, 509–530. [Google Scholar] [CrossRef] [PubMed]
- Tewary, S.K.; Zheng, Y.G.; Ho, M.C. Protein arginine methyltransferases: Insights into the enzyme structure and mechanism at the atomic level. Cell. Mol. Life Sci. 2019, 76, 2917–2932. [Google Scholar] [CrossRef]
- Iwasaki, H.; Yada, T. Protein arginine methylation regulates insulin signaling in L6 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2007, 364, 1015–1021. [Google Scholar] [CrossRef]
- Ljubicic, V.; Khogali, S.; Renaud, J.-M.; Jasmin, B.J. Chronic AMPK stimulation attenuates adaptive signaling in dystrophic skeletal muscle. Am. J. Physiol. Physiol. 2012, 302, C110–C121. [Google Scholar] [CrossRef]
- Blanc, R.S.; Vogel, G.; Li, X.; Yu, Z.; Li, S.; Richard, S. Arginine Methylation by PRMT1 Regulates Muscle Stem Cell Fate. Mol. Cell. Biol. 2017, 37, e00457-16. [Google Scholar] [CrossRef]
- Kawabe, Y.; Wang, Y.X.; McKinnell, I.W.; Bedford, M.T.; Rudnicki, M.A. Carm1 regulates Pax7 transcriptional activity through MLL1/2 recruitment during asymmetric satellite stem cell divisions. Cell Stem Cell 2012, 11, 333–345. [Google Scholar] [CrossRef]
- Chen, S.L.; Loffler, K.A.; Chen, D.; Stallcup, M.R.; Muscat, G.E.O. The coactivator-associated arginine methyltransferase is necessary for muscle differentiation: CARM1 coactivates myocyte enhancer factor-2. J. Biol. Chem. 2002, 277, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Günther, S.; Looso, M.; Künne, C.; Krüger, M.; Kim, J.; Zhou, Y.; Braun, T. Prmt5 is a regulator of muscle stem cell expansion in adult mice. Nat. Commun. 2015, 6, 7140. [Google Scholar] [CrossRef]
- Dacwag, C.S.; Ohkawa, Y.; Pal, S.; Sif, S.; Imbalzano, A.N. The Protein Arginine Methyltransferase Prmt5 Is Required for Myogenesis because It Facilitates ATP-Dependent Chromatin Remodeling. Mol. Cell. Biol. 2007, 27, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Blanc, R.S.; Vogel, G.; Chen, T.; Crist, C.; Richard, S. PRMT7 Preserves Satellite Cell Regenerative Capacity. Cell Rep. 2016, 14, 1528–1539. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Lee, S.-J.; Lee, H.-J.; Kim, H.-B.; Vuong, T.A.; Cho, H.; Bae, G.-U.; Kang, J.-S. Prmt7 promotes myoblast differentiation via methylation of p38MAPK on arginine residue 70. Cell Death Differ. 2019, 27, 573–586. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, H.-J.; Kim, H.; Choi, D.; Cho, S.-C.; Seong, J.K.; Koo, S.-H.; Kang, J.-S. Skeletal muscle-specific Prmt1 deletion causes muscle atrophy via deregulation of the PRMT6-FOXO3 axis. Autophagy 2019, 15, 1069–1081. [Google Scholar] [CrossRef]
- Shin, H.-J.R.; Kim, H.; Oh, S.; Lee, J.-G.; Kee, M.; Ko, H.-J.; Kweon, M.-N.; Won, K.-J.; Baek, S.H. AMPK–SKP2–CARM1 signalling cascade in transcriptional regulation of autophagy. Nature 2016, 534, 553–557. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Shang, Y.; Guo, Y.; Li, Z. CARM1 contributes to skeletal muscle wasting by mediating FoxO3 activity and promoting myofiber autophagy. Exp. Cell Res. 2018, 374, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.M.; Dowhan, D.H.; Eriksson, N.A.; Muscat, G.E.O. CARM1/PRMT4 is necessary for the glycogen gene expression programme in skeletal muscle cells. Biochem. J. 2012, 444, 323–331. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Lee, H.-J.; Vuong, T.A.; Choi, K.-S.; Choi, D.; Koo, S.-H.; Cho, S.C.; Cho, H.; Kang, J.-S. Prmt7 Deficiency Causes Reduced Skeletal Muscle Oxidative Metabolism and Age-Related Obesity. Diabetes 2016, 65, 1868–1882. [Google Scholar] [CrossRef]
