Interleukine-17 Modulates Neurogenesis and Behavior Following Exposure to Trauma in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Inescapable Electric Foot Shock
2.3. Behavioral Assays
2.3.1. Freezing Test
2.3.2. Elevated Plus Maze
2.3.3. Open Field Test
2.3.4. Social Interaction
2.4. IL-17A and IL-17F ELISA
2.5. Immunohistochemistry Staining
2.6. Real-Time PCR Array for Neurogenesis-Related Genes
2.7. Statistical Analysis
3. Results
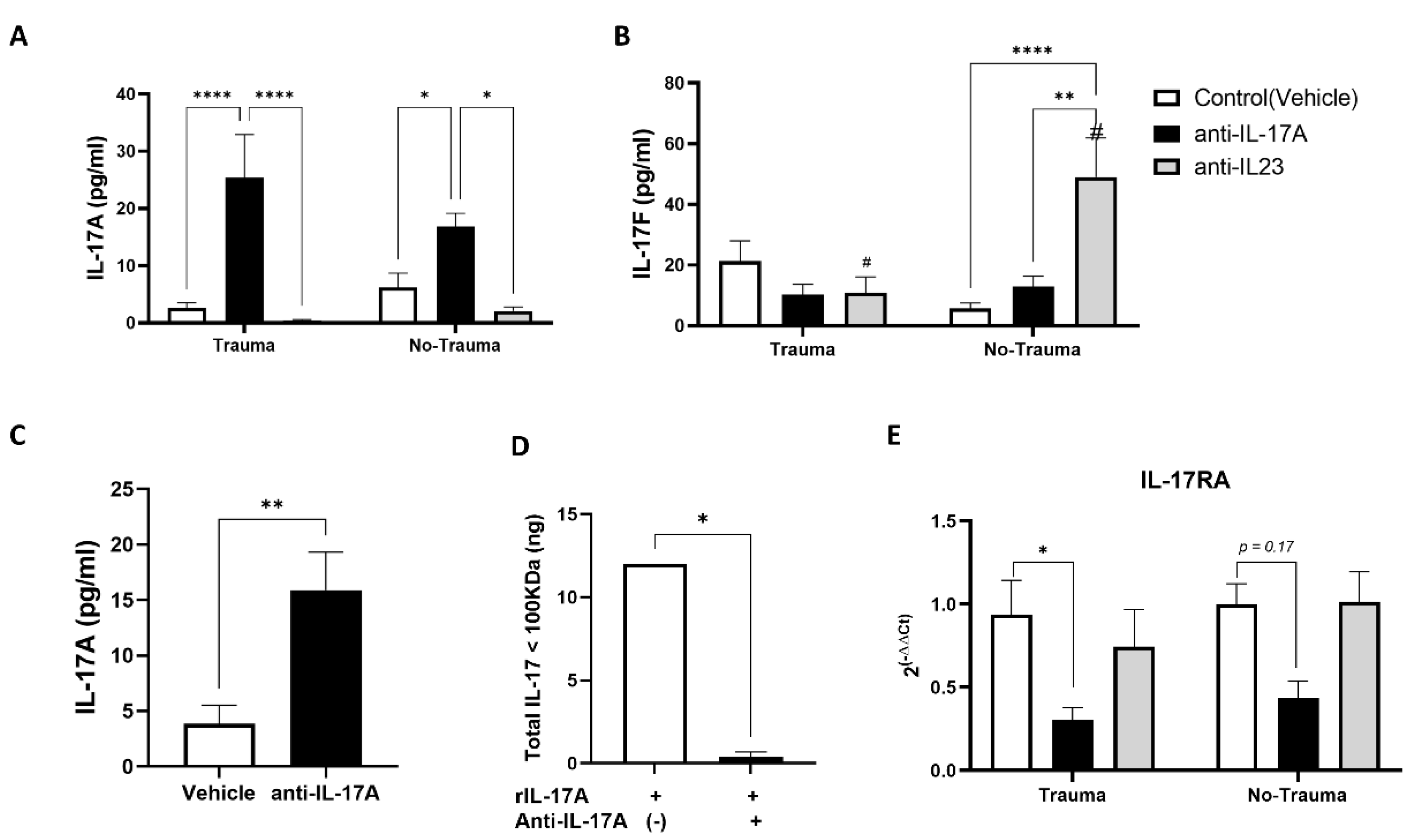
3.1. Long Term Changes in Serum Levels of IL-17A and IL-17F Following Exposure to Anti-IL-17A and Anti-IL-23
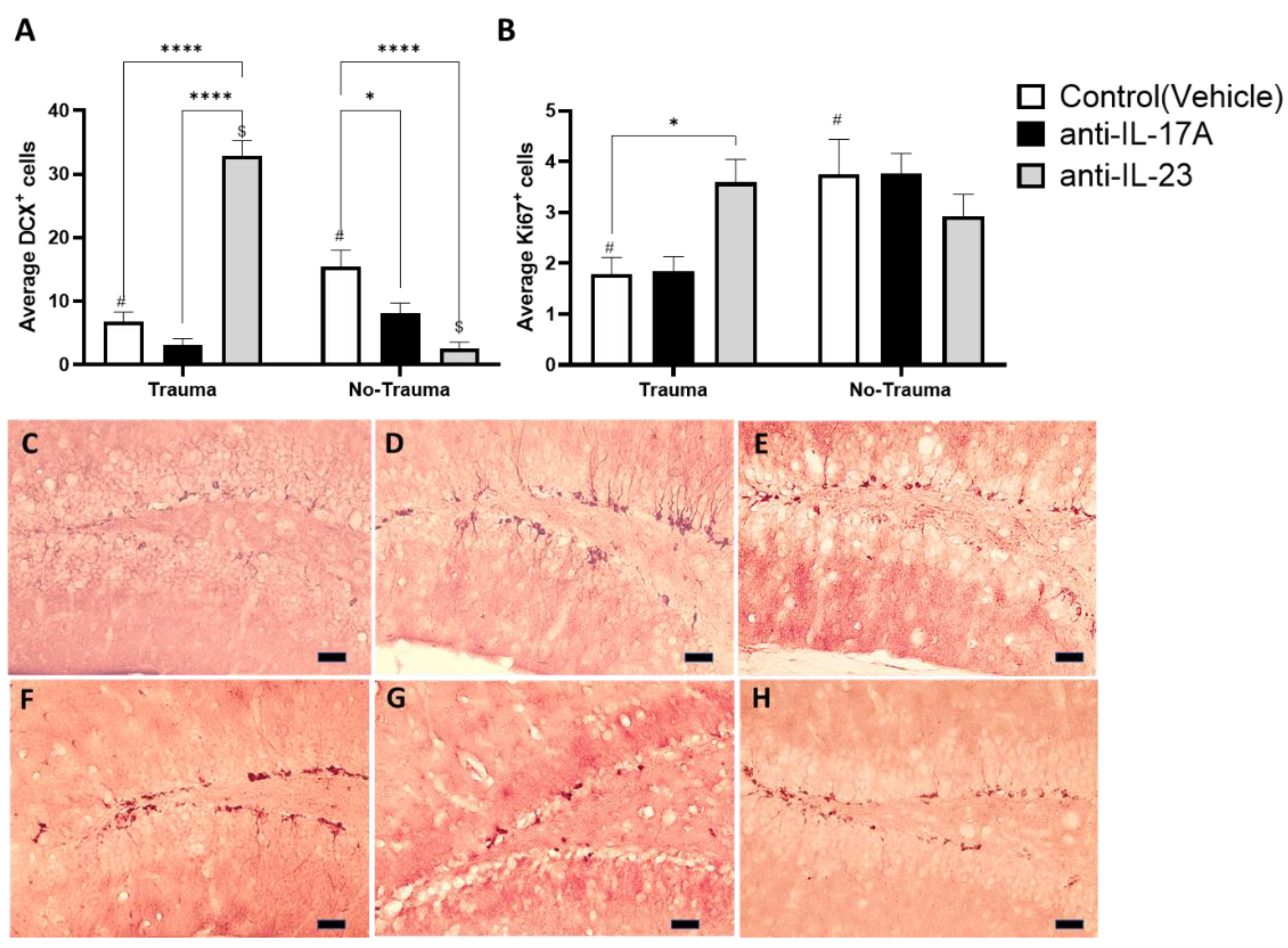
3.2. Trauma Exposure and Antibody Treatment Altered Hippocampal Neurogenesis
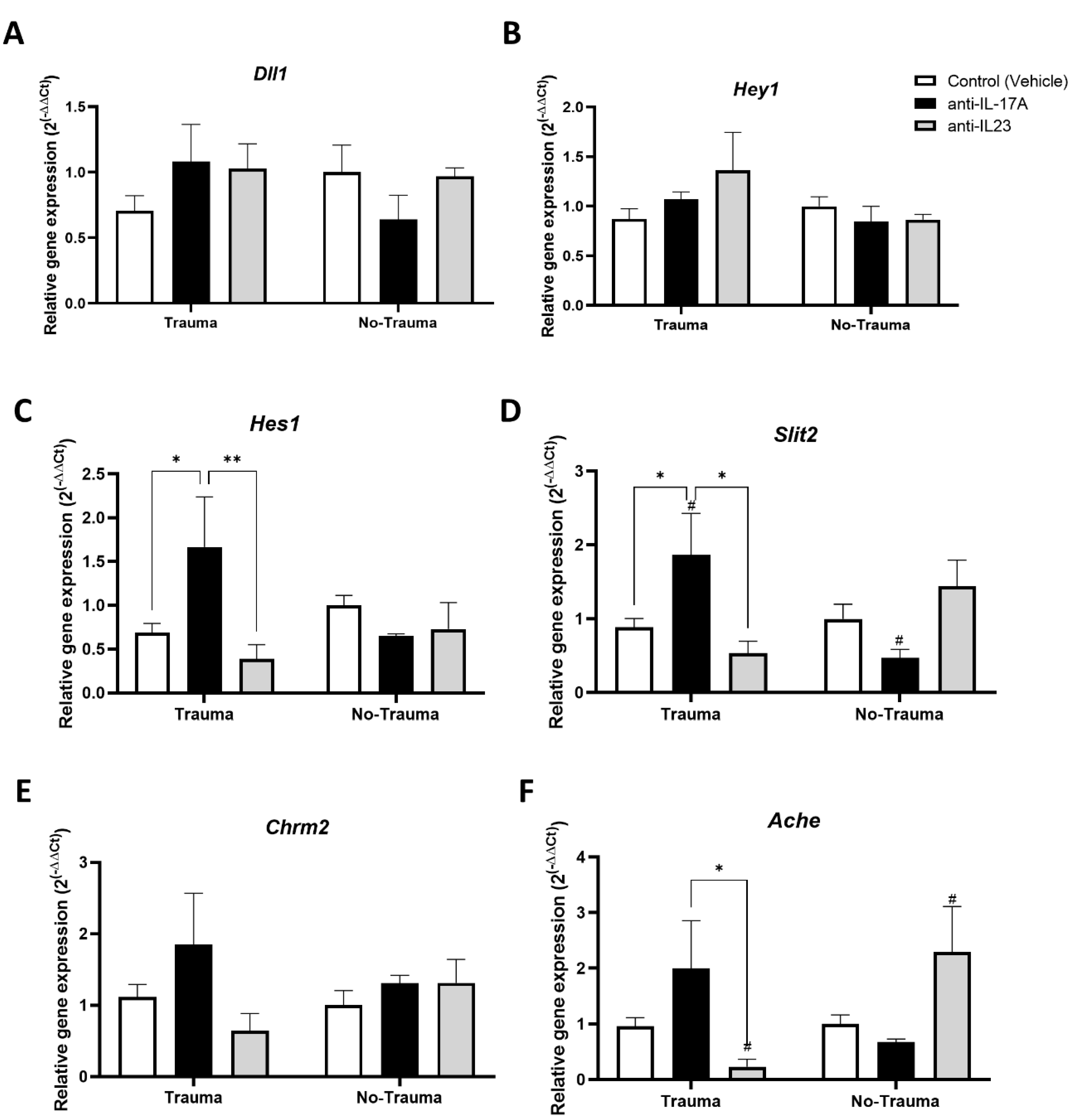
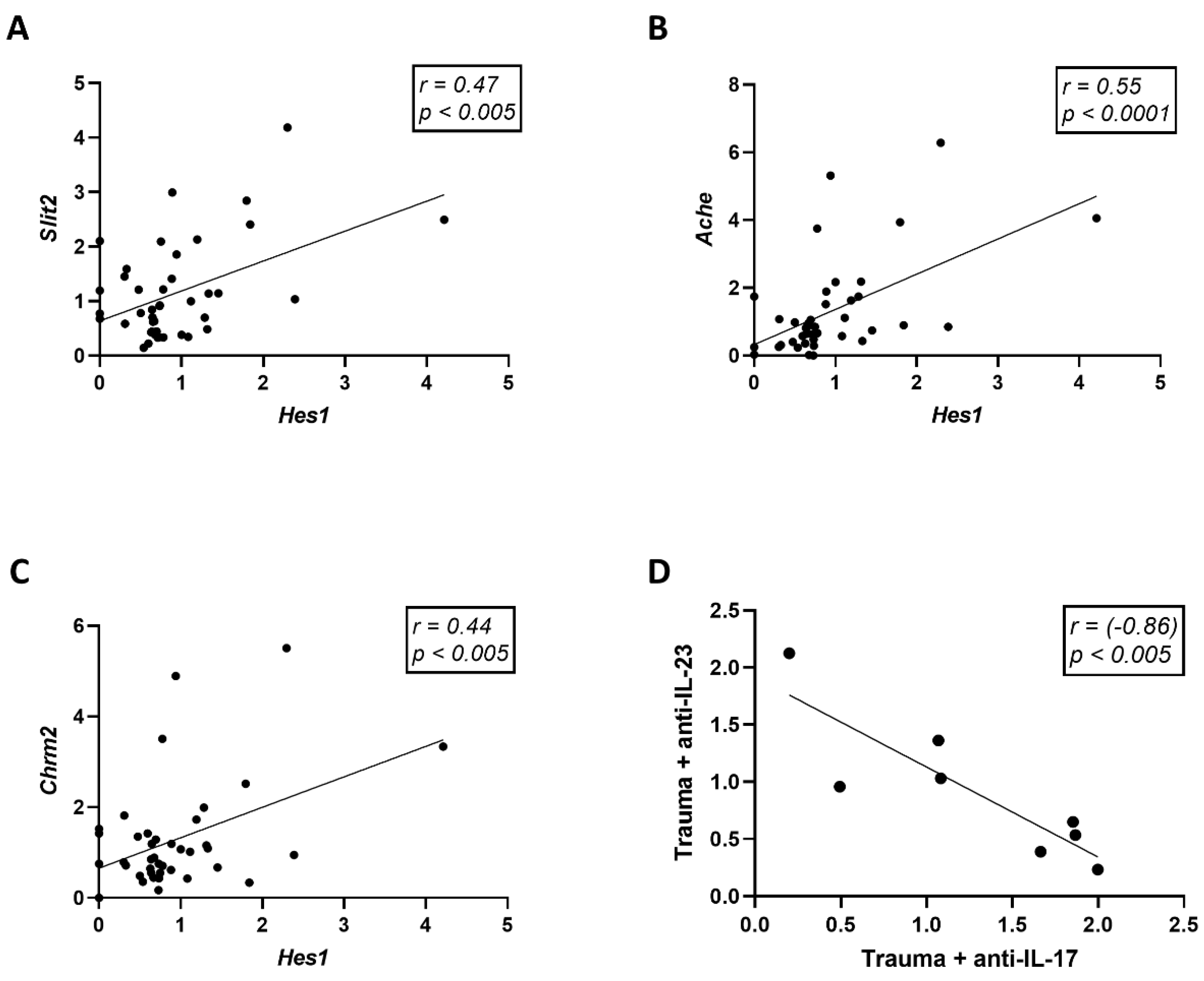
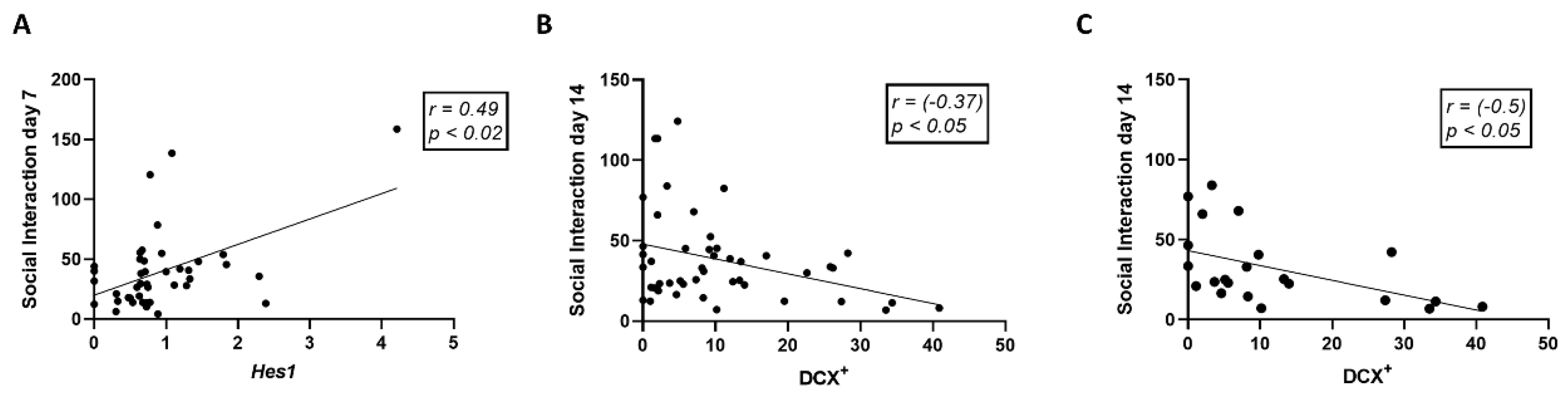
3.3. Trauma Exposure and Antibody Treatment Altered the Expression of Neurogenesis-Related Genes in the Hippocampus
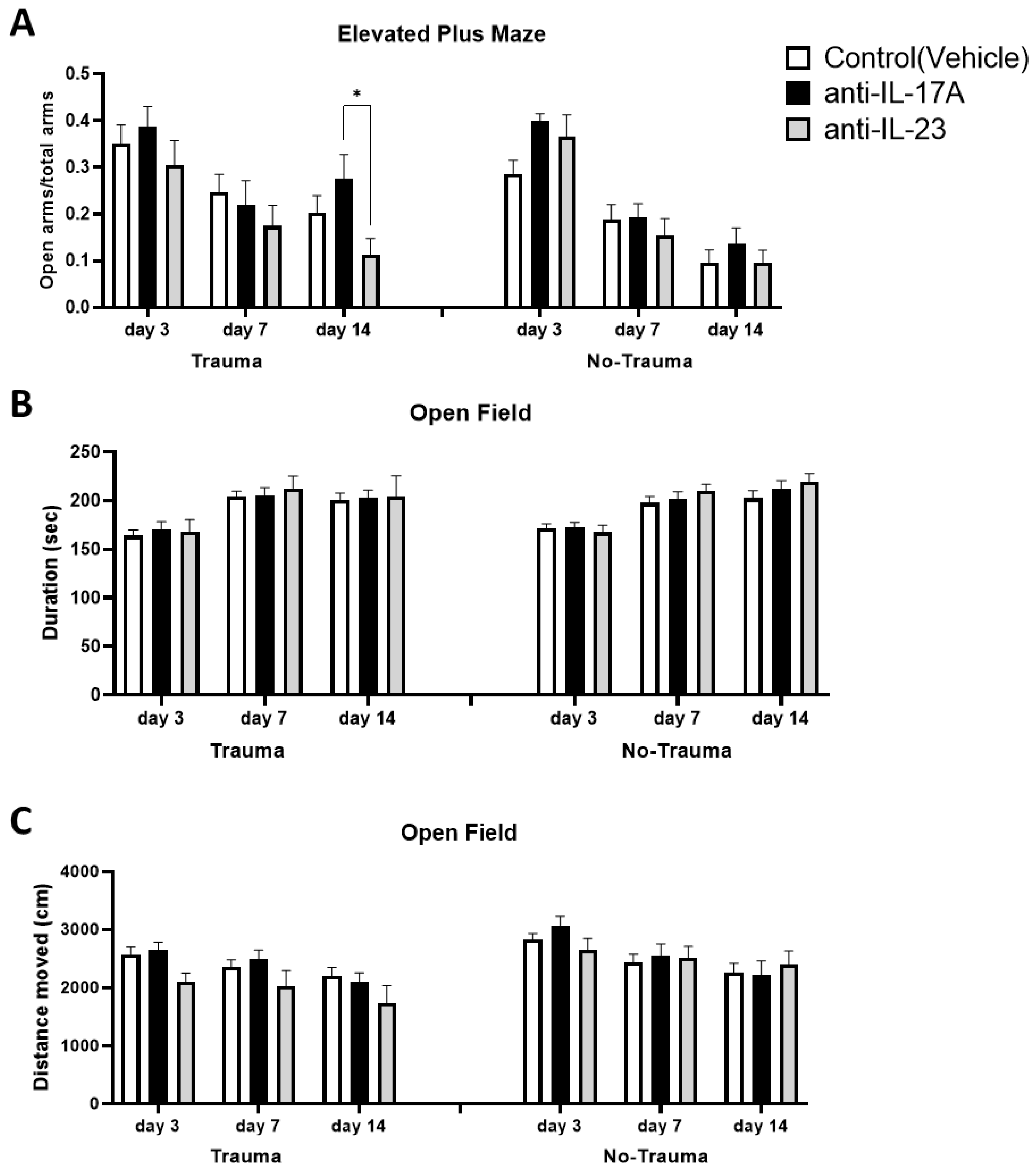
3.4. Trauma Exposure and Antibody Treatment Does Not Affect General Locomotion and Anxiety
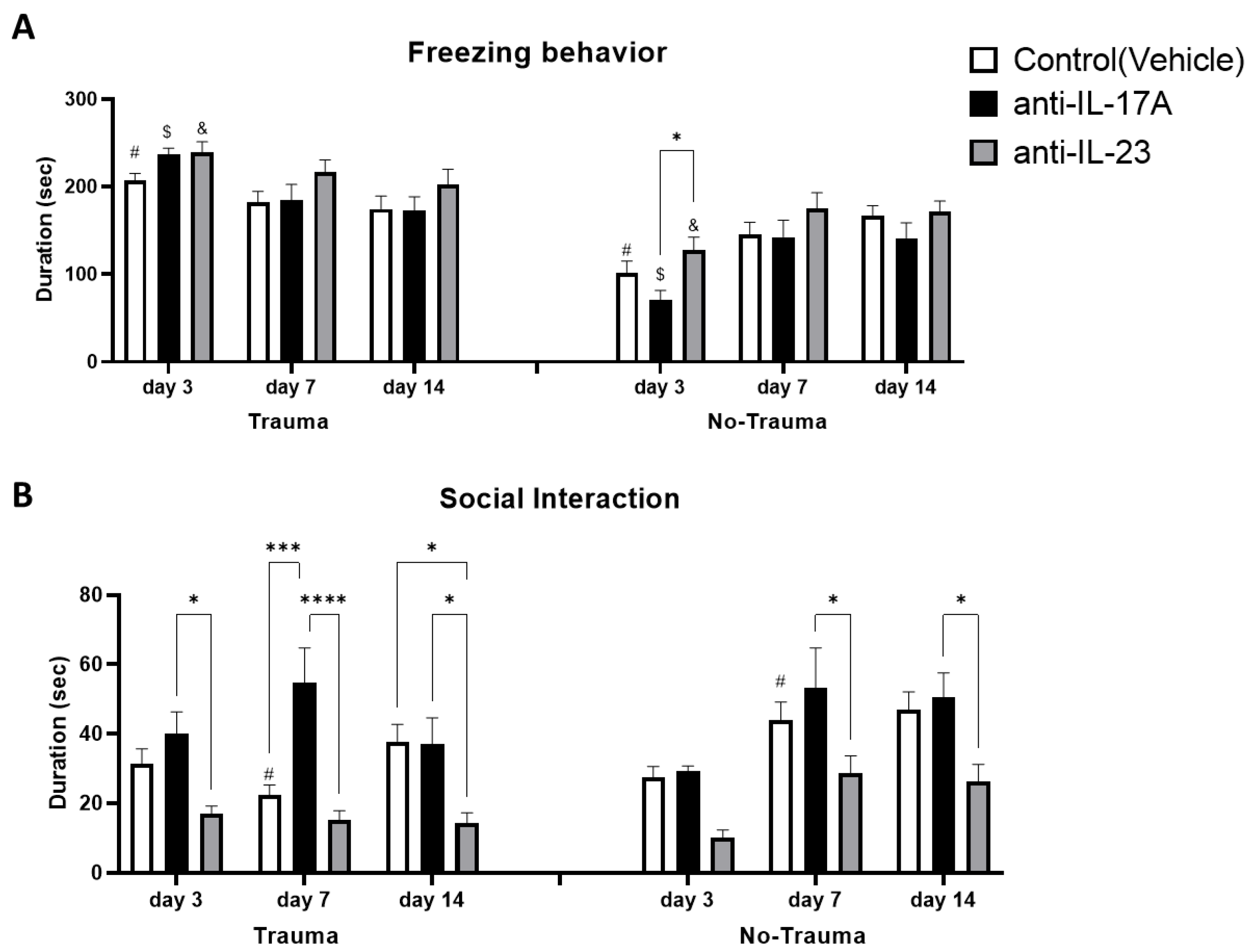
3.5. Trauma Exposure Increased Trauma Related Anxiety
3.6. Treatment with Anti-IL-17A Prevented Trauma-Induced Social Behavior Deficits While Anti-IL-23 Exacerbated Social Deficits
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Björklund, A.; Lindvall, O. Self-repair in the brain. Nature 2000, 405, 892–893. [Google Scholar] [CrossRef]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.-I.