- Lin, M.; Xiong, W.-C.; Mei, L. Neuromuscular Junction Formation, Aging, and Disorders. Annu. Rev. Physiol. 2018, 80, 159–188. [Google Scholar] [CrossRef]
- Li, X.M.; Dong, X.P.; Luo, S.W.; Zhang, B.; Lee, D.H.; Ting, A.K.L.; Neiswender, H.; Kim, C.H.; Carpenter-Hyland, E.; Gao, T.M.; et al. Retrograde regulation of motoneuron differentiation by muscle beta-catenin. Nat. Neurosci. 2008, 11, 262–268. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Matsuoka, Y.; Miyake, M.; Konishi, H. Methylated amino acid residues of proteins of brain and other organs. J. Neurochem. 1975, 24, 893–902. [Google Scholar] [CrossRef]
- Hashimoto, M.; Murata, K.; Ishida, J.; Kanou, A.; Kasuya, Y.; Fukamizu, A. Severe Hypomyelination and Developmental Defects Are Caused in Mice Lacking Protein Arginine Methyltransferase 1 (PRMT1) in the Central Nervous System. J. Biol. Chem. 2016, 291, 2237–2245. [Google Scholar] [CrossRef]
- Bezzi, M.; Teo, S.X.; Muller, J.; Mok, W.C.; Sahu, S.K.; Vardy, L.A.; Bonday, Z.Q.; Guccione, E. Regulation of constitutive and alternative splicing by PRMT5 reveals a role for Mdm4 pre-mRNA in sensing defects in the spliceosomal machinery. Genes Dev. 2013, 27, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Chittka, A.; Nitarska, J.; Grazini, U.; Richardson, W.D. Transcription Factor Positive Regulatory Domain 4 (PRDM4) Recruits Protein Arginine Methyltransferase 5 (PRMT5) to Mediate Histone Arginine Methylation and Control Neural Stem Cell Proliferation and Differentiation. J. Biol. Chem. 2012, 287, 42995–43006. [Google Scholar] [CrossRef]
- Huang, J.; Vogel, G.; Yu, Z.; Almazan, G.; Richard, S. Type II Arginine Methyltransferase PRMT5 Regulates Gene Expression of Inhibitors of Differentiation/DNA Binding Id2 and Id4 during Glial Cell Differentiation*. J. Biol. Chem. 2011, 286, 44424–44432. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Mori, Y.; Chu, D.L.; Koyama, Y.; Miyata, S.; Tanaka, H.; Yachi, K.; Kubo, T.; Yoshikawa, H.; Tohyama, M. CARM1 Regulates Proliferation of PC12 Cells by Methylating HuD. Mol. Cell. Biol. 2006, 26, 2273–2285. [Google Scholar] [CrossRef][Green Version]
- Selvi, B.R.; Swaminathan, A.; Maheshwari, U.; Nagabhushana, A.; Mishra, R.; Kundu, T.K. CARM1 regulates astroglial lineage through transcriptional regulation of Nanog and posttranscriptional regulation by miR92a. Mol. Biol. Cell 2015, 26, 316–326. [Google Scholar] [CrossRef]
- Penney, J.; Seo, J.; Kritskiy, O.; Elmsaouri, S.; Gao, F.; Pao, P.-C.; Su, S.C.; Tsai, L.-H. Loss of Protein Arginine Methyltransferase 8 Alters Synapse Composition and Function, Resulting in Behavioral Defects. J. Neurosci. 2017, 37, 8655–8666. [Google Scholar] [CrossRef]
- Lee, P.K.M.; Bin Goh, W.W.; Sng, J.C.G. Network-based characterization of the synaptic proteome reveals that removal of epigenetic regulatorPrmt8restricts proteins associated with synaptic maturation. J. Neurochem. 2017, 140, 613–628. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, M.H.; Kim, K.R.; Jung, C.Y.; Lee, S.Y.; Kim, H.; Koh, K.; Vuong, T.A.; Jung, S.M.; Yang, H.W.; et al. Protein arginine methylation facilitates KCNQ channel-PIP2 interaction leading to seizure suppression. Elife 2016, 5, e17159. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Vuong, T.A.; Wen, X.; Jeong, H.-J.; So, H.-K.; Kwon, I.; Kang, J.-S.; Cho, H. Methylation determines the extracellular calcium sensitivity of the leak channel NALCN in hippocampal dentate granule cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Statland, J.M.; Barohn, R.J.; McVey, A.L.; Katz, J.S.; Dimachkie, M.M. Patterns of Weakness, Classification of Motor Neuron Disease, and Clinical Diagnosis of Sporadic Amyotrophic Lateral Sclerosis. Neurol. Clin. 2015, 33, 735–748. [Google Scholar] [CrossRef]
- Ikenaka, K.; Atsuta, N.; Maeda, Y.; Hotta, Y.; Nakamura, R.; Kawai, K.; Yokoi, D.; Hirakawa, A.; Taniguchi, A.; Morita, M.; et al. Increase of arginine dimethylation correlates with the progression and prognosis of ALS. Neurology 2019, 92, e1868–e1877. [Google Scholar] [CrossRef] [PubMed]
- Chio, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G.; Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.-C. Synaptic Paths to Neurodegeneration: The Emerging Role of TDP-43 and FUS in Synaptic Functions. Neural Plast. 2018, 2018, 8413496. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Takumi, T. TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J. Cell Sci. 2005, 118, 5755–5765. [Google Scholar] [CrossRef]
- Tan, A.Y.; Manley, J.L. The TET Family of Proteins: Functions and Roles in Disease. J. Mol. Cell Biol. 2009, 1, 82–92. [Google Scholar] [CrossRef]
- Dormann, D.; Rodde, R.; Edbauer, D.; Bentmann, E.; Fischer, I.; Hruscha, A.; Than, M.E.; Mackenzie, I.R.A.; Capell, A.; Schmid, B.; et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. Embo. J. 2010, 29, 2841–2857. [Google Scholar] [CrossRef] [PubMed]
- Dormann, D.; Rodde, R.; Edbauer, D.; Bentmann, E.; Fischer, I.; Hruscha, A.; Than, M.E.; Mackenzie, I.R.A.; Capell, A.; Schmind, B.; et al. Arginine methylation next to the PY-NLS modulates Transportin binding and nuclear import of FUS. EMBO J. 2012, 31, 4258–4275. [Google Scholar] [CrossRef]
- Gal, J.; Zhang, J.; Kwinter, D.M.; Zhai, J.; Jia, H.; Jia, J.; Zhu, H. Nuclear localization sequence of FUS and induction of stress granules by ALS mutants. Neurobiol. Aging 2011, 32, 2323.e27–2323.e40. [Google Scholar] [CrossRef]
- Kwiatkowski, T.J., Jr.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T.; et al. Mutations in the FUS/TLS Gene on Chromosome 16 Cause Familial Amyotrophic Lateral Sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef]
- Winton, M.J.; Igaz, L.M.; Wong, M.M.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M.Y. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J. Biol. Chem. 2008, 283, 13302–13309. [Google Scholar] [CrossRef]
- Johnson, B.S.; Snead, D.; Lee, J.J.; McCaffrey, M.; Shorter, J.; Gitler, A.D. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J. Biol. Chem. 2009, 284, 20329–20339. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Kametani, F.; Arai, T.; Akiyama, H.; Hasegawa, M. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum. Mol. Genet. 2009, 18, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.E.; Schuman, E.M. The Central Dogma Decentralized: New Perspectives on RNA Function and Local Translation in Neurons. Neuron 2013, 80, 648–657. [Google Scholar] [CrossRef]
- Rappsilber, J.; Friesen, W.J.; Paushkin, S.; Dreyfuss, G.; Mann, M. Detection of Arginine Dimethylated Peptides by Parallel Precursor Ion Scanning Mass Spectrometry in Positive Ion Mode. Anal. Chem. 2003, 75, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Tradewell, M.L.; Yu, Z.; Tibshirani, M.; Boulanger, M.-C.; Durham, H.D.; Richard, S. Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations. Hum. Mol. Genet. 2011, 21, 136–149. [Google Scholar] [CrossRef]