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of Hippocampal Pyramidal Neurons after Ischemic Brain Injury by Recruitment of Endogenous Neural Progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Temple, S. The development of neural stem cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Understanding the Real State of Human Adult Hippocampal Neurogenesis from Studies of Rodents and Non-human Primates. Front. Neurosci. 2020, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Kang, E.; Liu, C.Y.; Ming, G.-L.; Song, H. Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 2008, 18, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell. Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef]
- Berger, T.; Lee, H.; Young, A.; Aarsland, D.; Thuret, S. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer’s Disease. Trends Mol. Med. 2020, 26, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Liu, H.; Liu, Y. Adult Neurogenesis Following Ischemic Stroke and Implications for Cell-Based Therapeutic Approaches. World Neurosurg. 2020, 138, 474–480. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Fitzsimons, C.P.; Salta, E.; Maletic-Savatic, M. Adult neurogenesis, human after all (again): Classic, optimized, and future approaches. Behav. Brain Res. 2020, 381, 112458. [Google Scholar] [CrossRef]
- Tanaka, M.; Li, H.; Zhang, X.; Singh, J.; Dalgard, C.L.; Wilkerson, M.; Zhang, Y. Region- and time-dependent gene regulation in the amygdala and anterior cingulate cortex of a PTSD-like mouse model. Mol. Brain 2019, 12, 25. [Google Scholar] [CrossRef]