- Scaramuzzino, C.; Monaghan, J.; Milioto, C.; Jr, N.A.L.; Maltare, A.; Aggarwal, T.; Casci, I.; Fackelmayer, F.O.; Pennuto, M.; Pandey, U.B. Protein Arginine Methyltransferase 1 and 8 Interact with FUS to Modify Its Sub-Cellular Distribution and Toxicity In Vitro and In Vivo. PLoS ONE 2013, 8, e61576. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Neumann, M.; Arzberger, T.; Abou-Ajram, C.; Funk, E.; Hartmann, H.; Edbauer, D.; Kremmer, E.; Göbl, C.; Resch, M.; et al. Monomethylated and unmethylated FUS exhibit increased binding to Transportin and distinguish FTLD-FUS from ALS-FUS. Acta Neuropathol. 2016, 131, 587–604. [Google Scholar] [CrossRef]
- Kang, J.; Lim, L.; Lu, Y.; Song, J. A unified mechanism for LLPS of ALS/FTLD-causing FUS as well as its modulation by ATP and oligonucleic acids. PLOS Biol. 2019, 17, e3000327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Choi, J.-M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699.e16. [Google Scholar] [CrossRef]
- Evich, M.; Stroeva, E.; Zheng, Y.G.; Germann, M.W. Effect of methylation on the side-chain pKa value of arginine. Protein Sci. 2016, 25, 479–486. [Google Scholar] [CrossRef]
- Hofweber, M.; Hutten, S.; Bourgeois, B.; Spreitzer, E.; Niedner-Boblenz, A.; Schifferer, M.; Ruepp, M.-D.; Simons, M.; Niessing, D.; Madl, T.; et al. Phase Separation of FUS Is Suppressed by Its Nuclear Import Receptor and Arginine Methylation. Cell 2018, 173, 706–719.e13. [Google Scholar] [CrossRef]
- Qamar, S.; Wang, G.; Randle, S.J.; Ruggeri, F.S.; Varela, J.A.; Lin, J.Q.; Phillips, E.C.; Miyashita, A.; Williams, D.; Strohl, F.; et al. FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine Cation-π Interactions. Cell. 2018, 173, 720–734.e15. [Google Scholar] [CrossRef]
- Nott, T.; Petsalaki, E.; Farber, P.; Jervis, D.; Fussner, E.; Plochowietz, A.; Craggs, T.; Bazett-Jones, D.P.; Pawson, T.; Forman-Kay, J.D.; et al. Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Mol. Cell 2015, 57, 936–947. [Google Scholar] [CrossRef]
- Ryan, V.; Dignon, G.L.; Zerze, G.H.; Chabata, C.V.; Silva, R.; Conicella, A.E.; Amaya, J.; Burke, K.A.; Mittal, J.; Fawzi, N.L. Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation. Mol. Cell 2018, 69, 465–479.e7. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.H.; Ryu, H.H.; Jun, Y.W.; Liu, T.; Li, Y.; Lim, C.S.; Lee, Y.S.; Kaang, B.K.; Jang, D.J.; Lee, J.A. Sequestration of PRMT1 and Nd1-L mRNA into ALS-linked FUS mutant R521C-positive aggregates contributes to neurite degeneration upon oxidative stress. Sci. Rep. 2017, 7, 40474. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, S.; Summerer, A.K.; Thömmes, C.M.; Pan, X.; Voigt, A.; Schulz, J.B.; Rasse, T.M.; Dormann, D.; Haass, C.; Kahle, P.J. Nuclear import factor transportin and arginine methyltransferase 1 modify FUS neurotoxicity in Drosophila. Neurobiol. Dis. 2015, 74, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.P.; Alves, C.J.; Chadi, G. Early gene expression changes in spinal cord from SOD1G93A Amyotrophic Lateral Sclerosis animal model. Front. Cell. Neurosci. 2013, 7, 216. [Google Scholar] [CrossRef]