- Egoswami, S.; Erodríguez-Sierra, O.; Ecascardi, M.; Epare, D. Animal models of post-traumatic stress disorder: Face validity. Front. Neurosci. 2013, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Samuelson, K.W. Post-traumatic stress disorder and declarative memory functioning: A review. Dialogues Clin. Neurosci. 2011, 13, 346–351. [Google Scholar] [PubMed]
- Bremner, J.D. The Relationship Between Cognitive and Brain Changes in Posttraumatic Stress Disorder. Ann. N. Y. Acad. Sci. 2006, 1071, 80–86. [Google Scholar] [CrossRef]
- Kikuchi, A.; Shimizu, K.; Nibuya, M.; Hiramoto, T.; Kanda, Y.; Tanaka, T.; Watanabe, Y.; Takahashi, Y.; Nomura, S. Relationship between post-traumatic stress disorder-like behavior and reduction of hippocampal 5-bromo-2′-deoxyuridine-positive cells after inescapable shock in rats. Psychiatry Clin. Neurosci. 2008, 62, 713–720. [Google Scholar] [CrossRef]
- Ishikawa, R.; Fukushima, H.; Frankland, P.W.; Kida, S. Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. eLife 2016, 5, e17464. [Google Scholar] [CrossRef]
- Kheirbek, M.; Klemenhagen, K.C.; Sahay, A.; Hen, R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012, 15, 1613–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donovan, A.; Chao, L.L.; Paulson, J.; Samuelson, K.W.; Shigenaga, J.K.; Grunfeld, C.; Weiner, M.W.; Neylan, T.C. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology 2015, 51, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Nagarkatti, P.; Zhong, Y.; Ginsberg, J.P.; Singh, N.P.; Zhang, J.; Nagarkatti, M. Dysregulation in microRNA Expression Is Associated with Alterations in Immune Functions in Combat Veterans with Post-Traumatic Stress Disorder. PLoS ONE 2014, 9, e94075. [Google Scholar] [CrossRef]
- Moshfegh, C.; Elkhatib, S.K.; Collins, C.W.; Kohl, A.J.; Case, A.J. Autonomic and Redox Imbalance Correlates With T-Lymphocyte Inflammation in a Model of Chronic Social Defeat Stress. Front. Behav. Neurosci. 2019, 13, 103. [Google Scholar] [CrossRef] [Green Version]
- Ojo, J.O.; Greenberg, M.B.; Leary, P.; Mouzon, B.; Bachmeier, C.; Mullan, M.; Diamond, D.M.; Crawford, F. Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front. Behav. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Wacleche, V.S.; Landay, A.; Routy, J.-P.; Ancuta, P. The Th17 Lineage: From Barrier Surfaces Homeostasis to Autoimmunity, Cancer, and HIV-1 Pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef]
- Tfilin, M.; Turgeman, G. Interleukine-17 Administration Modulates Adult Hippocampal Neurogenesis and Improves Spatial Learning in Mice. J. Mol. Neurosci. 2019, 69, 254–263. [Google Scholar] [CrossRef]
- Ramikie, T.S.; Ressler, K.J. Mechanisms of Sex Differences in Fear and Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, J.; Toth, M.; Der-Avakian, A.; Risbrough, V.B. Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol. Psychiatry 2018, 83, 895–907. [Google Scholar] [CrossRef]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent models of post-traumatic stress disorder: Behavioral assessment. Transl. Psychiatry 2020, 10, 1–28. [Google Scholar] [CrossRef]
- Wilson, R.; Cohen, J.M.; Jose, R.J.P.; De Vogel, C.; Baxendale, H.; Brown, J.S. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2014, 8, 627–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klune, J.R.; Bartels, C.; Luo, J.; Yokota, S.; Du, Q.; Geller, D.A. IL-23 mediates murine liver transplantation ischemia-reperfusion injury via IFN-γ/IRF-1 pathway. Am. J. Physiol. Liver Physiol. 