- Scekic-Zahirovic, J.; Sendscheid, O.; El Oussini, H.; Jambeau, M.; Sun, Y.; Mersmann, S.; Wagner, M.; Dieterlé, S.; Sinniger, J.; Dirrig-Grosch, S.; et al. Toxic gain of function from mutant FUS protein is crucial to trigger cell autonomous motor neuron loss. EMBO J. 2016, 35, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Litt, M.; Felsenfeld, G. Methylation of histone H4 by arginine methyltransferase PRMT1 is essential in vivo for many subsequent histone modifications. Genes Dev. 2005, 19, 1885–1893. [Google Scholar] [CrossRef]
- Scaglione, A.; Patzig, J.; Liang, J.; Frawley, R.; Bok, J.; Mela, A.; Yattah, C.; Zhang, J.; Teo, S.X.; Zhou, T.; et al. PRMT5-mediated regulation of developmental myelination. Nat. Commun. 2018, 9, 2840. [Google Scholar] [CrossRef]
- Xu, W.; Chen, H.; Du, K.; Asahara, H.; Tini, M.; Emerson, B.M.; Montminy, M.; Evans, R.M. A Transcriptional Switch Mediated by Cofactor Methylation. Science 2001, 294, 2507–2511. [Google Scholar] [CrossRef]
- Brust, J.C. Current Diagnosis & Treatment Neurology, 3rd ed.; McGraw Hill Professional: New York, NY, USA, 2018. [Google Scholar]
- Hauser, S.L.; Goodin, D.S. Multiple Sclerosis and Other Demyelinating Diseases. In Harrison’s Principles of Internal Medicine, 19e; Kasper, D.L., Fauci, A.S., Hauser, S.L., Longo, D.L., Jameson, J.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Heldal, A.T.; Owe, J.F.; Gilhus, N.E.; Romi, F. Seropositive myasthenia gravis: A nationwide epidemiologic study. Neurology 2009, 73, 150–151. [Google Scholar] [CrossRef]
- Webb, L.M.; Amici, S.A.; Jablonski, K.A.; Savardekar, H.; Panfil, A.R.; Li, L.; Zhou, W.; Peine, K.; Karkhanis, V.; Bachelder, E.M.; et al. PRMT5-Selective Inhibitors Suppress Inflammatory T Cell Responses and Experimental Autoimmune Encephalomyelitis. J. Immunol. 2017, 198, 1439–1451. [Google Scholar] [CrossRef]
- Parry, R.V.; Ward, S.G. Protein arginine methylation: A new handle on T lymphocytes? Trends Immunol. 2010, 31, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; West, K.O.; Sanghvi, S.; Laliotis, G.; Agost, L.M.; Lynch, K.W.; Tsichlis, P.N.; Singh, H.; Patrick, K.L.; Guerau-de-Arellano, M. PRMT5 Promotes Symmetric Dimethylation of RNA Processing Proteins and Modulates Activated T Cell Alternative Splicing and Ca2+/NFAT Signaling. Immunohorizons 2021, 5, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.M.; Sengupta, S.; Edell, C.; Piedra-Quintero, Z.L.; Amici, S.A.; Janiret Narvaez, M.; Bevins, M.; Kennemer, A.; Laliotis, G.; Tsichlis, P.N.; et al. Protein arginine methyltransferase 5 promotes cholesterol biosynthesis–mediated Th17 responses and autoimmunity. J. Clin. Investig. 2020, 130, 1683–1698. [Google Scholar] [CrossRef]
- Adachi, H.; Waza, M.; Katsuno, M.; Tanaka, F.; Doyu, M.; Sobue, G. Pathogenesis and molecular targeted therapy of spinal and bulbar muscular atrophy. Neuropathol. Appl. Neurobiol. 2007, 33, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Orr, H.T. Polyglutamine neurodegeneration: Expanded glutamines enhance native functions. Curr. Opin. Genet. Dev. 2012, 22, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzino, C.; Casci, I.; Parodi, S.; Lievens, P.M.; Polanco, M.J.; Milioto, C.; Chivet, M.; Monaghan, J.; Mishra, A.; Badders, N.; et al. Protein Arginine Methyltransferase 6 Enhances Polyglutamine-Expanded Androgen Receptor Function and Toxicity in Spinal and Bulbar Muscular Atrophy. Neuron 2015, 85, 88–100. [Google Scholar] [CrossRef]