2018, 315, G991–G1002. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Murakami, Y.; Wang, M.; Maeda, K.; Matsumoto, K. The effects of chronic valproate and diazepam in a mouse model of posttraumatic stress disorder. Pharmacol. Biochem. Behav. 2006, 85, 324–331. [Google Scholar] [CrossRef]
- Qiu, Z.-K.; Zhang, L.-M.; Zhao, N.; Chen, H.-X.; Zhang, Y.-Z.; Liu, Y.-Q.; Mi, T.-Y.; Zhou, W.-W.; Li, Y.; Yang, R.; et al. Repeated administration of AC-5216, a ligand for the 18kDa translocator protein, improves behavioral deficits in a mouse model of post-traumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 40–46. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immun. 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sueda, R.; Kageyama, R. Regulation of active and quiescent somatic stem cells by Notch signaling. Dev. Growth Differ. 2019, 62, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Xin, W.; He, P.; Turner, D.; Yin, J.; Gan, Y.; Shi, F.-D.; Wu, J. Interleukin-17 inhibits Adult Hippocampal Neurogenesis. Sci. Rep. 2014, 4, 7554. [Google Scholar] [CrossRef] [Green Version]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinna, S.; Strober, B. Anti-interleukin-17 treatment of psoriasis. J. Dermatol. Treat. 2015, 27, 1–5. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Madden, K.B.; Morris, S.C.; Holmes, J.M.; Boiani, N.; Katona, I.M.; Maliszewski, C.R. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 1993, 151, 1235. [Google Scholar]
- Stein, M.L.; Villanueva, J.M.; Buckmeier, B.K.; Yamada, Y.; Filipovich, A.H.; Assa’Ad, A.H.; Rothenberg, M.E. Anti–IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J. Allergy Clin. Immunol. 2008, 121, 1473–1483.e4. [Google Scholar] [CrossRef] [Green Version]
- Hymowitz, S.G.; Filvaroff, E.H.; Yin, J.; Lee, J.; Cai, L.; Risser, P.; Maruoka, M.; Mao, W.; Foster, J.; Kelley, R.F.; et al. IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001, 20, 5332–5341. [Google Scholar] [CrossRef]
- Chong, W.P.; Mattapallil, M.J.; Raychaudhuri, K.; Bing, S.J.; Wu, S.; Zhong, Y.; Wang, W.; Chen, Z.; Silver, P.B.; Jittayasothorn, Y.; et al. The Cytokine IL-17A Limits Th17 Pathogenicity via a Negative Feedback Loop Driven by Autocrine Induction of IL-24. Immunity 2020, 53, 384–397.e5. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, M.; Yang, S.; Choi, C.W.; Lee, K.S.; Youn, S.W. Persistent expression of interleukin-17 and downstream effector cytokines in recalcitrant psoriatic lesions after ustekinumab treatment. J. Dermatol. 2021, 48, 876–882. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Wang, W.; Chen, Z.; Wang, S.; Li, J.; Li, G.; Gao, C.; Sun, X. Astragaloside IV Exerts Cognitive Benefits and Promotes Hippocampal Neurogenesis in Stroke Mice by Downregulating Interleukin-17 Expression via Wnt Pathway. Front. Pharmacol. 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Han, R.; Guo, F.; Chen, H.; Wang, W.; Chen, Z.; Liu, W.; Sun, X.; Gao, C. Antagonistic effects of IL-17 and Astragaloside IV on cortical neurogenesis and cognitive behavior after stroke in adult mice through Akt/GSK-3β pathway. Cell Death Discov. 