- Tibshirani, M.; Tradewell, M.L.; Mattina, K.R.; Minotti, S.; Yang, W.; Zhou, H.; Strong, M.; Hayward, L.J.; Durham, H.D. Cytoplasmic sequestration of FUS/TLS associated with ALS alters histone marks through loss of nuclear protein arginine methyltransferase 1. Hum. Mol. Genet. 2014, 24, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Simandi, Z.; Pajer, K.; Karolyi, K.; Sieler, T.; Jiang, L.-L.; Kolostyak, Z.; Sari, Z.; Fekecs, Z.; Pap, A.; Patsalos, A.; et al. Arginine Methyltransferase PRMT8 Provides Cellular Stress Tolerance in Aging Motoneurons. J. Neurosci. 2018, 38, 7683–7700. [Google Scholar] [CrossRef] [PubMed]
- Coady, T.H.; Lorson, C.L. SMN in spinal muscular atrophy and snRNP biogenesis. Wiley Interdiscip. Rev. RNA 2011, 2, 546–564. [Google Scholar] [CrossRef]
- Côté, J.; Richard, S. Tudor Domains Bind Symmetrical Dimethylated Arginines. J. Biol. Chem. 2005, 280, 28476–28483. [Google Scholar] [CrossRef]
- Cheng, D.; Côté, J.; Shaaban, S.; Bedford, M.T. The Arginine Methyltransferase CARM1 Regulates the Coupling of Transcription and mRNA Processing. Mol. Cell 2007, 25, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, H.; Deschênes-Furry, J.; Boisvenue, S.; Côté, J. KH-type splicing regulatory protein interacts with survival motor neuron protein and is misregulated in spinal muscular atrophy. Hum. Mol. Genet. 2008, 17, 506–524. [Google Scholar] [CrossRef] [PubMed]
- Olaso, R.; Joshi, V.; Fernandez, J.; Roblot, N.; Courageot, S.; Bonnefont, J.P.; Melki, J. Activation of RNA metabolism-related genes in mouse but not human tissues deficient in SMN. Physiol. Genom. 2006, 24, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hubers, L.; Valderrama-Carvajal, H.; LaFramboise, J.; Timbers, J.; Sanchez, G.; Côté, J. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy-like neuronal defects. Hum. Mol. Genet. 2010, 20, 553–579. [Google Scholar] [CrossRef]
- Sanchez, G.; Dury, A.Y.; Murray, L.M.; Biondi, O.; Tadesse, H.; EL Fatimy, R.; Kothary, R.; Charbonnier, F.; Khandjian, E.W.; Côté, J. A novel function for the survival motoneuron protein as a translational regulator. Hum. Mol. Genet. 2012, 22, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Bondy-Chorney, E.; Laframboise, J.; Paris, G.; Didillon, A.; Jasmin, B.J.; Côté, J. A novel role for CARM1 in promoting nonsense-mediated mRNA decay: Potential implications for spinal muscular atrophy. Nucleic Acids Res. 2015, 44, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Branscombe, T.L.; Frankel, A.; Lee, J.H.; Cook, J.R.; Yang, Z.; Pestka, S.; Clarke, S. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J. Biol. Chem. 2001, 276, 32971–32976. [Google Scholar] [CrossRef]
- Boisvert, F.M.; Cote, J.; Boulanger, M.C.; Cleroux, P.; Bachand, F.; Autexier, C.; Richard, S. Symmetrical dimethylarginine methylation is required for the localization of SMN in Cajal bodies and pre-mRNA splicing. J. Cell. Biol. 2002, 159, 957–969. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Gish, G.; Braunschweig, U.; Li, Y.; Ni, Z.; Schmitges, F.W.; Zhong, G.; Liu, K.; Li, W.; Moffat, J.; et al. SMN and symmetric arginine dimethylation of RNA polymerase II C-terminal domain control termination. Nature 2015, 529, 48–53. [Google Scholar] [CrossRef]
- Hirano, M.; Quinzii, C.M.; Mitsumoto, H.; Hays, A.P.; Roberts, J.K.; Richard, P.; Rowland, L.P. Senataxin mutations and amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 12, 223–227. [Google Scholar] [CrossRef]
- Quan, X.; Yue, W.; Luo, Y.; Cao, J.; Wang, H.; Wang, Y.; Lu, Z. The protein arginine methyltransferase PRMT5 regulates Aβ-induced toxicity in human cells and Caenorhabditis elegans models of Alzheimer’s disease. J. Neurochem. 2015, 134, 969–977. [Google Scholar] [CrossRef]