2020, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xue, R.; Zhang, X.; Chen, S.; Wan, Y.; Wu, W. Sleep deprivation inhibits proliferation of adult hippocampal neural progenitor cells by a mechanism involving IL-17 and p38 MAPK. Brain Res. 2019, 1714, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, R.; Zhang, Q.; Li, J.; Kang, X.; Wang, H.; Huan, L.; Zhang, L.; Li, F.; Yang, S.; et al. Hes1, a Notch signaling downstream target, regulates adult hippocampal neurogenesis following traumatic brain injury. Brain Res. 2014, 1583, 65–78. [Google Scholar] [CrossRef]
- Keohane, A.; Ryan, S.; Maloney, E.; Sullivan, A.; Nolan, Y.M. Tumour necrosis factor-α impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol. Cell. Neurosci. 2010, 43, 127–135. [Google Scholar] [CrossRef]
- Gobshtis, N.; Tfilin, M.; Fraifeld, V.E.; Turgeman, G. Transplantation of mesenchymal stem cells causes long-term alleviation of schizophrenia-like behaviour coupled with increased neurogenesis. Mol. Psychiatry 2019, 26, 4448–4463. [Google Scholar] [CrossRef]
- Borovcanin, M.M.; Janicijevic, S.M.; Jovanovic, I.P.; Gajovic, N.M.; Jurisevic, M.M.; Arsenijevic, N.N. Type 17 Immune Response Facilitates Progression of Inflammation and Correlates with Cognition in Stable Schizophrenia. Diagnostics 2020, 10, 926. [Google Scholar] [CrossRef]
- Reed, M.D.; Yim, Y.S.; Wimmer, R.D.; Kim, H.; Ryu, C.; Welch, G.M.; Andina, M.; King, H.O.; Waisman, A.; Halassa, M.M.; et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 2020, 577, 249–253. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Jiang, R.; Hong, X.; Peng, J.; Chen, W.; Jiang, J.; Li, J.; Huang, D.; Dai, H.; et al. Interleukin-17 activates and synergizes with the notch signaling pathway in the progression of pancreatic ductal adenocarcinoma. Cancer Lett. 2021, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Yoshihara, T.; Ohtsuka, T.; Kageyama, R. Hes1 expression in mature neurons in the adult mouse brain is required for normal behaviors. Sci. Rep. 2019, 9, 8251. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence | |
|---|---|---|
| Forward | Reverse | |
| Hprt | TGTTGTTGGATATGCCCTTG | TTGCGCTCATCTTAGGCTTT |
| Hes1 | CCAGCCAGTGTCAACACGA | AATGCCGGGAGCTATCTTTCT |
| Chrm2 | CTGAAGGTGGCGGTTGACTT | TGGTTTGGCTATTACCAGTCCT |
| Dll1 | TCATCACACCCTGGCAGACAGAT | ACGGAGAAGGTTGCTCTGTGTC |
| Ache | GAAGGCCGAGTTCCAC | GGCTCGGTCGTATTATATCC |
| Hey1 | CCAGCCAGTGTCAACACGA | AATGCCGGGAGCTATCTTTCT |
| Slit2 | AGGGAAGATGAGTGGCATTG | GTGCCTGAGACCAGCAAAAT |
| Il-17ra | GGACTGTGTGAACCGCTCTC | CCTGTGAAGTCAGTGGGAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willinger, Y.; Turgeman, G. Interleukine-17 Modulates Neurogenesis and Behavior Following Exposure to Trauma in Mice. Cells 2022, 11, 343. https://doi.org/10.3390/cells11030343
Willinger Y, Turgeman G. Interleukine-17 Modulates Neurogenesis and Behavior Following Exposure to Trauma in Mice. Cells. 2022; 11(3):343. https://doi.org/10.3390/cells11030343
Chicago/Turabian StyleWillinger, Yehoshua, and Gadi Turgeman. 2022. "Interleukine-17 Modulates Neurogenesis and Behavior Following Exposure to Trauma in Mice" Cells 11, no. 3: 343. https://doi.org/10.3390/cells11030343
APA StyleWillinger, Y., & Turgeman, G. (2022). Interleukine-17 Modulates Neurogenesis and Behavior Following Exposure to Trauma in Mice. Cells, 11(3), 343. https://doi.org/10.3390/cells11030343