- Li, M.; An, W.; Xu, L.; Lin, Y.; Su, L.; Liu, X. The arginine methyltransferase PRMT5 and PRMT1 distinctly regulate the degradation of anti-apoptotic protein CFLAR(L) in human lung cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 64. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Bonnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Mendell, J.R.; Lloyd-Puryear, M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2013, 48, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.E.C.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Inoue, R.; Miyaka, M.; Kakimoto, Y. Accelerated protein turnover in the skeletal muscle of dystrophic mice. Biochim. et Biophys. Acta Gen. Subj. 1985, 843, 78–82. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Renton, A.E.; Majounie, E.; Waite, A.; Simon-Saánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Freibaum, B.D.; Lu, Y.; Lopez-Gonzalez, R.; Kim, N.C.; Almeida, S.; Lee, K.-H.; Badders, N.; Valentine, M.; Miller, B.L.; Wong, P.C.; et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 2015, 525, 129–133. [Google Scholar] [CrossRef]
- Lee, K.-H.; Zhang, P.; Kim, H.J.; Mitrea, D.M.; Sarkar, M.; Freibaum, B.D.; Cika, J.; Coughlin, M.; Messing, J.; Molliex, A.; et al. C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell 2016, 167, 774–788.e17. [Google Scholar] [CrossRef]
- Premasiri, A.; Gill, A.L.; Vieira, F.G. Type I PRMT Inhibition Protects Against C9ORF72 Arginine-Rich Dipeptide Repeat Toxicity. Front. Pharmacol. 2020, 11, 1404. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Vemulapalli, V.; Patananan, A.N.; Huang, G.L.; Di Lorenzo, A.; Richard, S.; Comb, M.J.; Guo, A.; Clarke, S.G.; Bedford, M.T. Loss of the major Type I arginine methyltransferase PRMT1 causes substrate scavenging by other PRMTs. Sci. Rep. 2013, 3, 1311. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Cho, Y.; Bae, G.-U.; Kim, S.-N.; Kim, Y.K. Protein arginine methyltransferases: Promising targets for cancer therapy. Exp. Mol. Med. 2021, 53, 788–808. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Hardiman, O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013, 9, 617–628. [Google Scholar] [CrossRef] [PubMed]
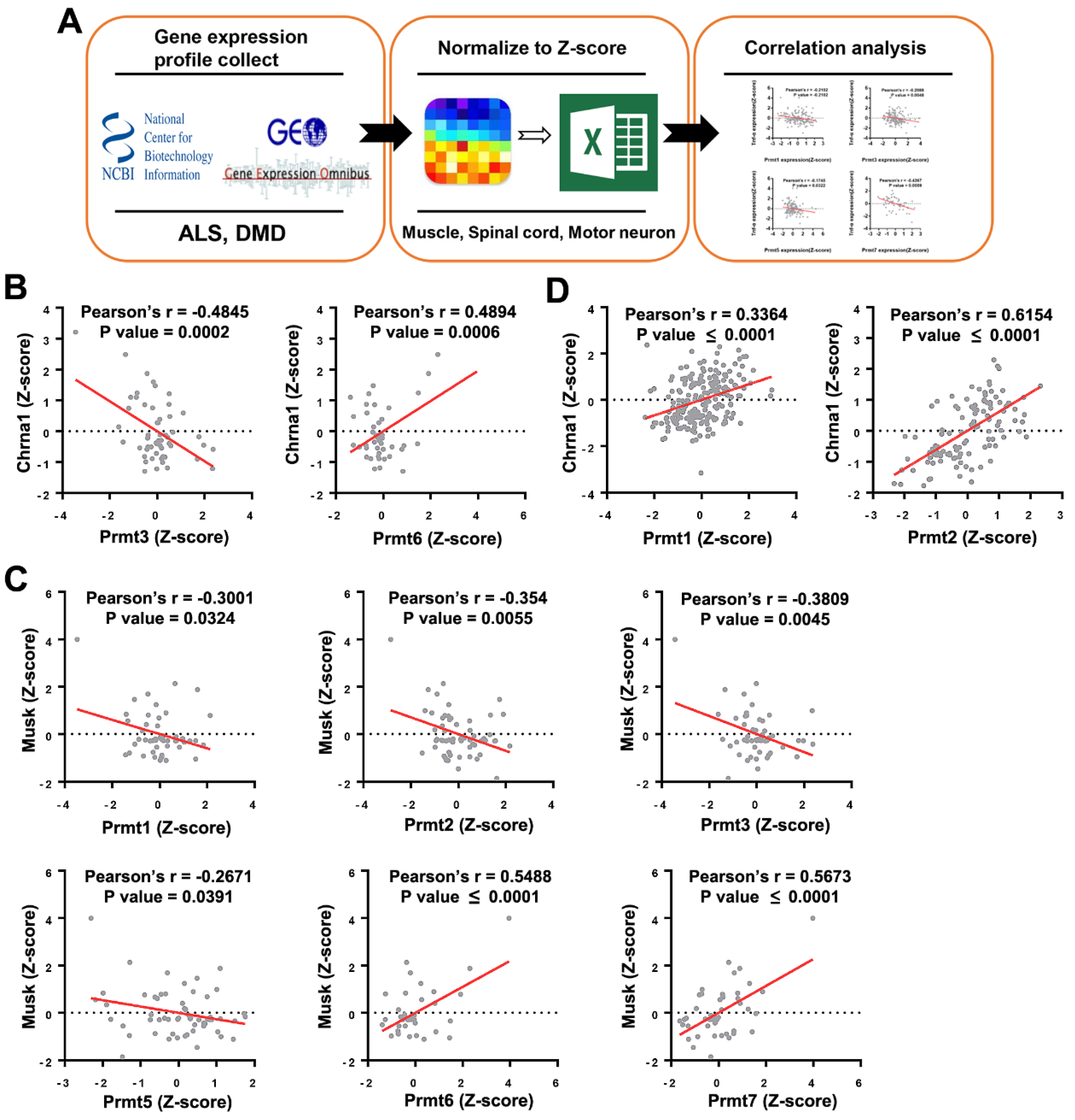
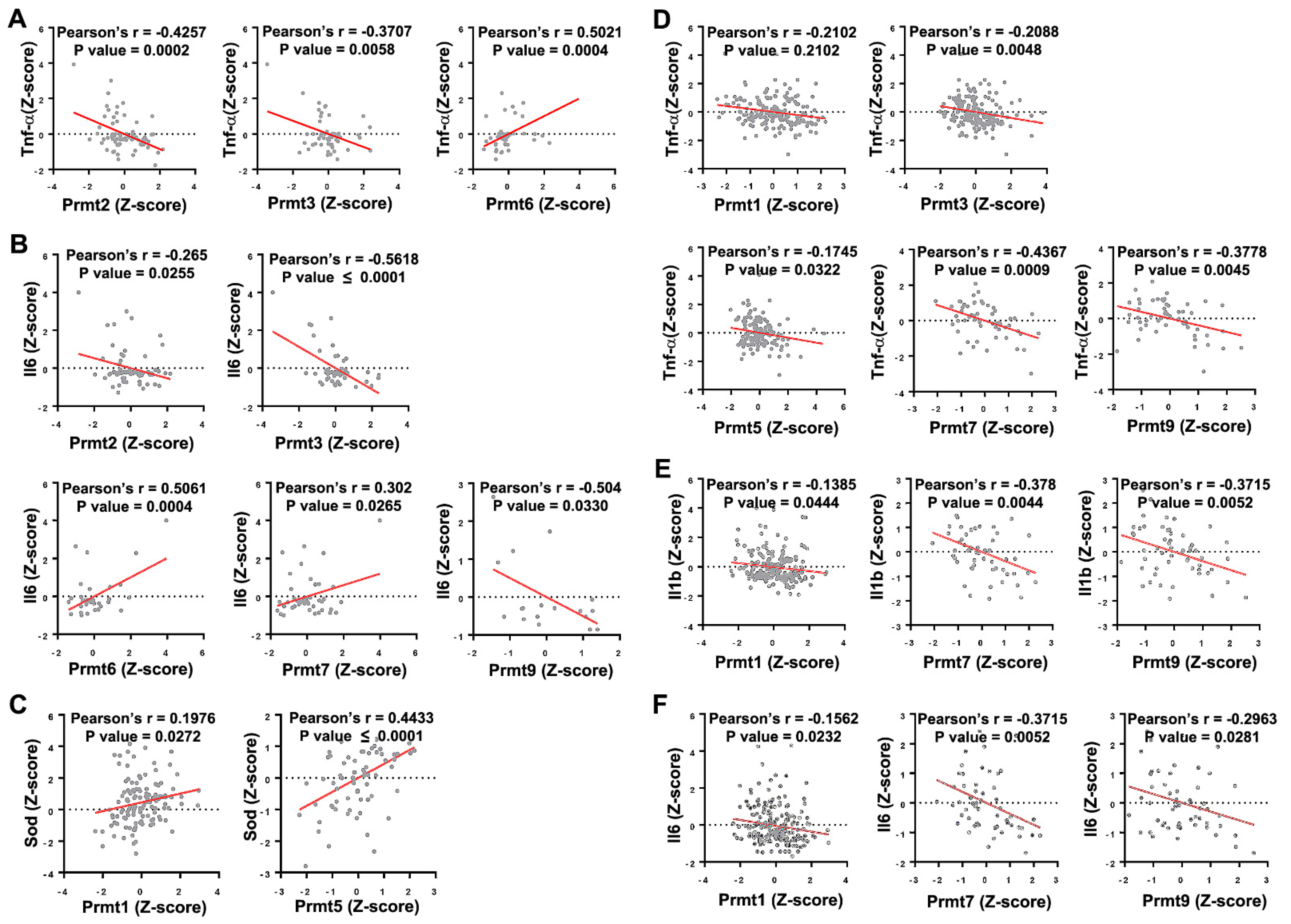
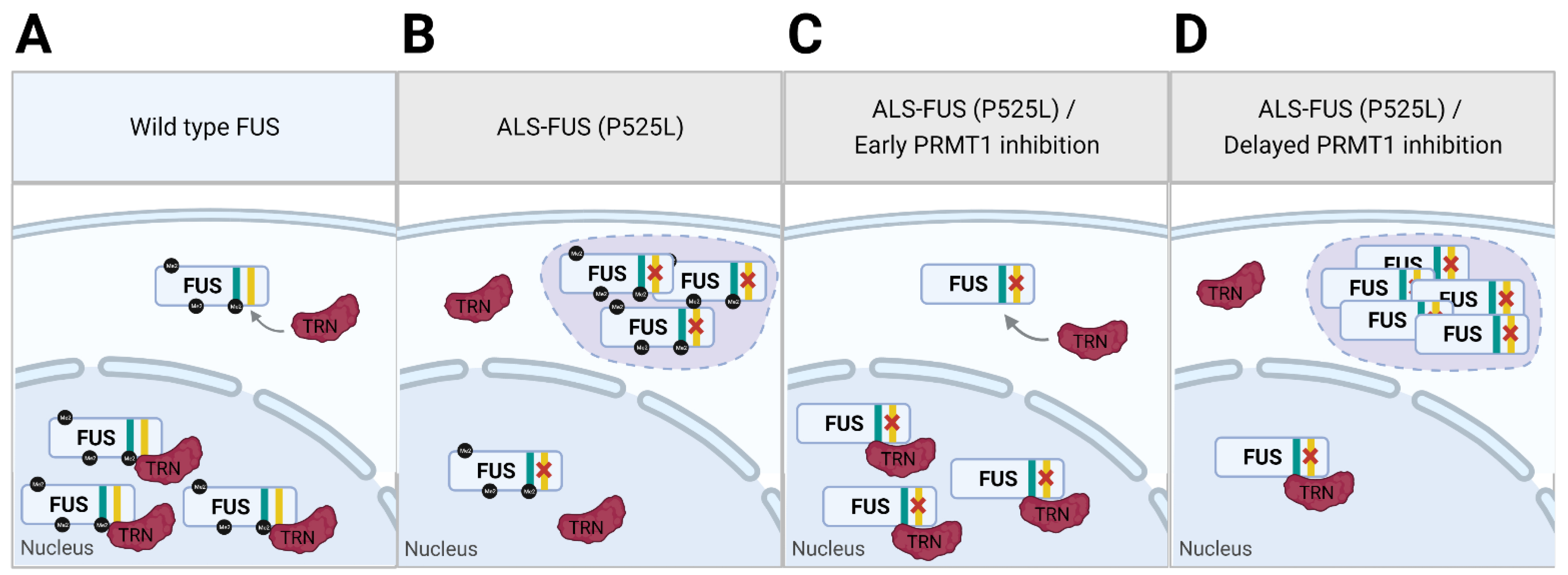
| PRMT | Method | Model | Effect on NMD Phenotype |
|---|---|---|---|
| General methyltransferase inhibitor | AdOx | Hela cells | Rescues nuclear import of FUS mutants (R524S, R522G, R525L) [64] |
| General methyltransferase inhibitor | AdOx | Primary rat hippocampal neurons | Rescues nuclear import of FUS mutant (P525L) [64] |
| General methyltransferase inhibitor | AdOx | Primary motor neurons | Diminishes cytoplasmic FUS mutants (R521H, R521G, R521C) [72] |
| General methyltransferase inhibitor | AdOx | ALS patient-derived lymphoblastoid cells | Rescues nuclear import of FUS mutant (R518G) [73] |
| PRMT1 | siRNA KD | Hela cells | Partial rescue of nuclear import of FUS mutant (P525L) [64] |
| PRMT1 | KO | MEF | Diminishes cytoplasmic FUS mutants (R521H, R521G, R521C) [72] |
| PRMT1 | siRNA KD | HEK293 | Diminishes cytoplasmic FUS mutants (R521H, R521G, R521C) [72] |
| PRMT1 | siRNA KD | Primary motor neurons | Increases cytoplasmic FUS mutants (R521H, R521G, R521C) [72] |
| PRMT1 | Inhibitor (AMI-1) | ALS patient-derived lymphoblastoid cells | Rescues nuclear import of FUS mutant (R518G) [73] |
| PRMT1 | shRNA KD | Cortical neurons | Enhances neurite shortening by FUS-R521C under oxidative stress [82] |
| PRMT1 | Overexpression | Cortical neurons | Prevents neurite shortening by FUS-R521C under oxidative stress [82] |
| PRMT1 | Inhibitor (MS023) | NSC-34 | Abrogates PR15-induced toxicity [123] |
| DART1 (PRMT1/PRMT8 ortholog) | siRNA KD | Drosophila | Enhances neurodegeneration of eyes induced by wild-type FUS or FUS-R521H [73] |
| DART1 (PRMT1/PRMT8 ortholog) | siRNA KD | Drosophila | Enhances neurodegeneration of eyes induced by wild-type FUS or FUS-P525L [83] |
| PRMT5 | Inhibitor (CMP5 or HLCL65) | Mouse memory T cells | Suppresses memory T cell expansion [92] |
| PRMT5 | Inhibitor (CMP5) or shRNA KD | Human memory T cells | Suppresses memory T cell activation and expansion, partly through downregulation of IL-2 [92] |
| PRMT5 | Inhibitor (CMP5) | OVA-induced DTH mouse | Suppresses T cell-mediated inflammatory response [92] |
| PRMT5 | Inhibitor (HLCL65) | MOG-induced EAE mouse | Suppresses clinical signs of EAE through diminishing T cell-mediated inflammatory response [92] |
| PRMT5 | CD4+ T-cell specific KO | MOG-induced EAE mouse | Suppresses clinical signs of EAE through diminishing T cell-mediated inflammatory response [95] |
| PRMT6 | Overexpression | MN-1 | Exacerbates cytotoxicity due to polyglutamine-expanded AR [98] |
| DART8 (PRMT6 ortholog) | RNAi KD | Drosophila | Suppresses neurodegenerative phenotype due to polyglutamine-expanded AR [98] |
| Drug | Description | Phase | Disease | ClnicalTrials.gov Identifier | Study Date |
|---|---|---|---|---|---|
| GSK3326595 | PRMT5 inhibitor | Phase 1 | Solid tumor Lymphoma | NCT02783300 | 30 August 2016–29 April 2025 |
| Phase 1/2 | Neoplasm | NCT03614728 | 16 October 2018–23 April 2025 | ||
| Phase 2 | Breast cancer | NCT04676516 | 21 March 2021–31 December 2022 | ||
| JNJ-64619178 | PRMT5 inhibitor | Phase 1 | Neoplasm | NCT03573310 | 13 July 2018–30 December 2022 |
| PRT543 | PRMT5 inhibitor | Phase 1 | Solid tumor Lymphoma Leukemia | NCT03886831 | 11 February 2019–11 August 2022 |
| PF-06939999 | PRMT5 inhibitor | Phase 1 | Solid tumor | NCT03854227 | 14 March 2019–21 September 2023 |
| PRT811 | PRMT5 inhibitor | Phase 1 | Solid tumor Lymphoma Glioma | NCT04089449 | 6 November 2019–October 2022 |
| AMG 193 | PRMT5 inhibitor | Phase 1/2 | Solid tumor | NCT05094336 | 30 December 2021–13 November 2024 |
| GSK3368715 | Type I PRMT inhibitor | Phase 1 | Neoplasm | NCT03666988 | 22 October 2018–4 March 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; An, S.; Lee, S.-J.; Kang, J.-S. Protein Arginine Methyltransferases in Neuromuscular Function and Diseases. Cells 2022, 11, 364. https://doi.org/10.3390/cells11030364
Lee J, An S, Lee S-J, Kang J-S. Protein Arginine Methyltransferases in Neuromuscular Function and Diseases. Cells. 2022; 11(3):364. https://doi.org/10.3390/cells11030364
Chicago/Turabian StyleLee, Jinwoo, Subin An, Sang-Jin Lee, and Jong-Sun Kang. 2022. "Protein Arginine Methyltransferases in Neuromuscular Function and Diseases" Cells 11, no. 3: 364. https://doi.org/10.3390/cells11030364
APA StyleLee, J., An, S., Lee, S.-J., & Kang, J.-S. (2022). Protein Arginine Methyltransferases in Neuromuscular Function and Diseases. Cells, 11(3), 364. https://doi.org/10.3390/cells11030364






